Abstract
We hypothesized that inhibition of ventilatory motor output leads to increased pharyngeal compliance during NREM sleep, independent of lung volume.
Methods
Eighteen subjects were studied using noninvasive positive pressure ventilation (NPPV) to inhibit ventilatory motor output during stable NREM sleep. Nasopharyngoscopy was used to measure the retro palatal cross-sectional area / pressure relationship (CSA/Pph) in 8 subjects. The effect of NPPV on neck circumference (NC) and end-expiratory lung volumes (EELV) was studied in 10 additional subjects using strain gauge plethysmography and Respitrace, respectively.
Results
1. The CSA/Pph was increased during expiration under passive compared to active breathing (11.7±7.1 vs. 8.5±5.6 mm2/cmH2O, respectively; p<0.05) but not during inspiration. 2. NC correlated with the CSA/Pph during passive expiration (R2=0.77, p<0.05). 3. NC and EELV did not change between active and passive breaths (p=NS).
Conclusions
1) Inhibiting the ventilatory motor output increases the pharyngeal compliance. 2) Increased passive expiratory pharyngeal compliance was not associated with changes in NC or EELV.
Keywords: ventilatory motor output, retro palatal airway, expiratory, upper airway compliance
1. INTRODUCTION
The pathogenesis of obstructive sleep apnea involves an interaction between unfavorable pharyngeal anatomy and ventilatory control instability (Badr et al., 1995; Isono et al., 1997; Rowley et al., 2002; Schwab et al., 1995). Anatomic factors that promote pharyngeal narrowing include large neck circumference, cervical soft tissue (Whittle et al., 1999), and other structures such as vessels and bony structures. Many of these factors promote pharyngeal narrowing by decreasing the caliber of the upper airway or by increasing the upper airway surrounding pressure (Badr et al., 1995 and Shiota et al., 2007). Neuromuscular activity is an important determinant of pharyngeal patency, especially in an anatomically compromised airway (Borowiecki et al., 1978). When ventilatory motor output oscillates during periodic breathing, pharyngeal narrowing or obstruction occurs at the nadir of ventilatory motor output, especially in individuals with a highly collapsible airway (Onal et al., 1986).
Pharyngeal occlusion during obstructive apnea is often described as an inspiratory phenomenon, caused solely by negative collapsing pressure. However, several lines of evidence indicate that expiratory narrowing may be a significant contributor to pharyngeal obstruction during sleep (Badr et al., 1995; Morrell et al., 1998a; Sanders and Moore., 1983; Schneider., 2002). More recently, we found that reduced ventilatory motor output, manifested by central hypopnea, leads to pharyngeal narrowing during expiration only (Sankri-Tarbichi et al., 2009). In contrast this study investigated the effect of inhibition of ventilatory drive, manifested by central apnea, to understand the determinants of upper airway narrowing during sleep. More specifically, we studied elimination of ventilatory drive without changes in lung volume.
The relative contribution of reduced neuromuscular activity versus reduced lung volume has been elusive since most interventions change both variables in the same direction. We hypothesized that inhibition of ventilatory motor output during NREM sleep would result in increased pharyngeal compliance during expiration independent of lung volume. To this end, we measured retro palatal compliance during noninvasive positive pressure ventilation, under active and passive conditions, without the confounding effect of reduced lung volume.
2. METHODS
2.1 Subjects
The Human Investigation Committee of the Wayne State University School of Medicine and the John D. Dingell Veterans Affairs Medical Center approved the experimental protocol. We studied 18 subjects (eight females); mean (±SD) age was 30.1±11.8yrs, BMI was 27.9±6.6kg/m2, and neck circumference was 32.7±5.8 cm. Subjects were screened for sleep-disordered breathing by a baseline polysomnography performed at a separate visit. Female subjects were not pregnant nor on birth control pills. Subjects were asked to restrict their sleep the night before the study to total sleep of 4–6 hours, and study was done under spontaneous natural sleep.
2.2. Equipment and measurements
The subjects were connected to the circuit with an airtight silicone rubber mask strapped to the face to prevent leaks. The mask was connected to a Plateau Exhalation Valve (Respironics, Inc., Pittsburgh, PA) via a pneumotachometer. All signals were displayed on a digital polysomnography recorder (Grass technology-AstroMed®) and a Comet amplifier. The signals were recorded also on POWER LAB® digital data acquisition/analysis software. Electroencephalograms (EEG), electrooculograms (EOG) and chin electromyograms (EMG) were recorded using the international 10–20 system of electrode placement. Every subject was studied with screening polysomnography for sleep staging. Baseline sleep studies were scored according to the criteria of the American Academy of Sleep Medicine (Iber et al., 2007).
