Abstract
The degradation of collagens, the most abundant proteins of the extracellular matrix, is involved in numerous physiological and pathological conditions including cancer invasion. An important turnover pathway involves cellular internalization and degradation of large, soluble collagen fragments, generated by initial cleavage of the insoluble collagen fibers. We have previously observed that in primary mouse fibroblasts, this endocytosis of collagen fragments is dependent on the receptor urokinase plasminogen activator receptor-associated protein (uPARAP)/Endo180. Others have identified additional mechanisms of collagen uptake, with different associated receptors, in other cell types. These receptors include β1-integrins, being responsible for collagen phagocytosis, and the mannose receptor. We have now utilized a newly developed monoclonal antibody against uPARAP/Endo180, which down-regulates the receptor protein level on treated cells, to examine the role of uPARAP/Endo180 as a mediator of collagen internalization by a wide range of cultured cell types. With the exception of macrophages, all cells that proved capable of efficient collagen internalization were of mesenchymal origin and all of these utilized uPARAP/Endo180 for their collagen uptake process. Macrophages internalized collagen in a process mediated by the mannose receptor, a protein belonging to the same protein family as uPARAP/Endo180. β1-Integrins were found not to be involved in the endocytosis of soluble collagen, irrespectively of whether this was mediated by uPARAP/Endo180 or the mannose receptor. This further distinguishes these pathways from the phagocytic uptake of particulate collagen.
Keywords: Cell Surface Protein, Cysteine Protease, Endocytosis, Extracellular Matrix, Fibroblast, Integrin, Matrix Metalloproteinase (MMP), Protein Turnover, Blocking Antibody, Collagen Degradation
Introduction
Remodeling of the extracellular matrix is required for a wide range of physiological and pathological conditions such as morphogenesis, organ growth, wound healing, arthritis, fibrosis and tumor growth, and metastasis (1–4). Collagens are the most abundant components of the extracellular matrix with collagen type I as the quantitatively dominating subtype. Thus, collagen constitutes about 25–30% of the dry weight of a human (5). The collagen in the body is undergoing continuous renewal and normally the collagen turnover rate is carefully controlled. Depending on the tissue type or extraneous events the collagen turnover rate can change dramatically. Therefore, highly efficient biological systems are needed in both the formation and degradation of collagen throughout life.
In normal healthy tissue, collagen is fully hydroxylated and forms insoluble, cross-linked fibers and sheets of triple helical structures that are resistant to attack by most proteases (6). A number of proteases are nevertheless potentially capable of initiating the collagen degradation process through the cleavage of intact collagen fibers. These proteases are the matrix metalloproteinases (MMPs)3 MMP-1, MMP-2, MMP-8, MMP-13, MMP-14, MMP-15, and MMP-16 and the cysteine protease cathepsin K (7–9). So far, most studies of collagen turnover have focused on the extracellular collagen degradation mediated solely by these proteases. Recently, however, we have established an important interplay between the extracellular collagenases and a collagen degradation pathway that involves endocytic uptake and intracellular degradation of well defined collagen fragments. In particular, we have demonstrated that the collagen fragments generated through the initial MMP-mediated cleavage of collagen fibers can be efficiently endocytosed and degraded in the lysosomes in a process mediated by the specific endocytic receptor, the urokinase plasminogen activator receptor-associated protein (uPARAP)/Endo180. This sequential degradation mechanism is required for complete and efficient collagen turnover by mouse skin fibroblasts (10, 11). We have also demonstrated that the uptake of solubilized, intact collagen mimics the efficient uptake of the defined MMP-generated collagen fragments (11). In addition to this composite collagen degradation pathway involving non-phagocytic collagen internalization, an alternative collagen uptake mechanism also exists. This latter pathway is dependent on β1-integrin activity and concerns the phagocytic uptake of large collagen particles (12–14). At present, the elucidation of the exact role of the different collagen receptors is an important issue.
Through the generation of uPARAP/Endo180 knock-out mice, we have previously demonstrated that uPARAP/Endo180 is an indispensable component for internalization and lysosomal degradation of solubilized collagen by primary mouse fibroblasts, chondrocytes, and osteoblasts (10, 15, 16). The uptake mechanism of uPARAP/Endo180 has been shown to function through constitutively recycling, clathrin-mediated endocytosis (17, 18).
Even though phenotypic studies of unchallenged uPARAP/Endo180 knock-out mice have only revealed rather modest phenotypic consequences (16),4 lack of uPARAP/Endo180 can lead to a dramatic collagen accumulation in pathological conditions with high collagen turnover such as cancer (19), thereby confirming a role of uPARAP/Endo180 in the process of in vivo extracellular matrix turnover. This role of uPARAP/Endo180 was further substantiated by the recent identification of a uPARAP/Endo180 null mutation in cattle, which leads to a severe syndrome characterized by dramatic bone defects (20). These studies demonstrate the importance of the uPARAP/Endo180-dependent collagen degradation pathway in vivo and illuminate the need for further investigations of this mechanism.
uPARAP/Endo180 is expressed in a variety of different tissues (21, 22) but a high uPARAP/Endo180 expression is in particular observed at sites of tissue remodeling, such as in the osteoblasts and chondrocytes of the developing bones (16, 23, 24). uPARAP/Endo180 is frequently up-regulated in invasive cancers (25–29), and most often expression is observed in the stromal cells. However, the total spectrum of cell types expressing uPARAP/Endo180 is not yet known and it is also unclear whether in all cases the receptor is active in and required for collagen turnover (30).
In this paper, we describe an investigation of the uptake of solubilized collagen in several cell types of human and murine origin. By preventing collagen uptake with a highly efficient mAb against uPARAP/Endo180 and using cells with deficiency for this and other collagen receptors, we can now define the non-phagocytic pathway of collagen degradation as a distinct mechanism in molecular terms. Depending on the cell type, this pathway is governed by either uPARAP/Endo180 or another member of the same protein family, the mannose receptor.
EXPERIMENTAL PROCEDURES
Reagents
The monoclonal mouse anti-uPARAP/Endo180 antibodies (mAbs) 5f4 and 2h9F12 were raised against purified recombinant soluble uPARAP/Endo180 and produced as previously described (28). Isotype-matched control anti-TNP antibody was produced as described in Ref. 31. The following proteins were purchased from commercial sources AS indicated: acid-extracted collagen type I from rat tail collagen, monoclonal antibody against murine β1-integrin (clone HA2/5), and phycoerythrin-conjugated anti-CD206 antibody (BD Biosciences), Oregon Green-labeled gelatin, Oregon Green-labeled collagen type IV, holotransferrin and cysteine protease inhibitor E64d (Calbiochem, San Diego, CA), interleukin 1β and tumor necrosis factor-α (Peprotech, Rocky Hill, NJ), iodine-125 (PerkinElmer Life Sciences), monoclonal antibody against human β1-integrin (clone 4B4) (Beckman Coulter, Brea, CA), cyclic RGD-peptide (Peptides International, Louisville, KT), mannose-BSA (Dextra Laboratories, Reading, United Kingdom), CellMask Orange (Invitrogen), granulocyte macrophage colony-stimulating-factor (GM-CSF) (Sigma), FITC-conjugated anti-mouse antibody (DAKO, Glostrup, Denmark), and proteinase K (Roche Applied Science).
Cultured Cells
For the generation of human macrophages, monocytes were isolated from human blood as described (32). The monocytes were seeded in a 24-well cell culture plate and differentiated into macrophages using GM-CSF at a concentration of 5 ng/ml over a 7-day span. The cells were cultured in AIM-V supplemented with 10% FCS and medium was replenished on day 4.
Animal experiments were approved the NIDCR Animal Care and Use Committee. For the isolation of activated murine macrophages, wild type and littermate mannose receptor−/− mice in C57BL/6J background (33) were injected intraperitoneally with 2 ml of a 2% Brewers TG solution (BD Biosciences). Mice were killed after 72 h and peritoneal cells were collected by lavage using 10 ml of cold PBS. The lavage fluid was centrifuged, the supernatant aspirated, and the cell pellet resuspended in complete RPMI 1640 medium with 10% DMSO, and the cells were frozen for later use. Cells were then frozen directly for later use. After thawing, cells were seeded in the wells of a 24-well cell culture plate in RPMI with 10% FCS and 1% penicillin/streptomycin, and the adherent cells were used for analysis.
The following established cell lines were purchased from the indicated sources or from sources described previously: human gingival fibroblast cell line HGF-1 (ATCC number CRL-2014), murine sarcoma cell line NCTC 2472 (ATCC number CCL-11), human osteosarcoma cell line MG63 (ATCC number CRL-1427), murine fibroblast cell line NIH3T3 (ATCC number CRL-1658), human prostate carcinoma cell line LNCaP (ATCC number CRL-1740), human lung fibroblast cell line MRC-5 (ATCC number CCL-171), murine melanoma cell line B16F10 (ATCC number CRL-6475), and human colorectal adenocarcinoma cell line CaCo-2 (ATCC number HTB-37) were all purchased from American Type Culture Collection. Human fibrosarcoma cell line HT1080, human rhabdomyosarcoma cell line RD, and the human cervix carcinoma cell line HeLa (34), human breast adenocarcinoma cell line MCF-7 (35), human embryonic kidney cell line HEK-293 (36), murine Lewis lung carcinoma (37), and human breast adenocarcinoma cell line MDA-MB-231 (38) cell lines were cultured according to ATCC guidelines. Murine β1-integrin-deficient fibroblasts (GD25) and GD25 fibroblasts re-transfected with β1-integrin (GD25-β) were kind gifts from Reinhard Fässler (39). Primary skin fibroblasts were isolated from newborn uPARAP/Endo180−/− or littermate wild type (uPARAP/Endo180+/+) mice as previously described (15). Fibroblasts were cultured in DMEM with 10% FCS and 1% penicillin/streptomycin.
