Abstract
HLA-B27 is highly associated with ankylosing spondylitis (AS), but the mechanism is unknown. Among the HLA-B27 alleles, B*2709, which differs by one amino acid from the susceptible B*2705, is not associated with the disease. Here, we analyze the reactivity, in patients with AS and in healthy controls carrying the B*2709 or B*2705 alleles, to an EBV epitope derived from LMP2 (236-244) and to a sequence-related self-peptide from vasoactive intestinal peptide receptor 1 (VIP1R 400-408). We found that both B*2705+ and B*2709+ subjects possess LMP2 236-244–specific, HLA-B27–restricted T cells, whereas only the B*2705+ individuals respond significantly to VIP1R 400-408. These results prompted us to compare, by IFN-γ ELISPOT analysis, the T-cell response to VIP1R 400-408 in patients with AS versus B*2705 healthy controls. The data show that VIP1R 400-408–specific reactivity is a major feature of the patients with AS. These findings show, for the first time to our knowledge, a widespread reactivity in patients with AS against a self-epitope that exhibits some features of a putative “arthritogenic” peptide.
Introduction
HLA-B27 and spondyloarthropathies constitute one of the strongest examples of HLA-associated disease. Population data and animal models point to the HLA-B27 molecules as directly involved in disease pathogenesis, and yet the role played by the HLA molecules remains elusive (1–3). In humans, a recent breakthrough has come from the observation that two HLA-B27 subtypes, B*2709 in Caucasians and B*2706 in Asians, are not or are rarely present in patients (4, 5). A common feature of the two subtypes is the lack of an Asp at position 116 (Tyr116 for B*2706; His116 for B*2709) compared with the strongly disease-associated B*2705 (6–8), which has been hypothesized to present one or more “arthritogenic” peptide(s) (9). HLA-B*2709 differs from the B*2705 only at position 116 located at the bottom of pocket F thus influencing the COOH-terminus of the bound peptides (10). This is reminiscent of the observations that a single polymorphism at position 57 in HLA-DQB1 molecules confers susceptibility or protection to insulin-dependent diabetes mellitus (IDDM) (11) and that in rheumatoid arthritis (RA), a stretch of HLA-DRB1 polymorphic residues determines disease susceptibility (12). This suggests a common mechanism in the pathogenesis of some HLA-associated autoimmune diseases wherein the HLA antigen–presenting properties play a causative role.
HLA-B27–restricted T cells reactive with self-antigens or arthritis-implicated pathogens have occasionally been described in patients with AS and in those with reactive arthritis (ReA), but their meaning in disease pathogenesis remains uncertain owing to failure to detect such reactivity more generally (13–16).
If the divergent association with the disease among HLA-B27 subtypes correlates with their specificity in presenting self-epitopes, a preliminary approach to the question could be to analyze the T-cell response to the same HLA-B27–specific epitope in the two genetic contexts, B*2705 and B*2709. Given the extent of overlapping of the peptide repertoires of the two molecules (10, 17), a suitable starting point appeared from our previous report that the two HLA-B27 subtypes present an EBV epitope (LMP2 236-244 RRRWRRLTV) and some of its analogues differently to CD8+ T- cell lines (18). B*2705+ and B*2709+ subjects were therefore analyzed for their reactivity against the LMP2 peptide and a sequence-related self-peptide (RRKWRRWHL). This peptide, which is derived from VIP1R (400–408), a seven-transmembrane G protein–coupled receptor (19, 20), was chosen because it differs from LMP2 236-244 epitope, apart from a conservative substitution at P3, only at the COOH-terminus, the part of the peptide most influenced by the HLA-B27 polymorphism at position 116. Accordingly, the VIP1R 400-408 peptide was found to possess a higher binding affinity for the B*2709 subtype.
Interestingly, B*2709+ and B*2705+ subjects diverge in their response to the two peptides: both possess LMP2-specific CD8+ T cells, whereas only the B*2705+ subjects respond to the VIP1R 400-408 peptide. These results prompted us to compare the reactivity to VIP1R 400-408 in patients with AS and in HLA-B*2705–matched healthy controls using the IFN-γ ELISPOT analysis. We found a significant VIP1R 400-408 response only in the patients with AS.
Methods
B27-positive donors.
