Abstract
Drug efflux represents an important protection mechanism in bacteria to withstand antibiotics and environmental toxic substances. Efflux genes constitute 6–18% of all transporters in bacterial genomes, yet the expression and functions of only a handful of them have been studied. Among the 20 efflux genes encoded in the Escherichia coli K-12 genome, only the AcrAB-TolC system is constitutively expressed. The expression, activities, and physiological functions of the remaining efflux genes are poorly understood. In this study we identified a dramatic up-regulation of an additional efflux pump, MdtEF, under the anaerobic growth condition of E. coli, which is independent of antibiotic exposure. We found that expression of MdtEF is up-regulated more than 20-fold under anaerobic conditions by the global transcription factor ArcA, resulting in increased efflux activity and enhanced drug tolerance in anaerobically grown E. coli. Cells lacking mdtEF display a significantly decreased survival rate under the condition of anaerobic respiration of nitrate. Deletion of the genes responsible for the biosynthesis of indole, tnaAB, or replacing nitrate with fumarate as the terminal electron acceptor during the anaerobic respiration restores the decreased survival of ΔmdtEF cells. Moreover, ΔmdtEF cells are susceptible to indole nitrosative derivatives, a class of toxic byproducts formed and accumulated within E. coli when the bacterium respires nitrate under anaerobic conditions. Taken together, we conclude that the multidrug efflux pump MdtEF is up-regulated during the anaerobic physiology of E. coli to protect the bacterium from nitrosative damage through expelling the nitrosyl indole derivatives out of the cells.
Keywords: Bacterial Metabolism, Drug Resistance, Membrane, Metabolic Regulation, Multidrug Transporters, Anaerobic Respiration, Nitrosative Stress
Introduction
Drug efflux represents one of the important mechanisms that account for the antibiotic and multidrug resistance in bacteria. The process is mediated by multidrug efflux pumps, a class of membrane protein transporters that actively extrude a wide spectrum of structurally unrelated compounds out of cells. In addition to antibiotics, bacterial efflux pumps are also found to be able to export detergents, dyes, organic solvents, bile acids, and various host-defense molecules present in their environment (for reviews, see Refs. 1–7).
Among the five classes of multidrug efflux pumps, the resistance nodulation division (RND)3 family pumps are especially effective in Gram-negative bacteria because of their tripartite composition that allows expulsion of drugs directly from the cytosol to the outside of the cells (1). An example of this high performance RND-type efflux pump is the Escherichia coli housekeeping efflux system, AcrAB-TolC, in which AcrB forms an inner membrane channel, TolC forms an outer membrane channel, and the periplasmic protein AcrA connects the two components (8). The constitutive activity of this pump renders E. coli a low level, intrinsic resistance to a wide range of toxic molecules (5, 8).
In addition to the AcrAB-TolC pump, the E. coli K-12 genome encodes another 19 efflux genes (9). However, their expression is not induced under the ordinary laboratory growth conditions, i.e. 37 °C in nutrient medium with aeration (9). The environmental or physiological conditions that trigger the expression of these remaining efflux genes as well as the cellular functions of their gene products remain unknown.
In this study we investigate the expression of all efflux genes in E. coli K-12 under the condition of O2 deprivation, an environmental signature of the mammalian gut and the anaerobic micro habitat of bacteria in nature. We identified a dramatic up-regulation of an RND type efflux pump MdtEF under this condition, which resulted in increased efflux activity and enhanced drug tolerance in anaerobically grown E. coli. We show that the up-regulated MdtEF promotes the growth of E. coli during its anaerobic physiology by actively removing nitrosyl indole derivatives, which are generated and accumulated during the anaerobic respiration of nitrate, out of the cells.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Plasmids
The bacterial strains and plasmids constructed in this study are listed in Table 1. Gene deletion mutants and FLAG-tagged E. coli strains were constructed based on the method of Datsenko and Wanner (10) or Yu et al. (11). E. coli cells were cultured in Luria Bertani (LB) broth or M9 minimum medium supplemented with nutrients. Antibiotic concentrations in the growth media were 100 μg/ml ampicillin, 20 μg/ml kanamycin, 25 μg/ml chloramphenicol, or 10 μg/ml tetracycline.
TABLE 1.