Cell and Organ Extracts and Western Blotting
Cultured MG-63 cells and fibroblasts from uPARAP/Endo180+/+ or littermate uPARAP/Endo180−/− mice were lysed in a Triton X-100 lysis buffer (10 mm Tris-HCl, 140 mm NaCl, pH 7.4, 1% Triton X-100, including protease inhibitor cocktail III (Calbiochem)). Hearts and lungs were isolated from uPARAP+/+ mice or littermate uPARAP−/− mice after perfusion of the mice with 10 ml of PBS. The organs were ground in liquid nitrogen using a mortar and pestle and homogenized in a CHAPS lysis buffer (0.1 m Tris-HCl, 50 mm NaCl, pH 7.5, 2% CHAPS, protease inhibitor mixture III) using a Polytron homogenizer. Cell lysates and organ homogenates were clarified by centrifugation at 20,000 × g for 15 min at 4 °C. Protein concentrations were determined using a BCA assay (Pierce) according to the manufacturer's instructions. SDS-PAGE was performed with 15 μg of cell lysate or organ homogenate loaded in each well followed by electroblotting onto PVDF membranes. For the analysis of mAb 5f4 specificity, Western blotting was performed with fibroblast lysates as previously described (15) using mAb 5f4 at a final concentration of 2 μg/ml. For the analysis of organ homogenates the PVDF membrane was incubated with 20 ng/ml of 125I-labeled mAb 5f4 at 4 °C overnight followed by automated phosphorimager analysis as previously described (11). Band intensities were quantified using the Image Gauge software (Fujifilm, Tokyo, Japan).
Endocytosis Studies
Labeling of proteins with 125I and internalization studies of radiolabeled ligands by cultured cells was performed essentially as described in Ref. 15. All cells, except for macrophages, were seeded in 24-well tissue culture plates at a density of 1 × 105 cells/well. Macrophages were seeded at a density of 2 × 105 cells/well. Cells were cultured overnight to ensure maximal surface adherence. For the functional blocking of uPARAP/Endo180, monoclonal antibody (mAb) 5f4 was added to a concentration of 10 μg/ml (unless another concentration is indicated) during this cell culture period. Isotype-matched control antibody, anti-TNP, was likewise added during this period. The cells were subsequently washed gently in serum-free cell culture medium at 37 °C and were then preincubated for 30 min in assay buffer (the appropriate cell culture medium with 20 mm Hepes, pH 7.4, and 15 mg/ml BSA). In experiments with functional blocking of uPARAP/Endo180, this preincubation buffer included mAb 5f4 or negative control antibody (anti-TNP) at a concentration of 10 μg/ml (unless another concentration is indicated). In experiments, which included functional blocking of integrins, the preincubation buffer included 2 or 10 μg/ml of the indicated antibody or the indicated concentration of cyclic RGD peptide. A relatively short preincubation period in the presence of integrin-blocking reagents (30 min, without preceding overnight culture) proved necessary to prevent detachment of the cells (results not shown). In separate experiments, it was ascertained that a similar short preincubation procedure with 5f4 antibody (omitting the overnight preculture with antibody) did not influence the blocking efficiency markedly compared with experiments with overnight preincubation (data not shown). The assay buffer was removed and replaced by assay buffer containing 133 ng/ml of 125I-labeled protein and the indicated blocking reagents, followed by incubation at 37 °C. The cells were allowed to internalize ligand for 4–5 h and then washed three times with ice-cold PBS, and incubated for <5 min in trypsin-EDTA with 50 μg/ml of proteinase K to remove surface-bound material. The detached cells were centrifuged at 1,000 × g for 1 min at 4 °C, and the radioactivity in the pellet (internalized material) and supernatant (surface-released material) was measured in a γ-counter. In each analysis, a maximum of 4 cell lines were examined, and to minimize assay to assay variations MG63 cells were always included as a reference cell line and measured values were normalized to those of MG63. For each cell line collagen internalization was measured multiple times. For determination of the cellular protein level of uPARAP/Endo180, the cells were incubated with radiolabeled anti-uPARAP/Endo180 mAb 2h9F12 for 4 h and the total amount of cell-associated mAb 2h9F12 was measured.
Incubation of cultured cells with Oregon Green-labeled gelatin or collagen type IV followed by fluorescence microscopy with a Zeiss LSM 510 microscope was performed as described previously for fluorescence-labeled collagen type IV (10), using an incubation period of 18–20 h in the absence of protease inhibitor or in the presence of 20 μm E64d. The cell surface of the murine fibroblasts was stained as described in Ref. 10 using a monoclonal antibody against uPAR and the cell surface of MG63 cells was stained with 1 μg/ml of CellMask Orange for 5 min before fixation of the cells.
Effect of Antibody on uPARAP/Endo180 Protein Level
Wild type mouse primary skin fibroblasts or human MG63 cells were seeded in 6-well plates at a density of 6 × 105 cells/well. Cells were cultured overnight at 37 °C, after which mAb 5f4 (10 μg/ml) was added. Control samples were cultured in the presence of 10 μg/ml of isotype-matched control antibody (anti-TNP). After 24 h, cells were washed briefly in the wells with PBS with 5 mm EDTA, followed by addition of a fresh aliquot (2 ml) of the same buffer. After incubation for 30 min at 37 °C, cells were detached by pipetting in the same aliquot of incubation buffer and transferred to Eppendorf tubes. The tubes were then centrifuged for 5 min at 400 × g. 80 μl of lysis buffer (10 mm Tris-HCl, 140 mm NaCl, pH 7.4, with 1% Triton X-100 and protease inhibitor mixture III) was added to each cell pellet, followed by resuspension of cells. Tubes were then placed on ice for 20 min, vortexed, and centrifuged for 20 min at 4 °C, 13,000 × g, after which the supernatant was transferred to new tubes. Protein concentrations were determined using a BCA assay and equal protein amounts from each sample of the supernatants were analyzed by Western blotting using 125I-labeled anti-uPARAP/Endo180 antibody as described above, except that mAb 2h9F12 was used. SDS-PAGE analysis and Coomassie staining were included to ensure that equal protein amounts were indeed loaded.
Collagen Degradation Assay
A collagen matrix was reconstituted by polymerization and drying of acid-extracted collagen type I as described in Ref. 40 with inclusion of trace amounts of radiolabeled collagen type I as described in Ref. 11. Fibroblasts were cultured on the collagen matrix in DMEM including 1 nm interleukin 1β and 10 nm tumor necrosis factor-α in the absence of mAb 5f4 or presence of 50 μg/ml of mAb 5f4. After 5 days, the conditioned media was collected and analyzed by SDS-PAGE followed by automated phosphorimager analysis. For the generation of collagen fragments, collagen type I was cleaved with recombinant MMP-13 as previously described (11).
Flow Cytometry Analysis of uPARAP/Endo180 and Mannose Receptor Expression
Dissociated cells were washed twice in PBS buffer with 5% FCS, and incubated for 30 min at 4 °C in 5 μg/ml of unlabeled primary antibody or 0.625 μg/ml of phycoerythrin-conjugated CD206 antibody. The cells were washed in PBS buffer containing 5% FCS and in the case of CD206 analyzed directly on a FACScalibur flow cytometer (BD Biosciences). For analysis of uPARAP/Endo180 expression, cells were washed as above and incubated for 30 min at 4 °C in a 1:100 dilution of secondary FITC-conjugated anti-mouse antibody. Cells were subsequently washed in PBS buffer + 5% FCS and analyzed by flow cytometry.
RESULTS
Blocking Collagen Uptake through uPARAP/Endo180
To enable the specific inhibition of the uPARAP/Endo180-dependent collagen uptake mechanism, a panel of mAbs was generated by immunization of uPARAP/Endo180-deficient (−/−) mice with recombinant human uPARAP/Endo180. The utilization of mice with a deletion of the gene of interest allows for the generation of mAbs capable of recognizing human uPARAP/Endo180 as well as the interspecies conserved parts of the protein (41–43). We successfully generated eight mAbs against human uPARAP/Endo180, of which seven cross-reacted with the murine protein (results not shown). The specificity of one of these antibodies, designated 5f4, is shown in Fig. 1A. Both in cultured fibroblasts (left panel) and mouse organ extracts such as heart and lung (right panel), the antibody displayed a very high specificity because only one protein band was detected, migrating with an apparent molecular mass of 180 kDa. This protein was indeed uPARAP/Endo180, because no protein was detected in parallel samples derived from uPARAP/Endo180−/− mice. The seven mAbs were tested for the ability to inhibit the uptake of radiolabeled collagen type I by primary mouse skin fibroblasts, a process reflecting a central step in the combined proteolytic and endocytic collagen degradation pathway (11, 15). One of them, mAb 5f4, proved able to very efficiently reduce the collagen internalization process (Fig. 1B). Although the assay conditions with protease treatment of the cells did inevitably leave a small amount of bound collagen on the outside of the cells (see “Experimental Procedures”), thus preventing a complete isolation of the internalized radioactivity, the antibody reduced the amount of radioactivity to less than 25%. Even more importantly, this level was very close to that obtained with fibroblasts from littermate uPARAP/Endo180 knock-out mice. The same antibody did not affect the cellular internalization of holotransferrin, an irrelevant, non-collagen protein that is internalized through clathrin-coated pits by means of the transferrin receptor (44) (Fig. 1C). Thus, mAb 5f4 does not affect the general endocytosis machinery of the cells. One additional antibody was also inhibitory, whereas the remainders were negative (results not shown). The inhibitory property of mAb 5f4 was further characterized and collagen internalization experiments with mouse fibroblasts revealed an approximate IC50 of 0.04 μg/ml (Fig. 1D), thus indicating a very potent blocking reagent.
