Abstract
CO-releasing molecules (CO-RMs) were previously shown by us to be more potent bactericides than CO gas. This suggests a mechanism of action for CO-RM, which either potentiates the activity of CO or uses another CO-RM-specific effect. We have also reported that CORM-2 induces the expression of genes related to oxidative stress. In the present study we intend to establish whether the generation of reactive oxygen species by CO-RMs may indeed result in the inhibition of bacterial cellular function. We now report that two CO-RMs (CORM-2 and ALF062) stimulate the production of ROS in Escherichia coli, an effect that is abolished by addition of antioxidants. Furthermore, deletion of genes encoding E. coli systems involved in reactive oxygen species scavenging, namely catalases and superoxide dismutases, potentiates the lethality of CORM-2 due to an increase of intracellular ROS content. CORM-2 also induces the expression of the E. coli DNA repair/SOS system recA, and its inactivation enhances toxicity of CORM-2. Moreover, fluorescence microscopy images reveal that CORM-2 causes DNA lesions to bacterial cells. We also demonstrate that cells treated with CORM-2 contain higher levels of free iron arising from destruction of iron-sulfur proteins. Importantly, we show that CO-RMs generate hydroxyl radicals in a cell-free solution, a process that is abolished by scavenging CO. Altogether, we provide a novel insight into the molecular basis of CO-RMs action by showing that their bactericidal properties are linked to cell damage inflicted by the oxidative stress that they are able to generate.
Keywords: Bacteria, Drug Action, Iron-Sulfur Protein, Oxidative Stress, Reactive Oxygen Species (ROS), CO-RM, Carbon Monoxide
Introduction
Carbon monoxide (CO)2 is generated in the human body by the degradation of heme mediated by heme oxygenase (HO) enzymes. The endogenous CO production has benefits in the neural, cardiovascular, and renal systems, having a cytoprotective effect in several pathologic conditions such as atherosclerotic vascular disease and inflammation (1). Nevertheless, high concentrations of CO are toxic resulting primarily from the binding of CO to hemoglobin and to cytochrome c oxidase, the terminal electron acceptor of the respiratory chain (2, 3). In mice brains it was observed that CO toxicity, associated with the binding of CO to the terminal oxidase, increases the generation of mitochondrial reactive oxygen species (ROS), namely hydrogen peroxide, augments the level of oxidized proteins and lipid peroxidation of the plasma, and decreases the ratio reduced/oxidized glutathione. As expected, these effects are counteracted by the presence of superoxide dismutase and iron chelators (4). One of the three isoforms of mammalian heme oxygenase enzymes, HO-1, is inducible by several stress agents, including heme, heat shock, pro-inflammatory cytokines, and hypoxia. Because these agents share the ability to directly or indirectly generate ROS, the elevated HO-1 expression is considered as a cellular indicator of oxidative stress (3, 5). On the other hand, HO-1-derived CO has been reported to have remarkable benefits on microbial sepsis, as HO-1-deficient mice display enhanced susceptibility to polymicrobial infections and the administration of exogenous CO rescues the HO-1-deficient mice from sepsis-induced lethality (6). Exogenous administration of CO, via CO-releasing molecules (CO-RMs), also shown to be able to kill bacteria (7), and the physiological consequences of the action of CO-RMs on bacteria addressed by global transcriptional analysis revealed that CO-RMs induce a significant alteration on the bacterial transcriptome. In particular, induction of genes encoding two main redox-sensing regulators that control the bacterial oxidative response, SoxS and OxyR, occurs in cells exposed to CORM-2, and Escherichia coli strains lacking oxyR and soxS genes exhibit enhanced susceptibility to CORM-2, altogether suggesting that CO potentiates the generation of reactive oxygen species (8).
The generation of intracellular ROS causes deterioration of DNA, lipids, and proteins and several targets have been linked to loss of cell integrity and/or viability (9). DNA is especially prone to iron-catalyzed oxidation, as iron binds directly to the phosphodiester backbone where OH radicals are subsequently generated (10, 11). In particular, guanine residues on DNA are targets of oxidation as well as thiol groups and iron-sulfur clusters of proteins (9, 12). Besides loss of function, denaturation of iron-sulfur clusters contributes itself to toxicity because the iron that is released upon oxidation of the clusters provokes additional oxidative damage via the Fenton reaction (11, 13).
Nevertheless, bacteria have several mechanisms to avoid this outcome. The typical responses to ROS include DNA repair systems (e.g. RecA), the action of scavenging molecules (e.g. glutathione) and detoxification systems, like superoxide dismutase and catalase (9). Moreover, oxidative conditions have been shown to trigger the Isc and Suf systems, which are responsible for the de novo assembly of iron-sulfur clusters in nascent polypeptides (14, 15).
In the present work we have exposed bacterial cultures to CO-releasing molecules and determined the effect of these compounds on cell viability upon addition of antioxidants, measured the level of ROS, analyzed the deletion of ROS scavenging systems, observed the degree of DNA damage, evaluated the status of iron and iron-sulfur enzymes, and tested the generation of oxygen radicals by the CO-releasing molecules. Altogether, the data revealed that the bactericidal action of CO-releasing molecules relies on the generation of deleterious intracellular oxidative stress conditions.
