Abstract
Elucidating factors regulating Crohn's disease-associated nucleotide-binding oligomerization domain 2 (Nod2) responses is critical to understanding the mechanisms of intestinal immune homeostasis. Stimulation of primary monocyte-derived macrophages by muramyl dipeptide (MDP), a component of bacterial peptidoglycan and specific Nod2 ligand, produces cytokines, including IL-1β. We found that IL-1β blockade profoundly inhibits MDP-induced cytokine production in human monocyte-derived macrophages, demonstrating a key role for IL-1β autocrine secretion in Nod2-mediated responses. Importantly, although MAPK activation has previously been attributed directly to Nod2 signaling, we determined that the IL-1β autocrine loop is responsible for the majority of MDP-induced MAPK activation. Because the critical effects of IL-1β autocrine secretion on MAPK activation are observed as early as 10 min after Nod2 stimulation, we hypothesized that secretion of IL-1β from preexisting intracellular pro-IL-1β stores is necessary for optimal MDP-mediated cytokine induction. Consistently, we detected IL-1β secretion within 10 min of MDP treatment. Moreover, caspase-1 inhibition significantly attenuates MDP-mediated early MAPK activation. Importantly, selective JNK/p38 activation is sufficient to rescue the decreased cytokine secretion during Nod2 stimulation in the absence of autocrine IL-1β. Finally, we found that the IL-1β autocrine loop significantly enhances responses by a broad range of pattern recognition receptors. Taken together, MDP stimulation activates Nod2 to process and release preexisting pro-IL-1β stores in a caspase-1-dependent fashion; this secreted IL-1β, in turn, contributes to the majority of MDP-initiated MAPK activation and leads to subsequent cytokine secretion. Our findings clarify mechanisms of IL-1β contributions to Nod2 responses and elucidate the dominant role of IL-1β in MDP-initiated MAPK and cytokine secretion.
Keywords: Cytokine, Inflammation, Macrophages, MAP Kinases (MAPKs), Pattern Recognition Receptor, Human
Introduction
Crohn's disease (CD)2 is an inflammatory bowel disease characterized by dysregulated intestinal immune homeostasis (1). Of the genes identified to date, loss-of-function polymorphisms in nucleotide oligomerization domain 2 (Nod2) are associated with the highest genetic risk for developing CD (1). The mechanisms regulating Nod2 are, however, not fully understood. Muramyl dipeptide (MDP) is the minimal active bacterial peptidoglycan component that specifically stimulates Nod2 (1). MDP treatment activates NF-κB and MAPK pathways and induces cytokines, including IL-1β, in multiple human and mouse cell populations, including myeloid-derived cells (1–4). Autocrine IL-1β is implicated in amplifying Toll-like receptor (TLR) cytokine induction, in particular by TLR4 (5–7) and TLR9 (5, 6, 8, 9), however, the mechanism of this contribution is not well understood. The few reports addressing the IL-1β autocrine loop in Nod2 signaling have yielded mixed results. Mouse studies demonstrate that Nod2-induced IL-1β is required in in vivo mouse models for optimal IL-6 induction (10), LPS response enhancement (11), and Nod2-mediated peritonitis, although not Nod2-mediated uveitis (12). Studies in human monocytes find that IL-1β autocrine secretion is not required for Nod2/TLR4 synergy (13) but is needed for IL-32/Nod2 synergy (14). Thus far, the mechanism(s) through which the IL-1β autocrine loop contributes to Nod2 responses is not known.
The Nod2-induced IL-1β autocrine loop is of particular interest because the IL-1β pathway is relevant to inflammatory bowel disease as evidenced by: (i) CD association of loss-of-function polymorphisms in a region that includes NLRP3, a protein required for IL-1β processing (15), and (ii) association of decreased IL-1 receptor (IL-1R) signaling with more severe colitis in mouse and human studies (9, 16, 17). Given the importance of both Nod2 and IL-1R regulation for intestinal immune homeostasis (18) and the putative IL-1β contribution to Nod2-induced cytokine secretion, we sought to further define this contribution and determine the mechanism(s) of the IL-1β autocrine loop contribution to Nod2 responses. Because Nod2 and IL-1R are relevant to human diseases and because there are significant differences in Nod2-mediated cytokine induction between mouse and human myeloid cells (2, 18–25), we utilized primary human monocyte-derived macrophages (MDMs). We found that the IL-1β autocrine loop is a major contributor to MDP-mediated secretion of both proinflammatory and antiinflammatory cytokines. Importantly, in the absence of the IL-1β autocrine loop, MDP stimulation of MDMs results in minimal activation of MAPK pathways. We found that Nod2 activation leads to rapid caspase-1-dependent secretion of IL-1β and subsequent early MAPK activation and secretion of additional cytokines. Consistent with this, activation of JNK and p38 was sufficient to partially restore cytokine secretion by Nod2 signaling in the absence of the IL-1β loop. These results establish a dominant role for the IL-1β autocrine loop in PRR responses and define mechanisms for how this autocrine loop modifies and amplifies Nod2 signaling.
EXPERIMENTAL PROCEDURES
Patient Recruitment and Genotyping
Informed consent was obtained per protocol approved by the Yale University institutional review board. We performed Nod2 genotyping by TaqMan SNP genotyping (Applied Biosystems, Foster City, CA) or Sequenom platform (Sequenom Inc., San Diego, CA). Unless otherwise indicated, we utilized cells from healthy individuals who were not Nod2Leu1007insC homozygote or compound heterozygote carriers.
Primary MDM Cell Culture
Human MDMs were generated as described previously (19).