FIGURE 1.
Anti-uPARAP/Endo180 mAb 5f4 inhibits cellular collagen internalization. A, mAb 5f4 specifically recognizes uPARAP/Endo180. Detergent extracts of cultured fibroblasts isolated from newborn mice (left panel) or mouse heart and lung (right panel) were analyzed by Western blotting, using mAb 5f4 as the primary antibody. In each panel, samples from wild type (uPARAP+/+) and uPARAP/Endo180-deficient (uPARAP−/−) mice were run in parallel lanes. The electrophoretic mobility of Mr marker proteins is indicated to the left. uPARAP/Endo180 migrates with an apparent molecular mass of 180 kDa. B, mAb 5f4 inhibits collagen internalization by murine fibroblasts. Primary fibroblasts from wild type (uPARAP/Endo180+/+) mice (dark gray columns) or littermate uPARAP/Endo180−/− mice (light gray columns) were allowed to internalize radiolabeled soluble collagen I for 4 h in the absence of antibody or in the presence of 10 μg/ml of mAb 5f4 (hatched columns). C, anti-uPARAP/Endo180 mAb 5f4 does not affect internalization of holotransferrin. Murine fibroblast cultures were assayed as in B, but using radiolabeled holotransferrin instead of collagen I. D, anti-uPARAP/Endo180 mAb 5f4 inhibits collagen internalization in a dose-dependent manner. Murine primary fibroblast cultures were allowed to internalize radiolabeled soluble collagen I in the absence of antibody, in the presence of mAb 5f4 at concentrations ranging from 0.01 to 100 μg/ml, or in the presence of 100 μg/ml of a monoclonal isotype-matched irrelevant control antibody (anti-TNP). uPARAP/Endo180−/− fibroblasts (light gray column) were included in the analysis for comparison. A half-maximum inhibition was obtained with an approximate concentration of 0.04 μg/ml of mAb 5f4. In B–D, the internalized radioactivity in untreated uPARAP/Endo180+/+ fibroblasts was defined as 100% internalization. E, anti-uPARAP/Endo180 mAb 5f4 inhibits collagen internalization by human MG63 cells. An experiment was performed as in B, but using the human osteosarcoma cell line MG63 instead of primary murine fibroblasts. Internalization by MG63 cells in the absence of antibody was defined as 100% internalization. Note that a similar inhibitory efficacy of mAb 5f4 was observed with human and mouse cells. For B–E, all samples were analyzed in triplicates. Error bars indicate S.D.
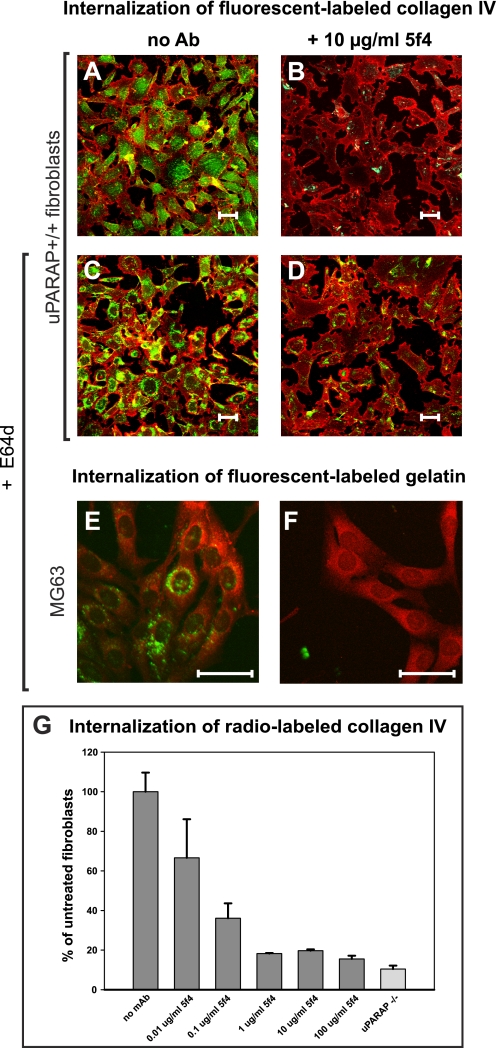
To directly visualize the effect of mAb 5f4 on the resulting degradation process, a different experimental system was applied, using fluorescence-labeled collagens. Like collagen type I, collagen type IV and gelatin (denatured collagen type I) are internalized by fibroblasts in a completely uPARAP/Endo180-dependent manner and can be used in fluorescence-labeled form for studies on endocytosis (10, 11). uPARAP/Endo180 deficient (−/−) fibroblasts or littermate wild type (uPARAP/Endo180+/+) fibroblasts were incubated with Oregon Green-labeled collagen type IV in the absence or presence of mAb 5f4 and cellular uptake was monitored using fluorescence microscopy (Fig. 2). In the absence of mAb 5f4, internalized collagen was observed in uPARAP/Endo180+/+ fibroblasts in vesicular structures representing endosomes and lysosomes (Fig. 2A). As expected, no or little internalized collagen was found in the uPARAP/Endo180−/− fibroblasts (results not shown). The intracellular routing was indeed part of a degradation pathway because an even more pronounced vesicular accumulation of collagen was observed when the lysosomal cysteine protease inhibitor E64d was added (Fig. 2C). Both in the absence and presence of E64d, this accumulation of collagen was dramatically reduced when uPARAP/Endo180+/+ cells were incubated in the presence of mAb 5f4 (Fig. 2, B and D), directly demonstrating the effect of this mAb on the total degradation pathway. An antibody titration experiment with radiolabeled collagen type IV (Fig. 2G) revealed that uptake of this type of collagen had the same high sensitivity to mAb 5f4 as that noted above with collagen type I.
FIGURE 2.
Anti-uPARAP/Endo180 mAb 5f4 prevents the accumulation of collagen in intracellular vesicles. A–D, the intracellular accumulation of fluorescent-labeled collagen type IV in murine fibroblasts is prevented by anti-uPARAP/Endo180 mAb 5f4. Oregon Green-labeled collagen IV was added to primary fibroblast cultures derived from uPARAP/Endo180+/+ mice, after which the cells were cultured for 18 h in the absence of the lysosomal protease inhibitor E64d (A and B) or in the presence of 20 μm E64d (C and D). The internalization of collagen was carried out in the absence of antibody (A and C) or in the presence of 10 μg/ml of mAb 5f4 (B and D). E and F, anti-uPARAP/Endo180 mAb 5f4 prevents intracellular accumulation of fluorescent-labeled gelatin in the human osteosarcoma cell line MG63. Oregon Green-labeled gelatin was added to cultured MG63 cells in the presence of E64d. Internalization was carried out for 20 h in the absence of antibody (E) or presence of 10 μg/ml of mAb 5f4 (F). G, cellular uptake of collagen IV is equally sensitive to mAb 5f4 as the uptake of collagen I. The mAb 5f4 dose dependence of collagen IV uptake was measured using radiolabeled collagen ligand. The conditions were the same as used in Fig. 1D, except that the ligand was 125I-labeled collagen IV.
To test whether mAb 5f4 also blocked collagen turnover in human cells, we utilized the human osteosarcoma cell line MG63, a cell line known to express high levels of uPARAP/Endo180 (18, 45). An internalization experiment using radiolabeled collagen type I revealed a blocking effect of mAb 5f4 with this human cell line that was similar to that found with uPARAP/Endo180+/+ mouse fibroblasts and a similar IC50 value was determined (Fig. 1E). Furthermore, when fluorescence-labeled gelatin was added to MG63 cells, the internalized protein accumulated in the endosomal/lysosomal compartments (Fig. 2E), whereas in the presence of mAb 5f4, this accumulation was clearly inhibited (Fig. 2F). Both with human and murine cells, even very low concentrations of antibody were sufficient to block the uptake of the fluorescence-labeled ligand (supplemental Fig. S1), in complete accordance with the titration studies performed with radiolabeled collagens. Thus, the uptake of all of the collagen ligands tested in these cells displayed a strong sensitivity to mAb 5f4 against uPARAP/Endo180.
These observations raised the question about the mechanism through which mAb 5f4 could counteract the function of uPARAP/Endo180 in collagen internalization. First, we utilized a purified system with surface plasmon resonance to test any blocking effect of the antibody against direct interaction between uPARAP/Endo180 and collagen. Using an established BIAcore setup with recombinant uPARAP/Endo180 immobilized on a distinct catching monoclonal antibody, mAb 2h9F12 (11), we first ensured that this catching antibody did not prevent the subsequent binding of mAb 5f4 to uPARAP/Endo180 (results not shown). In the next step, we injected solubilized collagen type I into flow cells with immobilized uPARAP that was either unoccupied or saturated with mAb 5F4 (supplemental Fig. S2). Somewhat surprisingly, this experiment showed that mAb 5f4 had no inhibitory effect on collagen binding.