EXPERIMENTAL PROCEDURES
Bacterial Strains, Growth Conditions, and Viability Assays
The bacterial strains used in this work are described in Table 1. All E. coli cells, grown aerobically in minimal medium salts (7) at 37 °C and 150 rpm, were cultured until reaching an optical density at 600 nm (OD600 nm) of 0.3 at which point the CO-releasing molecules were added. Tricarbonyldichlororuthenium (II) dimer (CORM-2) from Sigma and tetraethylammonium molybdenum pentacarbonylbromide (ALF062) from Alfama (Oeiras, Portugal) (7) were used as CO donors; these compounds were freshly prepared as 10 mm stock solutions by dissolution in dimethyl sulfoxide and methanol, respectively. Ru(II)Cl2(DMSO)4 from Strem Chemicals (Bischheim, France) was used as the inactive form of CORM-2 (iCORM-2) and the inactivated form of ALF062, named iALF062, was prepared by overnight vigorous mixing of the compound with methanol. When required, together with CO-RMs, reduced hemoglobin (20 μm; Sigma), reduced glutathione (5 mm; Calbiochem, Lisbon, Portugal), glutamate (50 mm; Sigma), cysteine (600 μm; Sigma), and methionine (3 mm; Sigma) were added to the growth culture. To determine the cell viability of E. coli strains, the number of colony forming unit(s) was evaluated in the presence of the compounds and their inactive forms. The percentage of survival was determined as the number of colonies obtained after CO-RMs treatment divided by the number of colonies formed in the cultures treated with the inactive form. Wild type E. coli cells were treated with 250 μm CORM-2 and 50 μm ALF062, whereas for the analysis of the E. coli mutant strains and their parent strains CORM-2 was used at a concentration of 150 μm. The experiments were performed in duplicate with at least two independent cultures. The results are presented as averaged values with error bars representing S.E.
TABLE 1.
E. coli strains used in this study
| E. coli strains | Description | Source or Ref. |
|---|---|---|
| K12 MG1655 | Wild type strain | Laboratory stock |
| DSH7 | Parental strain | 38 |
| ΔkatEG | DSH19, DSH7 katE1, katG17::Tn10 | 38 |
| ΔsodAB | DSH56, DSH7 φ(sodA′-′lacZ)49 Cmr, φ(sodB′-′kan)/Δ2Kmr) | 38 |
| BW25113 | Parental strain | 39 |
| ΔiscS | BW25113 iscS::Kanr | 39 |
| ΔtonB | BW25113 tonB::Kanr | 39 |
| ΔiscR | BW25113 iscR::Kanr | 39 |
| ΔsufS | BW25113 sufS:Kanr | 39 |
| ΔrecA | BW25113 recA::Kanr | 39 |
Endogenous ROS Production
Cells of E. coli were treated for 1 h, in the presence of 250 μm CORM-2, 50 μm ALF062, or their respective inactive forms. The E. coli mutant strains were treated with 150 μm CORM-2 and iCORM-2 for 1 and 4 h. Cells were harvested, washed twice with phosphate buffer (PBS), and resuspended in the same buffer. The probe 2′,7′-dichlorofluorescein diacetate (Sigma) was then added to cell suspensions, at a final concentration of 10 μm, and fluorescence intensity (FI) was acquired during 2 h using a Varian Eclipse 96-well spectrofluorimeter (wavelength of excitation at 485 nm and emission at 538 nm). To determine the ROS variation, the FI of cultures treated with control compounds were subtracted from those of cells treated with CO-RMs. The FI was normalized in relationship to the A600 nm of each culture. These experiments were done in duplicate with at least two different biological samples.
Quantitative Real-time RT-PCR
For real-time reverse transcription (RT)-PCR experiments, 0.5 μg of E. coli total RNA, from two independent cultures, grown with 250 μm CORM-2 and control compound for 1 h, was used to synthesize cDNA with the First Strand cDNA Synthesis Kit for RT-PCR from Roche Applied Science. Real-time PCR experiments were performed in a LightCycler Instrument using LightCycler® FastStart DNA Master SYBR Green I Kit according to the manufacturer's instructions (Roche Applied Science). The amplification reactions were carried out using the previously synthesized cDNA as the initial template, and each reaction mixture contained 0.5 μm specific oligonucleotides (recA_up, 5′-CCTTGCGGCACGTATGATGA-3′ and recA_low, 5′-GGCGCAGCGATTTTGTTCTT-3′), 2 mm MgCl2, and hot-start PCR mixture (Roche Applied Science). The expression ratio of the target gene was determined relatively to a reference gene, the E. coli 16S rRNA, whose transcription remains invariant under the tested conditions. The samples were assayed in triplicate.
DNA Damage Assays
For DNA damage assays, E. coli cultures were exposed for 1 h to 8 mm H2O2 (as control), 250 μm CORM-2 and iCORM-2, and treated and analyzed according to the manufacturer's instructions of the hOGG1 FlareTM assay kit (Trevigen, UK). Briefly, cells were centrifuged, washed, and resuspended in PBS to obtain a bacterial concentration of 108 colony forming units/ml. The cells were mixed with low agarose, immersed in a lysis solution for 1 h, and then submitted to electrophoresis for 1 h at 12 V in TAE buffer. The SYBR® Green I dye was used for DNA visualization and the images were acquired with a fluorescence microscope (Leica).
Reaction of CORM-2 with Free Thiol Groups
The reduction of Ellman reagent (DTNB, 5,5′-dithiobis-(2-nitrobenzoic acid); Sigma) by free thiol groups releases 5-thio-2-nitrobenzoic acid, which can be monitored spectrophotometrically at 412 nm (16). Increasing amounts of CORM-2, iCORM-2, and iodocetamide were mixed with 100 μm glutathione (GSH) in 100 mm phosphate buffer (pH 7). After 1 h at room temperature, 500 μm DTNB was added and the absorbance at 412 nm was measured. Iodocetamide is a irreversible thiol-modifying compound and was used as a positive control. Samples and standard curves were done in duplicate. Control reactions performed in the absence of GSH showed no absorbance.
EPR Studies
Free Iron Detection
The intracellular free iron content was determined by EPR in whole cells of E. coli grown for 1 h with 250 μm CORM-2 and iCORM-2, collected by centrifugation, resuspended in 9 ml of fresh minimal medium salts, and treated with 1 ml of 0.2 m deferoxamine mesylate (Sigma) for 10 min at 37 °C. Cells were then washed once with ice-cold 20 mm Tris-HCl (pH 7.4) and resuspended in 0.4 ml of the same buffer supplemented with 10% (v/v) glycerol. Aliquots of 0.3 ml were transferred to EPR tubes and immediately frozen in liquid nitrogen. The EPR spectra were obtained on a Bruker EMX spectrometer equipped with an Oxford Instruments continuous flow helium cryostat and recorded at 9.7 GHz microwave frequency, 20 milliwatt microwave power, modulation of amplitude of 2 millitesla at 95 K. The iron content of each sample was determined by measuring the amplitude of the EPR signal and normalizing it for the total protein concentration, which was determined by the bicinchoninic acid method (17). The results represent the average of five independent cultures.