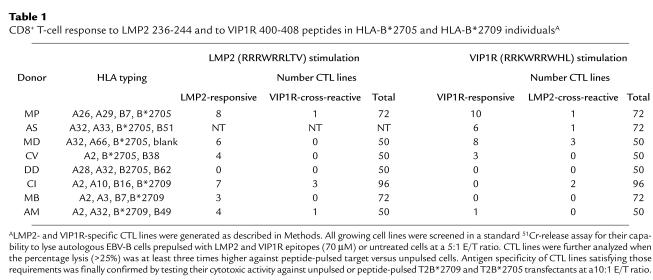
Eight patients with AS and 14 healthy individuals participated in this study. Serological and genomic analyses have been performed to determine the HLA class I haplotype or HLA-B27 subtyping. Table 1 indicates the HLA typing of subjects used to generate LMP2- and VIP1R-specific T-cell lines. Lymphoblastoid cell lines from these individuals were generated by in vitro transformation of B cells using the standard type 1 EBV isolate B95.8 (7).
Table 1.
CD8+ T-cell response to LMP2 236-244 and to VIP1R 400-408 peptides in HLA-B*2705 and HLA-B*2709 individualsA
Synthetic peptides.
Peptides were synthesized on an automated synthesizer (AMS 422; ABIMED, Langenfeld, Germany) using Fmoc chemistry. Purity higher than 90% was confirmed by HPLC analysis. Peptides were dissolved in DMSO and assayed for concentration by the BCA assay (Pierce Chemical Co., Rockford, Illinois, USA).
Generation of LMP2- and VIP1R-specific cytotoxic T lymphocyte lines.
PBMCs from B27+ individuals were isolated on a gradient of Lymphoprep and depleted of the CD4+ fraction by Dynabeads M-450 CD4 (Dynal AS, Oslo, Norway). Cells were incubated at 2 × 104 cells per well in 96-well flat-bottom microplates and stimulated at a 0.5:1 stimulator/responder ratio with autologous EBV-B cells prepulsed overnight with LMP2 or VIP1R peptides (8.5 μM) and γ-irradiated (200 Gy). Cells were grown in RPMI 1640 containing 10% heat inactivated pooled human serum, 2 mM L-glutamine, 10 U/mL penicillin, and 100 μg/mL streptomycin. On day 3, 10 U/mL of human rIL-2 (Boehringer-Mannheim, Indianapolis, Indian, USA) were added to each well. Ten days later, cytotoxic T lymphocyte (CTL) lines were restimulated as just indicated. After 1 week, they were tested for VIP1R and LMP2 specificity in a standard 51Cr-release assay using as targets autologous EBV-B cells and T2-B*2709 and T2-B*2705 transfectants pulsed or not with the indicated peptides. Phenotypic analysis of peptide specific CTL lines was performed by staining with mAb’s: OKT3, OKT4, and OKT8 (Orthodiagnostics, Stanford, California, USA).
Target cells and 51chromium release assay.
Specific reactivity of CTL lines to LMP2, VIP1R, or other peptides was evaluated using a standard 4-hour 51Cr-release procedure. Briefly, 3 × 103 51Cr-labeled targets (peptide pulsed or not) were incubated with effector T cells at the indicated E/T ratio. The percentage of specific lysis was calculated as 100 × (experimental cpm – spontaneous cpm)/(maximum cpm – spontaneous cpm).
Cell surface class I/peptide stabilization assay.
Peptide binding to HLA-B*2705 and -B*2709 cell surface molecules was evaluated using a stabilization assay on T2 transfectants (10). Cells were resuspended in AIM-V serum–free medium (1.5 × 106 cells/mL) and incubated overnight with the peptides or medium alone at 37°C. The cells were stained with the mAb ME1 (21), followed by FITC-conjugated goat anti-mouse IgG antibody. FACScan flow cytometry (Becton Dickinson Immunocytometry Systems, Mountain View, California, USA) analysis was used to calculate the mean fluorescence intensity (MFI) of each sample. The relative binding of each peptide was determined as follows: % of maximum fluorescence = 100 × (MFIsample peptide – MFIwithout peptide)/(MFIRRLPIFSRL – MFIwithout peptide). Reference peptide H2 (RRLPIFSRL) from TIS 11B 325-333 has been eluted from both HLA-B*2709 and B*2705 molecules (10, 17).