MDM Stimulation
For IL-1β blockade, IL-1R antagonist (IL-1Ra) (GenScript, Piscataway, NJ) and/or anti-IL-1β-blocking antibody (R&D Systems, Minneapolis, MN) was added 1 h prior to treatments with MDP (Bachem, King of Prussia, PA), IL-1β (eBioscience, San Diego, CA), lipid A (Peptides International, Louisville, KY), poly(I:C), CpG DNA (Calbiochem), TriDAP, flagellin, or CL097 (Invivogen). For inhibition studies, Ac-YVAD-cmk (Bachem), actinomycin D (Sigma-Aldrich), or dimethyl sulfoxide control was added 1 h prior to pretreatments. Anisomycin (Calbiochem) was used for JNK and p38 activation. Supernatants were assayed for IL-1β (Pierce), TNF-α, IL-8, or IL-6 (BD Biosciences), or for IL-1Ra and IL-10 (R&D Systems) by ELISA.
MAPK Activation
Phosphorylation of MAPKs was determined by flow cytometry using Alexa Fluor 647-labeled phospho-JNK, phospho-ERK, or phospho-p38 (Cell Signaling, Danvers, MA) along with isotype controls.
mRNA Expression Analysis
Following stimulation, total RNA was isolated and reverse-transcribed, and quantitative PCR was performed as in Ref. 2 on the ABI Prism 7000 (Applied Biosystems). Each sample was run in duplicate and normalized to GAPDH. Primer sequences were as follows: IL-1β forward primer, ACAGATGAAGTGCTCCTTCCA; IL-1β reverse primer, GTCGGAGATTCGTAGCTGGAT; IL-8 forward primer, ATGACTTCCAAGCTGGCCGTGGCT; IL-8 reverse primer, ATGACTTCCAAGCTGGCCGTGGCT; TNF-α forward primer, CTACTCCCCAGGTCCTCTTCA; TNF-α reverse primer, CAAAGTAGACCTGCCCAGAC; GAPDH forward primer, CATGTTCCAATATGATTCCACC; GAPDH reverse primer, CCTGGAAGATGGTGATGG.
Statistical Analysis
Significance with treatment was assessed using a paired one-tailed Student t test. p < 0.05 was considered significant.
RESULTS
Blockade of the IL-1β Autocrine Loop Profoundly Inhibits Pro- and Antiinflammatory Cytokine Induction Mediated by Nod2
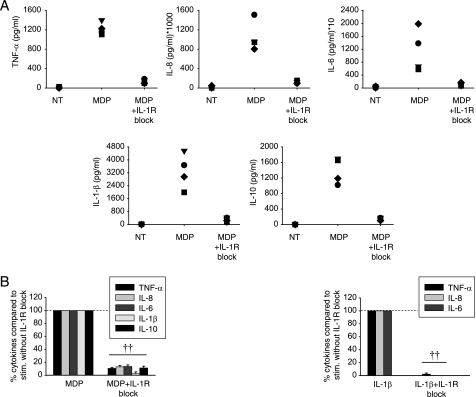
We first sought to define the magnitude of the IL-1β autocrine loop contribution to Nod2-induced cytokines in primary human MDMs from healthy controls. We found that blockade of IL-1β signaling with IL-1Ra and anti-IL-1β upon MDP stimulation of Nod2 results in a dramatic loss of proinflammatory cytokine secretion in human MDMs from healthy individuals (Fig. 1), such that cytokine secretion is ∼10% of the levels observed in the presence of the IL-1β autocrine loop. Antiinflammatory cytokines can be regulated differently than proinflammatory cytokines (26, 27). Therefore, we investigated secretion of IL-10 and found that the IL-1β autocrine loop also significantly contributes to induction of IL-10 upon MDP treatment of MDM (Fig. 1). In contrast to cells from Nod2 WT healthy controls and Nod2 WT CD patients, MDP stimulation of MDMs from CD-associated homozygote or compound heterozygote Nod2Leu1007insC carriers does not induce cytokines, including IL-1β (supplemental Fig. 1), indicating that the Nod2-initiated IL-1β autocrine loop does not operate in these individuals. Responses to TLR4 stimulation (lipid A) are intact in these cells (supplemental Fig. 1), demonstrating a specific defect to Nod2 stimulation. To determine whether the IL-1β autocrine loop contributes uniquely to Nod2 signaling in MDM or also participates in Nod2-induced cytokine secretion in other cell types, we examined human epithelial cell lines. We found that the concentration of cytokines secreted from these cells is less than from primary human macrophages. Nevertheless, human epithelial hepatoma HepG2 cells show a significant decrease in IL-8 production upon IL-1R blockade, although not to the same degree as in primary MDMs. This effect is not seen in intestinal epithelial Caco-2 cells (supplemental Fig. 2). Our results highlight the profound contribution of the IL-1β autocrine loop to overall MDP-induced pro- and antiinflammatory cytokine secretion in human MDMs.
FIGURE 1.
The IL-1β autocrine loop contributes to Nod2-mediated induction of pro- and antiinflammatory cytokines in MDMs. A, human MDMs from healthy controls were stimulated with 100 μg/ml MDP or 100 μg/ml MDP with IL-1R signaling blockade (0.5 μg/ml IL-1Ra and 1 μg/ml anti-IL-1β antibody) for 24 h. Supernatants were assayed for TNF-α, IL-8, IL-6, IL-1β, or IL-10. NT, not treated. B, summarized data (n = 12) are represented as the percent TNF-α, IL-8, IL-6, IL-1β, or IL-10 secretion by cells upon IL-1R signaling blockade normalized to cells in the absence of the blockade (represented by the dotted line at 100%) + S.E. (error bars). IL-1β stimulation is included to ensure efficacy of the IL-1R signaling blockade. Significance compared with cells in the absence of the IL-1R blockade is shown. ††, p < 1 × 10−5. Lines over adjacent bars indicate identical p values for these bars.