EPR Spin-trap Analysis
The 5-tert-butoxycarbonyl 5-methyl-1-pyrroline N-oxide (BMPO) from Applied Bioanalytical Labs (Bradenton, FL) was used as a spin trap for the detection of reactive oxygen species. This spin trap allows one to distinguish between the superoxide anion and the hydroxyl radical (18). Experiments were conducted with 5 mm BMPO, 2 mm CORM-2 and iCORM-2, and when required 1 mm hemoglobin. EPR spectra were obtained for samples of ALF062 (199 μm in H2O plus 0.7% methanol) mixed with BMPO (25 mm), which were previously bubbled with oxygen or degassed with nitrogen. Samples were loaded into a quartz flat cell and EPR spectra were recorded, at room temperature, on the Bruker EMX spectrometer and recorded at 9.8 GHz microwave frequency, 2 milliwatt microwave power, and modulation of amplitude of 0.1 millitesla.
Enzymatic Assays
E. coli cultures treated for 1 h with 250 μm CORM-2 and iCORM-2 were washed and resuspended in the respective assay buffer before being disrupted in a French press. The aconitase activity was measured, under anaerobic conditions, using the indirect method described by Gardner et al. (19), which follows the production of NADPH at 340 nm (ϵ340 nm = 6220 m−1 cm−1). To this end, samples were added to the reaction mixture that contained 50 mm Tris-HCl (pH 7.6), 0.6 mm MnCl2, 0.2 mm NADP+, and 1 unit of isocitrate dehydrogenase (Sigma). The reaction was initiated with 30 mm sodium citrate. One unit of aconitase activity was defined as 1 μmol of NADPH formed per min at 25 °C. The activity of glutamate synthase was determined by the formation of NADP+, following the decrease in absorbance at 340 nm (ϵ340 nm = 6220 m−1 cm−1) (20). The activity was measured in reaction mixtures containing the lysate samples in 100 mm Tris-HCl (pH 7.8) buffer plus 10 mm glutamine and 0.2 mm NADPH, and initiated by addition of 5 mm α-ketoglutarate. One unit of glutamate synthase activity was defined as 1 nmol of NADPH consumed per min at 25 °C. The glutamate dehydrogenase activity was determined as the above described glutamate synthase activity, using 5 mm ammonium chloride instead of glutamine. Activities were assayed in a Shimadzu UV-1700 and normalized to the total cellular protein lysate content determined by bicinchoninic acid assays (17).
RESULTS
Bactericidal CO-RMs Induce ROS
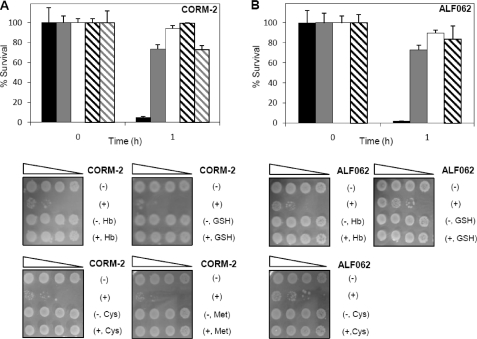
Previous studies have shown that CO-RMs alter the transcription of several genes involved in oxidative stress response, suggesting that CO-RMs elicit the production of reactive oxygen species (8). To infer if biological antioxidants attenuate the bactericidal effect of CO-RMs, we have evaluated the viability of E. coli cells upon treatment with two CO donors, namely CORM-2 and ALF062 (Fig. 1), in the presence of exogenous reduced glutathione (GSH) and cysteine. The data showed that GSH was able to restore the viability of the microbial cells by ∼95%. Furthermore, supplementation of the culture medium with cysteine allowed a cell growth recovery of ∼80% (Fig. 2, A and B). These results revealed that the presence of antioxidants causes reduction of the CO-RMs efficiency.
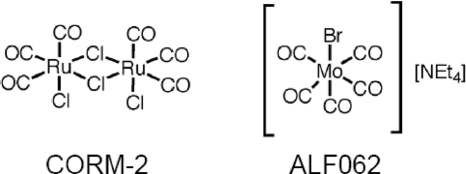
FIGURE 1.
Chemical structure of CO-RMs used in this study.
FIGURE 2.
Lethality of CO-releasing molecules is abolished by antioxidants. E. coli cells were grown under aerobic conditions and exposed to 250 μm CORM-2 (A) and 50 μm ALF062 (B) for 1 h, in medium containing the CO-releaser (black bars), and supplemented with hemoglobin (Hb, gray bars), glutathione (GSH, white bars), cysteine (Cys, black striped bars), and methionine (Met, gray striped bars). Survival percentage was calculated in relationship to cultures treated with the respective inactive compound. The results represent the average of two independent cultures performed in duplicate, and error bars represent the mean ± S.E. Sensitivity tests performed in cultures treated for 1 h with CO-RMs (+) and inactive forms (−) are also shown.
Because E. coli cells submitted to oxidative stress develop a methionine auxotrophy (21) and it was previously detected that CORM-2 interferes with the E. coli methionine metabolism (8), we have now analyzed the growth behavior of cells exposed to CORM-2 in the presence of methionine. We observed that addition of this amino acid abolishes the action of the bactericide as viability of bacteria treated with CORM-2 increased significantly in the methionine-supplemented culture (Fig. 2A).