Protocol-1: A pediatric fiberoptic bronchoscope was used to visualize the pharyngeal airway in 8 subjects (3 females) during NREM sleep. The scope was inserted via one of the patent nares after lubrication and local anesthesia. Topical anesthesia (4% liquid lidocaine) was applied to each nostril to anaesthetize the pharynx; 2% lidocaine jelly was used to lubricate the nostril during advancing the scope. The fiberoptic scope was then passed through the opposite nostril and positioned as described above. The nasal mask was then carefully lowered onto the face and secured. At this point, the exact position of the fiberoptic scope was adjusted and the scope plus the attached video camera were placed in a clamp suspended above the subject’s head. The mask was carefully sealed, including the hole through which the scope was inserted. A continuous image of the retro palatal lumen was obtained from a video camera connected to the scope (Endovision 3000, Pentax Precision Instrument). The video images and respiratory signals were digitalized at 10 frames/sec and 25 Hz, respectively, by using specially developed software. Pharyngeal pressure (Pph) was measured at the palatal rim in all subjects using transducer tipped catheter (Millar, Inc.). The remaining transducers were then attached, and further fine adjustments to the orientation of the scope were made. The subjects were then allowed to go to sleep.
Protocol-2: Similar to protocol 1, the ventilatory set up was performed and a strain gauge plethysmograph (EC6-Hokanson Inc., Bellevue, WA) was used to measure the change in neck circumference above the thyroid cartilage in 5 subjects (2 females) during NREM sleep. The transducer was placed around the neck in supine position and then taped on the neck after marking its site in two points.
Protocol-3: to measure the change in end-expiratory lung volumes (EELV) a respiratory inductive plethysmograph (RIP) (Respitrace 200 Inc., Miami Beach, FL) was used in 5 subjects (3 females) during sleep (figure 3). The RIP with two belts was used in the DC mode and was calibrated against the integrated volume signal of an unheated pneumotachometer. The thoracic coil was placed at the level of the nipples and the abdominal coil was placed at the umbilical level. To assess the corresponding intra-luminal pressure at end expiration (EEP), the supraglottic pressure (Psg) was measured using transducer tipped catheter (Millar, Inc) (figure 3).
