Abstract
Through the coordinated action of diverse actin-binding proteins, cells simultaneously assemble actin filaments with distinct architectures and dynamics to drive different processes. Actin filament cross-linking proteins organize filaments into higher order networks, although the requirement of cross-linking activity in cells has largely been assumed rather than directly tested. Fission yeast Schizosaccharomyces pombe assembles actin into three discrete structures: endocytic actin patches, polarizing actin cables, and the cytokinetic contractile ring. The fission yeast filament cross-linker fimbrin Fim1 primarily localizes to Arp2/3 complex-nucleated branched filaments of the actin patch and by a lesser amount to bundles of linear antiparallel filaments in the contractile ring. It is unclear whether Fim1 associates with bundles of parallel filaments in actin cables. We previously discovered that a principal role of Fim1 is to control localization of tropomyosin Cdc8, thereby facilitating cofilin-mediated filament turnover. Therefore, we hypothesized that the bundling ability of Fim1 is dispensable for actin patches but is important for the contractile ring and possibly actin cables. By directly visualizing actin filament assembly using total internal reflection fluorescence microscopy, we determined that Fim1 bundles filaments in both parallel and antiparallel orientations and efficiently bundles Arp2/3 complex-branched filaments in the absence but not the presence of actin capping protein. Examination of cells exclusively expressing a truncated version of Fim1 that can bind but not bundle actin filaments revealed that bundling activity of Fim1 is in fact important for all three actin structures. Therefore, fimbrin Fim1 has diverse roles as both a filament “gatekeeper” and as a filament cross-linker.
Keywords: Actin, Cell Division, Cytoskeleton, Endocytosis, Yeast, Arp2/3 Complex, Actin Capping Protein, Cofilin, Cytokinesis, Fimbrin
Introduction
The fission yeast Schizosaccharomyces pombe assembles multiple actin structures with distinct architecture and dynamics, including parallel bundles of filaments for polarization, antiparallel bundles for cytokinesis, and short branched networks for endocytosis (1–4). A major challenge for the cell is to simultaneously coordinate the self-assembly of diverse actin structures from a single pool of monomers within a crowded cytoplasm. Specification of actin organization may be initially established by different actin nucleating factors, including formins and Arp2/3 complex (5–8). Subsequently, partially overlapping sets of actin-binding proteins associate with and are required for generation, maintenance, and turnover of actin filaments within all three structures (4). Furthermore, actin-binding proteins have distinct roles for different processes. For instance, coronin promotes branching by recruiting the Arp2/3 complex to new, ATP-loaded, actin filaments but also synergizes with cofilin to turnover older ADP-rich filaments (9–11). Similarly, cofilin severs filaments and debranches Arp2/3 networks but can also stimulate filament nucleation (12–14).
Endocytic actin patches in yeast are short-lived, highly mobile structures nucleated by the Arp2/3 complex (3, 15–18). A diverse set of actin-binding proteins that includes actin capping protein, type I myosin motor, bundling proteins, and severing proteins associates with actin patches and contributes to rapid filament assembly and turnover (4, 19). Endocytic scaffold proteins and actin-binding proteins accumulate to defined levels at actin patches in a tightly controlled sequence (3, 20–22). However, the precise biochemical and organizational contribution of many of these proteins is unclear.
We previously discovered that the actin-bundling protein fimbrin Fim1 prevents access of the filament stabilizing protein tropomyosin Cdc8 to actin patches (23), which has important functional consequences. First, inhibition of Cdc8 permits severing by cofilin Adf1, which facilitates rapid filament turnover in patches (23). Second, Fim1 prevents Cdc8 from inhibiting the type-I motor Myo1, ensuring maximal Myo1 activity in actin patches (24). Antagonization of Cdc8 does not depend on the bundling activity of Fim1 in vitro (23). Based on these findings and because patches are thought to be composed primarily of branched filaments (25), we hypothesized that the bundling activity of Fim1 is dispensable for its role at actin patches in favor of its ability to regulate the localization of Cdc8 (23, 24).
We, therefore, utilized a combination of in vitro and in vivo approaches to examine the ability of Fim1 to bundle actin filaments, the polarity of filaments in Fim1-generated bundles, the role of Fim1 in Arp2/3 complex-mediated actin polymerization, and the necessity of bundling by Fim1 for diverse actin structures. We found that Fim1 creates both parallel and antiparallel bundles, and Fim1 efficiently bundles Arp2/3 complex-mediated branched filament networks, which appear to inhibit Arp2/3 complex filament branching. Capping protein relieves Fim1 inhibition of Arp2/3 complex in vitro. In disagreement with our original hypothesis, we found that the bundling activity of Fim1 is necessary for proper actin patch dynamics and at the contractile ring. We also discovered a novel role for bundling by Fim1 in actin cables. Together, these results indicate that, like other actin-binding proteins, Fim1 has multiple roles at different actin structures and is part of a complicated network of interactions between diverse actin-binding proteins.
EXPERIMENTAL PROCEDURES
Strains, Growth Conditions, Transformation, and Cellular Methods
We used standard growth media (YE5S complete medium and EMM5S minimal medium). Expression under the nmt1 promoter was regulated by the presence or absence of 10.0 μg/ml thiamine (Sigma). Supplemental Table 1 lists the S. pombe strains used in this study. For tetrad dissection, cells were sporulated on standard mating (SPA5S) plates for 36 h, then spread on complete medium (YE5S) plates, and tetrads were picked with a dissection scope. Plates were incubated at 25 °C for 10 h or until tetrads germinated, at which point individual spores were separated.
FimA2 Strain Construction
An S. pombe strain exclusively expressing FimA2-mCherry was constructed as follows. FimABD2-mCherry with a Nat resistance cassette was amplified from genomic DNA prepared from the wild type Fim1-mCherry strain (VS888–3) using a forward primer homologous to the Fim1 promoter and FimABD2 (5′-ACTGCAAGCCACCCAAAGCACACATCGTGTGGTTTCGTTTACTATACATTTTTTGGTCAAATTTTACTTTTAAAGAAATGTTAAATGAAGAGGAAAAGCC-3′) and a reverse primer homologous to the Fim1 sequence 68–150 nucleotides downstream of the stop codon (5′-TATCTATAATATGACATACCACTAATACACTAGCGAGTCAAAAGCGACGTACTCACTTCCGAAGTTGTTCGCAATATAAG-3′) using an Expand High Fidelity Plus PCR system (Roche Applied Science). Amplified product was inserted into the fim1Δ strain (JW142) by homologous recombination after lithium acetate transformation. Recovered colonies were assessed for 1) resistance to Nat, 2) red fluorescence, and finally 3) PCR amplification of ABD2-mCherry-Nat from genomic DNA.
Cell Microscopy
Cells were observed by differential interference contrast and epifluorescence microscopy with an Orca-ER camera (Hamamatsu, Bridgewater, NJ) on an IX-81 microscope (Olympus, Tokyo, Japan) fitted with a 60× 1.4 numerical aperture Plan-apo objective. Nuclei and septa were visualized simultaneously using 4,6-diamidino-2-phenylindole (nuclei) and calcofluor (septa) as described (26). Actin filaments were visualized using the general actin marker GFP-CHD(rng2)2 (27).
To quantify endocytic actin patch dynamics and behavior of Myo52–3×YFP particles, cells grown in exponential phase in Edinburgh minimal (EMM) liquid media were spotted onto EMM pads containing 25% gelatin on glass slides and sealed (Vasaline, Lanoline and Parafin wax). Images were acquired with a 100× 403/1.4 NA objective on a Zeiss Axiovert 200M equipped with a Yokogawa CSU-10 spinning-disk unit (McBain) illuminated with a 50-milliwatt 473-nm DPSS laser (Cobolt). Time-lapse acquisitions were captured at 75-ms intervals on a Cascade 512B EM-CCD camera (Photometrics) with MetaMorph (Molecular Devices). Analysis of patch dynamics was quantified for 50 randomly chosen patches for each strain using ImageJ software (rsbweb.nih.gov) as previously described (28). Only patches that originated and disappeared during the course of the movie were quantified.
For experiments reported in Figs. 6, C and D and 7A, mCherry-tagged Fim1 and FimA2 patches and cells expressing GFP-CHD were imaged by spinning disk confocal microscopy using an Ultraview VoX system (PerkinElmer Life Sciences) installed on a Nikon TiE microscope equipped with 100×/1.4 NA lens. Time-lapse series of images through the middle of the cell were acquired at the rate of 1 frame/s, and Z-series spanning the entire cell depth were acquired at 0.6-μm intervals.
FIGURE 6.