Ten-day IFN-γ ELISPOT assay.
The standard ELISPOT procedure is here modified by an antigen-driven step before a final restimulation with antigen-pulsed, γ-irradiated antigen-presenting cells (APCs) (22). PBMCs from patients with AS who are HLA-B27+ or from healthy subjects were incubated at 2 × 106 cells/mL with each peptide (8.5 μM) or in medium alone. Five days later, 10 U/mL of IL-2 was added. On day 8, cells were restimulated at 1:1 ratio with thawed PBMCs cultured overnight with each peptide (35 μM) or in medium alone and γ-irradiated (30 Gy). Antigen-restimulated, medium control or PHA-treated (0.5 μg/mL; Wellcome, Dartford, United Kingdom) PBMCs were seeded at serial dilutions (2 × 105, 1 × 105, and 5 × 104 cells per well), run in triplicates, in 96-well nitrocellulose plates (MAHA S4510; Millipore Corp., Bedford, Massachusetts, USA) and incubated at 37°C. The plates have been precoated overnight at 4°C with 50 μL of anti–IFN-γ mAb NIB42 (25 μg/mL; PharMingen, San Diego, California, USA). After 48 hours, the assays were arrested by shaking off the contents and washing the wells. Next, 50 μL of 9 μg/mL biotinylated anti-IFN-γ mAb 4S.B3 (PharMingen) were added to each well. After 2 hours of incubation at room temperature, plates were washed and 50 μL/well of avidin-peroxidase (1:500; PharMingen) was added. Plates were incubated at room temperature for a further 2 hours. After washing, 50 μL of aminoethyl carbazole (AEC) solution was added. The colorimetric reaction was stopped after 20–30 minutes by distilled water wash. Detection and enumeration of spots were performed with a stereomicroscope (×20).
Results
A higher CD8+ T-cell reactivity to VIP1R (400-408 distinguishes individuals who are B*2705+ from those who are B*2709+.
The VIP1R 400-408 peptide was selected from protein data banks for its sequence similarity with the LMP2 peptide 236-244 (18, 23). CD4+ -depleted PBMCs from five individuals who were HLA-B*2705+ (three patients: MP, AS, and MD; and two healthy controls: CV and DD) and from three HLA-B*2709+ donors (CI, MB, and AM) were stimulated with each peptide (Table 1). After two rounds of stimulation, CD8+ T cells were tested in a standard cytotoxicity assay against autologous EBV-B cells incubated overnight with LMP2 and VIP1R (70 μM) or in the absence of peptide. T-cell lines lysing the peptide-pulsed targets at least three times more than controls were further analyzed against peptide-pulsed T2B*2705 or T2B*2709. LMP2-stimulation elicited specific CD8+ T-cell responses in all subjects but one (DD). VIP1R 400-408 elicited a relevant CD8+ T-cell cytolytic activity especially in patients with AS. Of two B*2705+ healthy controls, one (CV) showed a lower reactivity against both peptides, and one (DD) failed to produce CD8+ T-cell lines with an unambiguous LMP2- or VIP1R-mediated activity. Among the three B*2709+ individuals, VIP1R activation produced a specific T-cell line only in one case (donor AM). Two VIP1R-stimulated T-cell lines from donor CI failed to react against target cells pulsed with VIP1R 400-408 but had a clear-cut LMP2 reactivity. This is probably due to the natural processing and presentation of the endogenously synthesized LMP2 epitope by the autologous EBV-B cells used as APCs (23). In this regard, we had previously obtained LMP2 236-244–specific T-cell lines from this donor using unpulsed, autologous EBV-B cells as APCs (data not shown).