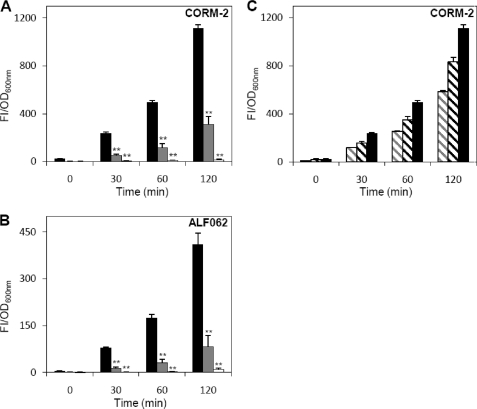
Next, we have examined the level of endogenous reactive oxygen species formed in cells treated with CORM-2 and ALF062. The data revealed a significant increase of ROS content in cells exposed to either compound (Fig. 3, A and B). Importantly, the level of ROS formed was dependent on the CORM-2 concentration utilized (Fig. 3C). Furthermore, a strong decrease of ROS production was observed upon addition of glutathione (Fig. 3, A and B). Quite importantly, the generation of ROS by CO-RMs was shown to be strictly dependent on CO because cells treated with inactive compounds, namely iCORM-2 and iALF062, which are CO-depleted molecules, exhibited ROS levels similar to that of untreated cells (FI/OD600 nm ∼30 at 120 min). Furthermore, when the medium was supplemented with the CO scavenger molecule hemoglobin, the ROS formation decreased more than 80% for both CORM-2 and ALF062 (Fig. 3, A and B).
FIGURE 3.
CO-RMs increase intracellular ROS content. ROS were quantified in E. coli cells treated, for 1 h, with 250 μm CORM-2 (A) and 50 μm ALF062 (B) with the CO releasers (black bars) and presence of hemoglobin (gray bars) or glutathione (white bars). In C, cells were treated with increased concentrations of CORM-2, 150 μm (gray striped bars), 200 μm (black striped bars), and 250 μm (black bars). The FI are represented as the subtraction of cultures treated with the inactive compound from cultures exposed to CO-releasing molecules and normalized in relationship to the OD600 nm of the respective culture. Each bar represents the average of three independent measurements with the corresponding standard errors (**, p < 0.01).
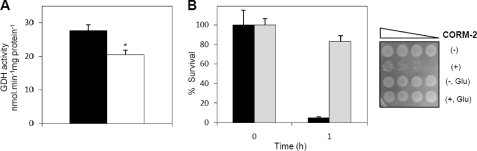
Because the activity of glutamate dehydrogenase was recently reported to decrease under oxidative stress (22), we tested this activity that was found to be indeed lowered by 26% in cells treated with CORM-2 (Fig. 4A). Moreover, supplementation of E. coli culture with glutamate, which is the substrate of GDH, abolishes the effect of CORM-2 (Fig. 4B).
FIGURE 4.
Glutamate dehydrogenase activity is lower in cells treated with CORM-2. A, activity of GDH in cell extracts of E. coli treated for 1 h with iCORM-2 (black bars) and CORM-2 (white bars). The error bars represent the mean ± S.E. (*, p < 0.05). B, effect of 250 μm CORM-2 on E. coli viability of aerobically grown cells exposed to the CO releaser in the absence (black bars) and presence of glutamate (Glu, gray bars). The percentage of survival was calculated in relationship to cultures treated with the respective inactive compound, and error bars represent the mean ± S.E. Sensitivity tests performed in cultures treated, for 1 h, with CORM-2 (+) and iCORM-2 (−) are also presented.
Altogether, these results showed that cells exposed to CORM-2 are under oxidative stress. As both CORM-2 and ALF062 caused similar oxidative stress conditions in E. coli cells the subsequent experiments were conducted only with CORM-2.
Deletion of Superoxide Dismutase and Catalase Genes Increase CORM-2 Lethality
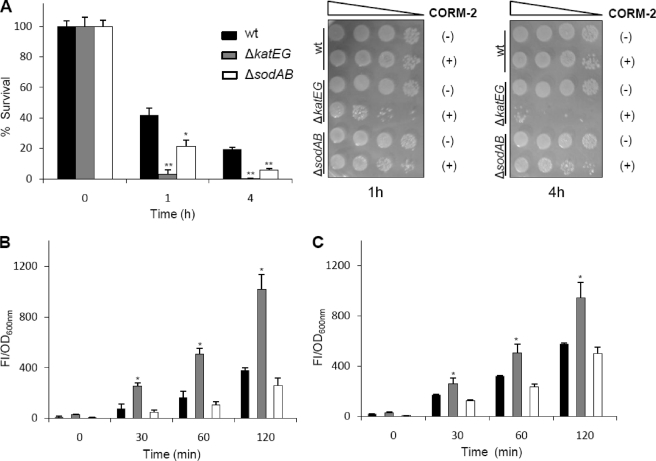
The observation that CO-RMs increase ROS production led us to hypothesize that strains deleted in the major antioxidant systems should be more prone to killing by CO-RMs. Therefore, we have analyzed the phenotype of E. coli strains deleted in catalases (ΔkatEG) and superoxide dismutases (ΔsodAB), in the presence of CORM-2.
The data regarding viability of ΔkatEG and ΔsodAB mutants showed that these strains have increased susceptibility to CORM-2 when compared with the wild type, and that the effect is higher for the catalase-deficient strains (Fig. 5A). In agreement, CORM-2-treated cells of ΔkatEG have higher amounts of ROS (Fig. 5, B and C). However, the ROS content of the ΔsodAB mutant strain did not vary, a result that may be due to the fact that superoxide anions do not directly oxidize the 2′,7′-dichlorofluorescein fluorescent probe (23). Therefore, it was concluded that the addition of CORM-2 to bacterial cells leads to an intracellular oxidative stress status that is exacerbated in the absence of oxidative defense systems.
FIGURE 5.