IL-1β Autocrine Loop Augments MDP-induced Cytokine Secretion by Regulating Cytokine mRNA
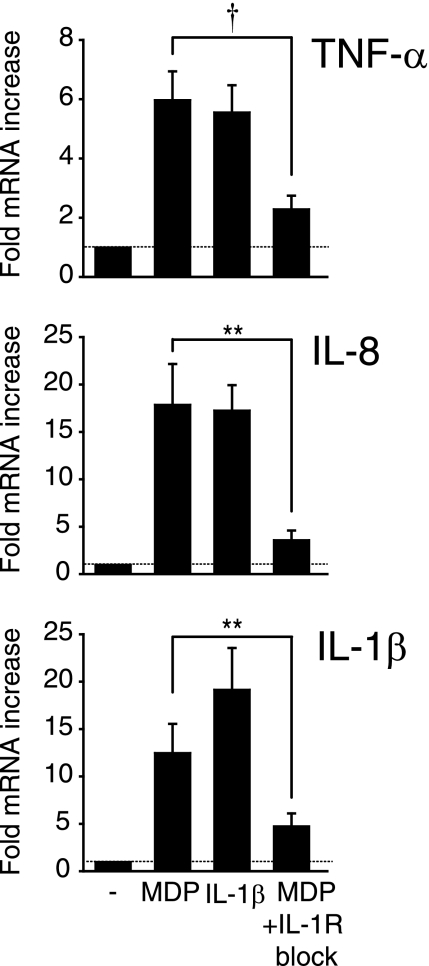
To address the mechanism through which the IL-1β autocrine loop contributes to MDP-mediated cytokine secretion, we first asked whether the loop functions upstream of protein induction by enhancing mRNA cytokine expression. We found that IL-1R blockade significantly reduces TNF-α, IL-8, and IL-1β mRNA levels following MDM stimulation with MDP (Fig. 2), indicating that the IL-1β autocrine loop contributes to Nod2-mediated cytokine induction at the mRNA level.
FIGURE 2.
The IL-1β autocrine loop regulates cytokine mRNA upon MDP stimulation of MDMs. Human MDMs from healthy controls (n = 12) were stimulated with 100 μg/ml MDP, 10 ng/ml IL-1β, or 100 μg/ml MDP with 0.5 μg/ml IL-1Ra and 1 μg/ml anti-IL-1β antibody for 4 h. TNF-α, IL-8, and IL-1β mRNA expression were assessed by real-time PCR. Data are represented as the -fold induction of cytokine mRNA compared with untreated cells (represented by the dotted line at 1) + S.E. (error bars). **, p < 0.01; †, p < 1 × 10−4.
IL-1β Autocrine Loop Plays a Dominant Role in MDP-initiated MAPK Activation
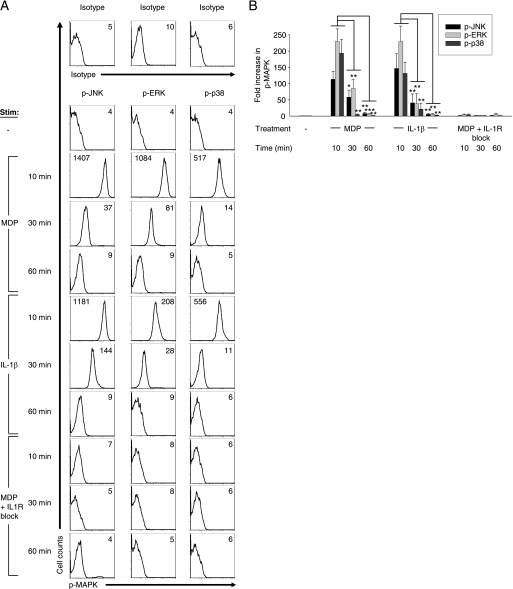
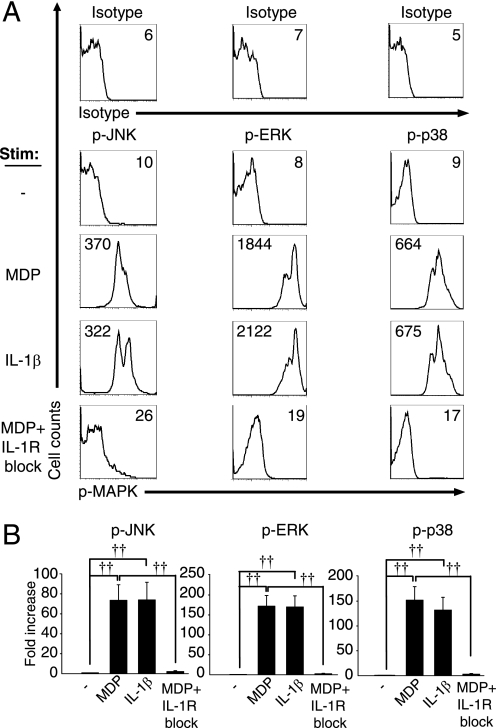
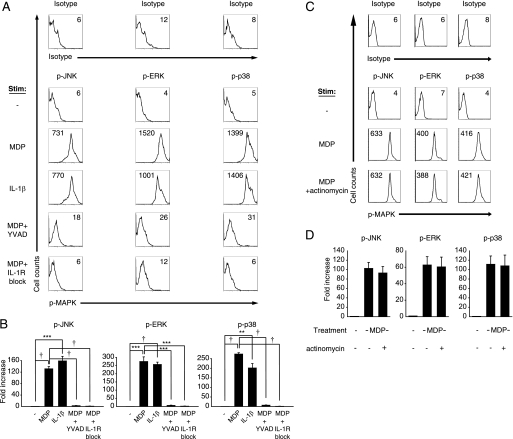
MDP treatment of human and mouse myeloid-derived cells activates the MAPK pathway (4, 22, 28–30), and MAPKs are critical for cytokine induction (31). Given the dramatic role for the IL-1β autocrine loop in MDP-induced cytokine secretion, we questioned whether the MAPK activation observed upon MDP treatment is directly initiated by Nod2 or is caused by the IL-1β autocrine loop. We assessed the phosphorylation of JNK, ERK, and p38 in the context of Nod2, IL-1R, or IL-1R-independent Nod2 signaling (Nod2 signaling in the absence of the IL-1β autocrine loop). Based on prior reports (22) and our time course experiments (see Fig. 4), we chose 10 min as the optimal time point of MAPK activation upon MDP stimulation. We found that JNK, ERK, and p38 are activated upon MDP and IL-1β stimulation of MDMs, whereas IL-1R signaling blockade upon MDP stimulation dramatically reduced the activation of these MAPKs (Fig. 3). The attenuated MAPK activation upon blocking the IL-1β autocrine loop persists over a prolonged period of time, such that the decrease is not merely one of kinetics (Fig. 4). Taken together, MDP-induced MAPK activation in MDMs is due to MAPK activation mediated by the IL-1β autocrine loop, rather than directly by Nod2.