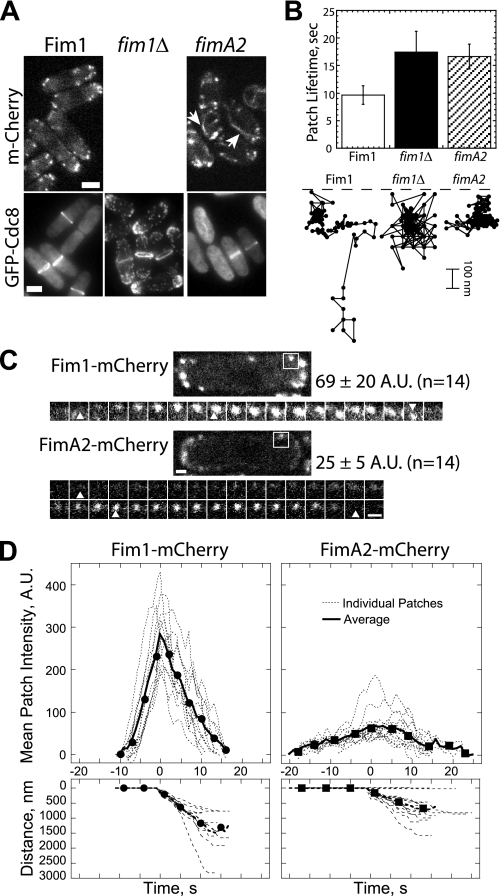
The cross-linking activity of fimbrin is important for endocytic actin patches. A, top, fluorescence micrographs of mCherry-tagged full-length Fim1 or fimbrin fragment FimA2. Arrows indicate actin cable-like association of FimA2. Bottom, tropomyosin GFP-Cdc8 in cells expressing full-length Fim1-mCherry or fimbrin fragment FimA2-mCherry or fim1Δ cells. Bars, 5 μm. B, actin patch dynamics of cells expressing the general actin marker GFP-CHD(rng2). Corresponding supplemental Movie S12 is available online. Top, plot of actin patch lifetime for full-length fim1, fim1Δ, and fimA2 cells (n = 30 patches for each strain). Bottom, the position over time (0.075 s between positions) for representative full-length fim1, fim1Δ, and fimA2 actin patches. Dashed lines indicate cell cortex. C and D, comparison of the dynamics of Fim1-mCherry and FimA2-mCherry in actin patches. C, fluorescent micrographs of cells expressing Fim1-mCherry (top) and FimA2-mCherry (bottom) and the corresponding montages of a time series of a single actin patch (white boxes) at 1 frame/s. Numbers to the right are mean total cell fluorescence intensities (±S.D.). Arrowheads mark patch appearance, initiation of movement, and disappearance. Bars, 1 μm. D, individual patch (thin dashed lines) and average patch (thick lines) time courses of fluorescence intensity (top) and distance moved (bottom) for 12 Fim1-mCherry (left) and 14 FimA2-mCherry (right) actin patches.
FIGURE 7.
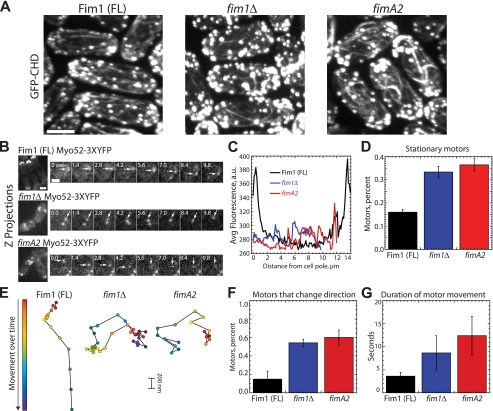
Actin cable integrity is likely impaired in cells lacking full-length fimbrin. A, comparison of actin cables in full-length (FL) Fim1-mCherry, fim1Δ, and fimA2 cells. Maximum intensity projections of a Z-series through cells expressing the general actin filament marker GFP-CHD(rng2). Bar, 3.5 μm. B, left, maximum intensity Z projections of Myo52–3×YFP distribution in full-length Fim1-mCherry, fim1Δ, or fimA2 cells. Right, time-lapse micrographs of Myo52–3×YFP particles in full-length Fim1-mCherry, fim1Δ, or fimA2. Seconds are indicated at the upper left. Bar, 1 μm. Corresponding supplemental Movie S13 is available online. C, Myo52–3×YFP fluorescence value distribution across the length of interphase cells (n = 25 for each strain). Bar, 5 μm. D, the fraction of Myo52–3×YFP particles that remain stationary throughout their lifetimes in full-length Fim1-mCherry, fim1Δ, or fimA2 cells (n = 25 particles for each strain). Values are the mean ± S.D. E, the position over time (0.8 s between marks) for representative Myo52–3×YFP particles. Patch start position is indicated in red, and end position is indicated by blue/violet. F, the fraction of Myo52–3×YFP moving particles that change direction in full-length Fim1-mCherry, fim1Δ, or fimA2 cells (n = 25 for each strain). Values are the mean ± S.D. G, the average duration of Myo52–3×YFP particle movement in full-length Fim1-mCherry, fim1Δ, or fimA2 cells (n = 25 for each strain). Values are the mean ± S.D.
Fluorescence intensity and position of mCherry-labeled patches over time were tracked in single sections through the middle of the cell using an eight-pixel-wide circular selection tool. Intensity values were subtracted for cytoplasmic background; all data were aligned to the start of patch movement or time of patch appearance (for non-moving patches) and averaged at each time point. Total cell fluorescence intensities were measured from sections through the middle of the cells and subtracted for background intensity. Images were processed using ImageJ (rsbweb.nih.gov).
Plasmid Constructs
Bacterial expression constructs for fimbrin truncation mutants were prepared by PCR amplification (iProof; Bio-Rad) and restriction digest cloning into a pET-21a vector (pET-21a-Fim Truncation-His). Inserts were sequenced to confirm fidelity of PCR amplification. The fission yeast tropomyosin Cdc8 expression plasmid pREP4x-cdc8 has been described (29). The bacterial expression plasmid for cofilin Adf1 (pMW-SpCofilin) and GST-Wsp1 (pGEX2-(SpWsp1p)VCA) have been described (3, 12).
The bacterial expression plasmid for fission yeast-capping protein with a His6 affinity tag was prepared as follows. Acp1 was amplified from pET3a-SpAcp1/2 (30), with a His6 affinity tag added to the forward primer (5′-(GACTCATATGCATCACCATCACCATCACGAAAAGGAGGCAATTTACAAAC-3′) and cloned into pET3a, then confirmed by sequencing. Acp2 was cut from pET3a-SpAcp2 (30), and ends were blunted with Klenow (New England Biolabs, Ipswich, MA) and inserted into pET3a-HIS-Acp1 using EcoRV (New England Biolabs). Orientation was confirmed by restriction digest; the construct selected contained Acp1 and Acp2 ORF sequences oriented in the same direction each expressed under the T7 promoter.
Protein Purification
Tropomyosin (Cdc8) was purified from fission yeast cells expressing pREP4X-cdc8 by successive steps of boiling, ammonium sulfate precipitation, and ion-exchange chromatography as described (29). Full-length recombinant fission yeast fimbrin Fim1 and GST-Wsp1 were purified as previously described (3, 23). Native fission yeast Arp2/3 complex was purified from a protease-deficient strain (TP150) by binding to GST-N-WASP-VCA as previously described (3). Human fascin was purified as previously described (35). Recombinant fission yeast cofilin Adf1 was expressed from pMW-SpCofilin in Escherichia coli BL21-Codon Plus(DE3)-RP and purified by ammonium sulfate precipitation, S-200 gel filtration, and ion-exchange chromatography as described (12, 31). Frozen cofilin slowly lost activity, so Adf1 was used without freezing.
Tetramethylrhodamine-6-maleimide (TMR)-Fim1 was prepared as described for OG488-AtFim1 (32), with a few modifications. First, TMR (Sigma) was used in place of OG488-iodoacetamide. Second, Talon Metal Affinity Resin (Clontech, Mountain View, CA) was used instead of glutathione-Sepharose. Third, because the His6 tag was not cleaved from Fim1 after the coupling reaction was stopped with DTT, the column was resettled and washed with Talon extraction buffer (50 mm NaH2PO4, pH 8.0, 500 mm NaCl, 10% glycerol, 10 mm imidazole, 10 mm β-mercaptoethanol), and protein was eluted from the column with Talon elution buffer (50 mm NaH2PO4, pH 8.0, 500 mm NaCl, 10% glycerol, 250 mm imidazole, 10 mm β-mercaptoethanol). Eluted protein was dialyzed overnight versus Source Q Buffer A (20 mm Tris-HCl, pH 8.5, 50 mm NaCl, 5% glycerol, 0.01% NaN3, 1 mm DTT). Dialyzed protein was loaded onto a 5.0-ml Mono Q column (GE Healthcare) and eluted with a linear gradient from 0 to 500 mm NaCl. Pure protein was dialyzed into fimbrin storage buffer (20 mm HEPES, 1 mm EDTA, pH 8.0, 200 mm KCl, 0.01% NaN3, 10% glycerol, 1 mm DTT), flash-frozen in liquid nitrogen, and stored at −80 °C. Because TMR does not absorb at A280, protein concentration was checked as previously described (23). Percent labeled was determined using a correction factor (A280 TMR/Amax TMR); yield was >90% labeled.
Truncated versions of Fim1 and recombinant His6-Acp1/2 were purified from E. coli BL21-Codon Plus(DE3)-RP (Stratagene) by expressing with 0.5 mm isopropyl β-d-thiogalactopyranoside (Sigma) for 16 h at 16 °C. Cells were harvested by sedimentation, washed with phosphate-buffered saline, and stored at −80 °C. Pellets were resuspended in extraction buffer (50 mm NaH2PO4, pH 8.0, 500 mm NaCl, 10% glycerol, 10 mm imidazole, 10 mm β-mercaptoethanol) supplemented with 0.5 mm phenylmethylsulfonyl fluoride and protease inhibitors and homogenized in an Emulsiflex-C3 (Avestin, Ottawa, ON, Canada). The homogenate was clarified at 30,000 × g and 50,000 × g for 20 min each, incubated with Talon Metal Affinity Resin for 1 h at 4 °C, and loaded onto a disposable column. After a 50-ml wash with extraction buffer, protein was eluted with Talon elution buffer (50 mm NaH2PO4, pH 8.0, 500 mm NaCl, 10% glycerol, 250 mm imidazole, 10 mm β-mercaptoethanol) and dialyzed overnight versus Source Q Buffer A (20 mm Tris-HCl, pH 8.5, 50 mm NaCl, 5% glycerol, 0.01% NaN3, 1 mm DTT). Dialyzed protein was loaded onto a 5.0 ml Source Q column (GE Healthcare) and eluted with a linear gradient from 0 to 500 mm NaCl. Pure protein was dialyzed into storage buffer (20 mm HEPES, 1 mm EDTA, pH 8.0, 200 mm KCl, 0.01% NaN3, 10% glycerol, 1 mm DTT), flash-frozen in liquid nitrogen, and stored at −80 °C.