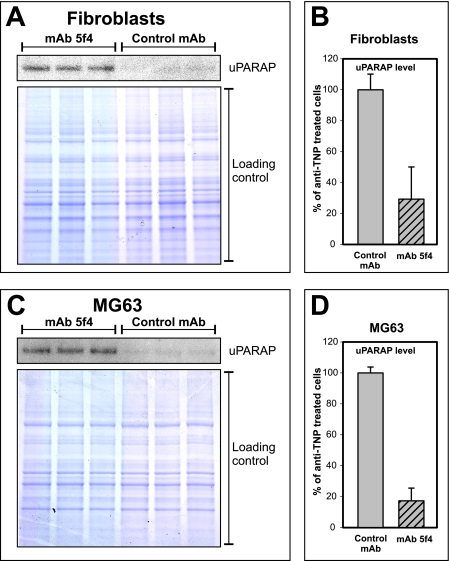
Consequently, we tested whether the antibody had an influence on the cellular uPARAP/Endo180 protein level. Mouse primary fibroblasts and human MG63 cells were cultured in the presence or absence of mAb 5f4, after which the cellular level of uPARAP/Endo180 was assessed by Western blotting (Fig. 3). Strikingly, with both cell types, cultivation in the presence of the antibody led to a strongly reduced level of collagen receptor. Thus, the effect of mAb 5f4 is similar to that observed with certain other antibodies against cell surface receptors that function through an apparent down-regulation of their target antigen (46, 47), an effect likely to reflect an antibody-induced failure of receptor recycling.
FIGURE 3.
Down-regulation of the uPARAP/Endo180 protein level by mAb 5f4. Primary mouse fibroblasts (A) or human MG63 osteosarcoma cells (C) were cultured in the presence of mAb 5f4 or anti-TNP control mAb, after which detergent lysates of the cells were analyzed by Western blotting using 125I-labeled anti-uPARAP/Endo180 mAb 2h9F12 (upper panels) or SDS-PAGE and Coomassie staining as loading control (lower panels). The quantification of band intensities in fibroblast lysates (A) is depicted in B, and the band intensities in MG63 lysates (C) is depicted in D.
We have previously demonstrated that the intracellular collagen turnover pathway is operative when fibroblasts act to remodel an insoluble collagen matrix. Thus, uPARAP/Endo180 is essential for the clearance of the initial collagen cleavage products that are released through the action of MT1-MMP when fibroblasts invade a trypsin-resistant collagen matrix (Fig. 4A) (11). This clearance defect results in the accumulation of defined collagen fragments in the conditioned medium of the uPARAP/Endo180−/− fibroblasts (Fig. 4B, lane 4) but not of the uPARAP/Endo180+/+ fibroblasts (Fig. 4B, lane 3). Strikingly, incubation of uPARAP/Endo180+/+ fibroblasts with the mAb 5f4 led to the same accumulation of collagen fragments that were observed for the uPARAP/Endo180−/− fibroblasts (Fig. 4B, lane 5). This demonstrates that, also in a composite cell culture assay of collagen degradation, it is possible to mimic uPARAP/Endo180 deficiency by incubation of uPARAP/Endo180+/+ fibroblasts with mAb 5f4.
FIGURE 4.
Anti-uPARAP/Endo180 mAb 5f4 treatment mimics uPARAP/Endo180 deficiency in a composite assay of collagen degradation. A, schematic illustration of the experimental setup. A collagen matrix was reconstituted by polymerization of acid-extracted collagen type I in which trace amounts of radiolabeled collagen type I were included. Fibroblasts were cultured on top of the collagen matrix for 5 days after which the conditioned culture supernatants were harvested and analyzed by non-reducing SDS-PAGE, followed by phosphorimaging analysis of the polyacrylamide gel for the localization of specific collagen fragments released from the collagen matrix. B, anti-uPARAP/Endo180 mAb 5f4 leads to extracellular accumulation of collagen degradation products. The culture supernatants from the following cells are shown: third lane, untreated uPARAP/Endo180+/+ cells; fourth lane, untreated uPARAP/Endo180−/− cells; fifth lane, uPARAP/Endo180+/+ cells cultured in the presence of 50 μg/ml of mAb 5f4. The following purified reference proteins are shown: first lane, 125I-labeled, intact collagen type I; second lane, 125I-labeled, cleaved collagen type I. The positions of ¼ and ¾ collagen degradation fragments are indicated at right.
The Role of uPARAP/Endo180 in Collagen Internalization by Different Cell Types
A panel of human and murine cell lines of epithelial or mesenchymal origin (Table 1) were screened for their ability to internalize radiolabeled collagen. Simultaneously, for each cell line, the level of uPARAP/Endo180 expression was determined using a distinct, high affinity anti-uPARAP/Endo180 mAb, designated 2h9F12. As shown by previous BIAcore studies (see Ref. 11 and results not shown), the interaction between uPARAP/Endo180 and this antibody is stable even at low pH, thereby precluding endosomal dissociation of the complex. Consequently, the total amount of cell-bound mAb 2h9F12 on different cell types enables determination of the relative amounts of uPARAP/Endo180 molecules. For each cell line collagen internalization was measured and the collagen internalization could then be plotted against the determined uPARAP/Endo180 levels (Fig. 5A).
TABLE 1.
Established cell lines included in the study of collagen uptake
| Species | Origin | Ref. | |
|---|---|---|---|
| Epithelial cell lines | |||
| LNCaP | Human | Prostate carcinoma | 72 |
| MDA-MB-231 | Human | Breast adenocarcinoma | 73 |
| CaCo-2 | Human | Colorectal adenocarcinoma | 74 |
| HeLa | Human | Cervix carcinoma | 75 |
| MCF-7 | Human | Breast adenocarcinoma | 76 |
| HEK-293 | Human | Embryonic kidney | 77 |
| Lewis Lung Carcinoma (LLC) | Murine | Lung carcinoma | 78 |
| B16F10 | Murine | Skin melanoma | 79 |
| Mesenchymal cell lines | |||
| HGF-1 | Human | Healthy gingiva | 80 |
| MG63 | Human | Osteosarcoma | 81 |
| MRC-5 | Human | Lung fibroblast | 82 |
| HT1080 | Human | Fibrosarcoma | 83 |
| RD | Human | Rhabdomyosarcoma | 84 |
| NCTC 2472 | Murine | Sarcoma | 85 |
| NIH/3T3 | Murine | Embryonic fibroblast | 86 |
FIGURE 5.
uPARAP/Endo180 expression correlates with collagen internalization in a variety of cell types. A, correlation between uPARAP/Endo180 expression and collagen internalization. Fifteen epithelial (filled dots) and mesenchymal cell lines (empty dots), in addition to uPARAP/Endo180+/+ and uPARAP/Endo180−/− fibroblasts, were assayed for the expression of uPARAP/Endo180 and the ability to internalize radiolabeled collagen. The level of uPARAP/Endo180 expression was determined as the amount of cell bound radiolabeled anti-uPARAP/Endo180 mAb 2h9F12. Collagen internalization was assayed as described in the legend to Fig. 1. MG63 cells were used as a reference cell line for normalization of both collagen internalization and the level of uPARAP/Endo180 expression. Each cell line is represented as one point in the scatterplot, with the relative uPARAP/Endo180 level assigned to the x axis and the collagen internalization activity assigned to the y axis. A correlation analysis revealed a Pearson correlation coefficient r = 0.77 and p = 0.00018. A regression line is included in the graph. B, no correlation between uPARAP/Endo180 expression and holotransferrin internalization. The same cell lines as in A were also assayed for the ability to internalize radiolabeled holotransferrin. MG63 cells were used as a reference cell line for the normalization of holotransferrin internalization. A scatterplot similar to the one in A was generated but with holotransferrin internalization assigned to the y axis. All samples were analyzed in triplicates. Error bars indicate S.D.
Strikingly, this analysis revealed a strong correlation between the uPARAP/Endo180 expression of the cells and their ability to internalize collagen (p = 0.00018). As expected, the mesenchymal cell types proved much more efficient in internalizing collagen than the epithelial cells (Fig. 5A, open dots). With the exception of rhabdomyosarcoma (RD) cells, all mesenchymal cells were capable of efficient collagen internalization (with a read-out of more than 40% of the reference cell line MG63). The epithelial cell lines expressed low levels of uPARAP/Endo180 (Fig. 5A, filled dots) and all were incapable of efficient internalization of collagen. To make certain that the uPARAP/Endo180 level correlated with collagen internalization specifically, and not just with the general endocytic activity of the cells, we also examined the uptake of holotransferrin (Fig. 5B). This analysis did not reveal any correlation between the uPARAP/Endo180 level and the general endocytic activity of the cell lines.
To examine whether uPARAP/Endo180 also on these cell types was directly responsible for collagen uptake, blocking uPARAP/Endo180 mAb 5f4 was utilized. Internalization of radiolabeled collagen type I was carried out in the presence or absence of mAb 5f4. In the presence of mAb 5f4, collagen uptake was markedly reduced for all cell lines capable of efficient collagen internalization (Fig. 6). As mentioned above, the mesenchymal RD cells expressed low levels of uPARAP/Endo180 and internalized low levels of collagen. The presence of mAb 5f4 did not reduce collagen internalization for this cell line. Of the epithelial cell lines, HeLa cells had the highest level of uPARAP/Endo180 expression, and, in fact, this epithelial cell line was the only one that displayed reduced collagen internalization in the presence of mAb 5f4.