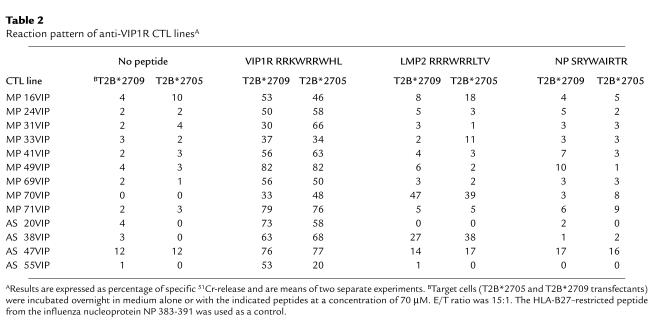
It was considered important to assess the degree of mutual cross-reactivity between LMP2- and VIP1R-driven T-cell responses. As shown in Table 1, few LMP2-specific T cells (five of 32; 15.6%) recognized VIP1R peptide as well. Likewise, among the VIP1R-activated T cells, only 25% (seven of 28) appeared to recognize also the LMP2 epitope. To explore this finding further, more VIP1R-responding T-cell lines were derived from two patients (MP and AS) and analyzed in a cytotoxicity assay. Again, the results clearly demonstrated that the majority of T cells were specific for VIP1R peptide (Table 2). Only two (MP 70VIP and AS 38VIP) of 13 T-cell lines lysed target cells pulsed with either peptide. Moreover, none of them recognized the influenza HLA-B27–restricted peptide NP 383-391 (Table 2) (24). Thus, these data demonstrate that only a subset of CTLs recognize both peptides and therefore that the autoreactivity toward VIP1R 400-408 is mostly independent of the immune response to Epstein Barr virus.
Table 2.
Reaction pattern of anti-VIP1R CTL linesA
A stronger binding affinity of VIP1R 400-408 to the B*2709 compared with the B*2705 molecules affects its presentation in the two HLA contexts.
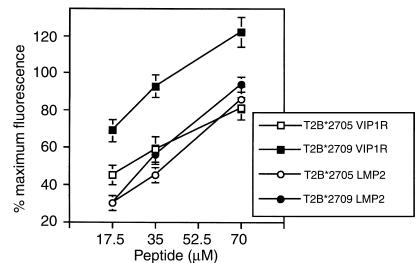
The observations described here prompted us to investigate whether VIP1R 400-408 binds comparably to the two HLA molecules. T2-B*2705 or T2-B*2709 transfectants were used in a cell surface class I stabilization assay in the presence of VIP1R 400-408 and LMP2 236-244. Notably, although the binding affinity of LMP2 236-244 to the two subtypes is not very different, VIP1R 400-408 appears to stabilize the HLA-B*2709 molecules with higher efficiency (Figure 1).
Figure 1.
Relative binding of VIP1R 400-408 (RRKWRRWHL) and LMP2 236-244 (RRRWRRLTV) to B*2709 and B*2705 molecules. T2 transfectants were incubated with LMP2 or VIP1R peptide at the indicated concentrations for 18–20 hours. Peptide H2 (RRLPIFSRL) was used as positive reference. Results are expressed as percentage of maximum fluorescence in which the fluorescence values with 35 μM of the reference peptide for each B27 molecule are taken as 100%. Values represent the mean ± SD of three separate experiments.
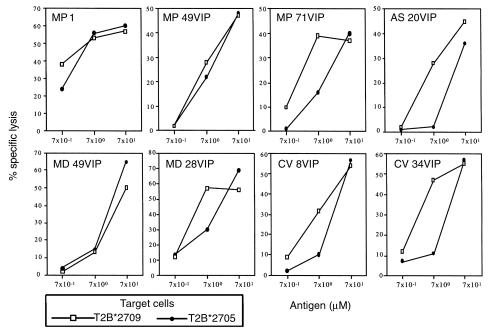
Randomly selected VIP1R 400-408–specific T-cell lines were therefore analyzed for their ability to lyse either B*2705 or B*2709 T2 transfectants loaded with increasing amounts of VIP1R peptide (Figure 2). At lower peptide concentration (7 μM), the majority of these cell lines, although all derived from B*2705+ subjects, lysed T2B*2709 targets more efficiently than B*2705 ones. Taken together, the data indicate that VIP1R peptide 400-408 fulfills at least two criteria for a potential arthritogenic peptide: it binds differently to disease-susceptible and -resistant HLA-B27 molecules, and it elicits a B27-restricted–specific T-cell response especially in the susceptible haplotype. We therefore asked whether the apparent difference in the magnitude of the response to VIP1R 400-408 in the patients with AS versus the B*2705-matched healthy controls described earlier here (Table 1) extends to a larger panel of subjects.
Figure 2.