Bacterial strains and plasmids used in this study
| Genotypes | Source | |
|---|---|---|
| Strain | ||
| MC4100 | F_ araD139_(argF-lac)U169 rpsL150(Strr) relA1 flbB5301 deoC1 ptsF25 rbsR | Ref. 15 |
| MG1655 | Wild type | Ref. 13 |
| PK4811 | MG1655 Δfnr::sp | Ref. 13 |
| PK7514 | MG1655ΔarcA::cat | Ref. 13 |
| AY0416 | MG1655 ΔlacZ::kan | This study |
| AY0417 | MG1655 Δfnr::sp ΔlacZ::kan | This study |
| AY0433 | MG1655 ΔlacZ::kan ΔarcA::bla | This study |
| AY0434 | MG1655 Δfnr::sp ΔlacZ::kan ΔarcA::bla | This study |
| AY0451 | MG1655Φ(mdtE-FLAG)::kan | This study |
| AY0452 | MG1655ΔarcA::cat Φ(mdtE-FLAG)::kan | This study |
| AY0458 | MG1655Φ(mdtF-FLAG)::kan | This study |
| AY0459 | MG1655ΔarcA::cat Φ(mdtF-FLAG)::kan | This study |
| AY0461 | MG1655ΔmdtEF::kan ΔtnaAB::bla | This study |
| NKE13 | KAM3 (TG1 acrB::MudI markerless) | Ref. 9 |
| NKE356 | KAM3 ΔmdtEF | Ref. 9 |
| NKE138 | MG1655 ΔmdtEF::kan | Ref. 15 |
| Plasmids | ||
| pNN387 | Single-copy vector, Cpr,a NotI-HindIII cloning site upstream of promoterless lacZ | Ref. 15 |
| pAY0906 | pNN387 (tnaC gene promoter-lacZ) | This study |
Construction of Ptna-lacZ Transcription Reporter in the Single Copy Plasmid pNN387
A DNA fragment corresponding to the −338 to +39-bp region relative to the ATG start codon of the first gene of the tna operon, tnaC, was amplified using MG1655 genomic DNA as the template and primers specific to this DNA fragment but flanked with NotI and HindIII restriction sites at the 5′- and 3′-end, respectively. After digestion with NotI and HindIII (Promega), the gel-purified fragment was ligated into the plasmid pNN387 (12), which was treated with the same restriction enzymes such that the expression of the lacZ gene on the plasmid is driven by the tna promoter. Positive clones were screened by colony PCR and verified by DNA sequencing. The plasmid was designated as pAY0906 and transformed into AY0416 (MG1655 but ΔlacZ) for β-galactosidase activity assays.
β-Galactosidase Activity Assay
β-Galactosidase activity assay was measured following the description by Mettert and Kiley (13). Briefly, cells were grown aerobically or anaerobically at 37 °C in M9 medium supplemented with nutrients and 25 μg/ml chloramphenicol. Anaerobic culturing of E. coli was achieved by inoculating the growth medium with a small number of inocula (initial cell density as 103 cells/ml) and by using screw-capped Pyrex culture tubes filled with growth medium and no aeration. The residual O2 dissolved in growth medium is preferably and rapidly consumed by the small portion of initial inocula, resulting in an anaerobic environment for cell multiplication and growth. When A600 of the cultures reached ∼0.3, tetracycline (10 μg/ml) was added to terminate protein synthesis and cell growth, and the cultures were placed on ice until assayed. The assay was performed from at least three independent isolates, and results were presented as the mean in Miller units.
Reverse Transcription (RT)-Quantitative PCR (qPCR)
RT-qPCR was performed using the guidelines of Bustin et al. (14). Bacteria were cultured in conditions identical to that for assaying β-galactosidase activity. RNA was isolated using Promega SV Total RNA Isolation System (Promega). Reverse transcription was performed using Moloney murine leukemia virus reverse transcriptase (Promega) and specific primers in a 25-μl reaction system. Sequences of the primers are listed in supplemental Table S1. Quantitative PCR was performed using the same specific primers mixed with the SYBR Green PCR master mix (Applied Biosystems) in a 20-μl reaction system. The reaction was performed in an ABI StepOnePlus real time PCR system. The E. coli rrsA was chosen as the reference gene to normalize the relative expression of the target genes. The results are expressed as -fold changes of the expression of target genes, and results are presented as the mean of two independent assays.
Western Blot
The strain containing C-terminal FLAG-tagged mdtE on the chromosome was grown in LB medium to log phase or stationary phase under either aerobic or anaerobic conditions as indicated. Log phase cells were harvested when the A600 reached 0.3, and stationary phase cells were harvested when the A600 reached 6.0. Cell extracts were separated by SDS-PAGE followed by transfer to the nitrocellulose membrane (Bio-Rad) for 1.5 h. After blocking with 5% milk solution in 1% TBST, the membrane was incubated with monoclonal anti-FLAG antibody (Sigma) followed by secondary antibody (goat anti-mouse IgG HRP conjugate, Bio-Rad). Protein bands corresponding to MdtE-FLAG were visualized using the ECL Western blotting analysis kit (GE Healthcare) followed by x-ray film development.
Drug Efflux Assay
Overnight cultures of NKE13 (KAM3) and NKE356 (KAM3, but ΔmdtEF) (9) were serially diluted to a cell density of 103 cells/ml in LB broth. Cells were grown under anaerobic or aerobic conditions at 37 °C to an A600 of 0.3. Cells were then collected and washed twice with 100 mm potassium phosphate buffer (pH 7.5 containing 5 mm MgSO4) and then resuspended in the same buffer to an A600 of 18. Assays were performed in 96-well flat-bottomed black plates (IWAKI, Japan) using a final volume of 100 μl of cells with the addition of doxorubicin (Sigma) at a final concentration 28.6 μm. The fluorescence of doxorubicin was measured in a microplate reader, model SH-8100 (Corona Electric Co., Japan) using excitation light at 480 nm and emission at 600 nm. An increase in fluorescence intensity indicates the expulsion of doxorubicin by an efflux pump.
Drug Tolerance Assay
Assays of drug tolerance were modified from that of Kobayashi et al. (15). Overnight cultures of NKE13 (KAM3) and NKE356 (KAM3, but ΔmdtEF) were serially diluted and inoculated into LB broth supplemented with erythromycin to a cell density of 103 cells/ml. Cells were grown anaerobically or aerobically, and the A600 was measured every 30 min. Values of A600 were derived from the mean of two independent isolates and were utilized to plot the growth curves. Tolerance to the synthetic indole red under anaerobic conditions was assayed similarly, except indole red (final concentration as 80 μm) was supplemented into the medium instead of erythromycin. Indole red was synthesized according to the description of Astolfi et al. (16). Indole red tolerance assay in aerobic logarithmic and stationary phases was following the description of Kobayashi et al. (15).