Bactericidal effect of CORM-2 is enhanced upon deletion of ROS scavenger systems. A, cell viability of the parental E. coli strain (black bars) and mutant strains, ΔkatEG (gray bars) and ΔsodAB (white bars), were determined upon treatment with 150 μm CORM-2 and iCORM-2 for 1 and 4 h. For the same times, sensitivity tests were performed in cultures treated with CORM-2 (+) and iCORM-2 (−). ROS intracellular content of parental (black bars), ΔkatEG (gray bars), and ΔsodAB (white bars) strains were determined in cells exposed 1 (B) and 4 h (C) to 150 μm CORM-2 and iCORM-2. The FI are represented as a subtraction of the cultures treated with iCORM-2 from cultures treated with CORM-2 and normalized in relationship to OD600 nm of each culture. The error bars represent the mean ± S.E. of the average values obtained from at least two independent biological samples performed in duplicate (*, p < 0.05; **, p < 0.01).
CORM-2 Induces DNA Damage
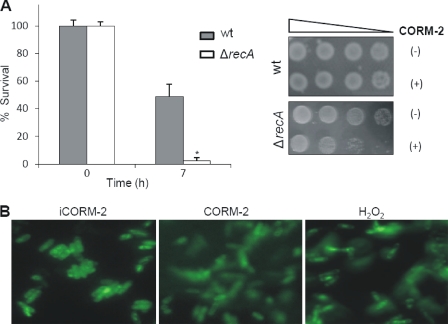
DNA is one of the main targets of ROS and because we found that CO-RMs increase the ROS production in E. coli cells, we examined if the effect of CORM-2 was potentiated in a recA mutant strain. RecA participates in the double strand break repair, catalyzing the homologous recombination and function as a regulatory protein to induce the SOS response to DNA damage (24). We observed that CORM-2 increases the expression of recA by ∼2.5-fold and that the deletion of recA yielded an E. coli strain that exhibited increased susceptibility to CORM-2-treated cells (Fig. 6A). Hence, we have analyzed if CORM-2-derived ROS caused DNA lesions. To this end, DNA damage was assayed in cells treated with CORM-2, iCORM-2, and H2O2 (which was used as a control) using the glycosylase OGG1 enzyme that detects 8-oxoguanine, a base modification that usually occurs upon DNA damage. The images acquired by fluorescence microscopy of cells treated with CORM-2 and hydrogen peroxide revealed the presence of long comet tails characteristic of damaged DNA. In contrast, cells exposed to the inactive compound, iCORM-2, exhibited images where the supercoiled form of undamaged DNA was visualized (Fig. 6B). Thus, the data revealed that CORM-2 is a DNA-damaging drug, which is due to the ROS being generated.
FIGURE 6.
CORM-2 causes damages to bacterial DNA. A, cell viability of E. coli recA mutant (white bars) and its parental strain (gray bars) grown under aerobic conditions and exposed to 150 μm CORM-2 and iCORM-2 was determined after 7 h. Error bars represent the mean ± S.E. (*, p < 0.05). Sensitivity tests were also performed in cultures treated with CORM-2 (+) and iCORM-2 (−). B, fluorescent microscopy of E. coli cells challenged, for 1 h, with 250 μm CORM-2, iCORM-2, and H2O2.
CORM-2 Interferes with Iron Homeostasis
The formation of ROS, particularly the generation of hydroxyl radicals, is usually associated with the presence of free iron that acts as a catalyst in the Fenton reaction. Hence, the intracellular levels of free iron upon exposure to CORM-2 were evaluated by means of whole cell EPR. The cells treated with CORM-2 have a 4-fold higher content of free iron than cells treated with the compound devoid of CO (supplemental Fig. S1).
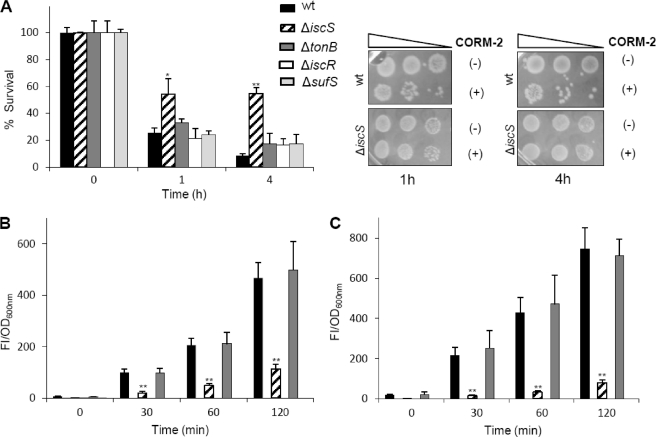
The higher level of free iron detected in cells treated with CORM-2 may come from extracellular sources due to alterations in iron import or have an intracellular origin arising from destruction of iron containing proteins. To test the first hypothesis, we have examined the viability of cells lacking tonB, which is one of the major iron import systems of E. coli (25). However, no effect was observed on the viability of CORM-2-treated E. coli tonB mutant (Fig. 7A), and no differences were detected in ROS production in the same mutant when compared with its parental strain (Fig. 7, B and C). These results indicate that exogenous iron does not play a significant role in oxidative damage-mediated cell killing.
FIGURE 7.
CORM-2 efficiency depends on iron-sulfur cell content. A, viability of E. coli parental strain (black bars), ΔiscS (striped bars), ΔtonB (dark gray bars), ΔiscR (white bars), and ΔsufS (light gray bars) determined upon exposure to 150 μm CORM-2 and iCORM-2, for 1 and 4 h. Sensitivity tests were also performed in ΔiscS cells treated with CORM-2 (+) and iCORM-2 (−) for 1 and 4 h. ROS intracellular content evaluated in the parental strain (black), ΔiscS (striped), and ΔtonB (dark gray) upon exposure for 1 (B) and 4 h (C) to 150 μm CORM-2 and iCORM-2. The FI represent the subtraction of the cultures treated with iCORM-2 from cultures treated with CORM-2 normalized in relationship to OD600 nm of the respective culture. The error bars are the mean ± S.E. of the average values obtained from two independent biological samples performed in duplicate (*, p < 0.05; **, p < 0.01).