FIGURE 4.
Decreased MAPK activation upon Nod2 signaling in the absence of the IL-1β autocrine loop persists over time. Human MDMs from healthy controls (n = 6–8) were stimulated with 100 μg/ml MDP, 10 ng/ml IL-1β, or 100 μg/ml MDP with 0.5 μg/ml IL-1Ra and 1 μg/ml anti-IL-1β antibody for 10, 30, or 60 min and analyzed by flow cytometry for the expression of phospho-JNK, phospho-ERK, or phospho-p38. A, shown are representative flow cytometry plots with the indicated mean fluorescence intensity values. Stimulated cells stained with isotype controls are shown. B, summarized data are represented as the -fold phospho-MAPK induction normalized to untreated cells + S.E. (error bars). *, p < 0.05; **, p < 0.01; ***, p < 0.001. p-, phospho-.
FIGURE 3.
The IL-1β autocrine loop contributes to the majority of MAPK activation upon MDP treatment of MDMs. Human MDMs from healthy controls (n = 19–23) were stimulated with 100 μg/ml MDP, 10 ng/ml IL-1β, or 100 μg/ml MDP with 0.5 μg/ml IL-1Ra and 1 μg/ml anti-IL-1β antibody for 10 min and analyzed by flow cytometry for the expression of phospho-JNK, phospho-ERK, or phospho-p38. A, shown are representative flow cytometry plots with mean fluorescence intensity values indicated. Stimulated cells stained with isotype controls are shown. B, summarized data are represented as the -fold phospho-MAPK induction normalized to untreated cells + S.E. (error bars). ††, p < 1 × 10−5. p-, phospho-.
MDP Treatment of MDMs Induces Rapid Caspase-1-dependent Activation of MAPK Pathways
We observed the autocrine IL-1β effects on MDP-mediated MAPK induction within the first 10 min of MDP treatment (Fig. 3), prior to when IL-1β transcription, translation, and secretion would be expected to occur. On the other hand, processing and secretion of preformed pro-IL-1β can occur by 5 min in mouse macrophages (32). Therefore, and in view of prior reports demonstrating Nod2 activation of caspase-1 (13, 33–35), our results indicate that Nod2 stimulation may be activating caspase-1, leading to the rapid processing of preformed pro-IL-1β, which in turn mediates early MAPK activation. To address this, we inhibited caspase-1 and observed significantly attenuated MAPK activation within the first 10 min following MDP treatment (Fig. 5, A and B), similar to what we observed on blocking the interaction of IL-1β with its receptor (Fig. 3). To verify further that the early IL-1β-dependent activation of MAPKs upon MDP stimulation was due to preexisting IL-1β stores, we stimulated macrophages with MDP while inhibiting transcription. Under these conditions, we still observed intact activation of JNK, ERK, and p38 10 min after MDP stimulation of MDMs (Fig. 5, C and D). Therefore, MDP-induced processing and secretion of existing stores of pro-IL-1β by caspase-1 are critical for early contributions of the IL-1β autocrine loop to MAPK activation upon Nod2 stimulation.
FIGURE 5.
MDP-initiated caspase-1 activation is required for early MAPK activation. Human MDMs from healthy controls (n = 4) were stimulated with 100 μg/ml MDP, 10 ng/ml IL-1β, or 100 μg/ml MDP for 10 min after pretreatment with 20 μm Ac-YVAD-cmk (to inhibit caspase-1) (A and B) or 100 μg/ml MDP or 100 μg/ml MDP for 10 min after pretreatment with 10 μg/ml actinomycin D (C and D) and analyzed by flow cytometry for the expression of phospho-JNK, phospho-ERK or phospho-p38. Stimulation of cells with 100 μg/ml MDP in the presence of 0.5 μg/ml IL-1Ra and 1 μg/ml anti-IL-1β antibody served as a control for IL-1R signaling block. A and C, shown are representative flow cytometry plots with mean fluorescence intensity values indicated. Stimulated cells stained with isotype controls are shown. B and D, summarized data are represented as the -fold phospho-MAPK induction normalized to untreated cells + S.E. (error bars). **, p < 0.01; ***, p < 0.001; †, p < 1 × 10−4. p-, phospho-.