Viability Assay
Cell viability was assayed by viable plate counting. Overnight cultures of E. coli MG1655, NKE138 (MG1655, but ΔmdtEF), or AY0462 (MG1655, but ΔmdtEF ΔtnaAB) were inoculated to a cell density of 103 cells/ml in LB, LB supplemented with 10 mm KNO3, or LB supplemented with 40 mm fumarate as indicated and grown under anaerobic conditions. At various time points, the A600 of cell cultures was measured, and serial dilutions were performed followed by plating on LB agar plates. The plates were incubated at 37 °C for 24 h, and the colony forming units were determined. Colony forming unit values were utilized to extrapolate the viable cells/ml in the original cultures. Results are presented as the mean from two independent assays.
Measurement of Indole Production
Overnight cultures of E. coli MG1655 were diluted to a cell density of 103 cells/ml in LB broth. Cells were grown under anaerobic conditions at 37 °C until an A600 of 0.3. Cells were separated from the growth medium by centrifugation. The supernatants were extracted twice with 3 ml of ethyl acetate, and the organic phase was analyzed by reverse-phase high performance liquid chromatography (HPLC) as described by Kobayashi et al. (15). Peaks were identified using purified indole (Sigma-Aldrich), and the indole concentration was calculated from the ratio of the detection peak area to the standard peak.
RESULTS
Expression of MdtEF Is Dramatically Increased under Anaerobic Conditions
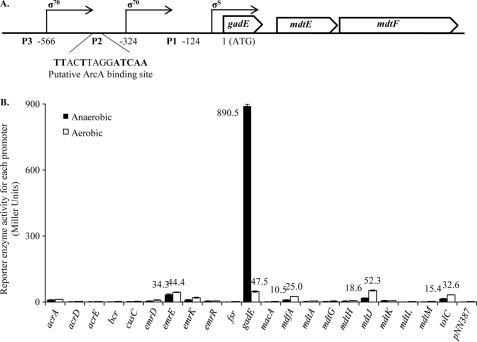
To investigate whether anaerobic growth conditions will trigger the expression of any other efflux systems in addition to the housekeeping AcrAB-TolC pump, we measured and compared the transcription of all 20 E. coli efflux genes from their respective promoter-lacZ fusions under aerobic and anaerobic conditions. β-Galactosidase activity assays showed that among these genes, transcription from the gadE promoter, which drives the expression of the multidrug efflux genes mdtE and mdtF in the operon of gadE-mdtEF (Fig. 1A), was increased more than 20-fold under anaerobic conditions than aerobic conditions (Fig. 1B). To confirm this result, we performed RT-qPCR to analyze the transcription product (mRNA levels) of these three genes. RT qPCR analyses showed that mRNA levels of all three genes in the gadE-mdtEF operon were increased to a similar extent (Fig. 2A) as observed in the promoter-lacZ transcription assay. To further examine this up-regulation at protein level, we constructed FLAG-tagged mdtE on the chromosome of E. coli MG1655 and performed Western blot analysis. As shown in Fig. 2B, a significant expression of MdtE-FLAG from cells cultured under anaerobic conditions was observed, whereas no MdtE-FLAG was detected when the same strain was cultured under aerobic conditions, confirming that MdtEF protein was significantly expressed during the anaerobic growth of E. coli. Because transcription of mdtEF was previously shown to also be up-regulated during the aerobic stationary growth of E. coli, we analyzed the level of MdtE-FLAG in cells cultured from this condition, and a similar significant band corresponds to MdtE-FLAG was observed (Fig. 2B). All these results together confirmed that the MdtEF efflux pump is significantly up-regulated under anaerobic growth conditions in E. coli.
FIGURE 1.
Transcription of 20 E. coli efflux genes under aerobic and anaerobic conditions. A, shown is a diagram of the gadE-mdtEF operon. Three promoter regions (P1, P2, and P3) and corresponding transcription starting sites (21) relative to the ATG start codon of gadE are indicated. A putative ArcA binding site is identified upstream of the −324 transcription starting site. B, transcription of the gadE-mdtEF was increased more than 20-fold under anaerobic than aerobic conditions. Single-copy reporter fusion plasmids (9) were transformed into E. coli strain AY0416 (MG1655 ΔlacZ) and assayed for β-galactosidase activities. E. coli cells were cultured under aerobic (white bar) or anaerobic (black bar) conditions until the A600 reached ∼0.3. β-Galactosidase activity was measured as previously described (13). The value of β-galactosidase activity is presented as the mean from three independent experiments. pNN387 indicates the vector control.
FIGURE 2.
Up-regulation of the gadE-mdtEF operon by ArcA under anaerobic conditions. FNR and ArcA are the two global regulatory factors in the anaerobic adaptation in E. coli. Effects of these two regulators on the up-regulation of mdtEF are examined. A, shown are -fold changes of the mRNA levels of MdtE, MdtF, and GadE in WT, Δfnr, and ΔarcA strains cultured under anaerobic conditions relative to that of WT cultured under aerobic conditions as determined by RT-qPCR. B, shown is a Western blot of MdtE-FLAG from cells cultured under aerobic log phase, anaerobic log phase ΔarcA strain under anaerobic log phase, as well as WT cultured under aerobic stationary conditions. Deletion of arcA causes a dramatic decrease of MdtE-FLAG under anaerobic conditions. C, shown is β-galactosidase activity of PgadE-lacZ transcriptional fusion under aerobic and anaerobic conditions and under anaerobic conditions in the Δfnr and ΔarcA strains.