We next evaluated if upon exposure of cells to CORM-2 denaturation of iron-sulfur clusters supplied intracellular iron for ROS generation. To this end, we have analyzed the impact of CORM-2 on the phenotype of E. coli strains deleted in genes involved in Fe-S assembly, namely the cysteine desulfurase iscS, a gene product that acts as the sulfur donor for biogenesis of Fe-S clusters, iscR, the regulator of the iscRSUA operon, and sufS, an iscS paralogue putatively required for Fe-S assembly under stress conditions (26).
Upon exposure to CORM-2 the behavior of strains lacking iscR and sufS remained essentially unaltered when compared with their respective parental strains (Fig. 7A). The viability of the CORM-2-treated ΔiscS strain was significantly higher and exhibited a lower content of ROS (Fig. 7, A–C). These results are in agreement with a decreased content of Fe-S centers originating from the absence of iscS, which translates into a diminished CORM-2 toxicity.
The higher level of intracellular iron in cells exposed to CORM-2 suggests that Fe-S containing enzymes are being degraded. This was corroborated by the lower activity of two enzymes, namely aconitase and glutamate synthase, measured in cells treated with the CO-releaser. We observed that exposure of cell extracts to this bactericide resulted in a significant decrease of the activity of the two enzymes (∼75 and 82% for aconitase and glutamate synthase, respectively), whereas no alterations occurred when using iCORM-2 (supplemental Fig. S2). Overall, these results revealed that the ROS-associated action of CORM-2 relies on the intracellular iron released from Fe-S clusters, with a concomitant decrease in the enzymatic activity of key metabolic enzymes of E. coli, and that iron-sulfur centers constitute a source of iron that potentiates the Fenton reaction.
In Vitro Reaction of CORM-2 with Free Thiol Groups
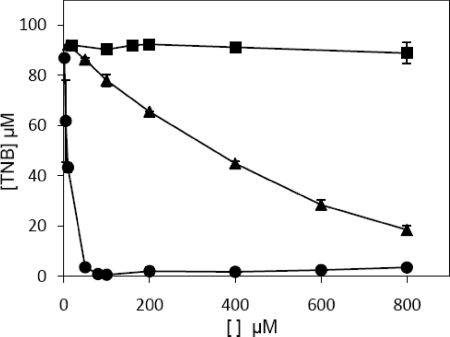
Because we observed that cysteine and methionine supplementation abolished the action of CORM-2 (Fig. 2A), we sought to investigate whether CORM-2 could react in vitro with thiol groups. To this end, Ellman reagent, DTNB, which is reduced by free thiol groups to 5-thio-2-nitrobenzoic acid, was added to a buffer solution containing CORM-2 in the presence of GSH. Although CORM-2 inhibited the reduction of Ellman reagent, similar concentrations of iCORM-2 did not impair DTNB reduction (Fig. 8). This result shows that thiol groups are no longer able to react with DNTB suggesting that CORM-2 generates species that promote oxidation of thiols.
FIGURE 8.
CORM-2 impairs reduction of thiols by Ellman reagent. Production of 5-thio-2-nitrobenzoic acid, upon incubation, for 1 h, of increased amounts of iodoacetamide (▴), CORM-2 (●), and iCORM-2 (■) with glutathione were determined in the presence of Ellman reagent. Error bars represent the mean ± S.E.
CORM-2 and ALF062 Generate per se Hydroxyl Radicals
We next asked whether CO-RMs were able to generate radicals in vitro, i.e. in the absence of cells.
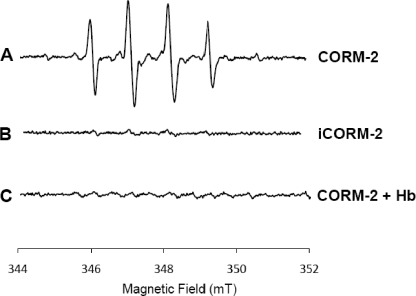
To this end, the EPR spectra of samples containing CORM-2 and the BMPO spin trap were done. The results showed that the mixture of CORM-2 with BMPO gives rise to an EPR signal attributable to formation of a BMPO-OH adduct (Fig. 9A), whereas no signal was detected when mixing iCORM-2 with the same spin trap (Fig. 9B). Notably, the signal arising from the BMPO-OH adduct was completely quenched upon incubation of CORM-2 with hemoglobin (Fig. 9C). Hence, these results indicate that in vitro CORM-2 produces radicals of the hydroxyl type and that CO is associated with its generation.
FIGURE 9.
CORM-2 generates ROS in vitro. EPR spectra of samples containing 2 mm CORM-2 (A), iCORM-2 (B), and CORM-2 plus hemoglobin (Hb, C) acquired in the presence of the spin trap, BMPO.
To further analyze this issue we did similar experiments with ALF062. The signal of the BMPO-OH adduct was observed upon bubbling O2 (supplemental Fig. S3), whereas in a degassed solution no EPR spectrum was seen. In this case it is clear that the OH radicals arise from the reaction of the electron-rich metal in the [Mo(CO)5Br]-[NEt4] complex with molecular oxygen in the protic solvent H2O.
The formation of OH radicals from CORM-2 solution and its dependence on CO is not so obvious. We propose that these OH radicals result from the reduction of O2 by ruthenium species that intermediate the water-gas shift reaction initiated with the attack of water on one of the CO ligands of the RuII(CO)3 moieties of CORM-2 (27–29). Addition of hemoglobin removes CO from RuII(CO)3 forming oxygen stable RuII(CO)2 complexes that are no longer attacked by water under these conditions. Thus, the formation of radicals is inhibited by hemoglobin through scavenging of CO.