MDP Stimulation of MDMs Induces Early IL-1β Secretion
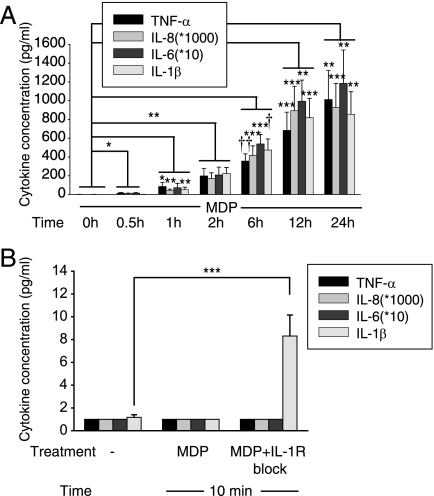
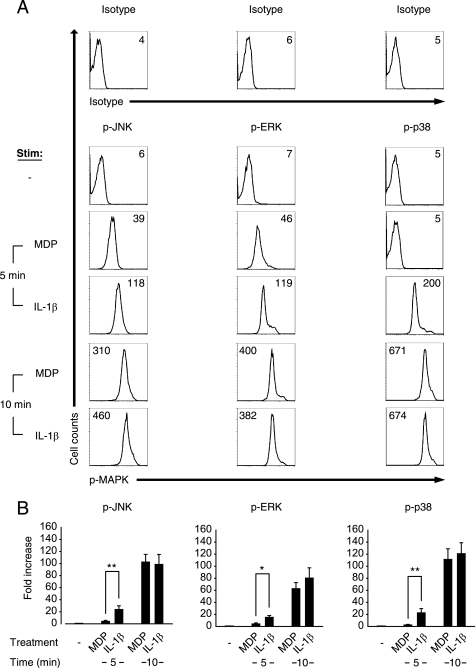
In light of the critical role for IL-1β in MDP-induced MAPK activation and downstream cytokine secretion, we sought to verify that IL-1β was secreted prior to other cytokines. Surprisingly, after MDP treatment, IL-1β was not detected at earlier time points in supernatants relative to TNF-α, IL-8, or IL-6 (Fig. 6A). Because IL-1β protein levels in supernatants reflect a combination of secretion and consumption of IL-1β, it is possible that IL-1β is secreted earlier through release of existing stores of pro-IL-1β but that we are unable to detect it due to its rapid consumption. To prevent this consumption, we measured IL-1β secretion 10 min after MDP stimulation in the presence of IL-1R blockade and detected IL-1β secretion prior to other cytokines (Fig. 6B). Given our findings, one would expect that MDP stimulation would require more time to activate MAPKs than would direct IL-1β stimulation because MDP needs to activate Nod2, then caspase-1 and then process and secrete IL-1β. We found that this is, in fact, the case, because IL-1β stimulation leads to increased MAPK activation relative to that of MDP stimulation at an earlier 5-min time point (Fig. 7). This is consistent with the time delay required for IL-1β release upon MDP treatment of MDMs. Taken together, MDP treatment leads to rapid caspase-dependent processing of preformed pro-IL-1β which results in IL-1β secretion prior to other cytokines, IL-1β autocrine signaling, and induction of cytokine expression.
FIGURE 6.
MDP treatment of MDMs leads to early IL-1β secretion. Human MDMs from healthy controls were stimulated with 100 μg/ml MDP (n = 8) (A) or 100 μg/ml MDP with IL-1R signaling blockade (0.5 μg/ml IL-1Ra and 1 μg/ml anti-IL-1β antibody) (n = 8) (B) for the indicated times. Data are represented as the cytokine concentrations in supernatants at the indicated time points + S.E. (error bars). Significance compared with no treatment is shown. *, p < 0.05; **, p < 0.01; ***, p < 0.001; †, p < 1 × 10−4; ††, p < 1 × 10−5. Multiplication factors for IL-8 and IL-6 protein concentration are indicated in the inset box.
FIGURE 7.
IL-1β stimulation of MDMs demonstrates earlier MAPK activation compared with MDP stimulation. Human MDMs from healthy controls (n = 6–8) were stimulated with 100 μg/ml MDP or 10 ng/ml IL-1β for 5 and 10 min and analyzed by flow cytometry for the expression of phospho-JNK, phospho-ERK, or phospho-p38. A, shown are representative flow cytometry plots with indicated mean fluorescence intensity values. Stimulated cells stained with isotype controls are shown. B, summarized data are represented as the -fold phospho-MAPK induction normalized to untreated cells + S.E. (error bars). *, p < 0.05; **, p < 0.01. p-, phospho-.
Selective JNK and p38 Activation Significantly Improves Suboptimal Cytokine Induction by Nod2 Signaling in the Absence of the IL-1β Autocrine Loop
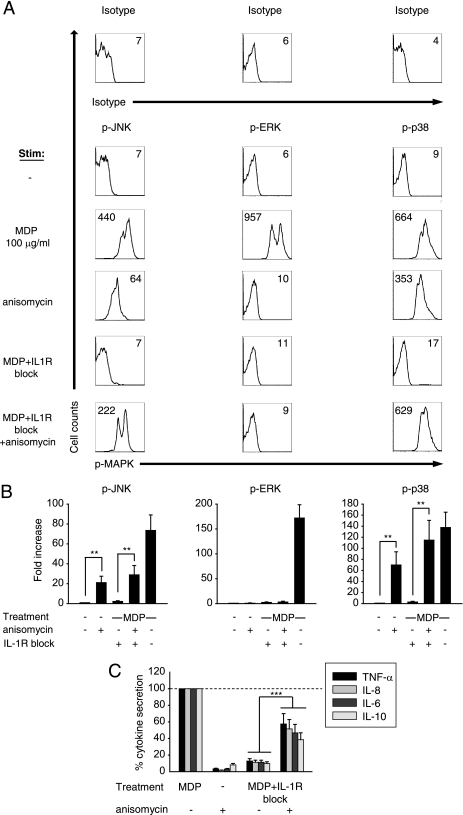
The dramatic decrease in Nod2-mediated MAPK activation in the absence of the IL-1β autocrine loop parallels the significant decrease in cytokine induction upon IL-1R signaling blockade during Nod2 stimulation. Therefore, we asked whether selective activation of MAPKs during Nod2 stimulation in the absence of the IL-1β loop is sufficient to restore cytokine induction. For these studies we utilized anisomycin, which selectively activates JNK and p38 (36). We first confirmed that the low level of JNK and p38 phosphorylation upon MDP signaling in the absence of the IL-1β loop was partially restored with anisomycin treatment (Fig. 8A). Importantly, we found that anisomycin treatment of MDP-stimulated MDMs during blockade of the IL-1β loop significantly rescues the decreased cytokine secretion (Fig. 8B). IL-1R-independent Nod2 signaling (MDP signaling in the absence of the IL-1β autocrine loop) led to cytokine secretion at 11% of the concentrations observed relative to that of MDP-stimulated cells; the addition of anisomycin to IL-1R-independent Nod2 signaling increased cytokines to 47% of that of MDP-stimulated cells (Fig. 8B). We verified these results with UV light, which, like anisomycin, activates JNK and p38 (37, 38) but on its own does not induce cytokine production in primary MDMs (supplemental Fig. 3). Accordingly, we observed similar rescue of decreased cytokine secretion with MDP stimulation in the absence of the IL-1β autocrine loop upon UV light activation of MDMs (supplemental Fig. 3). Taken together, selective activation of p38 and JNK upon Nod2 signaling in the absence of the IL-1β autocrine loop is sufficient to increase cytokine secretion significantly.