Comparison of CTL line reactivity against VIP1R 400-408 epitope presented by B*2709 and B*2705 molecules. Dose-response curves show the cytotoxic activity of eight representative VIP1R-specific CTL lines against T2B*2709- and T2B*2705-transfected cells pulsed with VIP1R 400-408. E/T ratio was 15:1. Spontaneous release of 51Cr-labeled cells was less than 15%. One of three separate experiments is shown. The lysis of untreated cells was less than 5%.
Patients with AS show a higher frequency of VIP1R 400-408–responsive T cells.
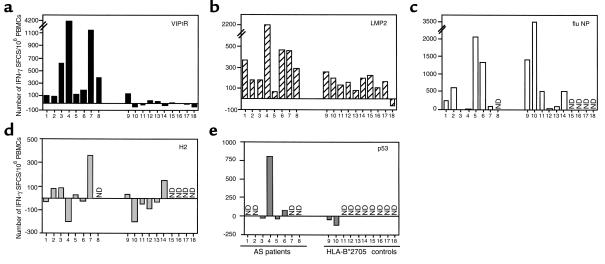
Eight patients with AS and ten B*2705 controls were analyzed by the IFN-γ ELISPOT assay. PBMCs were stimulated with VIP1R 400-408, LMP2 236-244, flu NP 383-391, H2 325-333, p53 266-274 (GRNSFEVRV), or without peptide for 8 days, before being restimulated for 48 hours in the presence of the same peptide. As positive controls, PBMCs cultured in medium for 8 days were treated with PHA for 48 hours (data not shown). The basal reactivity from medium control PBMCs was subtracted from the values obtained by antigen-stimulated cells. Despite the great variability, it is evident from Figure 3a that all patients with AS respond to VIP1R 400-408, with some of them showing a very high reactivity (nos. 3, 4, and 7). On the other hand, only a few healthy controls show a weak specific reactivity, with one subject (no. 9) of ten reaching a significant response. Stimulation with the sequence-related LMP2 236-244 peptide induces IFN-γ production in both groups (Figure 3b), although, on average, the responses are more pronounced in the group of patients with AS. Both groups show a comparable response to the HLA-B27–restricted peptide from influenza NP 383-391 (Figure 3c), although some individuals within each group apparently do not possess T cells reacting against this immunodominant peptide. The two groups, the healthy controls and the patients with AS, also show a comparable behavior when assayed with other self-derived peptides (Figure 3, d and e), with only some individuals possessing a low number of specific precursors. Interestingly, H2 appears to inhibit in some cases the basal self-reactivity. An unexpected high reactivity against p53 266-274 was found in one patient (no. 4). Because this peptide corresponds to a p53 mutational hot spot, this observation should be investigated further.
Figure 3.
Frequency of VIP1R and LMP2 T-cell responders evaluated by IFN-γ ELISPOT assay. Enumeration of IFN-γ spot-forming cells (SFCs) in a 10-day ELISPOT assay performed with peptide-restimulated PBMCs from eight patients with AS and in ten B*2705+ healthy subjects. (a) VIP1R 400-408; (b) LMP2 236-244; (c) NP 383-391 from influenza nucleoprotein; (d) H2 325-333 from TIS 11B; and (e) p53 266-274. For some individuals, the reactivity to control peptides (flu NP, H2, and p53) was not tested because of limited number of cells. ND, not done. Values are reported as the number of IFN-γ SFCs from peptide-restimulated PBMCs minus the number of IFN-γ SFCs from medium-grown PBMCs per 106 PBMCs. Comparison of the frequency of VIP1R- and LMP2-responder T cells between patients with AS and healthy controls was performed using the two-tailed nonparametric Mann-Whitney test (25). VIP1R 400-408: P = 0.0003; LMP2 236-244: P = 0.04.