DISCUSSION
Recently, CO-RMs based on transition metal carbonyls, were shown to have bactericidal properties against several pathogens including E. coli, Staphylococcus aureus, and Pseudomonas aeruginosa (7, 30). These effects have been attributed to CO release because the compounds lacking CO ligands are no longer bacterial inhibitors. Moreover, DNA microarrays indicate that CO is effectively delivered to intracellular targets causing a plethora of transcriptional modifications as judged by the analysis of the data acquired in cells of E. coli exposed to CO-RMs (8, 31). The present work adds relevant information as it shows that CO-RMs stimulate the in vivo production of ROS and the main cellular antioxidant glutathione confers protection against the killing effect of CO-RMs.
Importantly, ROS generation from CORM-2 increases with the concentration of the CO-RM and is abolished once the CO groups are absent or scavenged by hemoglobin, providing a clear indication that CO is required for the formation of reactive oxygen species. Our data also show that E. coli strains mutated in genes that encode ROS detoxifiers, namely in superoxide dismutases (sodAB) and catalases (katEG), exhibited a sharp decreased survival indicating that impairment of ROS detoxification potentiates the effect of CORM-2.
Furthermore, CORM-2 was found to activate recA transcription and we observed that the bactericidal effect of CORM-2 is accentuated by knocking out recA, most probably due to the inability of triggering the SOS response. Accordingly, we observed by a fluorimetric assay that cells treated with CORM-2 have a higher content of damaged DNA when compared with cells exposed to the inactive form of the compound. The presence of degraded DNA may be explained by the ROS generated by the bactericide as these species are known to cause DNA lesions (10).
The results also revealed that cells treated with CORM-2 have higher levels of free iron, which does not arise from exogenous sources but from the disruption of iron-sulfur containing proteins. One such damaged protein was shown to be aconitase, a member of the dehydratase family of proteins characterized by a solvent-exposed oxygen-labile [4Fe-4S]2+ cluster (32), which upon exposure to CORM-2 exhibited a significantly lower in vivo activity. Moreover, the iscS mutant showed higher resistance to CORM-2, possibly due to the lower abundance of Fe-S clusters in this mutant and to the activation of the suf operon through positive regulation of the apo-form of IscR (26, 33). Hence, the toxicity of CORM-2 is related to the content of denaturated Fe-S clusters.
Carbon monoxide is a classical inhibitor of the aerobic respiration and consequently it is a source of ROS, as demonstrated for mitochondria and embryonic kidney cells (34). However, our results show that CORM-2 generates hydroxyl radicals per se, and this ability seems not to be restricted to this CO-releasing compound because not only ALF062 but also other transition metal carbonyls, like Na[Mo(CO)3(histidinate)] and Na3[Mo(CO)3(citrate)], are reported to promote hydroxyl radical formation (28). More importantly, we proved that the radical formation from CORM-2 is intimately associated with the presence of CO ligands as no radical species were observed when the inactive compound devoid of CO, iCORM-2, was used and upon addition of the hemoglobin, a CO chelator.
Furthermore, in vitro experiments demonstrate that in the presence of CORM-2 thiol groups become unable to react with Ellman reagent, thus suggesting that CORM-2 oxidizes free thiol groups of amino acids, most probably due to its ability to produce ROS in vitro. These results are in agreement with the fact that thiol donors, like cysteine and methionine, are able to cancel the effects of CORM-2 on bacterial growth, which was observed by us in the present work and other authors when using CORM-3 against P. aeruginosa (30).
The work described herein shows that bacterial cells treated with CORM-2 have higher amounts of ROS. The fact that other authors did not observe any increase in H2O2 production in P. aeruginosa cells exposed to CORM-3 may be due to the experimental conditions utilized. Nevertheless, the addition of the antioxidant N-acetyl cysteine abolished CORM-3-mediated inhibition of bacterial growth (30). Moreover, they observed that P. aeruginosa cells treated with CORM-3 caused the impairment of the respiratory chain, which usually leads to generation of ROS (30).
Several reports have demonstrated that many antibiotics mediate cell death through hydroxyl radical formation in bacteria and that there exists a relationship between antibiotic susceptibility and production of ROS (35–37). Hence, like other antibiotics, the lethal effect of CO-releasing compounds seems to rely on the production of ROS.
In conclusion, we have demonstrated that CO-RMs stimulate, in a non-cellular medium, the formation of hydroxyl radicals in a CO-dependent mode. The radicals that are generated account for the bactericidal properties of CORM-2 by damaging DNA and iron-sulfur proteins; the consequent increase of intracellular free iron promotes ROS via the Fenton reaction and for survival bacteria need to bring into play cellular mechanisms of oxidant protection. Altogether, the herein presented results provide a molecular basis of the CO-RMs antibacterial action.
Supplementary Material
Acknowledgment
We are grateful to Prof. Nishioka for providing E. coli DSH7, DSH19, and DSH56 strains.
This work was supported by Fundação para a Ciência e Tecnologia project Grant PTDC/BIA-PRO/098224/2008 and Fundação para a Ciência e Tecnologia Studentships SFRH/BD/38457/2007 (to A. F. N. T.) and SFRH/BPD/69325/2010 (to L. S. N.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- CO
- carbon monoxide
- CO-RMs
- carbon monoxide-releasing molecules
- HO
- heme oxygenase
- ROS
- reactive oxygen species
- CORM-2
- tricarbonyldichlororuthenium (II) dimer
- ALF062
- tetraethylammonium molybdenum pentacarbonylbromide
- FI
- fluorescence intensities
- DTNB
- 5,5′-dithiobis-(2-nitrobenzoic acid)
- BMPO
- 5-tert-butoxycarbonyl 5-methyl-1-pyrroline N-oxide.