FIGURE 8.
Selective activation of JNK and p38 rescues the decreased cytokine secretion observed during Nod2 signaling in the absence of the IL-1β autocrine loop. A and B, human MDMs from healthy controls (n = 12) were stimulated with 100 μg/ml MDP or 100 μg/ml MDP with 0.5 μg/ml IL-1Ra and 1 μg/ml anti-IL-1β antibody in the presence or absence of 50 ng/ml anisomycin. A, shown are representative flow cytometry plots with indicated mean fluorescence intensity values. B, data are represented as the -fold MAPK activation normalized to untreated cells + S.E. (error bars). **, p < 0.01. C, human MDMs from healthy controls (n = 12) were stimulated with the indicated MDP doses in the presence or absence of 50 ng/ml anisomycin. Data are represented as cytokine concentrations in supernatant normalized to the MDP stimulation of cells without the IL-1R block (represented by the dotted line at 100%) + S.E. ***, p < 0.001. Lines over adjacent bars indicate identical p values for these bars.
IL-1R Autocrine Signaling Contributes to Cytokine Induction by a Broad Range of PRRs
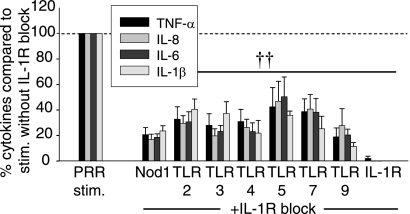
As MDMs encounter bacteria, Nod2 is stimulated in the context of multiple PRRs. IL-1R autocrine signaling has not been investigated downstream of other PRRs, with the exception of TLR4 (5–7) and TLR9 (5, 6, 8, 9), to our knowledge. Given the dramatic contribution of autocrine IL-1β to Nod2 responses, we examined whether the loop contributes to cytokine secretion by multiple PRR. We found that IL-1R signaling blockade dramatically decreases TNF-α, IL-8, IL-6 and IL-1β secretion upon stimulation of Nod1, TLR2, TLR3, TLR4, TLR5, TLR7, or TLR9 in MDMs from healthy controls (Fig. 9). Taken together, we established that IL-1R autocrine signaling is critical for cytokine responses through a broad range of PRR, indicating its major importance to innate immunity.
FIGURE 9.
IL-1R autocrine signaling significantly enhances cytokine secretion upon stimulation of MDM by multiple PRR. Human MDM from healthy controls (n = 12–16) were stimulated with 100 μg/ml TriDAP (Nod1 ligand), 10 μg/ml Pam3Cys (TLR2 ligand), 100 μg/ml poly(I:C) (TLR3 ligand), 0.1 μg/ml lipid A (TLR4 ligand), 5 ng/ml flagellin (TLR5 ligand), 1 μg/ml CL097 (TLR7 ligand), or 10 μg/ml CpG DNA (TLR9 ligand) with or without 0.5 μg/ml IL-1Ra for 24 h. IL-1β stimulation was included to ensure efficacy of the IL-1R signaling blockade. Summarized data are represented as the percent TNF-α, IL-8, IL-6, or IL-1β secretion by cells upon IL-1R signaling blockade normalized to cells in the absence of the blockade (represented by the dotted line at 100%) + S.E. (error bars). Significance compared with cells in the absence of the IL-1R blockade is shown. ††, p < 1 × 10−5. Lines over adjacent bars indicate identical p values for these bars.
DISCUSSION
In this study, we found that the IL-1β autocrine loop accounts for a dominant component of MDP-initiated pro- and antiinflammatory cytokine secretion. Although a role for IL-1β autocrine signaling in Nod2 cytokine induction has been reported in some (10–12), but not all studies (12, 13), the mechanisms of this contribution are not known. We found that, in fact, Nod2 stimulation leads to minimal MAPK activation in MDMs and that IL-1β autocrine secretion accounts for most of the MAPK activation observed upon MDP treatment. Thus, Nod2 and IL-1β have distinct roles following MDP stimulation in MDMs: Nod2 initiates caspase-1-dependent processing of preexisting pro-IL-1β stores, and this early secreted IL-1β feeds back in an autocrine fashion to activate MAPK pathways and induce cytokine secretion (Fig. 10). Selective activation of JNK and p38 under conditions of Nod2 signaling in the absence of the IL-1β autocrine loop is sufficient to increase pro- and antiinflammatory cytokine secretion significantly, thereby indicating that a major mechanism of the IL-1β autocrine loop contribution to Nod2-induced cytokines is by enhancing the strength of MAPK signaling. We determined that IL-1R autocrine signaling is critical in not only Nod2, but also Nod1 and TLR2-, 3-, 4-, 5-, 7-, and 9-mediated cytokine secretion, indicating a broad role for IL-1R autocrine signaling in optimal responses upon various microbial infections.
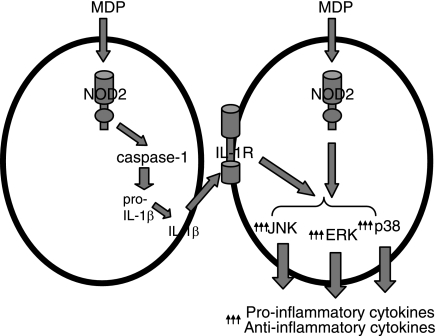
FIGURE 10.
Model for the role of autocrine IL-1β in MAPK and cytokine induction upon Nod2 stimulation in primary MDMs. MDP stimulation of Nod2 activates caspase-1 to process pro-IL-1β stores that rapidly feed back in an autocrine fashion to stimulate the IL-1R and substantially increase MAPK phosphorylation. This increased MAPK activation significantly enhances secretion of both anti- and proinflammatory cytokines.