Ca-ATP actin was purified from chicken skeletal muscle as described (33). Gel-filtered actin was labeled on Cys-374 with pyrenyl iodoacetamide or Oregon Green iodoacetamide (Invitrogen) (26). Immediately before each experiment, Ca-ATP actin was converted to Mg-ATP actin by the addition of 0.1 volume of 2 mm EGTA and 0.5 mm MgCl2 for 2 min at 25 °C.
Extinction coefficients for Cdc8 (A280 = 2980 m−1 cm−1), Fim1 (A280 = 55140 m−1 cm−1), FimA12 (A280 = 50670 m−1 cm−1), FimA2 (A280 = 27960 m−1 cm−1), FimA1 (A280 = 29700 m−1 cm−1), FimEFA1 (A280 = 34170 m−1 cm−1), Arp2/3 complex (A290 = 138570 m−1 cm−1), GST-Wsp1 (A280 = 80260 m−1 cm−1), capping protein (A280 = 72000 m−1 cm−1), and Adf1 (A280 = 13075 m−1 cm−1) were estimated with ProtParam from the amino acid composition.
Actin Filament Sedimentation
Actin filaments preassembled for 2 h at 25 °C from 15 μm Mg-ATP-actin monomers were incubated with a range of concentrations of Fim1 for 20 min at 25 °C and then spun at 100,000 × g (high speed) or 10,000 × g (low speed) for 20 min at 25 °C. Equal volumes of total (before centrifugation), supernatant, and pellet were separated by 12.5% SDS-polyacrylamide gel electrophoresis, stained with Coomassie Blue for 30 min, and destained for 16 h. Gels were analyzed by densitometry on an Odyssey Infrared Imager (LI-COR Biosciences, Lincoln, NE) as previously described (29). The amount of protein was quantified as depletion from the supernatant, converted to micromolar bound. Plots of the dependence of the concentration of bound Fim1 on the concentration of actin were fit with a quadratic function.
To determine the effect of nucleotide state on the affinity of Fim1 for actin filaments, ADP-Pi actin filaments were prepared by assembling ADP-actin in the presence of 25 mm potassium phosphate, pH 7.0 (15.37 mm H2KPO4 and 9.63 mm HK2PO4) with 19.9 mm KCl. Mg-ATP actin and Mg-ADP actin were assembled in the presence of 25 mm potassium sulfate with 3.5 mm KCl to keep the conductivity consistent (34).
Fluorescence Spectroscopy
Actin assembly was measured from the fluorescence of pyrene-actin with Spectramax Gemini XPS (Molecular Devices) and Safire2 (Tecan) fluorescent plate readers. Spontaneous assembly has been described in detail previously (33). Briefly, assembly of a mixture of unlabeled and pyrene-labeled Mg-ATP-actin monomers was initiated by the addition of 50 mm KCl, 1 mm MgCl2, 1 mm EGTA, 10 mm imidazole, pH 7.0, and other proteins to be assayed (Fim1, Arp2/3 complex, Wsp1, etc.). Final protein concentrations and percent pyrene-labeled actin are indicated in the figure legends. Calculation of spontaneous polymerization rates has been described (33).
Total Internal Reflection Fluorescence (TIRF) Microscopy
TIRF images of a mixture of either 2.0 μm Mg-ATP-actin supplemented with 1.0 μm Oregon Green-labeled actin (Fig. 1) or 1.0 μm Mg-ATP-actin supplemented with 0.5 μm Oregon Green-labeled Mg-ATP-actin (see Figs. 3–5), excited by evanescent wave fluorescence, were acquired every 10 s on an IX-71 microscope (Olympus) fit with through-the-objective TIRF illumination and an iXon EMCCD camera (Andor Technology, South Windsor, CT) as described (33). Branching rates were measured as total branches that appeared over a period of 10 min and normalized to total amount of polymer.
FIGURE 1.
Fimbrin produces both parallel and antiparallel bundles. TIRF microscopy observation of filament bundling is shown. 2 μm Mg-ATP-actin with 1.0 μm Mg-ATP-actin labeled with Oregon Green was assembled on slides coated with N-ethylmaleimide-myosin II. Corresponding supplemental Movies S1–S8 are available online. A, quantification of Fim1 bundling as measured by skewness of pixel intensity (supplemental Movie S1) is shown. Left, shown are histograms of the distribution of pixel intensities in 50 frames of a TIRF movie from 1000 to 1500 s after polymerization initiation in the absence or presence of 250 nm Fim1. The red line indicates the mode of the data. Right, Fim1 increases pixel skewness, indicating a shift toward brighter pixels due to bundling. Values are the mean ± S.D. B-H, time-lapse micrographs with time in seconds indicated in the upper right. Pink, blue, and yellow arrowheads indicate filament barbed ends. Yellow brackets indicate bundled filaments. Bars, 5 μm. B, actin filaments growing in the absence of Fim1 (supplemental Movie S2). C, two filaments bundled in a parallel fashion by 250 nm Fim1; 40.2% of two-filament bundles are parallel (supplemental Movie S3). D, two filaments bundled in an antiparallel fashion by 250 nm Fim1; 59.6% of two-filament bundles are antiparallel (supplemental Movie S4). E, a bundle containing both antiparallel (pink and blue arrowheads) and parallel (yellow and pink arrowheads) filaments (supplemental Movie S5). F, a parallel bundle containing at least four filaments in the presence of 250 nm fascin (supplemental Movie S6). G and H, bundles severed by the addition of 150 nm cofilin Adf1 (pink arrowhead). Severing events in G are marked by pink dots (supplemental Movie S7). Severed filament barbed ends rapidly grow upon the addition of new 1.0 μm of 33% Oregon Green-labeled G-actin (green arrowhead) in H (supplemental Movie S8).
FIGURE 3.
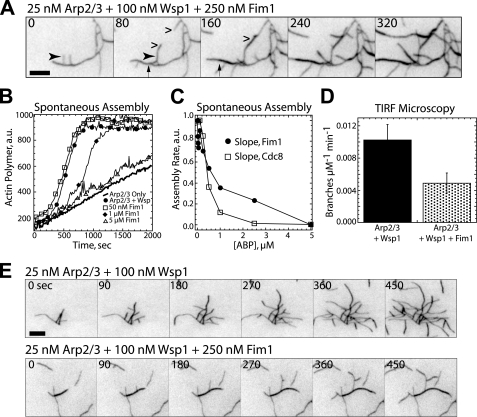
Fimbrin inhibits Arp2/3 complex-mediated actin filament assembly. A, shown is a TIRF microscopy observation of a branched actin filament network (25 nm Arp2/3 complex + 100 nm GST-SpWsp1VCA) bundled by 250 nm Fim1. The arrow indicates a branch bundling onto the mother filament. The arrowhead indicates neighboring parallel branches bundling onto each other. Wedges indicate antiparallel bundling between branches. Seconds are indicated at the upper left. Bar, 5 μm. Corresponding supplemental Movie S9 is available online. B and C, spontaneous assembly of 20% pyrene-labeled 2.5 μm Mg-ATP-actin monomers. B, assembly of actin monomers with 25 nm Arp2/3 complex alone (thick curve) or Arp2/3 complex and 100 nm Wsp1 in the absence (●) or presence of 50 nm (□), 1 μm (♦), or 5 μm Fim1 (△). C, dependence of the initial assembly rate on concentration of Fim1 or tropomyosin Cdc8. D and E, TIRF microscopy observation of Arp2/3 complex-mediated branching. D, a plot of the branching rate (branches μm−1 min−1) by Arp2/3 complex and Wsp1 in the absence or presence of 250 nm Fim1. Values are the mean ± S.D. E, time-lapse micrographs of actin filament branching by 25 nm Arp2/3 complex and Wsp1 alone (top) or in the presence of 250 nm Fim1 (bottom). Time in seconds is indicated at the top left. Bar, 5 μm. Corresponding supplemental Movie S10 is available online.
FIGURE 4.
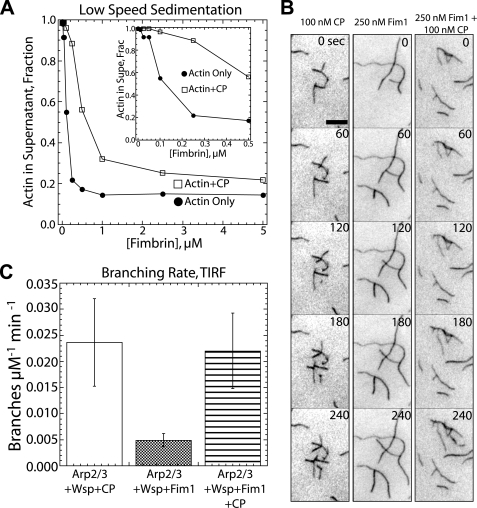
Capping protein restores rapid Arp2/3 complex-mediated actin polymerization in the presence of Fim1. A, CP reduces Fim1-mediated bundling efficiency. Dependence of actin in the low speed sedimentation supernatant (non-cross-linked actin) on the concentration of Fim1 in the absence (●) or presence (□) of 100 nm CP is shown. Inset, plot with a magnified x axis. B, TIRF microscopy time-lapse micrographs of actin filament branching by 25 nm Arp2/3 complex and 100 nm Wsp1 in the presence of 100 nm CP (left), 250 nm Fim1 (center), or CP and Fim1 (right). Time in seconds is indicated at the upper right. Bar, 5 μm. Corresponding supplemental Movie S11 is available online. C, plot of branching rate (branches μm−1 min−1) in the presence of 25 nm Arp2/3 complex with 100 nm Wsp1 and the indicated combinations of 100 nm CP and 250 nm Fim1. Values are the mean ± S.D.