The Global Transcription Regulator ArcA Activates the Expression of the gadE-mdtEF Operon
To investigate how the gadE-mdtEF operon is regulated during the anaerobic growth of E. coli, we examined the roles of fumarate nitrate reduction (FNR) and anaerobic respiration control (ArcA), two global transcriptional regulatory factors that mediate the transition from an aerobic to anaerobic life style in E. coli (17–19). We constructed PgadE-lacZ in Δfnr and ΔarcA strains and measured transcription from PgadE-lacZ in these strains. β-Galactosidase activity assay showed that Δfnr caused a moderate decrease in the transcription of PgadE-lacZ, whereas ΔarcA almost completely abolished transcription from PgadE (Fig. 2C). Δfnr ΔarcA double deletion had a similar effect as of ΔarcA, suggesting that ArcA primarily activates the expression of the gadE-mdtEF operon under anaerobic conditions. Consistent with this result, RT-qPCR showed a significant decrease of the mRNA levels of all three genes in this operon (Fig. 2A), and Western blot analysis showed a dramatic decrease of MdtE-FLAG in ΔarcA strain (Fig. 2B), confirming that the anaerobic up-regulation of the gadE-mdtEF operon is dependent on ArcA under anaerobic conditions.
It is known that the first gene product in the gadE-mdtEF operon, GadE, is an acid-resistant regulator and its expression is induced at low pH. We wondered if the up-regulation of mdtEF by ArcA was attributed to the effect of inevitable pH drop in the anaerobic culture. We then monitored pH fluctuation during the anaerobic culturing of E. coli MG1655 in M9 minimum medium and found that the pH indeed dropped from 7.0 to 6.2 at the time point when cells were harvested for β-galactosidase activity assay. However, this slight pH fluctuation is unlikely to induce the glutamate-dependent acid resistance system as the pH of the medium required to induce the system is at least below 4.5 and activation of the system is usually stationary phase-induced (20). Indeed, a bioinformatics search revealed a conserved ArcA binding site in the P2 promoter region (21) of the gadE-mdtEF operon (Fig. 1A), suggesting that ArcA may directly bind to the promoter region of the gadE-mdtEF operon and activate its expression under anaerobic conditions.
MdtEF Increases the Efflux Activity in Anaerobic E. coli Cells
MdtEF has been shown to be the inner membrane and periplasmic components of the RND efflux pump MdtEF-TolC system (9), and overexpression of the pump from a multicopy plasmid confers resistance to various drugs under aerobic conditions (9). To examine whether the anaerobically up-regulated MdtEF also enhances drug efflux in E. coli, we monitored the efflux of the fluorescent drug doxorubicin in whole cells. To exclude the effect of the housekeeping AcrAB-TolC efflux system, we utilized the drug-sensitive KAM3 strain in which acrAB was deleted (22). Compared with the result from E. coli grown under aerobic conditions, the fluorescence intensity of doxorubicin increased from cells grown under anaerobic conditions (Fig. 3), suggesting that anaerobic cells display a higher efflux of doxorubicin. Furthermore, when mdtEF was deleted, the fluorescence intensity in anaerobic cultures decreased to the level of that in KAM3 cells under aerobic conditions, suggesting that the increased efflux of doxorubicin in anaerobic E. coli KAM3 strain was dependent on the presence of MdtEF. These results indicate that the anaerobically up-regulated MdtEF indeed enhanced drug efflux in E. coli.
FIGURE 3.
MdtEF enhances the efflux of doxorubicin under anaerobic conditions. Bacterial cells were grown under anaerobic or aerobic conditions and were subsequently resuspended in 100 mm potassium phosphate buffer for the doxorubicin efflux assay. The fluorescence of doxorubicin was measured in a microplate reader using excitation light at 480 nm and emission at 600 nm. The fluorescence intensity of doxorubicin is higher when this compound is expelled by an efflux pump.
MdtEF Enhances Drug Tolerance of Anaerobic E. coli Cells
To examine whether the up-regulated MdtEF efflux pump also confers drug tolerance in anaerobic E. coli, we measured the growth of wild type and ΔmdtEF strains in the presence of erythromycin, a substrate of MdtEF (9), under anaerobic conditions. In the absence of drugs, the growth of KAM3 ΔmdtEF is identical with that of the parental KAM3 strain. However, in the presence of erythromycin, although the growth of both strains is slower than in the absence of drugs, the ΔmdtEF strain grows significantly slower than the KAM3 strain (Fig. 4), suggesting that MdtEF contributes to the tolerance of E. coli to erythromycin under anaerobic conditions. Under aerobic conditions, however, both ΔmdtEF and the KAM3 strain grow poorly in the presence of drug challenge, and ΔmdtEF does not affect the growth of cells (Fig. 4). This result is consistent with our expression data, which demonstrated that mdtEF was not expressed under aerobic conditions; thus, both KAM3 and KAM3 ΔmdtEF were susceptible to erythromycin, and deletion of mdtEF does not have an effect on the drug tolerance of cells under this condition. These results confirmed that MdtEF is not only expressed under anaerobic conditions but also is functionally active in drug efflux under this condition.
FIGURE 4.