Figure 3.
Polygraph record of a trial that illustrates breaths during noninvasive positive pressure ventilation (NPPV) followed by central apnea. End-expiratory lung volume (EELV) and end-expiratory pharyngeal pressure (EEP) were indicated by the dotted lines. RIP: respiratory inductive plethysmograph, Psg: supraglottic pressure, PETCO2, end-tidal CO2, PETCO2, end-tidal O2.
2.2. Mechanical ventilation
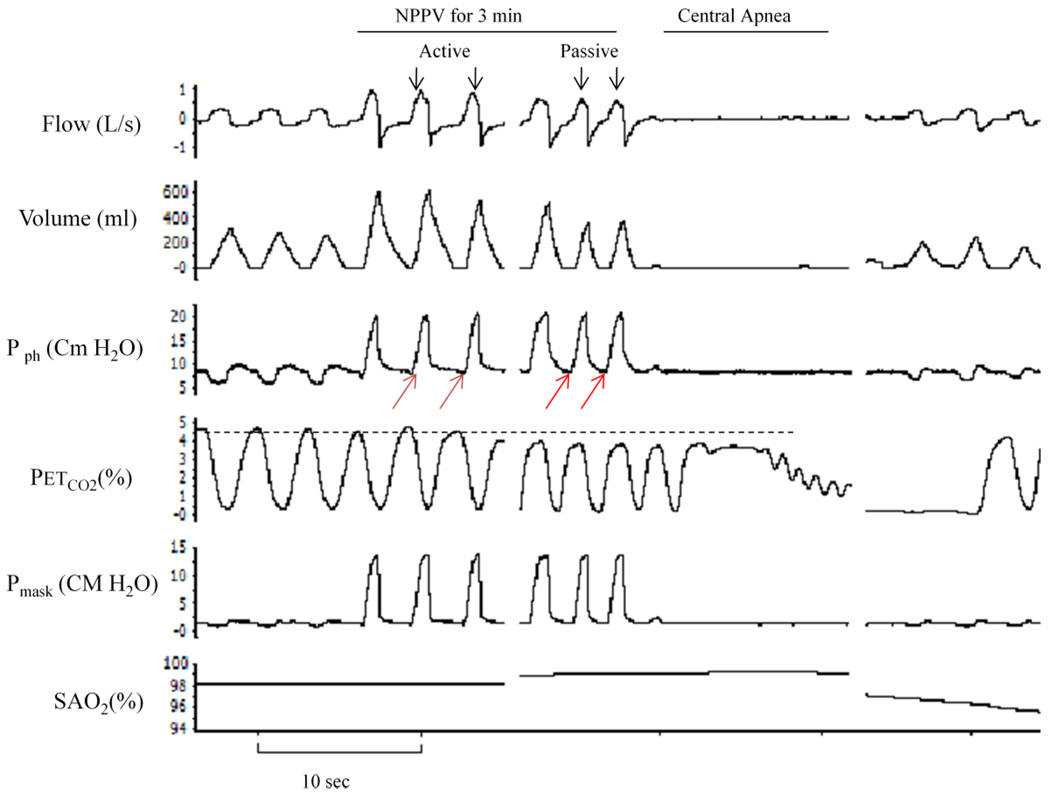
To inhibit ventilatory motor output during sleep, we used noninvasive positive pressure ventilation (NPPV) as previously described (Badr et al., 1995; Sankri-Tarbichi et al., 2009; Zhou et al., 2003). NPPV (Quantum PSV, Healthyne technologies, Marietta, GA) was applied for three minutes then terminated abruptly during expiration, with the expiratory pressure set at its lowest pressure throughout the study. The inspiratory positive airway pressure (IPAP) was increased gradually in 1–2 cmH2O increments at the beginning of each mechanical hyperventilation trial and was reduced to the lowest pressure at the end of each three minutes of NPPV. The hyperventilation trials were repeated at higher IPAP (1–2 cmH2O) until central apnea occurred. Passive mechanical ventilation was determined by the absence of sub-atmospheric negative pressure deflection on the pharyngeal pressure tracings (red arrows in figure1). The occurrence of central apnea upon termination of mechanical ventilation confirmed that breathing became passive. Therefore, our study includes trials that were followed by central apnea only. Spontaneous breathing for 5 minutes followed each mechanical ventilation trial.
Figure 1.
Polygraph record of a trial that illustrates breaths during noninvasive positive pressure ventilation (NPPV) followed by central apnea. Ventilatory motor output inhibition was confirmed by the absence of negative pressure deflection on pharyngeal pressure (red arrows) and by the occurrence of central apnea upon termination of NPPV. First three breaths at the beginning of mechanical ventilation (active breaths) were compared to the last three breaths preceding the termination of NPPV (passive breaths). The horizontal dotted line indicates the level of end-tidal CO2 at baseline. Pph, pharyngeal pressure; PETCO2, end-tidal CO2; SaO2, oxygen saturation.