FIGURE 5.
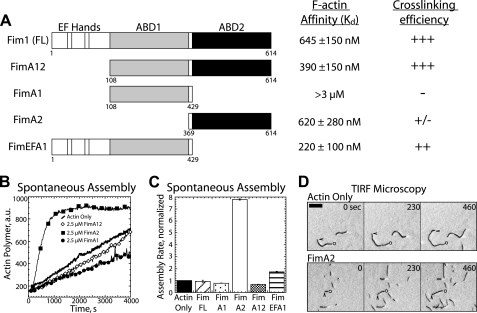
The two actin binding domains of Fim1 are not identical. A, left, domain organization of constructs used in this study. Numbers under constructs indicate amino acids. Right, affinity for filaments (Kd) is based on two separate high speed sedimentation assays, and the general ability to cross-link filaments is based on low speed sedimentation assays (see supplemental Fig. 2). B and C, spontaneous assembly of 20% pyrene-labeled 2.5 μm Mg-ATP-actin monomers. B, polymerization of actin alone (thick curve) or in the presence of 2.5 μm FimA12 (◊), 2.5 μm FimA2 (■), or 2.5 μm FimA1 (●). C, initial polymerization rates in the presence of the indicated constructs. Values are the mean ± S.D. D, TIRF microscopy time-lapse micrographs of increased filament nucleation in the presence of FimA2 as compared with actin alone. Time in seconds is indicated at the top right. Bar, 5 μm.
Measuring Off-rates by TIRF
The method was modified from Courson and Rock (35). Bundles were prepared by incubating 10 μm actin filaments with 3 μm TMR-Fimbrin at 4 °C for 2 h in assay buffer (AB: 25 mm imidazole, pH 7.5, 25 mm KCl, 1 mm EGTA, 4 mm MgCl2, and 10 mm DTT). Flow chambers were sequentially loaded with N-ethylmaleimide-myosin, 1 mg/ml BSA, actin (0.5 μm bundles or 0.1 μm filaments), AB, and finally observation buffer (AB plus 0.86 mg/ml glucose oxidase, 0.14 mg/ml catalase, 9 mg/ml glucose). All proteins added during the experiment were diluted in observation buffer.
RESULTS
Fission Yeast Fimbrin Bundles Actin Filaments with Mixed Polarities
To better understand the roles of fission yeast fimbrin Fim1 for various cellular processes, we biochemically characterized the actin binding and bundling properties of Fim1. In agreement with previous reports (36, 37), high and low speed cosedimentation assays revealed that Fim1 binds filaments with submicromolar affinity irrespective of nucleotide state (supplemental Fig. 1, A–C) and that Fim1 efficiently cross-links filaments into dense bundles composed of multiple filaments (supplemental Fig. 1, D–F).
To determine the orientation of the filaments in these bundles, we observed individual filaments growing in real time via TIRF microscopy (Fig. 1, supplemental Movie 1). We quantified the amount of bundling in two ways. First, we measured the distribution, or skewness, of pixel brightness in each sample. Samples containing actin alone have a Gaussian distribution of pixel intensity, whereas the intensity of pixels in bundled samples is shifted toward the right (skewed) (38, 39). In the presence of 250 nm Fim1, the histogram of pixel intensity skews toward brighter pixels as compared with actin alone (Fig. 1A, supplemental Movie 1). Second, we quantified the percentage of filaments that coalign with at least one other filament over at least 10 μm for at least 10 frames. In the absence of Fim1, filaments rarely align (∼3%) for more than two frames (Fig. 1B, supplemental Movie 2). However, in the presence of 250 nm Fim1, >80% of the observed filaments coalign with other filaments (Fig. 1, C–E).
Many actin cross-linking proteins including filamin, α-actinin, fascin, and other fimbrins have a strong selectivity for one filament orientation over the other (35, 40–46). Following the growing barbed ends of individual filaments revealed the orientation of filaments within bundles (Fig. 1, C–F, supplemental Movies 3–6). Fim1 creates bundles containing filaments in both parallel and antiparallel orientations without strong selectivity (Fig. 1, C and D, supplemental Movies 3 and 4). Of bundles containing only two filaments, 40.2% are parallel, whereas 59.6% are antiparallel (the remaining 0.2% could not be determined). Bundles with more than two filaments contain filaments in both orientations (Fig. 1E, supplemental Movie 5). Conversely, fascin creates bundles composed exclusively of parallel filaments (Fig. 1F, supplemental Movie 6) (35, 43). Because Fim1 binds to structures composed of both parallel and antiparallel filaments in vivo, this may be an essential feature of Fim1.
Fim1 plays roles in the contractile ring and in actin cables (23) (see below), where filaments are bundled (1, 2). Given that filament severing by cofilin Adf1 is critical for turnover of actin filaments (12, 23, 47, 48), we examined the ability of Adf1 to sever Fim1 bundles by TIRF microscopy. Adf1 is able to sever individual filaments in bundles (Fig. 1G, supplemental Movie 7). The addition of new actin monomers to severed bundles results in the rapid thickening of bundles as severed barbed ends elongate and new filaments are added to the bundle (Fig. 1H, supplemental Movie 8). Therefore, Adf1-mediated severing can stimulate either rapid filament turnover or bundle expansion depending upon the availability of monomeric actin.
Fim1 Is Stable in Bundles
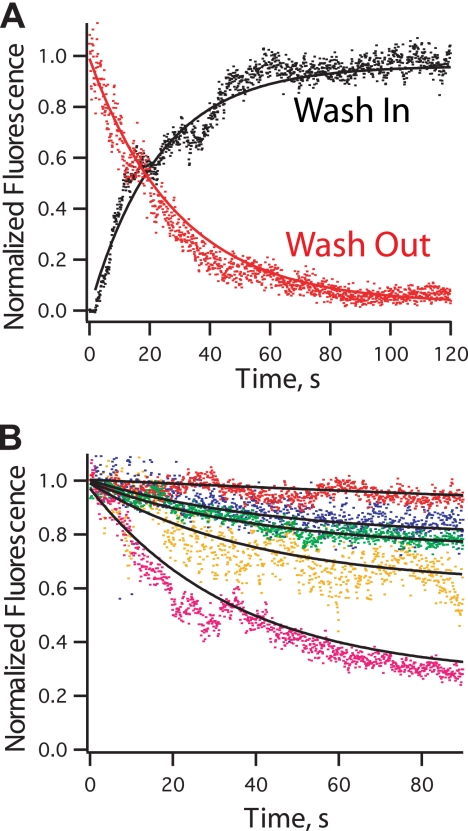
We used a TIRF microscope coupled to a flow chamber to assess the dissociation of Fim1 from single filaments and filament bundles (Fig. 2). First, we observed binding of labeled TMR-Fim1 to individual unlabeled actin filaments that were immobilized on a coverslip. TMR-Fim1 was washed over the filaments, and fluorescent intensity on those filaments was recorded (Fig. 2A, black curve). A single exponential curve fit revealed a dissociation constant of 0.043 ± 0.001 s−1. Then we washed TMR-Fim1 off of the same filaments with buffer (Fig. 2A, red curve). TMR-Fim1 rapidly dissociates from filaments with an identical off rate of 0.043 ± 0.001 s−1.
FIGURE 2.
Fimbrin is stably associated with actin filament bundles. Determination of the fimbrin off-rate from single filaments and bundles is shown. A, a solution of 0.1 μm TMR-fimbrin was washed over single actin filaments (black). A single exponential curve fit is in black. The dissociation constant measured from this experiment was 0.043 ± 0.001 s−1. Single filaments coated with TMR-fimbrin were washed with observation buffer (red). Fimbrin dissociated with an off-rate of 0.043 ± 0.001 s−1. B, TMR-Fimbrin bundles were washed with different concentrations of unlabeled fimbrin: 1 μm (blue, koff = 0.023 ± 0.003 s−1), 5 μm (green, koff = 0.025 ± 0.001 s−1), 10 μm (orange, koff = 0.025 ± 0.003 s−1), 20 μm Fim1 (magenta, koff = 0.028 ± 0.001 s−1), and no significant TMR-fimbrin dissociation with buffer only (red, koff = 0.0028 ± 0.002 s−1).
We examined the stability of Fim1 in bundles by immobilizing bundles assembled with TMR-Fim1 on a coverslip and washing in 0–20 μm unlabeled Fim1 (Fig. 2B). With buffer only, TMR-Fim1 is highly stable in bundles (koff = 0.0028 ± 0.002 s−1). TMR-Fim1 dissociates from bundles in the presence of a range of unlabeled Fim1 concentrations with koff = 0.023–0.028 s−1 (Fig. 2B). This indicates that increasing concentrations of free fimbrin do not increase the rate of bundle turnover but instead increase the amplitude of turnover.
Fim1 Inhibits Polymerization by the Arp2/3 Complex
Fim1 is abundant at endocytic actin patches, which are thought to be composed of Arp2/3 complex-branched filament networks rather than bundles (3, 25, 36, 49). We, therefore, examined the ability of Fim1 to bundle Arp2/3 complex-generated branched networks. TIRF microscopy assays revealed Fim1 efficiently bundles branched filament arrays (Fig. 3A, supplemental Movie 9). Fim1 produces both parallel and antiparallel bundles between adjacent branched networks. Fim1 also efficiently bundles filaments within a single branched “tree,” where all filaments can be traced back to an original mother filament. Branches bundle to each other (Fig. 3A, arrowhead) as well as back on to the mother filament (Fig. 3A, arrow). Because filaments within an actin tree are oriented in the same direction, the percentage of parallel bundles created by Fim1 increases from 59.6% with actin alone to 73% in the presence of Arp2/3 complex.