FIGURE 6.
uPARAP/Endo180 mediates collagen internalization in a variety of cell types. Collagen internalization by a variety of established cell lines is inhibited by mAb 5f4. Collagen internalization was measured for each cell line used in Fig. 5. Internalization was carried out in the absence of antibody (black columns) or presence of 10 μg/ml of mAb 5f4 (gray columns). The cell lines are ranked in the diagram according to their level of uPARAP/Endo180 expression, with the highest expressing cells at the left. MG63 cells were used as a reference cell line for the normalization of both collagen internalization and the level of uPARAP/Endo180 expression. All samples were analyzed in triplicates. Error bars indicate S.D.
β-Integrins Are Not Involved in the Endocytosis of Soluble Collagen
Although collagen internalization was found to be a completely uPARAP/Endo180-dependent process in our experimental systems, we could not exclude the possibility that other receptors could be involved as well. β1-Integrins are key mediators of cellular adhesion to collagen (48) and are required for the phagocytic uptake of collagen-coated beads (49). Therefore, and because a central issue to be elucidated is the distinction between the present route of collagen degradation and the phagocytic uptake of particulate collagen, we studied the possible function of β1-integrins in internalization of solubilized, radiolabeled collagen. This was examined using β1-integrin null mouse fibroblasts (GD25), as well as GD25 fibroblasts re-transfected with β1-integrin (GD25-β) (Fig. 7A). Both cell lines were capable of internalizing solubilized collagen and furthermore, collagen internalization could be markedly reduced by the presence of mAb 5f4 for both cell lines. Thus, also with these cells uPARAP/Endo180 has a decisive function in the collagen uptake mechanism. β1-Integrin null fibroblasts (GD25) internalized collagen very efficiently (155% of MG63 cells under our experimental conditions). The efficient internalization in GD25 cells clearly demonstrated that β1-integrins are not required for the uptake of solubilized collagen. Indeed, with the present fibroblast strains, β1-integrin re-transfected (GD25-β) cells internalized less collagen than GD25 cells. This difference in collagen uptake between the GD25 and GD25-β fibroblasts could be explained by the different levels of uPARAP/Endo180 expression (Fig. 7B) and was not caused by a general difference in their endocytic capacities (Fig. 7C).
FIGURE 7.
β1-Integrins are not involved in the internalization of soluble collagen. A, murine β1-integrin null fibroblasts are capable of internalizing collagen. Murine β1-integrin-deficient fibroblasts (GD25) (black columns) and β1-integrin-retransfected fibroblasts (GD25-β) (gray columns) were analyzed for their ability to internalize radiolabeled soluble collagen I in the absence of antibody or in the presence of 10 μg/ml of mAb 5f4 (hatched columns). The collagen internalization assay was performed as described in the legend to Fig. 1. B, uPARAP/Endo180 expression correlates with collagen internalization efficiency. The relative levels of uPARAP/Endo180 expression were determined for GD25 and GD25-β fibroblasts as described in the legend to Fig. 4. C, GD25 and GD25-β fibroblasts have the same overall endocytic capacity. An internalization assay was performed with radiolabeled holotransferrin as described in the legend to Fig. 1B. D, MG63 cells internalize collagen in a β1-integrin independent process. MG63 cells were analyzed for their ability to internalize labeled soluble collagen I in the absence of any exogenous reagents (first column), or in the presence of 10 μg/ml of mAb 5f4 (second column), 2 μg/ml of anti-β1 mAb 4B4 (third column), 10 μg/ml of anti-β1 mAb 4B4 (fourth column), 50 μm cyclic RGD peptide (fifth column), or 200 μm cyclic RGD peptide (sixth column). A–D, MG63 cells were in all cases included in parallel analyses and used as a reference for the normalization of the data. All samples were analyzed in triplicates. Error bars indicate S.D.
To examine the possibility that β1-integrin independent collagen endocytosis was restricted to murine fibroblasts we analyzed the human osteosarcoma cell line MG63 (Fig. 7D). To investigate the role of β1-integrin for collagen internalization in a human cell line we utilized blocking β1-integrin antibody 4B4 (12, 13, 50). Collagen internalization by MG63 cells was not affected by the addition of 4B4 (Fig. 7D, third and fourth columns). To confirm this result we also utilized cyclic RGD peptide (13, 36, 51) to block β1-integrin. In the presence of cyclic RGD peptide collagen internalization was likewise unaffected (Fig. 7D, fifth and sixth columns), demonstrating also that human cell line MG63 is capable of β1-integrin-independent collagen endocytosis.
β1-Integrin dependence was also tested for murine fibroblast cell line NIH/3T3 using blocking antibody and cyclic RGD peptide. This cell line was included in the analysis because of the reported β1-integrin-mediated phagocytic collagen uptake by this cell line (52, 53). However, in our assay of non-phagocytic collagen internalization we did not observe any β1-integrin dependence for this cell line (results not shown).
The Mannose Receptor Is Responsible for Collagen Endocytosis in Macrophages
In addition to fibroblasts, osteoblasts, and chondrocytes, which are key players in collagen turnover in the healthy body (16, 54, 55), macrophages play a decisive role in matrix degradation processes in inflammatory conditions (3, 56, 57). Interestingly, uPARAP/Endo180 expression has in certain cases been demonstrated on macrophages (58–60). This raised the question whether an uptake mechanism for solubilized collagen exists in macrophages and whether any such mechanism depends on uPARAP/Endo180 or different receptors. To examine the process of collagen endocytosis in macrophages, we isolated monocytes from human plasma and differentiated these cells to macrophages in vitro by cell culture for 8 days in the presence of GM-CSF. No uPARAP/Endo180 was detected on the undifferentiated monocytes, whereas a low level of uPARAP/Endo180 expression could indeed be observed after differentiation to macrophages (supplemental Fig. S3). However, the amounts of uPARAP/Endo180 detected were very low as compared with MG63 cells (Fig. 8A). The macrophages were assayed for their ability to internalize collagen and despite the very low level of uPARAP/Endo180 expression, they were capable of internalizing collagen at levels comparable with those of MG63 cells (Fig. 8B). Addition of mAb 5f4 did not affect collagen internalization significantly (Fig. 8B, fourth column), indicating that the macrophages were taking up the collagen in a process independent of uPARAP/Endo180. Interestingly, this process was also insensitive to blocking of β1-integrins, as shown with the same reagents as used above (Fig. 8C). To confirm that the internalized collagen was routed to the lysosomes for degradation, we repeated the collagen internalization assay with inclusion of E64d (Fig. 8D). We have previously shown that this inhibitor retards the lysosomal degradation of collagen, which in the case of murine fibroblasts, leads to an ∼2-fold increase in the amount of intracellular collagen under our experimental conditions (11). In the case of macrophages, addition of E64d led to an ∼4-fold increase in the amount of intracellular collagen. This large effect could be indicative of a high lysosomal collagen degradation activity in macrophages in the absence of inhibitor.
FIGURE 8.
The MR governs collagen internalization in macrophages. A–D, human blood monocytes were differentiated to macrophages in vitro. A, cultured macrophages express low amounts of uPARAP/Endo180. The relative level of uPARAP/Endo180 expression by cultured macrophages was based on the amount of cell-bound radiolabeled anti-uPARAP/Endo180 mAb 2h9F12 as described in the legend to Fig. 5. MG-63 cells were included as a normalization reference. B, macrophages internalize collagen in a uPARAP/Endo180-independent process. The internalization of radiolabeled collagen type I was measured for MG63 cells (first and third columns) and macrophages (second and fourth columns) in the absence (first and second columns) or presence (third and fourth columns) of 10 μg/ml of mAb 5f4. Internalization by MG63 cells in the absence of antibody was defined as 100% internalization. C, the uptake of collagen in macrophages is independent of β1-integrins. The internalization of radiolabeled collagen type I was measured in cultured human macrophages in the absence of exogenous reagents (first column), or in the presence of 10 μg/ml of anti-β1 mAb 4B4 (second column), or 200 μm cyclic RGD peptide (third column). D, internalized collagen is degraded by lysosomal cysteine proteases. The amount of collagen type I internalized by human macrophages was measured in the absence of protease inhibitor (first column) or presence of 20 μm E64d (second column). Internalization by untreated macrophages was defined as 100% internalization. E, macrophages internalize collagen in a mannose receptor-dependent process. Activated macrophages were isolated from the peritoneum of either MR−/− mice or littermate wild type (MR+/+) mice 72 h after thioglycollate injection. The internalization of collagen was measured for MR+/+ (first and third columns) and MR−/− macrophages (second and fourth columns) in the absence (first and second columns) or presence (third and fourth columns) of 10 μg/ml of mAb 5f4. Internalization by MR+/+ macrophages in the absence of antibody was defined as 100% internalization. F, positive control for mannose receptor-mediated endocytosis. Murine MR+/+ and littermate MR−/− macrophages were assayed for their ability to internalize radiolabeled mannose-BSA. For A–F, all samples were analyzed in triplicates. Error bars indicate S.D.