To understand the mechanisms through which PRRs mediate responses, it is important to dissect which pathways are initiated directly by PRRs and which might be through indirect contributions, such as autocrine loops. Many autocrine loops have been associated with a second, delayed phase of signaling and activation, which is usually less potent than the initial activation peak. For example, TNF-α stimulation of human epithelial cells initiates an autocrine IL-1α loop leading to a 4–24-h delay of NF-κB activation (39). However, the MDP-induced IL-1β autocrine loop observed in our study acts within a very short time period and is the dominant contributor to MDP-mediated MAPK activation. This rapid IL-1β processing is due to Nod2-mediated caspase-1 activation; direct association of Nod2 and caspase-1 has been reported in human THP-1 cells (33). In addition to caspase-1 (13), Nod2 also requires NALP3 and Rip2 to induce IL-1β properly (40). The rapid induction of autocrine loops, such as that of IL-1β, can provide for significant early signaling following stimulation of a particular receptor, thereby masking direct effects of the receptor. Given this, the differences in autocrine loops produced in distinct cell types might account for some of the differences in signaling outcomes following receptor stimulation between cell populations. For example, the contribution of the IL-1 autocrine loop to MDP-mediated cytokine induction was modest in epithelial HepG2 cells and absent in epithelial CaCo-2 cells (supplemental Fig. 2); therefore this loop plays a particularly important role in modulating Nod2 signaling in primary MDMs.
The dramatic contribution of the IL-1 autocrine loop to PRRs and particularly Nod2 responses (10, 12, 19) is notable given the association of loss-of-function Nod2 polymorphisms and loss-of-function NLRP3 polymorphisms with CD (15). Interestingly, impaired IL-1R signaling can lead to increased disease severity in CD patients (17) and to worsening of dextran sulfate sodium and Citrobacter rodentium-induced mouse colitis (9, 16). This contrasts with the simple interpretation that IL-1R signaling is associated with inflammatory disease. Similarly, deficiencies in MyD88 (41) and TLR5 (42) signaling in mice are associated with an increased risk of intestinal inflammation, indicating a complexity in the regulation of intestinal immune homeostasis by PRR-initiated pathways. The dramatic decrease in cytokine induction by the CD-associated protein Nod2 (Fig. 1) and by other PRRs (Fig. 9) in the absence of IL-1R autocrine signaling indicates that dysregulation in IL-1R signaling could have deleterious effects for proper regulation of PRR signaling in the context of the intestinal environment. Because blockade of IL-1β and IL-1R is being used as a therapeutic intervention for an increasing number of inflammatory disorders (43), one needs to consider the implications and potential adverse consequences of this blockade on a wide array of PRR responses. Our results identify mechanisms through which IL-1R autocrine signaling contributes to cytokine responses by Nod2 in primary human MDMs and provide insight into how these interactions ultimately contribute to intestinal immune homeostasis.
Supplementary Material
Acknowledgments
We thank the blood donors for their contribution and Maria-Luisa Alegre, Judy H. Cho, and Bruce H. Horwitz for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants R01DK077905, DK-P30-34989, and U19-AI082713 (to C. A.). This work was also supported by the Crohn's and Colitis Foundation of America (to C. A. and M. H.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
- CD
- Crohn's disease
- IL-1R
- IL-1 receptor
- IL-1Ra
- IL-1R antagonist
- MDM
- monocyte-derived macrophage
- MDP
- muramyl dipeptide
- Nod
- nucleotide-binding oligomerization domain
- PRR
- pattern recognition receptor
- TLR
- Toll-like receptor.
REFERENCES
- 1. Abraham C., Cho J. H. (2009) N. Engl. J. Med. 361, 2066–2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li J., Moran T., Swanson E., Julian C., Harris J., Bonen D. K., Hedl M., Nicolae D. L., Abraham C., Cho J. H. (2004) Hum. Mol. Genet. 13, 1715–1725 [DOI] [PubMed] [Google Scholar]
- 3. Watanabe T., Kitani A., Murray P. J., Strober W. (2004) Nat. Immunol. 5, 800–808 [DOI] [PubMed] [Google Scholar]
- 4. Park J. H., Kim Y. G., McDonald C., Kanneganti T. D., Hasegawa M., Body-Malapel M., Inohara N., Núñez G. (2007) J. Immunol. 178, 2380–2386 [DOI] [PubMed] [Google Scholar]
- 5. Cassatella M. A., Meda L., Bonora S., Ceska M., Constantin G. (1993) J. Exp. Med. 178, 2207–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pioli P. A., Weaver L. K., Schaefer T. M., Wright J. A., Wira C. R., Guyre P. M. (2006) J. Immunol. 176, 6647–6655 [DOI] [PubMed] [Google Scholar]
- 7. Dinarello C. A. (2002) Clin. Exp. Rheumatol. 20, S1–13 [PubMed] [Google Scholar]
- 8. Granowitz E. V., Vannier E., Poutsiaka D. D., Dinarello C. A. (1992) Blood 79, 2364–2369 [PubMed] [Google Scholar]
- 9. González-Navajas J. M., Law J., Nguyen K. P., Bhargava M., Corr M. P., Varki N., Eckmann L., Hoffman H. M., Lee J., Raz E. (2010) J. Exp. Med. 207, 2799–2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hruz P., Zinkernagel A. S., Jenikova G., Botwin G. J., Hugot J. P., Karin M., Nizet V., Eckmann L. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 12873–12878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shikama Y., Kuroishi T., Nagai Y., Iwakura Y., Shimauchi H., Takada H., Sugawara S., Endo Y. (2011) Innate Immun. 17, 3–15 [DOI] [PubMed] [Google Scholar]