2.3. Data Analysis
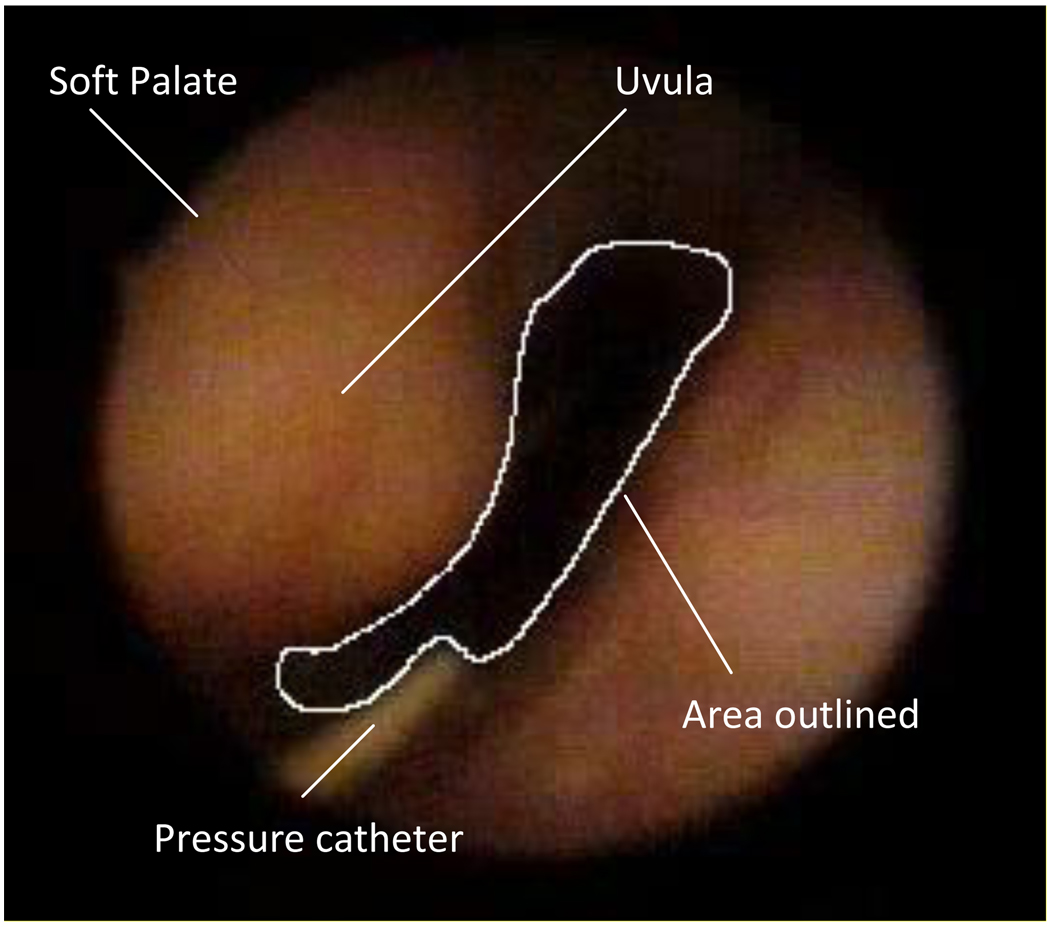
This study aimed to measure the cross-sectional area/pressure relationship (CSA/Pph) in the retro palatal airway during active and passive respiration. The absolute value of retro palatal CSA was obtained from five different phases of the respiratory cycle (BI: beginning inspiration, PI: peak inspiration, EI/BE: end inspiration and beginning expiration, PE: peak expiration, EE: end expiration) by manually outlining the retro palatal lumen using computer software (Sigma Scan, Jandel) in mm2 (Figure 2). The ability to reproduce this technique has been previously validated by our laboratory (Morrell et al., 1998a; Morrell et al., 1998b; Rowley et al., 1998; Rowley et al., 2002). For each image, the scanning software provided an area in pixels. We converted these relative areas to absolute areas by using the dimensions of the pressure catheter as a reference (Rowley et al., 1998; Rowley et al., 2002; Sankri-Tarbichi et al., 2009).
Figure 2.
Fiberoptic image of the retro palatal airway with anatomic landmarks identified.
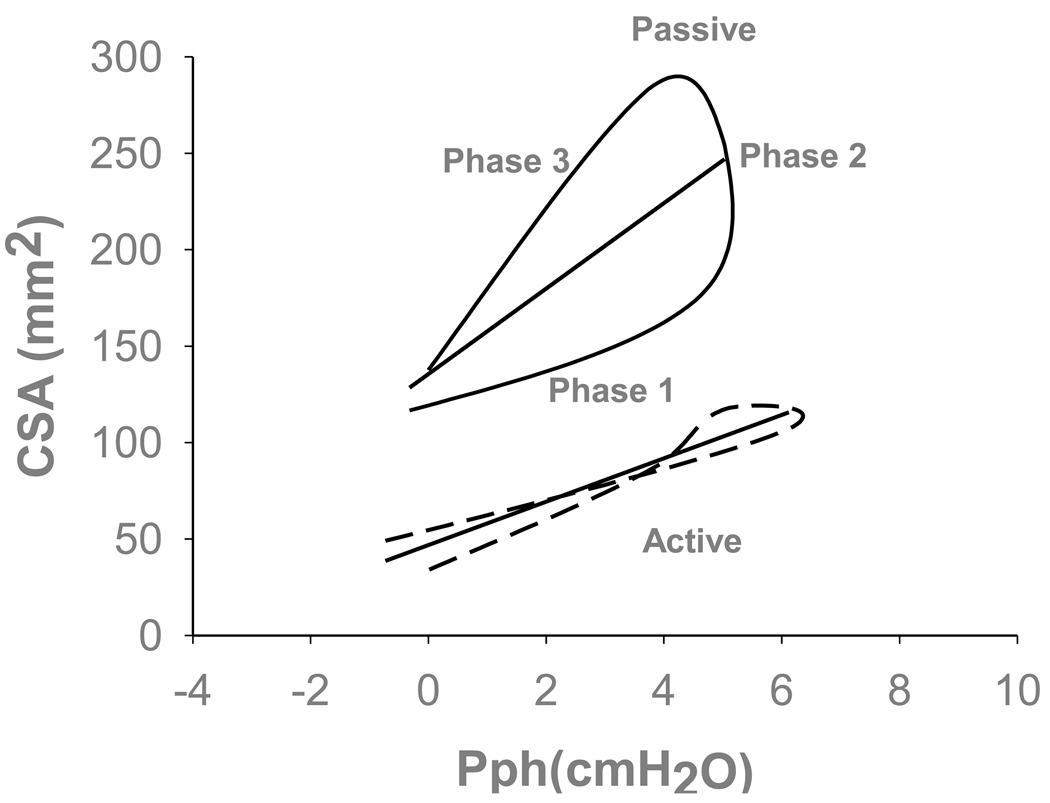
To assess the changes in upper airway compliance (CUA) during active and passive respiration, we used the CSA/Pph relationship as a surrogate for retro palatal compliance. We measured CSA/Pph at two different points within each mechanical ventilation trial that ended with central apnea; the first three breaths represented active mechanical ventilation breaths and the last three breaths prior to central apnea represented passive mechanical ventilation breaths. CSA/Pph relationship was calculated for three phases within each breath (Phases 1 through 3 as illustrated in figure 5). First phase was the inspiratory phase of CSA/Pph which was defined as the slope of the regression line that was drawn through the plot of CSA vs. Pph between the beginning of inspiration and peak inspiration. Second phase was post-inspiratory phase of CSA/Pph which was defined as the slope of the regression line that was drawn through the plot of CSA vs. Pph between the peak inspiration and end inspiration. Third was the expiratory phase of CSA/Pph which was defined as the slope of CSA vs. Pph between the beginning of expiration and end of expiration. The flow signal was used to define the beginning and end of each respiratory phase. All breath were analyzed during stable NREM sleep, breaths that were associated with arousal were not included in the analysis.