Various recent studies have demonstrated that the mannose receptor (MR), which belongs to the same receptor family as uPARAP/Endo180, is able to bind to collagen and promote the uptake of solubilized fluorescent-labeled collagen (61, 62). Interestingly, the above mentioned monocyte differentiation process led to the expression of the MR on the resulting macrophages, as verified by flow cytometry analysis (supplemental Fig. S3). To examine whether macrophages utilize the MR to internalize collagen, we isolated activated macrophages from the peritoneum of MR-deficient mice (MR−/−) or littermate wild type (MR+/+) mice. First, we showed that the routinely used MR ligand model, mannose-BSA, was internalized exclusively by the MR+/+ macrophages and not by the MR−/− macrophages (Fig. 8F), demonstrating that MR-dependent endocytosis processes are indeed ongoing during our standard assay conditions. Strikingly, in subsequent collagen internalization assays, the MR−/− macrophages displayed a markedly reduced ability to internalize collagen relative to the MR+/+ cells (Fig. 8E), indicating a dominant function of MR in collagen endocytosis in this cell type. In contrast, neither MR+/+ nor MR−/− macrophages were affected by addition of mAb 5f4 (Fig. 8E). Altogether, our results show that members of the receptor family comprising uPARAP/Endo180 and MR are dominant mediators of the endocytic uptake of solubilized collagen, with the identity of the active receptor differing between different cell types.
DISCUSSION
In this study we have examined the endocytosis of solubilized collagen in a panel of mesenchymal and epithelial cell lines and in human and murine macrophages. We have previously demonstrated that this endocytic process models the non-phagocytic uptake of the primary collagen cleavage products (“¼” and “¾” fragments), which are generated by the action of collagenolytic proteases in an organized degradation pathway (11). To enable analysis of the specific role of the collagen internalization receptor uPARAP/Endo180 in all of the cell types studied, we developed a monoclonal antibody against uPARAP/Endo180 that works to deplete the cells for the receptor. This antibody, designated mAb 5f4, very efficiently prevents the uPARAP/Endo180-dependent collagen uptake in both murine and human cells.
The analysis of a range of established cell lines revealed a striking correlation between the cellular collagen internalization capability and the cellular protein level of uPARAP/Endo180. For all cell lines, which displayed efficient collagen internalization, the addition of mAb 5f4 led to a pronounced reduction in the amount of internalized collagen. The epithelial cell lines internalized much less collagen than mesenchymal cells and expressed lower amounts of uPARAP/Endo180. Among the epithelial cell lines, HeLa cells displayed the highest expression level of uPARAP/Endo180 and interestingly, this cell line did internalize less collagen in the presence of mAb 5f4 and was the only epithelial cell type with this property.
Among the examined cell types we found that the gingival fibroblast cell line HGF-1 expressed the highest protein level of uPARAP/Endo180. As expected, this cell line displayed efficient and uPARAP/Endo180-dependent collagen internalization. An altered collagen metabolism by gingival fibroblasts is known to be involved in such pathological conditions as periodontal disease and gingival overgrowth, which are characterized by increased and decreased collagen degradation, respectively. In this cell type, it has long been recognized that intracellular collagen degradation is a central route of turnover, but so far, only integrins have been suggested as mediators of this degradation pathway (12, 49, 63). Our study reveals that uPARAP/Endo180 is required for the endocytosis of collagen by gingival fibroblasts, thus potentially opening new therapeutic options for future treatment of periodontal disease.
The distinction between different mechanisms of collagen uptake and the identification of the receptors involved are two important issues. The β1-integrins are an additional type of collagen receptors, which have been shown to be involved in processes of collagen internalization (12, 49, 53). However, those studies have focused entirely on a phagocytic uptake mechanism, i.e. the uptake of collagen-coated beads. A wide range of different cell types express β1-integrins (12, 48, 53), including many of the cell lines that we have found in this study to be capable of non-phagocytic, uPARAP/Endo180-dependent collagen uptake. Thus, some cell lines appear to possess the ability to internalize collagen in both phagocytic and non-phagocytic manners, notably including gingival fibroblasts and NIH/3T3 cells.
However, although our blocking studies with mAb 5f4 on the non-phagocytic pathway did document a central role of uPARAP/Endo180, they did not exclude that β1-integrins could also be involved in this collagen uptake process, because the two receptors might cooperate. To study this possibility, we examined the non-phagocytic collagen uptake by fibroblasts derived from β1-integrin-deficient mice (39). We found that these fibroblasts, termed GD25, displayed efficient collagen internalization and that this uptake was completely uPARAP/Endo180-dependent. That β1-integrins were indeed not involved in the uptake of collagen in our assay system was further substantiated by the fact that GD25 fibroblasts re-transfected with β1-integrin (GD25-β) (64) actually internalized less collagen than the GD25 fibroblasts. This difference is most likely a consequence of clonal variations between the two cell lines, resulting in different levels of uPARAP/Endo180 expression. The analyses using GD25 fibroblasts are in line with previous observations where antibody-mediated blocking of β1-integrin on murine skin fibroblasts did not affect the uptake of solubilized collagen (15).
The distinction between these mechanisms of collagen internalization is supported by a recent study where Shi et al. (14) examined the uptake of soluble collagen and insoluble polymerized collagen. These authors found that β1-integrins are not involved in endocytosis of soluble collagen, in agreement with our results, whereas they are indeed dominant mediators of the internalization of the collagen originating from an insoluble collagen matrix, inconsistent with the studies cited above on collagen phagocytosis. Furthermore, Shi et al. (14) found that uPARAP/Endo180 is indeed also involved in a particular turnover mechanism for insoluble matrix-collagen that is sensitive to matrix metalloprotease inhibitors. This points to a sequential degradation mechanism for matrix-collagen in agreement with our previous studies (11). Our present work suggests that this pathway may be a widespread mechanism in many cell types.
The existence of two distinct mechanisms for collagen uptake leads to the question about the role of these two collagen degradation pathways in vivo. Intracellular degradation of collagen has been known for several decades to be operative in vivo in a number of physiological and pathological contexts (63) but the identity of the responsible receptors has been limited to speculation. Recently, however, a number of studies have confirmed that uPARAP/Endo180 does indeed take part in collagen turnover processes in vivo. Crooked Tail Syndrome is a severe genetic disease observed in the inbred “Belgian Blue” strain of cattle, which results in massive bone defects that necessitate the euthanization of the affected animals. In a recent genetic study, the underlying cause of the disease was identified as a frameshift mutation in the gene encoding uPARAP/Endo180, leading to the expression of a non-functional protein (20).
Expression of uPARAP/Endo180 has also been demonstrated during bone development in normal mice (23) but in uPARAP/Endo180 knock-out mice in the absence of challenge, only a slightly reduced bone length has been observed (16).4 However, in combination with deficiency for MT1-MMP, an important collagenolytic protease, uPARAP/Endo180 deficiency was found to lead to fatal bone defects (16). Thus, the collagen turnover processes of uPARAP/Endo180 play a central role in bone development in mice as well as in cattle.
In a pathological context, uPARAP/Endo180 expression has been observed in the tumor stroma of multiple types of cancer, including breast cancer (27). The functional implication of uPARAP/Endo180 in breast cancer progression was investigated using a mouse model of genetically induced mammary cancer (MMTV-PymT model) (19). In this cancer model, uPARAP/Endo180 deficiency leads to delayed growth of the tumors and, interestingly, also to large accumulations of intratumoral collagen, demonstrating that uPARAP/Endo180 participates in the turnover of collagen in this pathological condition.
uPARAP/Endo180 belongs to a protein family comprising three other receptors, the MR, phospholipase A2 receptor, and DEC-205. All four receptors share a common protein domain composition, which includes a well conserved fibronectin type II domain (65, 66). This domain is believed to be the most important element for the interaction between uPARAP/Endo180 and collagen. MR has for many years been linked to the innate immune system as a receptor capable of binding to carbohydrates on the surface of certain pathogens, thereby promoting their phagocytosis (67). Recently, however, MR has also been reported to bind and internalize collagen in vitro (61, 62) and to be responsible for the clearance of denatured collagen from the circulation in vivo (68). MR is primarily expressed by macrophages but is also observed on certain other cell types such as the specialized endothelial cells of the liver sinusoids (67, 69).
Macrophages are known to be involved in promoting the degradation of the extracellular matrix in multiple pathological processes (3, 56, 57, 70). In this work we have examined collagen endocytosis by macrophages to study the relative role of uPARAP/Endo180 and MR in the non-phagocytic collagen degradation pathway. The differentiation of human blood monocytes to macrophages in vitro led to a dramatic up-regulation of MR and also a moderate up-regulation of uPARAP/Endo180. Further determination of the cellular level of uPARAP/Endo180 did, however, reveal that the macrophages expressed less than 20% of the reference cell line MG63. Strikingly, these macrophages proved capable of efficient collagen internalization, but were insensitive to addition of mAb 5f4 against uPARAP/Endo180. Previous studies using fluorescence-labeled solubilized collagen suggest that MR is the receptor responsible for collagen internalization in the case of macrophages (62). To clarify whether this was also the case in our assay system, we isolated peritoneal macrophages from MR−/− mice or from littermate MR+/+ mice. Collagen internalization assays with these primary cells revealed a clear dependence on MR for collagen internalization. Just like the situation observed with mesenchymal cells, we did not observe any β1-integrin dependence for the collagen uptake by cultured macrophages.
Altogether, these observations suggest that the mannose receptor could be critical for the degradation of collagen in pathological conditions, which involve increased macrophage influx and/or activation. Such conditions include cancer, fibrosis, and arthritis (3, 57, 70).