REFERENCES
- 1. Li C., Hossieny P., Wu B. J., Qawasmeh A., Beck K., Stocker R. (2007) Antioxid. Redox Signal. 9, 2227–2239 [DOI] [PubMed] [Google Scholar]
- 2. Haldane J. B. (1927) Biochem. J. 21, 1068–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu L., Wang R. (2005) Pharmacol. Rev. 57, 585–630 [DOI] [PubMed] [Google Scholar]
- 4. Zhang J., Piantadosi C. A. (1992) J. Clin. Invest. 90, 1193–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ryter S. W., Otterbein L. E., Morse D., Choi A. M. (2002) Mol. Cell. Biochem. 234–235, 249–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chung S. W., Liu X., Macias A. A., Baron R. M., Perrella M. A. (2008) J. Clin. Invest. 118, 239–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nobre L. S., Seixas J. D., Romão C. C., Saraiva L. M. (2007) Antimicrob. Agents Chemother. 51, 4303–4307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nobre L. S., Al-Shahrour F., Dopazo J., Saraiva L. M. (2009) Microbiology 155, 813–824 [DOI] [PubMed] [Google Scholar]
- 9. Cabiscol E., Tamarit J., Ros J. (2000) Int. Microbiol. 3, 3–8 [PubMed] [Google Scholar]
- 10. Imlay J. A., Linn S. (1988) Science 240, 1302–1309 [DOI] [PubMed] [Google Scholar]
- 11. Avery S. V. (2011) Biochem. J. 434, 201–210 [DOI] [PubMed] [Google Scholar]
- 12. Brzóska K., Meczyńska S., Kruszewski M. (2006) Acta Biochim. Pol. 53, 685–691 [PubMed] [Google Scholar]
- 13. Keyer K., Imlay J. A. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 13635–13640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ayala-Castro C., Saini A., Outten F. W. (2008) Microbiol. Mol. Biol. Rev. 72, 110–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson D. C., Dean D. R., Smith A. D., Johnson M. K. (2005) Annu. Rev. Biochem. 74, 247–281 [DOI] [PubMed] [Google Scholar]
- 16. Ellman G. L. (1959) Arch. Biochem. Biophys. 82, 70–77 [DOI] [PubMed] [Google Scholar]
- 17. Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. (1985) Anal. Biochem. 150, 76–85 [DOI] [PubMed] [Google Scholar]
- 18. Zhao H., Joseph J., Zhang H., Karoui H., Kalyanaraman B. (2001) Free Radic. Biol. Med. 31, 599–606 [DOI] [PubMed] [Google Scholar]
- 19. Gardner P. R. (2002) Methods Enzymol. 349, 9–23 [DOI] [PubMed] [Google Scholar]
- 20. Meister A. (1985) Methods Enzymol. 113, 327–337 [DOI] [PubMed] [Google Scholar]
- 21. Masip L., Veeravalli K., Georgiou G. (2006) Antioxid. Redox Signal. 8, 753–762 [DOI] [PubMed] [Google Scholar]
- 22. Mailloux R. J., Singh R., Brewer G., Auger C., Lemire J., Appanna V. D. (2009) J. Bacteriol. 191, 3804–3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. LeBel C. P., Ischiropoulos H., Bondy S. C. (1992) Chem. Res. Toxicol. 5, 227–231 [DOI] [PubMed] [Google Scholar]
- 24. Courcelle J., Hanawalt P. C. (2003) Annu. Rev. Genet. 37, 611–646 [DOI] [PubMed] [Google Scholar]
- 25. Moeck G. S., Coulton J. W. (1998) Mol. Microbiol. 28, 675–681 [DOI] [PubMed] [Google Scholar]
- 26. Schwartz C. J., Djaman O., Imlay J. A., Kiley P. J. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 9009–9014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Santos-Silva T., Mukhopadhyay A., Seixas J. D., Bernardes G. J., Romão C. C., Romão M. J. (2011) J. Am. Chem. Soc. 133, 1192–1195 [DOI] [PubMed] [Google Scholar]
- 28. Seixas J. D. (2010) in Development of CO-releasing Molecules for the Treatment of Inflammatory Diseases. Doctoral dissertation, Instituto de Tecnología Quimíca e Biológica da Universidade Nova de Lisboa [Google Scholar]
- 29. Johnson T. R., Mann B. E., Teasdale I. P., Adams H., Foresti R., Green C. J., Motterlini R. (2007) Dalton Trans. 15, 1500-1508 [DOI] [PubMed] [Google Scholar]
- 30. Desmard M., Davidge K. S., Bouvet O., Morin D., Roux D., Foresti R., Ricard J. D., Denamur E., Poole R. K., Montravers P., Motterlini R., Boczkowski J. (2009) FASEB J. 23, 1023–1031 [DOI] [PubMed] [Google Scholar]
- 31. Davidge K. S., Sanguinetti G., Yee C. H., Cox A. G., McLeod C. W., Monk C. E., Mann B. E., Motterlini R., Poole R. K. (2009) J. Biol. Chem. 284, 4516–4524 [DOI] [PubMed] [Google Scholar]
- 32. Gardner P. R., Fridovich I. (1991) J. Biol. Chem. 266, 19328–19333 [PubMed] [Google Scholar]
- 33. Jang S., Imlay J. A. (2010) Mol. Microbiol. 78, 1448–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. D'Amico G., Lam F., Hagen T., Moncada S. (2006) J. Cell Sci. 119, 2291–2298 [DOI] [PubMed] [Google Scholar]
- 35. Becerra M. C., Albesa I. (2002) Biochem. Biophys. Res. Commun. 297, 1003–1007 [DOI] [PubMed] [Google Scholar]
- 36. Dwyer D. J., Kohanski M. A., Collins J. J. (2009) Curr. Opin. Microbiol. 12, 482–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kohanski M. A., Dwyer D. J., Hayete B., Lawrence C. A., Collins J. J. (2007) Cell 130, 797–810 [DOI] [PubMed] [Google Scholar]
- 38. Yonezawa Y., Nishioka H. (1999) J. Toxicol. Environ. Health A 57, 237–245 [DOI] [PubMed] [Google Scholar]
- 39. Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M., Datsenko K. A., Tomita M., Wanner B. L., Mori H. (2006) Mol. Syst. Biol. 2, 2006.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.