Because Arp2/3 complex nucleates new filaments by binding to the sides of existing filaments (5, 8), we hypothesized that bundling might inhibit Arp2/3 complex by decreasing available binding sites. In the presence of the activator GST-Sp(Wsp1)VCA, hereafter referred to as Wsp1, Arp2/3 complex strongly promotes actin assembly (Fig. 3B) (3, 50). However, increasing concentrations of Fim1 inhibit stimulation of actin polymerization by Arp2/3 complex and Wsp1, eventually reducing the rate to that of Arp2/3 complex in the absence of Wsp1 (Fig. 3B). Quantification of the initial slope of the polymerization reactions revealed a concentration-dependent inhibition of polymerization by Fim1 (Fig. 3C). Conversely, Fim1 does not affect spontaneous polymerization of actin monomers alone (see Fig. 5C). As expected, Arp2/3 complex is also strongly inhibited by tropomyosin Cdc8 (Fig. 3C) (51).
As predicted, TIRF microscopy assays revealed that Fim1 significantly decreases the overall branching rate of Arp2/3 complex (Fig. 3, D and E, supplemental Movie 10). Conversely, Fim1 does not affect the rate of debranching (data not shown).
Capping Protein and Fimbrin Are Antagonistic in Vitro
Although fimbrin inhibits branching by Arp2/3 complex in vitro, Arp2/3 complex efficiently stimulates actin assembly at endocytic actin patches in cells (3, 22). We reasoned that filaments branched at a 70° angle must reach a critical length before they can be bundled. Actin capping protein (CP) is a major component of actin patches (30, 36), which might prevent bundling of branched filaments by restricting their length.
Initially, low speed sedimentation assays were used to assess whether the bundling efficiency of Fim1 is generally affected by filament length. CP significantly reduces Fim1 bundling efficiency by decreasing filament length, although CP does not completely prevent bundling (Fig. 4A). High concentrations of Fim1 bundle short-capped filaments to essentially the same extent as low concentrations of Fim1 bundle longer filaments (Fig. 4A).
We utilized TIRF microscopy to examine the effects of CP on Arp2/3 complex-mediated branched actin filament assembly in the presence of Fim1 (Fig. 4, B and C, supplemental Movie 11). Although CP reduces filament length by inhibiting barbed end elongation, as observed previously, CP increases branching by Arp2/3 complex and Wsp1 (52–54). Likewise, the addition of CP restores branching in the presence of Fim1 to similar levels observed in the absence of Fim1 (Fig. 4, B and C, supplemental Movie 11). We hypothesize that CP restores Arp2/3 complex-mediated filament branching by reducing filament length and, therefore, bundling by Fim1.
The Two Actin Binding Domains of Fim1 Are Not Identical
Arp2/3 complex-generated branched actin filaments in actin patches are not thought to form dense bundles (25), which is in part due to prevention of Fim1-mediated bundling by CP (Fig. 4). Furthermore, a major role of Fim1 is to exclude tropomyosin Cdc8 from endocytic patches (23, 24). Because the bundling activity of Fim1 is not required to prevent tropomyosin from binding actin filaments in vitro, we hypothesized that the bundling activity of Fim1 is dispensable in vivo (23). We, therefore, replaced fim1 at its endogenous genomic locus with a version of fimbrin that contains only one of two actin binding domains (see Figs. 6 and 7). However, we first biochemically characterized truncated constructs of Fim1 to determine the in vitro properties of each isolated actin binding domain (Fig. 5).
Fim1 bundles actin filaments as a monomer. Fimbrin is composed of an N-terminal headpiece followed by two ∼250-amino acid actin binding domains, each composed of two tandem calponin-homology (CH) domains (Fig. 5A) (36, 49). We cloned and purified a series of constructs that either lack the N-terminal headpiece (FimA12) or contain a single actin binding domain (FimA1 and FimA2) or the N-terminal headpiece with the N-terminal actin binding domain (FimEFA1) (Fig. 5A) (36, 49).
We tested the ability of each construct to bind and bundle actin filaments utilizing in vitro cosedimentation assays (Fig. 5A, supplemental Fig. 2, A and B). We determined the affinity of each construct for F-actin by incubating a single concentration of fimbrin with a range of concentrations of F-actin followed by high speed centrifugation. Full-length Fim1, FimA12, FimA2, and FimEFA1 all bind to and sediment with F-actin, although with different affinities. The affinity of full-length Fim1 for actin filaments is ∼650 nm. FimA12 binds to actin approximately twice as tightly as full-length fimbrin (Kd ∼390 nm), whereas FimA2 has approximately the same affinity for actin as full-length fimbrin (Kd ∼650 nm). FimA1, as has been previously reported (36), does not bind F-actin strongly (Kd > 3 μm). Interestingly, FimEFA1 binds to actin a bit better than full-length Fim1 (Kd ∼ 220 nm).
As expected, both full-length fimbrin and FimA12 (containing two actin binding domains) cross-link F-actin with similar efficiency in low speed cosedimentation assays (Fig. 5A, supplemental Fig. 2, B and C). Neither FimA1 nor FimA2 (each containing an isolated actin binding domain) strongly cross-links actin filaments. Surprisingly, FimEFA1 (containing the N-terminal headpiece and the isolated N-terminal actin binding domain) cross-links actin filaments.
We next examined the effects of fimbrin truncations on the spontaneous assembly of actin monomers (Fig. 5, B and C). Full-length Fim1, FimA12, and FimEFA1 do not affect the spontaneous polymerization of actin monomer. FimA1, which does not bind filaments well, slightly inhibits polymerization. Conversely, FimA2 significantly increases the actin polymerization rate. To examine the mechanism by which FimA2 increases actin assembly, we visualized actin filament assembly by TIRF microscopy (Fig. 5D). As compared with actin alone, FimA2 significantly increases the total number of filaments, suggesting that FimA2 increases nucleation (Fig. 5D). However, FimA2 does not entirely eliminate the initial lag step of bulk assembly assays (Fig. 5B), suggesting that FimA2 likely stabilizes the actin trimer rather than actin dimer. Neither Fim1 nor FimA2 affect the filament elongation rate (data not shown).
A Fimbrin Truncation Only Partially Substitutes for Full-length Fim1 in Vivo
In general, the importance of filament cross-linking has been assumed rather than rigorously tested. In fission yeast, cross-linked filaments are found at the contractile ring and cables (1, 2), but endocytic actin patches are not thought to be composed of bundles (25). Given the abundance of Fim1 in actin patches, which apparently lack bundled filaments (25), we hypothesized that the primary role of Fim1 in patches is to prevent ectopic localization of tropomyosin Cdc8 (23). Conversely, the bundling activity of Fim1 is likely to be important for the contractile ring and possibly cables. To determine whether the bundling activity of Fim1 is required for any of the actin structures, we directly compared three strains: 1) a strain exclusively expressing truncated FimA2 tagged with mCherry (fimA2) integrated at the endogenous locus, 2) a wild type strain carrying full-length Fim1 tagged with mCherry, and 3) a strain deleted of fim1 (fim1Δ) (Fig. 6A). In general we found that although FimA2 is able to prevent ectopic localization of Cdc8 to endocytic actin patches, the fimA2 strain otherwise phenocopies the fim1Δ strain, indicating that bundling is necessary for actin patches, the contractile ring, and actin cables.
Similar to full-length Fim1-mCherry (36, 49), FimA2-mCherry localizes primarily to endocytic actin patches (Fig. 6A, top). FimA2-mCherry also localizes weakly to cable-like structures.
We next observed tropomyosin GFP-Cdc8 in live cells to examine the ability of FimA2 to inhibit the binding of Cdc8 to endocytic actin patches (Fig. 6A, bottom). In wild type Fim1-mCherry cells, GFP-Cdc8 associates with the contractile ring but not with endocytic actin patches (23, 55). However, in the absence of Fim1 (fim1Δ), GFP-Cdc8 localizes to the contractile ring as well as ectopically to endocytic actin patches (23). Conversely, as expected from in vitro assays (23), GFP-Cdc8 is mostly restricted to the contractile ring in fimA2 cells (Fig. 6A, bottom).
To determine whether FimA2 is capable of fully replacing full-length Fim1 at endocytic actin patches, we tracked the lifetime and dynamics of individual patches using the general actin marker GFP-CHD(rng2) (Fig. 6B, supplemental Movie 12). In wild type cells, patches have a lifetime of about 9.5 s. Wild type patches originate near the cell cortex, where they remain for about 60% of their lifetime before propelling into the cell interior (3, 23). Conversely, patches in fim1Δ cells have a lifetime of 16 s, and only about 20% of patches internalize (23). Patches in fimA2 cells exist for 17 s, almost twice as long as in wild type cells. Furthermore, only 25% of patches in fimA2 internalize compared with >90% of wild type patches. Therefore, despite the absence of significant Cdc8 on actin patches in fimA2 cells, their behavior strongly resembles fim1Δ patches, indicating that fimbrin-mediated cross-linking is important for actin patch function.
We then compared the total intracellular concentration and dynamics of full-length Fim1-mCherry and FimA2-mCherry in actin patches using quantitative live cell imaging (Fig. 6, C and D) (22). Even though FimA2-mCherry is expressed under the control of the native promoter, total intracellular concentration of FimA2-mCherry is ∼3-fold lower compared with Fim1-mCherry (Fig. 6C), resulting in the accumulation of ∼4-fold less FimA2 in actin patches than Fim1 (Fig. 6D). The dynamics of FimA2-labeled patches is quite different than Fim1-labeled patches. FimA2 patches persist for ∼2-fold longer than Fim1 patches, and FimA2 patches have a pronounced internalization defect (Fig. 6D).