Discussion
The data reported here show a plausible model of the pathogenic process in AS based on the assumption that one or more autoantigens are involved (9) and that the HLA-B27 alleles diverge in their association with the disease by virtue of fine but relevant differences in presenting such epitope(s). Our approach has been to compare the reactivity to both a viral and a highly sequence-related self-antigen in disease-prone B*2705 and in disease-resistant B*2709 subjects in order to verify whether, in the two genetic contexts, the responses were distinguishable. We found that the two groups respond comparably to the EBV-derived peptide LMP2 236-244 but differently to the self-epitope VIP1R 400-408, with a preponderant response in patients with AS. These data were somehow surprising, as B*2709 molecules show a higher affinity for VIP1R peptide and a generally more efficient presentation compared with the B*2705 counterpart. Therefore, the low reactivity in the HLA-B*2709+ subjects against VIP1R 400-408 could not be attributed to an impairment of presentation. A broad range of self-antigens, such as GAD, retinal S antigen, glucagon, insulin, and proteolipid protein (PLP), are transcribed in the human thymus, suggesting a main role for central mechanisms in tolerance toward peripheral antigens (26, 27). Moreover, quantitative and qualitative differences in transcription, the strength of binding to HLA-molecules, and the affinity of T-cell receptors all play a role in the outcome of T-cell selection (27, 28). Given that VIP1R is expressed in the thymus (20, 29), these data suggest instead that the higher binding affinity of B*2709 molecules for VIP1R 400-408 favors a negative selection in B*2709+ individuals and therefore is responsible for the rare response in these subjects. But, how can we explain the higher reactivity against both LMP2- and VIP1R-derived peptides in patients with AS compared with B*2705 healthy controls? An enrichment for CD8+ T cells reactive against EBV has frequently been found in chronic rheumatic inflammatory diseases, and in AS, this appears to be true for the LMP2 epitope as well (30). This virus has been suggested as a possible trigger for some of these diseases (31). However, such a widespread EBV-specific T-cell response raises the possibility of its reactivation during an individual’s life, particularly in patients receiving long-term immuno-suppressive regimen (30).
However, the reactivity against the VIP1R-derived peptide can hardly be interpreted as a consequence of the response to the virus, since, of the many T-cell lines we have tested, only a few recognized both peptides. In addition, several patients show a higher number of IFN-γ–producing T cells when stimulated with VIP1R 400-408 rather than with LMP2 236-244. It appears therefore that a VIP1R 400-408–specific reactivity characterizes our panel of patients with AS. The specificity of this reaction is strongly supported by the observation that the viral flu NP 383-391 peptide elicits a specific T-cell response only in some subjects, regardless to which group they belong. In contrast, self-derived HLA-B27–specific peptides, like H2 and p53 266-274, do not appear to stimulate any relevant reactivity in both groups. It is therefore conceivable that B27-related arthritis requires the binding of a specific peptide or of a set of peptides to B27 and that VIP1R 400-408 possesses some of the structural properties that characterize the arthritogenic peptide(s). Although VIP1R is not a tissue-specific antigen, it cannot however be excluded that is itself involved in the pathogenic process. For instance, in Lyme arthritis, it has been proposed that autoreactivity against a peptide derived from LFA1, a molecule expressed all over the body, is involved in the pathogenesis of the autoimmune process (32). Moreover, a recent report has shown, in an animal model of RA, that the disease is caused by T- and B-cell recognition of a ubiquitously expressed self-antigen, the glucose-6-phosphate isomerase, demonstrating that at least some forms of arthritis may develop by a non–joint-specific antigen (33). The homing of the autoreactive CD8+ T cells might indeed be the discriminating factor. Interestingly, VIP1R promoter carries specific binding elements for transcription factors induced by inflammatory cytokines such as IL-1, IL-6, TNF, and IFN-γ, and it could be inducible during an inflammatory process (20).
In conclusion, given the arbitrary criterion used in this study to select the self-peptide, the involvement of VIP1-R 400-408 or related peptides in this disease remains only a hypothesis. Nevertheless, our data show, for the first time to our knowledge, the presence of a selective, widespread peptide-specific autoreactivity in patients with AS and represent, so far, one of the stronger pieces of evidence suggesting the involvement of an arthritogenic peptide(s) in disease pathogenesis.
Acknowledgments
The authors thank V. Barnaba, R. Tosi, and F. Bettosini for fruitful discussions and advice; G. Greco and L. Finocchi for synthetic peptides, and G. Sebastiani and A. Martinetti for providing blood samples. This work is supported by Telethon-Italy (grant E.760) and Istitutopasteur-Cenci Bolognetti Foundation. M.T. Fiorillo is a recipient of a fellowship from Telethon-Italy.