Subsets of macrophages have been identified as uPARAP/Endo180 positive in vivo in certain locations (58–60). To this end, we cannot rule out the possibility that our cultured macrophages represent a certain subtype of macrophages, whereas other subtypes of macrophages could display a higher level of uPARAP/Endo180 expression and be capable of internalizing collagen in a uPARAP/Endo180-dependent manner.
This study reveals that uPARAP/Endo180 and the mannose receptor are two major mediators of the non-phagocytic collagen degradation pathway, with uPARAP/Endo180 acting primarily on mesenchymal cells and MR on macrophages. It remains a possibility that other members of the same protein family, phospholipase A2 receptor and DEC-205 could have a similar function on other cell types. In fact, the phospholipase A2 receptor has been found to interact with collagen in vitro (71) and we speculate that this interaction could likewise be connected to intracellular degradation of collagen. Altogether, our work points to a previously underestimated role of the mannose receptor protein family members in the turnover of extracellular matrices. Because these breakdown processes are important in malignant as well as non-malignant diseases this concept opens a new range of therapeutic strategies.
Supplementary Material
Acknowledgments
The excellent technical assistance of Suzanne K. Møller and Katharina H. Stegmann is gratefully acknowledged.
This work was supported, in whole or in part, by the National Institutes of Health NIDCR Intramural Research Program (to A. M. and T. H. B.), the Danish Cancer Society, the Danish Medical Research Council, the Danish Cancer Research Foundation, the Lundbeck Foundation, the Danish National Research Foundation (Danish-Chinese Center for Proteases and Cancer), European Community's Seventh Framework Programme FP7/2007–2011 Grant 201279 (to N. B.), grants from the Lundbeck Foundation and the “Grosserer Alfred Nielsen og Hustrus” foundation (to L. H. E.), the Copenhagen University Hospital (to D. H. M. and H. J. J.), and the University of Copenhagen, Faculty of Science (to S. I.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
D. H. Madsen, H. J. Jürgensen, S. Ingvarsen, M. C. Melander, A. Hald, T. H. Bugge, N. Behrendt, and L. H. Engelholm, unpublished data.
- MMP
- matrix metalloprotease
- uPARAP
- urokinase plasminogen activator receptor-associated protein
- MT1-MMP
- membrane type 1 matrix metalloprotease
- TNP
- trinitrophenyl
- MR
- mannose receptor.
REFERENCES
- 1. Milner J. M., Cawston T. E. (2005) Curr. Drug Targets Inflamm. Allergy 4, 363–375 [DOI] [PubMed] [Google Scholar]
- 2. Holmbeck K., Szabova L. (2006) Birth Defects Res. C Embryo Today 78, 11–23 [DOI] [PubMed] [Google Scholar]
- 3. Friedman S. L. (2008) Gastroenterology 134, 1655–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rowe R. G., Weiss S. J. (2009) Annu. Rev. Cell Dev. Biol. 25, 567–595 [DOI] [PubMed] [Google Scholar]
- 5. Pataridis S., Eckhardt A., Mikulíková K., Sedláková P., Miksík I. (2008) J. Sep. Sci. 31, 3483–3488 [DOI] [PubMed] [Google Scholar]
- 6. Ottani V., Martini D., Franchi M., Ruggeri A., Raspanti M. (2002) Micron 33, 587–596 [DOI] [PubMed] [Google Scholar]
- 7. Saftig P., Hunziker E., Wehmeyer O., Jones S., Boyde A., Rommerskirch W., Moritz J. D., Schu P., von Figura K. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 13453–13458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hotary K., Li X. Y., Allen E., Stevens S. L., Weiss S. J. (2006) Genes Dev. 20, 2673–2686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shi J., Son M. Y., Yamada S., Szabova L., Kahan S., Chrysovergis K., Wolf L., Surmak A., Holmbeck K. (2008) Dev. Biol. 313, 196–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kjøller L., Engelholm L. H., Høyer-Hansen M., Danø K., Bugge T. H., Behrendt N. (2004) Exp. Cell Res. 293, 106–116 [DOI] [PubMed] [Google Scholar]
- 11. Madsen D. H., Engelholm L. H., Ingvarsen S., Hillig T., Wagenaar-Miller R. A., Kjøller L., Gårdsvoll H., Høyer-Hansen G., Holmbeck K., Bugge T. H., Behrendt N. (2007) J. Biol. Chem. 282, 27037–27045 [DOI] [PubMed] [Google Scholar]
- 12. Segal G., Lee W., Arora P. D., McKee M., Downey G., McCulloch C. A. (2001) J. Cell Sci. 114, 119–129 [DOI] [PubMed] [Google Scholar]
- 13. Lee J. W., Qi W. N., Scully S. P. (2002) J. Orthop. Res. 20, 66–75 [DOI] [PubMed] [Google Scholar]
- 14. Shi F., Harman J., Fujiwara K., Sottile J. (2010) Am. J. Physiol. Cell Physiol. 298, C1265–C1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Engelholm L. H., List K., Netzel-Arnett S., Cukierman E., Mitola D. J., Aaronson H., Kjøller L., Larsen J. K., Yamada K. M., Strickland D. K., Holmbeck K., Danø K., Birkedal-Hansen H., Behrendt N., Bugge T. H. (2003) J. Cell Biol. 160, 1009–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wagenaar-Miller R. A., Engelholm L. H., Gavard J., Yamada S. S., Gutkind J. S., Behrendt N., Bugge T. H., Holmbeck K. (2007) Mol. Cell. Biol. 27, 6309–6322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Isacke C. M., van der Geer P., Hunter T., Trowbridge I. S. (1990) Mol. Cell. Biol. 10, 2606–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Howard M. J., Isacke C. M. (2002) J. Biol. Chem. 277, 32320–32331 [DOI] [PubMed] [Google Scholar]
- 19. Curino A. C., Engelholm L. H., Yamada S. S., Holmbeck K., Lund L. R., Molinolo A. A., Behrendt N., Nielsen B. S., Bugge T. H. (2005) J. Cell Biol. 169, 977–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fasquelle C., Sartelet A., Li W., Dive M., Tamma N., Michaux C., Druet T., Huijbers I. J., Isacke C. M., Coppieters W., Georges M., Charlier C. (2009) PLoS Genet. 5, e1000666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu K., Yuan J., Lasky L. A. (1996) J. Biol. Chem. 271, 21323–21330 [DOI] [PubMed] [Google Scholar]
- 22. Behrendt N. (2004) Biol. Chem. 385, 103–136 [DOI] [PubMed] [Google Scholar]
- 23. Engelholm L. H., Nielsen B. S., Netzel-Arnett S., Solberg H., Chen X. D., Lopez Garcia J. M., Lopez-Otin C., Young M. F., Birkedal-Hansen H., Danø K., Lund L. R., Behrendt N., Bugge T. H. (2001) Lab. Invest. 81, 1403–1414 [DOI] [PubMed] [Google Scholar]
- 24. Howard M. J., Chambers M. G., Mason R. M., Isacke C. M. (2004) Osteoarthritis Cartilage 12, 74–82 [DOI] [PubMed] [Google Scholar]
- 25. Huijbers I. J., Iravani M., Popov S., Robertson D., Al-Sarraj S., Jones C., Isacke C. M. (2010) PLoS One 5, e9808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kogianni G., Walker M. M., Waxman J., Sturge J. (2009) Eur. J. Cancer 45, 685–693 [DOI] [PubMed] [Google Scholar]
- 27. Schnack N. B., Rank F., Engelholm L. H., Holm A., Danø K., Behrendt N. (2002) Int. J. Cancer 98, 656–664 [DOI] [PubMed] [Google Scholar]
- 28. Sulek J., Wagenaar-Miller R. A., Shireman J., Molinolo A., Madsen D. H., Engelholm L. H., Behrendt N., Bugge T. H. (2007) J. Histochem. Cytochem. 55, 347–353 [DOI] [PubMed] [Google Scholar]
- 29. Wienke D., Davies G. C., Johnson D. A., Sturge J., Lambros M. B., Savage K., Elsheikh S. E., Green A. R., Ellis I. O., Robertson D., Reis-Filho J. S., Isacke C. M. (2007) Cancer Res. 67, 10230–10240 [DOI] [PubMed] [Google Scholar]
- 30. Engelholm L. H., Ingvarsen S., Jürgensen H. J., Hillig T., Madsen D. H., Nielsen B. S., Behrendt N. (2009) Front. Biosci. 14, 2103–2114 [DOI] [PubMed] [Google Scholar]
- 31. Jögi A., Pass J., Høyer-Hansen G., Lund L. R., Nielsen B. S., Danø K., Rømer J. (2007) J. Thromb. Haemost. 5, 1936–1944 [DOI] [PubMed] [Google Scholar]
- 32. Honoré C., Rørvig S., Munthe-Fog L., Hummelshøj T., Madsen H. O., Borregaard N., Garred P. (2008) Mol. Immunol. 45, 2782–2789 [DOI] [PubMed] [Google Scholar]