MdtEF enhances the tolerance of E. coli cells to erythromycin under anaerobic conditions. KAM3 and KAM3 ΔmdtEF were inoculated into LB broth with or without erythromycin with the final cell density of 103 cells/ml and grown anaerobically or aerobically. The A600 of the cultures was measured every 30 min. The relatively long lag phase shown in the anaerobic growth curve was due to the low cell density of initiative inoculums that is necessary to ensure the consumption of residual O2 and initiation of an anaerobic environment for the growth of E. coli (details are under “Experimental Procedures”).
MdtEF Promotes Cell Survival during Anaerobic Respiration of Nitrate
The fact that MdtEF was up-regulated and active under anaerobic conditions without the presence of antibiotics and drugs suggests that MdtEF has physiological functions under this condition. To test this notion, we constructed ΔmdtEF in the E. coli K-12 wild type strain MG1655 and compared its growth with the WT under anaerobic conditions. A fairly slower growth rate was observed in the deletion strain compare with the WT (Fig. 5A), suggesting that MdtEF does contribute to the ordinary physiology of E. coli under anaerobic conditions. The fact that activation of mdtEF was subject to the regulation of ArcA, which regulates the expression of the genes involved in the anaerobic respiration in E. coli, prompted us to investigate whether MdtEF is also involved in the process of the anaerobic respiration. It is known that facultative anaerobes must utilize alternative terminal electron acceptors such as nitrate, sulfate, DMSO, fumarate, etc. to generate ATP under anaerobic conditions, and nitrate is the preferable alternative electron acceptor for this process (23). To examine whether MdtEF contributes to the physiology of anaerobic respiration of nitrate, we measured the growth of WT MG1655 and the isogenic ΔmdtEF strain in the presence of 10 mm nitrate under anaerobic conditions. However, no significant difference in growth rate between the two strains was observed as determined by optical density at 600 nm. Because the absorbance measurement does not reflect the viability of cell cultures, we performed viable plate counting to examine viable cell numbers in the two cultures. Surprisingly, we observed a significant decrease of cell viability in ΔmdtEF culture compared with that of the WT during prolonged growth (after 17 h) under this condition (Fig. 5B), suggesting that MdtEF contributes to the physiology of E. coli when cells respire nitrate. To examine whether the similar effect is observed during anaerobic respiration of other alternative electron acceptors, we performed the same viability assay in the presence of 40 mm fumarate. However, no difference on the viability of ΔmdtEF strain was observed compare with that of WT under this condition (Fig. 5C). These results suggest that MdtEF has a physiological function that is specifically associated with the anaerobic respiration of nitrate in E. coli.
FIGURE 5.
MdtEF promotes cell survival during anaerobic respiration of nitrate. A, ΔmdtEF E. coli cells grew more slowly than WT in rich medium LB under anaerobic conditions. B, deletion of mdtEF caused a significant decline of cell viability during the prolonged anaerobic respiration of nitrate and deletion of tnaAB, in which gene products are responsible for the biosynthesis of indole, restores this defect. C, deletion of mdtEF did not affect the survival of E. coli cells during anaerobic respiration of fumarate, another alternative electron acceptor used by E. coli in its anaerobic respiration. ΔtnaAB did not enhance the survival of ΔmdtEF under this condition. CFU, colony forming unit.
MdtEF Expels Nitrosyl Indole Derivatives during Anaerobic Respiration of Nitrate
Because MdtEF has been shown to function as an efflux pump in E. coli, we speculate that the anaerobically up-regulated MdtEF may function to expel certain cellular substances generated under this physiological condition. Kwon and Weiss et al. (24) have reported that a significant amount of indole derivatives, including indole red, indoxyl red, and indole trimer, were accumulated in anaerobically grown E. coli when the bacterium respires nitrate. These products were derived from nitrosylation and subsequent condensation of indole molecules by the reactive nitrogen species (RNS) produced during this physiological process (25–28). They showed that anaerobic accumulation of these toxic indole derivatives becomes more pronounced during prolonged anaerobic growth and leads to significant cell death during this growth stage (24). This observation correlates with our viable plate-counting assay, which demonstrated that a significant decrease of the viability of ΔmdtEF strain was also observed after long hours of cell growth. This led us to hypothesize that perhaps MdtEF is activated to export these toxic indole nitrosyl derivatives out of the E. coli cells.
To test this hypothesis, we synthesized the indole nitrosyl derivative, indole red (Fig. 6A), and performed the similar drug tolerance assay of cells to this compound. As shown in Fig. 6B, although both the KAM3 and KAM3 ΔmdtEF strains grew slower in the presence of 80 μm indole red than without the presence of the compound under anaerobic conditions, ΔmdtEF strain grew significantly slower than the parental KAM3 strain under this condition, suggesting MdtEF contributes to the tolerance of KAM3 to indole red. As expected, no growth difference was observed between the two strains when they were cultured aerobically in the presence of the same concentration of indole red (Fig. 6B), confirming the dependence of the expression of MdtEF to confer tolerance to indole red. To further confirm the capability of MdtEF to expel indole red, we performed the same tolerance assay under another circumstance where the expression of MdtEF is also induced; that is, the aerobic stationary growth of E. coli (15). It was shown that indeed the KAM3 strain subcultured from aerobic stationary phase cells displays increased tolerance to indole red in the same MdtEF-dependent manner (Fig. 6C). In contrast, cells subcultured from aerobic log phase cells showed no tolerance to indole red (Fig. 6C). These data together strongly support the notion that the function of the anaerobically up-regulated MdtEF is to expel the nitrosyl indole derivatives out of the cells.