Fimbrin-mediated Filament Bundling Is Important for Cell Division
The other major fission yeast actin-bundling protein α-actinin Ain1 localizes to the contractile ring (49). Although Ain1 is not essential for life, deletion of both ain1 (ain1Δ) and fim1 (fim1Δ) is synthetically lethal due to cytokinesis defects predicted to result from the loss of bundling activity in the contractile ring (49). To examine whether FimA2 can substitute for full-length fimbrin in ain1Δ cells, we crossed ain1Δ with full-length (Fim1-mCherry), fim1Δ, or fimA2 (FimA2-mCherry) (supplemental Fig. 3). We recovered Fim1-mCherry ain1Δ strains as expected, but all predicted double mutant fim1Δ ain1Δ cells are inviable. Similarly, all predicted double mutant cells from 24 tetrads of fimA2 ain1Δ are also inviable, indicating that FimA2 is not sufficient for life in the absence of α-actinin ain1. Therefore, the redundant bundling activities of Ain1 and Fim1 appear to be important for cytokinesis.
Fimbrin Is Involved in the Maintenance of Actin Cables
Although fimbrin Fim1 has not been detected in cables, fim1Δ cells have mild polarity defects, and FimA2-mCherry weakly localizes to cables (Fig. 6A) (36, 49). We did not observe gross actin cable defects in fim1Δ and fimA2 cells expressing the general actin marker GFP-CHD(rng2) (Fig. 7A) (23). However, whereas actin cables are primarily oriented parallel to the long axis of wild type cells, actin cables in fim1Δ and fimA2 cells appear qualitatively more randomly oriented (Fig. 7A).
To quantify the integrity of actin cables, we examined motility of the type V myosin motor Myo52 (Myo52–3×YFP), which walks exclusively along actin cables and has a role in cell polarity (Fig. 7, B–G, supplemental Movie 13) (56–58). In wild type cells with full-length Fim1 (Fim1-mCherry), Myo52–3×YFP particles are localized primarily to the cell poles or division site and move linearly along the length of the cell, with an average lifetime of ∼3.5 s (Fig. 7, B–G). In both fim1Δ and fimA2 cells, Myo52–3×YFP particles are dispersed throughout the cell (Fig. 7, B and C). Although more than 30% of Myo52–3×YFP particles remain stationary in fim1Δ and fimA2 cells (Fig. 7D), those that do move are frequently perpendicular to the long axis and often change direction (Fig. 7, E and F). Furthermore, the lifetime of Myo52–3×YFP particles in fim1Δ and fimA2 cells is more than double that of wild type cells (Fig. 7G). These data are consistent with Fim1 bundling activity playing a role in actin cable organization.
DISCUSSION
Filament cross-linking proteins are fundamental components of the actin cytoskeleton. In fission yeast, fimbrin Fim1 plays a role in endocytic actin patches and the contractile ring, although there is little evidence about a possible involvement in polarizing actin cables. Previously we discovered that a major role of Fim1 is to regulate the localization of tropomyosin Cdc8 (23), which allows cofilin-mediated filament severing and ensures type-I myosin motor Myo1 activation (23, 24). Because inhibition of Cdc8 does not require that Fim1 cross-links filaments (23), we hypothesized that the cross-linking activity of Fim1 might not be important for actin patches.
Through a combination of in vitro and in vivo approaches, we determined that Fim1 bundles filaments in both parallel and antiparallel orientations, and the ability of Fim1 to cross-link filaments is in fact important for patches, rings, and cables. Furthermore, in vitro Fim1 bundles Arp2/3 complex-mediated branched networks and inhibits Arp2/3 complex-mediated actin assembly. However, actin CP prevents Fim1 from efficiently bundling branched filaments and inhibiting Arp2/3 complex. We, therefore, postulate that CP contributes to rapid polymerization by Arp2/3 complex in the presence of Fim1 in vivo. The diverse roles of Fim1 in regulating tropomyosin localization and cross-linking filaments as well as affecting Arp2/3 complex-mediated polymerization highlight the importance of coordinating the balance of actin-binding proteins for diverse cellular roles (23, 24).
The Bundling Properties of Fimbrin Are Likely Tailored for Its Cellular Role
In general, the selectivity of particular filament orientations by bundling proteins in vitro matches their in vivo localization. For example, chicken and mammalian fimbrins strongly favor parallel bundles in vitro (42, 44, 45) and localize to parallel bundled filaments in the inner core of intestinal microvilli (59). Fascin also preferentially makes parallel bundles in vitro (Fig. 1F) (35, 41, 43) and localizes to cellular structures composed of parallel bundles including filopodia (60, 61). Fission yeast Fim1 localizes to endocytic patches composed of branched filaments with barbed ends thought to be oriented toward the membrane as well as the contractile ring composed of antiparallel bundles of filaments and likely actin cables composed of parallel filaments (Figs. 6A and 7) (2, 25, 36, 49). We find that in vitro Fim1 non-selectively bundles filaments in both orientations (Fig. 1).
It is possible that either the bundling protein defines filament orientation of a structure or that the pre-existing filament architecture selectively recruits particular actin-bundling proteins. These possibilities are not mutually exclusive. Nucleation factors establish the initial filament orientation that recruits particular bundling proteins, which would in turn strengthen and maintain the existing architecture. Many bundling proteins, which do not select a particular orientation, are also recruited to specific cellular structures. We, therefore, propose that bundling proteins are initially recruited to new filaments due to intrinsic properties conferred by the nucleation factor such as filament twist, flexibility, or nucleotide state (62). Once at a structure, actin-bundling proteins reinforce existing filament orientation, thus organizing the architecture of actin structures as they assemble rather than being passively recruited to a completed structure.
Role of Fimbrin in Arp2/3 Complex-nucleated Structures
Yeast endocytic actin patches are composed primarily of short-branched filaments nucleated by the Arp2/3 complex, similar to the leading edge of motile cells (4, 20, 63). Short-branched filaments at endocytic sites are thought to help promote vesicle internalization (20, 63, 64). Unlike lamellipodium of motile cells (65), fimbrin concentrates in yeast endocytic actin patches (3, 36, 49, 66, 67). In both budding and fission yeast, deletion of fimbrin severely disrupts actin patch dynamics (23, 68, 69).
Because Arp2/3 complex nucleates new filaments from the side of pre-existing mother filaments (70, 71), bundling could inhibit the Arp2/3 complex by masking mother filament binding sites. We found that Fim1 inhibits Arp2/3 complex in vitro (Fig. 3) and by a greater extent when pre-existing filaments are bundled by Fim1 before the addition of Arp2/3 complex (data not shown). However, we cannot rule out the possibility that Fim1 competes directly with Arp2/3 complex for binding to filament sides. Interestingly, heat shock protein HSP90 can bind to N-WASP and bundle branched filaments but does not inhibit polymerization by Arp2/3 complex and N-WASP (72).
Capping Protein Reduces Bundling by Fim1 and Relieves Inhibition of Arp2/3 Complex
Given that the majority of filaments in actin patches are probably not bundled (25) and that bundling inhibits Arp2/3 complex, mechanisms must be in place to reduce bundling in vivo. Branched filaments nucleated at a 70° angle must reach a critical length before they can bend back onto the mother filament or “zipper-up” with neighboring branches. Actin CP is a major actin patch component that keeps filaments short by tightly binding the barbed end of filaments (30, 73). The average length of filaments in an actin patch is estimated to be only 50–100 nm (20–40 actin monomers) (21, 22, 25). We found that CP significantly reduces bundling by Fim1 in vitro and restores rapid branching by Arp2/3 complex.
According to our model, deletion of CP should produce patches containing longer, bundled filaments. Additional actin does accumulate in patches of fission yeast cells lacking CP (30). Conversely, electron microscopy indicates that patches in budding yeast cells lacking a single subunit of CP appear normal (25). We would also predict that deletion of fim1 should rescue defects in cells lacking CP. However, fim1Δ and acp2Δ are synthetically lethal (30), and fim1Δ acp1Δ strains show gross defects in cell morphology and division (73). Interpretation of these interactions is complicated because Fim1 also has important roles at the contractile ring (23, 24) and actin cables (Fig. 7). Because deletion of CP (acp2Δ) partially suppresses contractile ring nucleation factor formin cdc12 mutants (30), defects in fim1Δ acp1Δ could be explained by their roles in cytokinesis as well as general perturbation of the actin cytoskeleton. It is also possible that other actin patch components help alleviate Fim1-mediated bundling and inhibition of Arp2/3 complex.
The Two Actin Binding Domains of Fim1 Play Different Roles in Filament Binding
The cross-linking core of fimbrin forms a horseshoe shape of two ABDs, ABD1 and ABD2 (Fig. 5A) (74–76). Sequence homology is generally higher between a single ABD of different fimbrin family members (60–80% homology) than between the two ABDs of a single fimbrin (20–30% homology) (77). Identical mutations in the two ABDs of budding yeast fimbrin Sac6 do not have the same effect in vivo, suggesting that the two ABDs are functionally distinct (78–80). Structural and biochemical studies also indicate that ABD1 and ABD2 do not bind filaments equivalently (Fig. 5A, supplemental Fig. 2, A and B) (76, 81, 82). In general, isolated ABD1s bind filaments much more weakly than ABD2s (81–83). ABD1 is more flexible than ABD2 and appears to cause a conformational change in subdomain 1 of actin upon binding to a filament (76, 84). The higher affinity ABD may function as a positioning head for the other to form a stronger contact, particularly when filaments are not perfectly in register (77, 81, 82).
As previously suggested, we found that Fim1 ABD1 does not bind filament efficiently in vitro (Fig. 5A, Kd > 3 μm) (36). However, ABD2 binds with submicromolar affinity (Fig. 5A, Kd = 620 nm) (36), supporting a model by which ABD2 initially binds to a filament, allowing ABD1 to find a nearby filament.