References
- 1.Brewerton DA, et al. Ankylosing spondylitis and HL-A 27. Lancet. 1973;1:904–907. doi: 10.1016/s0140-6736(73)91360-3. [DOI] [PubMed] [Google Scholar]
- 2.Schlosstein L, Terasaki PI, Bluestone R, Pearson CM. High association of an HL-A antigen, W27, with ankylosing spondylitis. N Engl J Med. 1973;288:704–706. doi: 10.1056/NEJM197304052881403. [DOI] [PubMed] [Google Scholar]
- 3.Lopez de Castro JA. The pathogenetic role of HLA-B27 in chronic arthritis. Curr Opin Immunol. 1998;10:59–66. doi: 10.1016/s0952-7915(98)80033-2. [DOI] [PubMed] [Google Scholar]
- 4.D’Amato M, et al. Relevance of residue 116 of HLA-B27 in determining susceptibility to ankylosing spondylitis. Eur J Immunol. 1995;25:3199–3201. doi: 10.1002/eji.1830251133. [DOI] [PubMed] [Google Scholar]
- 5.Gonzales-Roces S, et al. HLA-B27 polymorphism and worldwide susceptibility to ankylosing spondylitis. Tissue Antigens. 1997;49:116–123. doi: 10.1111/j.1399-0039.1997.tb02724.x. [DOI] [PubMed] [Google Scholar]
- 6.Breur-Vriesendorp BS, Dekker-Saeys AJ, Ivanyi P. Distribution of HLA-B27 subtypes in patients with ankylosing spondylitis: the disease is associated with a common determinant of various B27 molecules. Ann Rheum Dis. 1987;46:353–356. doi: 10.1136/ard.46.5.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Porto P, et al. Identification of a novel HLA-B27 subtype by restriction analysis of a cytotoxic γδ T cell clone. J Immunol. 1994;153:3093–3100. [PubMed] [Google Scholar]
- 8.Taurog, J.D. 1998. HLA-B27 subtypes, disease susceptibility, and peptide binding specificity. In The spondyloarthritides. A. Calin and J.D. Taurog, editors. Oxford University Press. Oxford, United Kingdom. 267–274.
- 9.Benjamin R, Parham P. Guilty by association: HLA-B27 and ankylosing spondylitis. Immunol Today. 1990;11:137–142. doi: 10.1016/0167-5699(90)90051-a. [DOI] [PubMed] [Google Scholar]
- 10.Fiorillo MT, et al. Susceptibility to ankylosing spondylitis correlates with the C-terminal residue of peptides presented by various HLA-B27 subtypes. Eur J Immunol. 1997;27:368–373. doi: 10.1002/eji.1830270205. [DOI] [PubMed] [Google Scholar]
- 11.Todd JA, Bell JI, McDevitt HO. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987;329:599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- 12.Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987;30:1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- 13.Gao XM, Wordsworth P, McMichael A. Collagen-specific cytotoxic T lymphocyte responses in patients with ankylosing spondylitis and reactive arthritis. Eur J Immunol. 1994;24:1665–1670. doi: 10.1002/eji.1830240731. [DOI] [PubMed] [Google Scholar]
- 14.Ugrinovic S, Mertz A, Wu P, Braun J, Sieper J. A single nonamer from the Yersinia 60-kDa heat shock protein is the target of HLA-B27–restricted CTL response in Yersinia-induced reactive arthritis. J Immunol. 1997;159:5715–5723. [PubMed] [Google Scholar]
- 15.Hermann E, Yu DTY, Meyer zum Buschenfelde KH, Fleischer B. HLA-B27-restricted T cells derived from synovial fluids of patients with reactive arthritis and ankylosing spondylitis. Lancet. 1993;342:646–650. doi: 10.1016/0140-6736(93)91760-j. [DOI] [PubMed] [Google Scholar]
- 16.Huang F, et al. A patient-derived cytotoxic T-lymphocyte clone and two peptide-dependent monoclonal antibodies recognize HLA-B27-peptide complexes with low stringency for peptide sequences. Infect Immun. 1996;64:120–127. doi: 10.1128/iai.64.1.120-127.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rotzschke O, et al. Dominant aromatic/aliphatic C-terminal anchor in HLA-B*2702 and B*2705 peptide motifs. Immunogenetics. 1994;39:74–77. doi: 10.1007/BF00171803. [DOI] [PubMed] [Google Scholar]