FIGURE 6.
Anaerobic E. coli confers tolerance to indole red in an MdtEF-dependent manner. A, the structure of indole red is shown. Synthesis of indole red was according to the description by Astolfi et al. (16). B, shown is a tolerance assay of KAM3 and KAM3 ΔmdtEF to indole red (80 μm) under anaerobic and aerobic conditions. Anaerobic E. coli displays tolerance to indole red in an MdtEF-dependent manner. C, shown is a tolerance assay of KAM3 and KAM3 ΔmdtEF to indole red (100 μm) in cells subcultured from log phase or stationary phase E. coli cells under aerobic conditions. Stationary phase E. coli cells display tolerance to indole red in an MdtEF-dependent manner. Tolerance assay was performed as described in Fig. 4, except indole red instead of erythromycin was added to the growth medium.
If that is the case, we speculate that blocking the indole production should restore the decreased viability of ΔmdtEF cells in the presence of nitrate. This can be achieved by deletion of tnaAB, whose gene products are responsible for the biosynthesis of indole from tryptophan in the ΔmdtEF background strain. The similar viability assay showed that indeed ΔmdtEF and ΔtnaAB double deletion strain displayed a substantially enhanced viability than in the single deletion of mdtEF strain during the prolonged anaerobic growth in the presence of 10 mm nitrate (Fig. 5B), confirming that the defect of cell viability in ΔmdtEF strain was at least partially attributed to indole production. As expected, deletion of tnaAB does not affect the viability of either WT or ΔmdtEF strain when the assay was performed in the presence of fumarate (Fig. 5C). It is noteworthy that viability of MG1655 ΔmdtEF ΔtnaAB was actually enhanced to a level even higher than that of WT MG1655 cells, suggesting that indole production indeed imposes a harmful effect to anaerobically cultured cells, and there are perhaps other mechanisms in addition to the efflux by MdtEF that can lead to detoxification of indole nitrosative derivatives (see “Discussion”). Nonetheless, this result validated the role of MdtEF in detoxifying the harmful effect of nitrosyl indole derivatives in the cell and promoting cell survival during prolonged anaerobic respiration of nitrate in E. coli.
Indole Production Is Significantly Elevated during Anaerobic Growth of E. coli
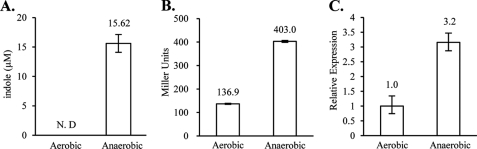
Because indole production is not limited to cells grown anaerobically, we raised the question of why significant induction of MdtEF to remove the indole nitrosyl products is necessary during the anaerobic respiration of nitrate. One reason is the production of RNS during this physiological process, which elicits the nitrosyl and subsequent condensation of indole molecules. Another possibility is that indole production might be elevated. To test this possibility, we first measured the concentration of indole in cells cultured under these two conditions. HPLC analysis of indole from aerobic and anaerobic log phase cells revealed that the indole concentration in anaerobic cells is about 16 μm, as opposed to no detectable levels of indole in aerobically grown MG1655 cells at the same cell density (Fig. 7A), confirming that indole production is indeed elevated during the anaerobic growth of E. coli. We further asked how indole production is significantly elevated under this condition by examining the expression of tryptophanase TnaA under anaerobic conditions. β-Galactosidase activity assay and RT-qPCR analyses showed that both the transcription of Ptna-lacZ and the mRNA of TnaA increased ∼3-fold in anaerobically grown cells than in aerobically grown cells (Fig. 7, B and C). These results combined rationalized the up-regulation of mdtEF in the anaerobic physiology of E. coli and its relevance to the anaerobic respiration of nitrate.
FIGURE 7.
Indole production is significantly elevated in anaerobic E. coli. A, the extracellular indole concentration of E. coli MG1655 grown under anaerobic conditions is significantly higher than that from aerobic cultures measured by HPLC analysis. B and C, transcription of PtnaA-lacZ and relative mRNA levels of TnaA are ∼3-fold higher under anaerobic conditions than aerobic conditions as determined by β-galactosidase activity assay (B) and RT qPCR (C). N.D. not determined.
DISCUSSION
Drug efflux has been shown to be associated with most clinical multidrug resistance by not only conferring simultaneous resistance to a wide range of antibiotics but also driving the acquisition of additional mechanisms of resistance (5). Efflux genes differ from other classical antibiotic resistance determinants in that efflux pump genes are widely distributed in the chromosome of all living organisms; their structures and genetic arrangement is highly conserved, and their expression is tightly regulated and often is not restricted to antibiotic exposure (29). These unique properties coupled with accumulating evidence in recent years demonstrating that in addition to antibiotics, efflux pumps efficiently extrude many environment and host derived toxic compounds, suggest that efflux pumps have a general function of detoxification in bacteria that is presumably tailored to their ecological niches (6, 7, 29, 30). Identifying the environmental or physiological conditions, which leads to the up-regulation of these genes, provides an effective means to uncover their physiological functions.