Interestingly, we found that inclusion of the EF-hand-like N-terminal headpiece of Fim1 significantly increases the affinity of ABD1 for actin filaments (Kd = 220 nm). Many fimbrin isoforms are calcium-sensitive (85), whereas Fim1 is not (36, 49, 86). The EF hands might, therefore, have an additional role. In crystal structures, the EF hands of some fimbrins face away from the filament between CH domains of ABD1, possibly acting as a wedge to position ABD1 (75). Deletion of the EF hands of other fimbrins reduces actin binding by ABD1 (75). We propose that inclusion of the EF hands increases the affinity of Fim1 ABD1 by changing the conformation of ABD1, aligning actin binding residues at positions where they can access the filament in the proper orientation. Structural studies are required to further explore this possibility.
The Bundling Activity of Fim1 Is Important for Actin Patches, the Contractile Ring, and Actin Cables
Both the contractile ring and actin cables are composed of bundled filaments (1, 2). However, despite high Fim1 levels (3, 22, 36, 49), endocytic actin patch filaments do not appear to be extensively bundled (25). We proposed that bundling by Fim1 is dispensable at the endocytic patches, where instead the ability of Fim1 to regulate cofilin-mediated severing by inhibiting the binding of tropomyosin Cdc8 is critical (23). The cross-linking activity of Fim1 is predicted to play an overlapping role with α-actinin Ain1 in the contractile ring because small amounts of Fim1 are detected in the ring (36, 49), and deletion of both fim1 and ain1 is synthetically lethal (49). Conversely, it is not known whether Fim1 cross-links filaments in actin cables. We, therefore, hypothesized that FimA2, which can bind but not bundle actin filaments (Fig. 5) (23), would be fully functional for actin patches but not the contractile ring and possibly actin cables. However, we found that the bundling activity of Fim1 appears to be required for all three processes (Figs. 6 and 7).
Although FimA2 inhibits localization of tropomyosin Cdc8 to actin patches, patches otherwise behave like those in fimbrin-deleted cells. Therefore, some patch filaments are probably cross-linked. Similarly, FimA2 is not sufficient for life in the absence of α-actinin Ain1 (supplemental Fig. 3), indicating that actin cross-linking activity is essential at the contractile ring.
Alternatively, it is important to note that because FimA2 is composed of only a single actin binding domain, there are half the ABDs per molecule of FimA2. Furthermore, FimA2 is ∼4-fold less abundant in actin patches compared with wild type Fim1 (Fig. 6D), so it is certainly possible that defects in FimA2 cells are due to less protein rather than deficient cross-linking activity. Conversely, FimA2 localizes to cables more strongly than wild type Fim1. Furthermore, as we do not yet fully understand the function of all other actin patch components, we cannot account for their behavior in fimbrin mutant cells.
A limited number of actin-binding proteins have been localized to actin cables, including coronin Crn1, tropomyosin Cdc8, and transgelin Stg1 (17, 87, 88). Neither of the canonical bundling proteins, Fim1 or Ain1, has been localized to actin cables (36, 49). However, both fim1Δ and fimA2 strains show mild polarity defects (Fig. 6A) (36, 49). We found that actin cables are functionally compromised in both fim1Δ and fimA2 cells, as they no longer provide effective tracks for the type V myosin motor Myo52. However, these defects can be interpreted multiple ways. The simplest explanation is that Fim1 does bundle filaments in cables but has not been detected due to low levels. In agreement with this, overexpression of either full-length Fim1 or FimA2 induces extra cable-like structures in a tropomyosin-dependent manner (36). Conversely, cable defects could be due to global defects in the actin cytoskeleton (23, 36, 49). Because turnover of actin patches is significantly slower in fim1Δ and fimA2 cells (Fig. 6B) (23), less actin may be available for assembling cables.
Conclusion
Fimbrin Fim1 has multiple important roles at different actin structures in fission yeast. We propose that Fim1 acts as a central regulator of actin structure organization both through its intrinsic function as an actin-bundling protein and through regulation of other proteins such as tropomyosin and myosin, which can in turn affect severing by cofilin and nucleation by Arp2/3 complex (23, 24, 51). However, interpretations remain clouded because the roles of many actin-binding proteins remain poorly understood. For example, budding yeast transgelin has vital roles working with fimbrin at actin patches (89), but the function of fission yeast transgelin is less clear (88). Similarly, although extensive work has detailed the mechanisms by which the contractile ring assembles, the function of several contractile ring-specific proteins, including the F-BAR protein Cdc15 and IQGAP Rng2, are multifaceted and only partially understood (37, 90–93). Because we believe that diverse actin structures self-assemble in a crowded cytoplasm from a common pool of nucleators, profilin-bound actin monomer, and actin-binding proteins, elucidating the functional interaction among actin-binding proteins remains a daunting but essential task.
Supplementary Material
Acknowledgments
We thank Jessica Henty and Chris Staiger (Purdue University) for technical assistance and members of the Kovar laboratory for strains, technical assistance, and insightful comments.
This work was supported, in whole or in part, by National Institutes of Health Grant RO1GM079265 (to D. R. K.) and RO1GM078450 (to R. S. R.) and Molecular and Cellular Biology Training Grant T32 GM007183 (to C. T. S.). This work was also supported by a William Rainey Harper Fellowship (to C. T. S.) and an American Heart Association Predoctoral Fellowship (to D. S. C.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1, Figs. S1–S3, and Movies S1–S13.
- CHD
- calponin homology (CH) domain
- TIRF
- total internal reflection fluorescence
- WASP
- Wiskott-Aldrich syndrome protein
- CP
- capping protein
- a.u.
- arbitrary units
- TMR
- tetramethylrhodamine-6-maleimide.
REFERENCES
- 1. Kamasaki T., Arai R., Osumi M., Mabuchi I. (2005) Nat. Cell Biol. 7, 916–917 [DOI] [PubMed] [Google Scholar]
- 2. Kamasaki T., Osumi M., Mabuchi I. (2007) J. Cell Biol. 178, 765–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sirotkin V., Beltzner C. C., Marchand J. B., Pollard T. D. (2005) J. Cell Biol. 170, 637–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kovar D. R., Sirotkin V., Lord M. (2011) Trends Cell Biol. 21, 177–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carlsson A. E., Wear M. A., Cooper J. A. (2004) Biophys. J. 86, 1074–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dominguez R. (2009) Crit. Rev. Biochem. Mol. Biol. 44, 351–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goode B. L., Eck M. J. (2007) Annu. Rev. Biochem. 76, 593–627 [DOI] [PubMed] [Google Scholar]
- 8. Pollard T. D., Beltzner C. C. (2002) Curr. Opin Struct. Biol. 12, 768–774 [DOI] [PubMed] [Google Scholar]
- 9. Gandhi M., Achard V., Blanchoin L., Goode B. L. (2009) Mol. Cell 34, 364–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kueh H. Y., Charras G. T., Mitchison T. J., Brieher W. M. (2008) J. Cell Biol. 182, 341–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cai L., Marshall T. W., Uetrecht A. C., Schafer D. A., Bear J. E. (2007) Cell 128, 915–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andrianantoandro E., Pollard T. D. (2006) Molecular Cell 24, 13–23 [DOI] [PubMed] [Google Scholar]
- 13. Ghosh M., Song X., Mouneimne G., Sidani M., Lawrence D. S., Condeelis J. S. (2004) Science 304, 743–746 [DOI] [PubMed] [Google Scholar]
- 14. Chan C., Beltzner C. C., Pollard T. D. (2009) Curr. Biol. 19, 537–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Toret C. P., Drubin D. G. (2006) J. Cell Sci. 119, 4585–4587 [DOI] [PubMed] [Google Scholar]
- 16. Doyle T., Botstein D. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 3886–3891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pelham R. J., Jr., Chang F. (2001) Nat. Cell Biol. 3, 235–244 [DOI] [PubMed] [Google Scholar]
- 18. Weaver A. M., Young M. E., Lee W. L., Cooper J. A. (2003) Curr. Opin. Cell Biol. 15, 23–30 [DOI] [PubMed] [Google Scholar]
- 19. Moseley J. B., Okada K., Balcer H. I., Kovar D. R., Pollard T. D., Goode B. L. (2006) J. Cell Sci. 119, 1547–1557 [DOI] [PubMed] [Google Scholar]
- 20. Kaksonen M., Toret C. P., Drubin D. G. (2006) Nat. Rev. Mol. Cell Biol. 7, 404–414 [DOI] [PubMed] [Google Scholar]
- 21. Berro J., Sirotkin V., Pollard T. D. (2010) Mol. Biol. Cell 21, 2905–2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sirotkin V., Berro J., Macmillan K., Zhao L., Pollard T. D. (2010) Mol. Biol. Cell 21, 2894–2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Skau C. T., Kovar D. R. (2010) Curr. Biol. 20, 1415–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clayton J. E., Sammons M. R., Stark B. C., Hodges A. R., Lord M. (2010) Curr. Biol. 20, 1423–1431 [DOI] [PubMed] [Google Scholar]