- 18.Fiorillo MT, et al. The naturally occurring polymorphism Asp116-His116 that differentiates the ankylosing spondylitis associated HLA-B*2705 from the non associated HLA-B*2709 subtype influences peptide-specific CD8 T cells recognition. Eur J Immunol. 1998;28:2508–2516. doi: 10.1002/(SICI)1521-4141(199808)28:08<2508::AID-IMMU2508>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 19.Sreedharan SP, Patel DR, Huang JX, Goetzl EJ. Cloning and functional expression of a human neuroendocrine vasoactive intestinal peptide receptor. Biochem Biophys Res Commun. 1993;193:546–553. doi: 10.1006/bbrc.1993.1658. [DOI] [PubMed] [Google Scholar]
- 20.Sreedharan SP, Huang JX, Cheung MC, Goetzl EJ. Structure, expression, and chromosomal localization of the type I human vasoactive intestinal peptide receptor gene. Proc Natl Acad Sci USA. 1995;92:2939–2943. doi: 10.1073/pnas.92.7.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis SA, Taylor C, McMichael A. Recognition of HLA-B27 and related antigens by a monoclonal antibody. Hum Immunol. 1982;5:49–53. doi: 10.1016/0198-8859(82)90030-1. [DOI] [PubMed] [Google Scholar]
- 22.McCutcheon M, et al. A sensitive ELISPOT assay to detect low-frequency human T lymphocytes. J Immunol Methods. 1997;210:149–166. doi: 10.1016/s0022-1759(97)00182-8. [DOI] [PubMed] [Google Scholar]
- 23.Brooks JM, Murray RJ, Thomas WA, Kurilla MG, Rickinson AB. Different HLA-B27 subtypes present the same immunodominant Epstein-Barr virus peptide. J Exp Med. 1993;178:879–887. doi: 10.1084/jem.178.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowness P, Allen RL, McMichael AJ. Identification of T cell receptor recognition residues for a viral peptide presented by HLA B27. Eur J Immunol. 1994;24:2357–2363. doi: 10.1002/eji.1830241015. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 26.Sospedra M, et al. Transcription of a broad range of self-antigens in human thymus suggests a role for central mechanisms in tolerance towards peripheral antigens. J Immunol. 1998;161:5918–5929. [PubMed] [Google Scholar]
- 27.Klein L, Klugmann M, Klaus-Armin N, Tuohy VK, Kyewski B. Shaping of the autoreactive T-cell repertoire by a splice variant of self protein expressed in thymic epithelial cells. Nat Med. 2000;6:56–61. doi: 10.1038/71540. [DOI] [PubMed] [Google Scholar]
- 28.Kersh G, Allen PM. Essential flexibility in the T-cell recognition of antigen. Nature. 1996;380:495–498. doi: 10.1038/380495a0. [DOI] [PubMed] [Google Scholar]
- 29.Xin Z, et al. Effect of vasoactive intestinal peptide (VIP) on cytokine production and expression of VIP receptors in thymocyte subsets. Regul Pept. 1997;72:41–54. doi: 10.1016/s0167-0115(97)01028-8. [DOI] [PubMed] [Google Scholar]
- 30.Scotet E, et al. Frequent enrichment for CD8 T cell reactive against common herpes viruses in chronic inflammatory lesions: towards a reassessment of the physiopathological significance of T cell clonal expansions found in autoimmune inflammatory processes. Eur J Immunol. 1999;29:973–985. doi: 10.1002/(SICI)1521-4141(199903)29:03<973::AID-IMMU973>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 31.Scotet E, et al. T cell response to Epstein-Barr virus transactivators in chronic rheumatoid arthritis. J Exp Med. 1996;184:1791–1800. doi: 10.1084/jem.184.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gross DM, et al. Identification of LFA-1 as candidate autoantigen in treatment-resistant Lyme arthritis. Science. 1998;281:703–706. doi: 10.1126/science.281.5377.703. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto I, Staub A, Benoist C, Mathis D. Arthritis provoked by linked T and B cell recognition of a glycolitic enzyme. Science. 1999;286:1732–1735. doi: 10.1126/science.286.5445.1732. [DOI] [PubMed] [Google Scholar]