Previous studies revealed that a number of environmental and physiological signals such as the transition to stationary growth phase (30), acidic pH (31), and presence of certain metabolites and metabolite precursors (32, 33), can induce the transcription of efflux genes in aerobically grown E. coli. Many regulatory factors involved in these processes (33–35) have also been identified. However, the connection between the up-regulation of the efflux genes and the physiology of the cells under those circumstances has not been established. In this report we combined the study of gene expression and efflux activities with the physiological context of E. coli cells to define the natural functions of the efflux pump MdtEF. We demonstrated that the global anaerobic regulatory factor ArcA up-regulates the expression of MdtEF to protect cells from the toxicity of nitrosative stress during the anaerobic respiration of nitrate. This finding is in agreement with the primary role of the global transcription factor ArcA, which controls expression of genes associated with the transition from an aerobic to anaerobic mode of energy metabolism (17). Our finding also explained why mdtEF was also up-regulated during stationary growth of E. coli in which indole was significantly accumulated and nitrosative stress was also induced (15, 36). It is noteworthy that although other transcription factors such as EvgSA and a supplement of indole can trigger the expression of the gadE-mdtEF operon under the aerobic log phase growth conditions, the anaerobic up-regulation of mdtEF demonstrated in this study seems independent of these factors, as deletion of EvgSA and tnaAB (responsible for indole biosynthesis) does not affect the anaerobic transcription of PgadE-lacZ (data not shown) under anaerobic conditions. Taken together, a working model was developed to summarize the regulation and physiological roles of MdtEF in adapting E. coli to its anaerobic niches as shown in Fig. 8.
FIGURE 8.
A working model for the regulation and physiological roles of MdtEF during anaerobic growth of E. coli. During anaerobic respiration of nitrate in E. coli, RNS were generated and reacted with high levels of indole to form toxic indole nitrosative derivatives. Multidrug efflux pump mdtEF is up-regulated by ArcA during this process to export these compounds out of cells, thus protect cells against nitrosative damage during the anaerobic respiration of nitrate.
Nitrosative damage elicited by RNS is encountered by bacteria in many of their natural habitats and in human host (25–27). Known bacterial defense mechanisms against this damage include enzymatic conversion of RNS to innocuous nitrogen species, such as nitrate and nitrous oxide (27, 36) and metabolizing nitrosyl cellular products to reusable carbon and nitrogen sources (37). Here we show that in addition to these two mechanisms, bacteria also actively remove the nitrosyl-damaged cellular components through the action of efflux pump MdtEF. This is in accordance with the extensive distribution of efflux genes in the genomes of all bacterial species and their general role of detoxification. In support of this notion, an RND efflux system MexEF-OprN was also shown to be activated by nitrosative stress in Pseudomonas aeruginosa (38) and perhaps protects cells against nitrosative damage in a similar fashion as the MdtEF-TolC system in E. coli.
Indole is an important intracellular signaling molecule for indole production bacteria (39). Its exact role in the anaerobic physiology of bacteria and how it is relevant to bacterial tolerance to antibiotics is not fully clear. A recent study showed that indole serves as a signal to enter the antibiotic vulnerable E. coli subpopulation and subsequently activate MdtEF efflux pumps in these cells, thus promoting the survival of the entire population of the bacterium in the face of antibiotic challenges (40). This finding reinforced the physiological relevance of indole production and activation of the MdtEF efflux pump. However, indole does not directly activate the expression of mdtEF either in aerobic stationary phase (15) or under anaerobic conditions, as neither ΔtanAB nor a supplement of extra indole to the growth medium affects the transcription of PgadE under anaerobic condition (data not shown). Indeed, indole as a normal metabolite of E. coli is exported through a different efflux system AcrEF-TolC (41) rather than the MdtEF-TolC system, supporting a role of MdtEF in adapting E. coli cells to altered or stress conditions rather than during its ordinary growth. This also suggests that E. coli cells bear an intricate mechanism that allows differentiation of the natural signaling molecule (indole) from the harmful, non-metabolizable substances (nitrosative indole derivatives), perhaps through mediating the binding affinity of efflux pumps to different substrates or exploiting different regulatory pathways to control the expression of different efflux genes.
Nonetheless, the fact that expression of mdtEF is regulated by different regulatory factors (33–35) under different environmental and physiological conditions (32–35) suggests that perhaps MdtEF plays an important and general role of detoxification in adapting E. coli to its various ecological niches. If that is the case, it would be intriguing to study whether this efflux pump is tailored to export different types of harmful substances under different stress conditions in future studies.
Supplementary Material
Acknowledgment
We are grateful to Dr. Patricia Kiley (University of Wisconsin-Madison) for critical reading of the manuscript.
This work was supported by the Hong Kong General Research Fund (HKU 781509M; to A. Y.) and by the Cooperative Research Program of “Network Joint Research Center for Materials and Devices” (to A. Y. and K. N.) as well as grants from the Japan Society for the Promotion of Science and the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to K. N.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1.
- RND
- resistance nodulation division
- qPCR
- quantitative PCR
- FNR
- fumarate nitrate reduction
- ArcA
- anaerobic respiration control
- RNS
- reactive nitrogen species.