- 12. Rosenzweig H. L., Martin T. M., Planck S. R., Galster K., Jann M. M., Davey M. P., Kobayashi K., Flavell R. A., Rosenbaum J. T. (2008) J. Leukoc. Biol. 84, 529–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferwerda G., Kramer M., de Jong D., Piccini A., Joosten L. A., Devesaginer I., Girardin S. E., Adema G. J., van der Meer J. W., Kullberg B. J., Rubartelli A., Netea M. G. (2008) Eur. J. Immunol. 38, 184–191 [DOI] [PubMed] [Google Scholar]
- 14. Netea M. G., Azam T., Ferwerda G., Girardin S. E., Walsh M., Park J. S., Abraham E., Kim J. M., Yoon D. Y., Dinarello C. A., Kim S. H. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 16309–16314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Villani A. C., Lemire M., Fortin G., Louis E., Silverberg M. S., Collette C., Baba N., Libioulle C., Belaiche J., Bitton A., Gaudet D., Cohen A., Langelier D., Fortin P. R., Wither J. E., Sarfati M., Rutgeerts P., Rioux J. D., Vermeire S., Hudson T. J., Franchimont D. (2009) Nat. Genet. 41, 71–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lebeis S. L., Powell K. R., Merlin D., Sherman M. A., Kalman D. (2009) Infect. Immun. 77, 604–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carter J. D., Valeriano J., Vasey F. B. (2003) J. Clin. Rheumatol. 9, 276–277 [DOI] [PubMed] [Google Scholar]
- 18. Hedl M., Abraham C. (2011) Gastroenterology 140, 231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hedl M., Li J., Cho J. H., Abraham C. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 19440–19445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim Y. G., Park J. H., Shaw M. H., Franchi L., Inohara N., Núñez G. (2008) Immunity 28, 246–257 [DOI] [PubMed] [Google Scholar]
- 21. Watanabe T., Asano N., Murray P. J., Ozato K., Tailor P., Fuss I. J., Kitani A., Strober W. (2008) J. Clin. Invest. 118, 545–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kobayashi K. S., Chamaillard M., Ogura Y., Henegariu O., Inohara N., Nuñez G., Flavell R. A. (2005) Science 307, 731–734 [DOI] [PubMed] [Google Scholar]
- 23. Wolfert M. A., Roychowdhury A., Boons G. J. (2007) Infect. Immun. 75, 706–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Girardin S. E., Boneca I. G., Viala J., Chamaillard M., Labigne A., Thomas G., Philpott D. J., Sansonetti P. J. (2003) J. Biol. Chem. 278, 8869–8872 [DOI] [PubMed] [Google Scholar]
- 25. Netea M. G., Ferwerda G., de Jong D. J., Werts C., Boneca I. G., Jéhanno M., Van Der Meer J. W., Mengin-Lecreulx D., Sansonetti P. J., Philpott D. J., Dharancy S., Girardin S. E. (2005) J. Biol. Chem. 280, 35859–35867 [DOI] [PubMed] [Google Scholar]
- 26. Donnelly R. P., Freeman S. L., Hayes M. P. (1995) J. Immunol. 155, 1420–1427 [PubMed] [Google Scholar]
- 27. Fiorentino D. F., Zlotnik A., Mosmann T. R., Howard M., O'Garra A. (1991) J. Immunol. 147, 3815–3822 [PubMed] [Google Scholar]
- 28. Yang Y., Yin C., Pandey A., Abbott D., Sassetti C., Kelliher M. A. (2007) J. Biol. Chem. 282, 36223–36229 [DOI] [PubMed] [Google Scholar]
- 29. Vidal V. F., Castéran N., Riendeau C. J., Kornfeld H., Darcissac E. C., Capron A., Bahr G. M. (2001) Eur. J. Immunol. 31, 1962–1971 [DOI] [PubMed] [Google Scholar]
- 30. Pauleau A. L., Murray P. J. (2003) Mol. Cell. Biol. 23, 7531–7539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lang R., Hammer M., Mages J. (2006) J. Immunol. 177, 7497–7504 [DOI] [PubMed] [Google Scholar]
- 32. Qu Y., Franchi L., Nunez G., Dubyak G. R. (2007) J. Immunol. 179, 1913–1925 [DOI] [PubMed] [Google Scholar]
- 33. Hsu L. C., Ali S. R., McGillivray S., Tseng P. H., Mariathasan S., Humke E. W., Eckmann L., Powell J. J., Nizet V., Dixit V. M., Karin M. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 7803–7808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marina-García N., Franchi L., Kim Y. G., Miller D., McDonald C., Boons G. J., Núñez G. (2008) J. Immunol. 180, 4050–4057 [DOI] [PubMed] [Google Scholar]
- 35. Damiano J. S., Oliveira V., Welsh K., Reed J. C. (2004) Biochem. J. 381, 213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rutault K., Hazzalin C. A., Mahadevan L. C. (2001) J. Biol. Chem. 276, 6666–6674 [DOI] [PubMed] [Google Scholar]
- 37. Klotz L. O., Pellieux C., Briviba K., Pierlot C., Aubry J. M., Sies H. (1999) Eur J. Biochem. 260, 917–922 [DOI] [PubMed] [Google Scholar]
- 38. Silvers A. L., Bachelor M. A., Bowden G. T. (2003) Neoplasia 5, 319–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Janes K. A., Gaudet S., Albeck J. G., Nielsen U. B., Lauffenburger D. A., Sorger P. K. (2006) Cell 124, 1225–1239 [DOI] [PubMed] [Google Scholar]
- 40. Pan Q., Mathison J., Fearns C., Kravchenko V. V., Da Silva Correia J., Hoffman H. M., Kobayashi K. S., Bertin J., Grant E. P., Coyle A. J., Sutterwala F. S., Ogura Y., Flavell R. A., Ulevitch R. J. (2007) J. Leukoc. Biol. 82, 177–183 [DOI] [PubMed] [Google Scholar]
- 41. Rakoff-Nahoum S., Paglino J., Eslami-Varzaneh F., Edberg S., Medzhitov R. (2004) Cell 118, 229–241 [DOI] [PubMed] [Google Scholar]
- 42. Vijay-Kumar M., Sanders C. J., Taylor R. T., Kumar A., Aitken J. D., Sitaraman S. V., Neish A. S., Uematsu S., Akira S., Williams I. R., Gewirtz A. T. (2007) J. Clin. Invest. 117, 3909–3921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gabay C., Lamacchia C., Palmer G. (2010) Nat. Rev. Rheumatol. 6, 232–241 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.