- 25. Young M. E., Cooper J. A, Bridgman P. C. (2004) J. Cell Biol. 166, 629–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kovar D. R., Kuhn J. R., Tichy A. L., Pollard T. D. (2003) J. Cell Biol. 161, 875–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martin S. G., Chang F. (2006) Curr. Biol. 16, 1161–1170 [DOI] [PubMed] [Google Scholar]
- 28. Carlsson A. E., Shah A. D., Elking D., Karpova T. S., Cooper J. A. (2002) Biophys. J. 82, 2333–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Skau C. T., Neidt E. M., Kovar D. R. (2009) Mol. Biol. Cell 20, 2160–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kovar D. R., Wu J. Q., Pollard T. D. (2005) Mol. Biol. Cell 16, 2313–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blanchoin L., Pollard T. D. (1999) J. Biol. Chem. 274, 15538–15546 [DOI] [PubMed] [Google Scholar]
- 32. Kovar D. R., Gibbon B. C., McCurdy D. W., Staiger C. J. (2001) Planta 213, 390–395 [DOI] [PubMed] [Google Scholar]
- 33. Neidt E. M., Skau C. T., Kovar D. R. (2008) J. Biol. Chem. 283, 23872–23883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mahaffy R. E., Pollard T. D. (2006) Biophys. J. 91, 3519–3528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Courson D. S., Rock R. S. (2010) J. Biol. Chem. 285, 26350–26357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nakano K., Satoh K., Morimatsu A., Ohnuma M., Mabuchi I. (2001) Mol. Biol. Cell 12, 3515–3526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takaine M., Numata O., Nakano K. (2009) EMBO J. 28, 3117–3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Higaki T., Kutsuna N., Sano T., Kondo N., Hasezawa S. (2010) Plant J. 61, 156–165 [DOI] [PubMed] [Google Scholar]
- 39. Khurana P., Henty J. L., Huang S., Staiger A. M., Blanchoin L., Staiger C. J. (2010) Plant Cell 22, 2727–2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sjöblom B., Salmazo A., Djinović-Carugo K. (2008) Cell. Mol. Life Sci. 65, 2688–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maekawa S., Endo S., Sakai H. (1982) J. Biochem. 92, 1959–1972 [DOI] [PubMed] [Google Scholar]
- 42. Bretscher A. (1981) Proc. Natl. Acad. Sci. U.S.A. 78, 6849–6853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ishikawa R., Sakamoto T., Ando T., Higashi-Fujime S., Kohama K. (2003) J. Neurochem. 87, 676–685 [DOI] [PubMed] [Google Scholar]
- 44. Glenney J. R., Jr., Kaulfus P., Matsudaira P., Weber K. (1981) J. Biol. Chem. 256, 9283–9288 [PubMed] [Google Scholar]
- 45. Matsudaira P., Mandelkow E., Renner W., Hesterberg L. K., Weber K. (1983) Nature 301, 209–214 [DOI] [PubMed] [Google Scholar]
- 46. Stossel T. P., Chaponnier C., Ezzell R. M., Hartwig J. H., Janmey P. A., Kwiatkowski D. J., Lind S. E., Smith D. B., Southwick F. S., Yin H. L. (1985) Annu. Rev. Cell Biol. 1, 353–402 [DOI] [PubMed] [Google Scholar]
- 47. Nakano K., Mabuchi I. (2006) Mol. Biol. Cell 17, 1933–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vavylonis D., Wu J. Q., Hao S., O'Shaughnessy B., Pollard T. D. (2008) Science 319, 97–100 [DOI] [PubMed] [Google Scholar]
- 49. Wu J. Q., Bähler J., Pringle J. R. (2001) Mol. Biol. Cell 12, 1061–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee W. L., Bezanilla M., Pollard T. D. (2000) J. Cell Biol. 151, 789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Blanchoin L., Pollard T. D., Hitchcock-DeGregori S. E. (2001) Curr. Biol. 11, 1300–1304 [DOI] [PubMed] [Google Scholar]
- 52. Akin O., Mullins R. D. (2008) Cell 133, 841–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Loisel T. P., Boujemaa R., Pantaloni D., Carlier M. F. (1999) Nature 401, 613–616 [DOI] [PubMed] [Google Scholar]
- 54. Blanchoin L., Pollard T. D., Mullins R. D. (2000) Curr. Biol. 10, 1273–1282 [DOI] [PubMed] [Google Scholar]
- 55. Skoumpla K., Coulton A. T., Lehman W., Geeves M. A., Mulvihill D. P. (2007) J. Cell Sci. 120, 1635–1645 [DOI] [PubMed] [Google Scholar]
- 56. Motegi F., Arai R., Mabuchi I. (2001) Mol. Biol. Cell 12, 1367–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Win T. Z., Gachet Y., Mulvihill D. P., May K. M., Hyams J. S. (2001) J. Cell Sci. 114, 69–79 [DOI] [PubMed] [Google Scholar]
- 58. Grallert A., Martín-García R., Bagley S., Mulvihill D. P. (2007) J. Cell Sci. 120, 4093–4098 [DOI] [PubMed] [Google Scholar]
- 59. Mooseker M. S., Tilney L. G. (1975) J. Cell Biol. 67, 725–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Otto J. J., Kane R. E., Bryan J. (1979) Cell 17, 285–293 [DOI] [PubMed] [Google Scholar]
- 61. Otto J. J., Kane R. E., Bryan J. (1980) Cell Motil. 1, 31–40 [DOI] [PubMed] [Google Scholar]
- 62. Oda T., Maéda Y. (2010) Structure 18, 761–767 [DOI] [PubMed] [Google Scholar]
- 63. Galletta B. J., Cooper J. A. (2009) Curr. Opin. Cell Biol. 21, 20–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Engqvist-Goldstein A. E., Drubin D. G. (2003) Annu. Rev. Cell Dev. Biol. 19, 287–332 [DOI] [PubMed] [Google Scholar]
- 65. Bretscher A., Weber K. (1980) J. Cell Biol. 86, 335–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Adams A. E., Botstein D., Drubin D. G. (1991) Nature 354, 404–408 [DOI] [PubMed] [Google Scholar]
- 67. Kübler E., Riezman H. (1993) EMBO J. 12, 2855–2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Goode B. L., Wong J. J., Butty A. C., Peter M., McCormack A. L., Yates J. R., Drubin D. G., Barnes G. (1999) J. Cell Biol. 144, 83–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Goodman A., Goode B. L., Matsudaira P., Fink G. R. (2003) Mol. Biol. Cell 14, 2617–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Beltzner C. C., Pollard T. D. (2008) J. Biol. Chem. 283, 7135–7144 [DOI] [PubMed] [Google Scholar]
- 71. Pollard T. D., Borisy G. G. (2003) Cell 112, 453–465 [DOI] [PubMed] [Google Scholar]
- 72. Park S. J., Suetsugu S., Sagara H., Takenawa T. (2007) Genes Cells 12, 611–622 [DOI] [PubMed] [Google Scholar]
- 73. Nakano K., Mabuchi I. (2006) Genes Cells 11, 893–905 [DOI] [PubMed] [Google Scholar]
- 74. Goldsmith S. C., Pokala N., Shen W., Fedorov A. A., Matsudaira P., Almo S. C. (1997) Nat. Struct. Biol. 4, 708–712 [DOI] [PubMed] [Google Scholar]
- 75. Hanein D., Volkmann N., Goldsmith S., Michon A. M., Lehman W., Craig R., DeRosier D., Almo S., Matsudaira P. (1998) Nat. Struct. Biol. 5, 787–792 [DOI] [PubMed] [Google Scholar]
- 76. Klein M. G., Shi W., Ramagopal U., Tseng Y., Wirtz D., Kovar D. R., Staiger C. J., Almo S. C. (2004) Structure 12, 999–1013 [DOI] [PubMed] [Google Scholar]
- 77. Lebart M. C., Hubert F., Boiteau C., Ventéo S., Roustan C., Benyamin Y. (2004) Biochemistry 43, 2428–2437 [DOI] [PubMed] [Google Scholar]
- 78. Adams A. E., Botstein D., Drubin D. G. (1989) Science 243, 231–233 [DOI] [PubMed] [Google Scholar]
- 79. Brower S. M., Honts J. E., Adams A. E. (1995) Genetics 140, 91–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Honts J. E., Sandrock T. S., Brower S. M., O'Dell J. L., Adams A. E. (1994) J. Cell Biol. 126, 413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Galkin V. E., Orlova A., Cherepanova O., Lebart M. C., Egelman E. H. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 1494–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Volkmann N., DeRosier D., Matsudaira P., Hanein D. (2001) J. Cell Biol. 153, 947–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Voigt B., Timmers A. C., Samaj J., Müller J., Baluska F., Menzel D. (2005) Eur. J. Cell Biol. 84, 595–608 [DOI] [PubMed] [Google Scholar]
- 84. Hanein D., Matsudaira P., DeRosier D. J. (1997) J. Cell Biol. 139, 387–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. de Arruda M. V., Watson S., Lin C. S., Leavitt J., Matsudaira P. (1990) J. Cell Biol. 111, 1069–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Strynadka N. C., James M. N. (1989) Annu. Rev. Biochem. 58, 951–998 [DOI] [PubMed] [Google Scholar]
- 87. Balasubramanian M. K., Helfman D. M., Hemmingsen S. M. (1992) Nature 360, 84–87 [DOI] [PubMed] [Google Scholar]
- 88. Nakano K., Bunai F., Numata O. (2005) FEBS Lett. 579, 6311–6316 [DOI] [PubMed] [Google Scholar]
- 89. Gheorghe D. M., Aghamohammadzadeh S., Smaczynska-de, Rooij II, Allwood E. G., Winder S. J., Ayscough K. R. (2008) J. Biol. Chem. 283, 15037–15046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bathe M., Chang F. (2010) Trends Microbiol. 18, 38–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Carnahan R. H., Gould K. L. (2003) J. Cell Biol. 162, 851–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pollard T. D., Wu J. Q. (2010) Nat. Rev. Mol. Cell Biol. 11, 149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Roberts-Galbraith R. H., Ohi M. D., Ballif B. A., Chen J. S., McLeod I., McDonald W. H., Gygi S. P., Yates J. R., 3rd, Gould K. L. (2010) Mol. Cell 39, 86–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.