REFERENCES
- 1. Krulwich T. A., Lewinson O., Padan E., Bibi E. (2005) Nat. Rev. Microbiol. 3, 566–572 [DOI] [PubMed] [Google Scholar]
- 2. Li X. Z., Nikaido H. (2009) Drugs 69, 1555–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nishino K., Yamaguchi A. (2008) IUBMB life 60, 569–574 [DOI] [PubMed] [Google Scholar]
- 4. Piddock L. J. V. (2006) Nat. Rev. Microbiol. 4, 629–636 [DOI] [PubMed] [Google Scholar]
- 5. Poole K. (2005) J. Antimicrob. Chemother. 56, 20–51 [DOI] [PubMed] [Google Scholar]
- 6. Poole K. (2008) Microbe 3, 179 [Google Scholar]
- 7. Allen H. K., Donato J., Wang H. H., Cloud-Hansen K. A., Davies J., Handelsman J. (2010) Nat. Rev. Microbiol. 8, 251–259 [DOI] [PubMed] [Google Scholar]
- 8. Takatsuka Y., Chen C., Nikaido H. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 6559–6565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nishino K., Yamaguchi A. (2001) J. Bacteriol. 183, 5803–5812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Datsenko K. A., Wanner B. L. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yu D., Ellis H. M., Lee E. C., Jenkins N. A., Copeland N. G., Court D. L. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 5978–5983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elledge S. J., Davis R. W. (1989) Genes Dev. 3, 185–197 [DOI] [PubMed] [Google Scholar]
- 13. Mettert E. L., Kiley P. J. (2007) J. Bacteriol. 189, 3036–3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bustin S. A., Benes V., Garson J. A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M. W., Shipley G. L., Vandesompele J., Wittwer C. T. (2009) Clin. Chem. 55, 611–622 [DOI] [PubMed] [Google Scholar]
- 15. Kobayashi A., Hirakawa H., Hirata T., Nishino K., Yamaguchi A. (2006) J. Bacteriol. 188, 5693–5703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Astolfi P., Panagiotaki M., Rizzoli C., Greci L. (2006) Org. Biomol. Chem. 4, 3282–3290 [DOI] [PubMed] [Google Scholar]
- 17. Georgellis D., Kwon O., Lin E. C. C. (2001) Science 292, 2314–2316 [DOI] [PubMed] [Google Scholar]
- 18. Kiley P. J., Beinert H. (2003) Curr. Opin. Microbiol. 6, 181–185 [DOI] [PubMed] [Google Scholar]
- 19. Spiro S. (1994) Antonie van Leeuwenhoek 66, 23–36 [DOI] [PubMed] [Google Scholar]
- 20. Richard H. T., Foster J. W. (2003) Adv. Appl. Microbiol. 52, 167–186 [DOI] [PubMed] [Google Scholar]
- 21. Sayed A. K., Foster J. W. (2009) Mol. Microbiol. 71, 1435–1450 [DOI] [PubMed] [Google Scholar]
- 22. Morita Y., Kodama K., Shiota S., Mine T., Kataoka A., Mizushima T., Tsuchiya T. (1998) Antimicrob. Agents Chemother. 42, 1778–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Unden G., Bongaerts J. (1997) Biochim. Biophys. Acta 1320, 217–234 [DOI] [PubMed] [Google Scholar]
- 24. Kwon Y. M., Weiss B. (2009) J. Bacteriol. 191, 5369–5376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Corker H., Poole R. K. (2003) J. Biol. Chem. 278, 31584–31592 [DOI] [PubMed] [Google Scholar]
- 26. Fang F. C. (2004) Nat. Rev. Microbiol. 2, 820–832 [DOI] [PubMed] [Google Scholar]
- 27. Poole R. K. (2005) Biochem. Soc. Trans. 33, 176–180 [DOI] [PubMed] [Google Scholar]
- 28. Weiss B. (2006) J. Bacteriol. 188, 829–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martinez J. L., Sánchez M. B., Martínez-Solano L., Hernandez A., Garmendia L., Fajardo A., Alvarez-Ortega C. (2009) FEMS Microbiol. Rev. 33, 430–449 [DOI] [PubMed] [Google Scholar]
- 30. Helling R. B., Janes B. K., Kimball H., Tran T., Bundesmann M., Check P., Phelan D., Miller C. (2002) J. Bacteriol. 184, 3699–3703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kailasan Vanaja S., Bergholz T. M., Whittam T. S. (2009) J. Bacteriol. 191, 1868–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hirakawa H., Inazumi Y., Senda Y., Kobayashi A., Hirata T., Nishino K., Yamaguchi A. (2006) J. Bacteriol. 188, 5851–5858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hirakawa H., Inazumi Y., Masaki T., Hirata T., Yamaguchi A. (2005) Mol. Microbiol. 55, 1113–1126 [DOI] [PubMed] [Google Scholar]
- 34. Nishino K., Senda Y., Yamaguchi A. (2008) J. Antibiot. 61, 120–127 [DOI] [PubMed] [Google Scholar]
- 35. Hirakawa H., Nishino K., Hirata T., Yamaguchi A. (2003) J. Bacteriol. 185, 1851–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weiss B. (2001) Mutat. Res. 461, 301–309 [DOI] [PubMed] [Google Scholar]
- 37. Rankin L. D., Bodenmiller D. M., Partridge J. D., Nishino S. F., Spain J. C., Spiro S. (2008) J. Bacteriol. 190, 6170–6177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fetar H., Gilmour C., Klinoski R., Daigle D. M., Dean C. R., Poole K. (2011) Antimicrob. Agents Chemother. 55, 508–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee J. H., Lee J. (2010) FEMS Microbiol. Rev. 34, 426–444 [DOI] [PubMed] [Google Scholar]
- 40. Lee H. H., Molla M. N., Cantor C. R., Collins J. J. (2010) Nature 467, 82–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kawamura-Sato K., Shibayama K., Horii T., Iimuma Y., Arakawa Y., Ohta M. (1999) FEMS Microbiol. Lett. 179, 345–352 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.