- 33. Lee S. J., Evers S., Roeder D., Parlow A. F., Risteli J., Risteli L., Lee Y. C., Feizi T., Langen H., Nussenzweig M. C. (2002) Science 295, 1898–1901 [DOI] [PubMed] [Google Scholar]
- 34. Lund L. R., Danø K. (1992) FEBS Lett. 298, 177–181 [DOI] [PubMed] [Google Scholar]
- 35. Hansen L. V., Gårdsvoll H., Nielsen B. S., Lund L. R., Danø K., Jensen O. N., Ploug M. (2004) Biochem. J. 380, 845–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hillig T., Engelholm L. H., Ingvarsen S., Madsen D. H., Gårdsvoll H., Larsen J. K., Ploug M., Danø K., Kjøller L., Behrendt N. (2008) J. Biol. Chem. 283, 15217–15223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nielsen B. S., Lund L. R., Christensen I. J., Johnsen M., Usher P. A., Wulf-Andersen L., Frandsen T. L., Danø K., Gundersen H. J. (2001) Am. J. Pathol. 158, 1997–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rømer J., Pyke C., Lund L. R., Eriksen J., Kristensen P., Rønne E., Høyer-Hansen G., Danø K., Brünner N. (1994) Int. J. Cancer 57, 553–560 [DOI] [PubMed] [Google Scholar]
- 39. Fässler R., Pfaff M., Murphy J., Noegel A. A., Johansson S., Timpl R., Albrecht R. (1995) J. Cell Biol. 128, 979–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Holmbeck K., Bianco P., Caterina J., Yamada S., Kromer M., Kuznetsov S. A., Mankani M., Robey P. G., Poole A. R., Pidoux I., Ward J. M., Birkedal-Hansen H. (1999) Cell 99, 81–92 [DOI] [PubMed] [Google Scholar]
- 41. Claesson M. H., Endel B., Ulrik J., Pedersen L. O., Skov S., Buus S. (1994) Scand. J. Immunol. 40, 257–264 [DOI] [PubMed] [Google Scholar]
- 42. Declerck P. J., Carmeliet P., Verstreken M., De Cock F., Collen D. (1995) J. Biol. Chem. 270, 8397–8400 [DOI] [PubMed] [Google Scholar]
- 43. Ingvarsen S., Madsen D. H., Hillig T., Lund L. R., Holmbeck K., Behrendt N., Engelholm L. H. (2008) Biol. Chem. 389, 943–953 [DOI] [PubMed] [Google Scholar]
- 44. Watts C., Marsh M. (1992) J. Cell Sci. 103, 1–8 [DOI] [PubMed] [Google Scholar]
- 45. Wienke D., MacFadyen J. R., Isacke C. M. (2003) Mol. Biol. Cell 14, 3592–3604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. von Figura K., Gieselmann V., Hasilik A. (1984) EMBO J. 3, 1281–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schwartz A. L., Ciechanover A., Merritt S., Turkewitz A. (1986) J. Biol. Chem. 261, 15225–15232 [PubMed] [Google Scholar]
- 48. Barczyk M., Carracedo S., Gullberg D. (2010) Cell Tissue Res. 339, 269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee W., Sodek J., McCulloch C. A. (1996) J. Cell. Physiol. 168, 695–704 [DOI] [PubMed] [Google Scholar]
- 50. Hegerfeldt Y., Tusch M., Bröcker E. B., Friedl P. (2002) Cancer Res. 62, 2125–2130 [PubMed] [Google Scholar]
- 51. Ruoslahti E. (1996) Annu. Rev. Cell Dev. Biol. 12, 697–715 [DOI] [PubMed] [Google Scholar]
- 52. Arora P. D., Fan L., Sodek J., Kapus A., McCulloch C. A. (2003) Exp. Cell Res. 286, 366–380 [DOI] [PubMed] [Google Scholar]
- 53. Arora P. D., Marignani P. A., McCulloch C. A. (2008) Am. J. Physiol. Cell Physiol. 295, C130–C137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Blair H. C., Zaidi M., Schlesinger P. H. (2002) Biochem. J. 364, 329–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang J. H., Thampatty B. P., Lin J. S., Im H. J. (2007) Gene 391, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rizas K. D., Ippagunta N., Tilson M. D., 3rd (2009) Cardiol. Rev. 17, 201–210 [DOI] [PubMed] [Google Scholar]
- 57. Szekanecz Z., Koch A. E. (2007) Curr. Opin. Rheumatol. 19, 289–295 [DOI] [PubMed] [Google Scholar]
- 58. Honardoust H. A., Jiang G., Koivisto L., Wienke D., Isacke C. M., Larjava H., Häkkinen L. (2006) Histopathology 49, 634–648 [DOI] [PubMed] [Google Scholar]
- 59. Sheikh H., Yarwood H., Ashworth A., Isacke C. M. (2000) J. Cell Sci. 113, 1021–1032 [DOI] [PubMed] [Google Scholar]
- 60. Ye Q., Xing Q., Ren Y., Harmsen M. C., Bank R. A. (2010) Eur. Cell Mater. 20, 197–209 [DOI] [PubMed] [Google Scholar]
- 61. Napper C. E., Drickamer K., Taylor M. E. (2006) Biochem. J. 395, 579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Martinez-Pomares L., Wienke D., Stillion R., McKenzie E. J., Arnold J. N., Harris J., McGreal E., Sim R. B., Isacke C. M., Gordon S. (2006) Eur. J. Immunol. 36, 1074–1082 [DOI] [PubMed] [Google Scholar]
- 63. Everts V., van der Zee E., Creemers L., Beertsen W. (1996) Histochem. J. 28, 229–245 [DOI] [PubMed] [Google Scholar]
- 64. Wennerberg K., Lohikangas L., Gullberg D., Pfaff M., Johansson S., Fässler R. (1996) J. Cell Biol. 132, 227–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. East L., Isacke C. M. (2002) Biochim. Biophys. Acta 1572, 364–386 [DOI] [PubMed] [Google Scholar]
- 66. Behrendt N., Jensen O. N., Engelholm L. H., Mørtz E., Mann M., Danø K. (2000) J. Biol. Chem. 275, 1993–2002 [DOI] [PubMed] [Google Scholar]
- 67. Martinez-Pomares L., Linehan S. A., Taylor P. R., Gordon S. (2001) Immunobiology 204, 527–535 [DOI] [PubMed] [Google Scholar]
- 68. Malovic I., Sørensen K. K., Elvevold K. H., Nedredal G. I., Paulsen S., Erofeev A. V., Smedsrød B. H., McCourt P. A. (2007) Hepatology 45, 1454–1461 [DOI] [PubMed] [Google Scholar]
- 69. Smedsrød B., Melkko J., Risteli L., Risteli J. (1990) Biochem. J. 271, 345–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mantovani A., Schioppa T., Porta C., Allavena P., Sica A. (2006) Cancer Metastasis Rev. 25, 315–322 [DOI] [PubMed] [Google Scholar]
- 71. Ancian P., Lambeau G., Lazdunski M. (1995) Biochemistry 34, 13146–13151 [DOI] [PubMed] [Google Scholar]
- 72. Horoszewicz J. S., Leong S. S., Chu T. M., Wajsman Z. L., Friedman M., Papsidero L., Kim U., Chai L. S., Kakati S., Arya S. K., Sandberg A. A. (1980) Prog. Clin. Biol. Res. 37, 115–132 [PubMed] [Google Scholar]
- 73. Cailleau R., Young R., Olivé M., Reeves W. J., Jr. (1974) J. Natl. Cancer Inst. 53, 661–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fogh J., Fogh J. M., Orfeo T. (1977) J. Natl. Cancer Inst. 59, 221–226 [DOI] [PubMed] [Google Scholar]
- 75. Scherer W. F., Syverton J. T., Gey G. O. (1953) J. Exp. Med. 97, 695–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Brooks S. C., Locke E. R., Soule H. D. (1973) J. Biol. Chem. 248, 6251–6253 [PubMed] [Google Scholar]
- 77. Graham F. L., Abrahams P. J., Mulder C., Heijneker H. L., Warnaar S. O., De Vries F. A., Fiers W., Van Der Eb A. J. (1975) Cold Spring Harbor Symp. Quant. Biol. 39, 637–650 [DOI] [PubMed] [Google Scholar]
- 78. Bertram J. S., Janik P. (1980) Cancer Lett. 11, 63–73 [DOI] [PubMed] [Google Scholar]
- 79. Fidler I. J. (1973) Nat. New Biol. 242, 148–149 [DOI] [PubMed] [Google Scholar]
- 80. McAllister B. S., Leeb-Lundberg L. M., Javors M. A., Olson M. S. (1993) Arch. Biochem. Biophys. 304, 294–301 [DOI] [PubMed] [Google Scholar]
- 81. Billiau A., Edy V. G., Heremans H., Van Damme J., Desmyter J., Georgiades J. A., De Somer P. (1977) Antimicrob. Agents Chemother. 12, 11–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jacobs J. P., Jones C. M., Baille J. P. (1970) Nature 227, 168–170 [DOI] [PubMed] [Google Scholar]
- 83. Rasheed S., Nelson-Rees W. A., Toth E. M., Arnstein P., Gardner M. B. (1974) Cancer 33, 1027–1033 [DOI] [PubMed] [Google Scholar]
- 84. McAllister R. M., Melnyk J., Finkelstein J. Z., Adams E. C., Jr., Gardner M. B. (1969) Cancer 24, 520–526 [DOI] [PubMed] [Google Scholar]
- 85. Sanford K. K., Merwin R. M., Hobbs G. L., Young J. M. (1959) J. Natl. Cancer Inst. 23, 1035–1059 [PubMed] [Google Scholar]
- 86. Jainchill J. L., Aaronson S. A., Todaro G. J. (1969) J. Virol. 4, 549–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.