Figure 5.
Representative example of retro palatal cross sectional area (CSA) and pharyngeal pressure (Pph) relationships for active and passive breaths (dotted and solid lines, respectively). Note that the passive breath displayed hysteresis relative to the triggered active breath on the same positive pressure. The total slope of passive breath is significantly higher than active breath. Partitioned slope of the passive breath is higher during expiration relative to the active breath. Phase 1 (inspiratory) was defined as the slope of the regression line between the beginning of inspiration and peak inspiration. Phase 2 (post-inspiratory) was defined as the slope of the regression line between the peak inspiration and end inspiration. Phase 3 (expiratory) was defined as the slope of CSA vs. Pph between the beginning of expiration and end of expiration. The straight lines represent the total area-pressure relationship for each individual representative breaths defined as the slope of the regression line between the beginning of inspiration and end expiration.
The ventilatory parameters were measured breath by breath and analysis was conducted on the subjects’ mean values. The ventilatory parameters included inspired tidal volume (VT), inspiratory time (TI), total breath time (TTOT), breathing frequency (f), and PETCO2. To ascertain possible changes in end-expiratory lung volume between the active and passive breaths, we measured the end expiratory lung volumes (EELV) using RIP for three consecutive breaths at the beginning, and end of mechanical ventilation trials.
To assess the effect of NPPV on neck circumference between the active and passive breaths, the change in neck circumference was measured using strain gauge within each respiratory cycle (from beginning of inspiration to end expiration) for three consecutive breaths at the beginning, and end of mechanical ventilation trials. The flow signal was used to define the beginning and end of each respiratory phase.
2.4. Statistical analysis
A commercially available computer statistical package was used to analyze the data (Sigma Stat 3.5, SPSS). A paired two tails t-test was used to compare the mean values of each ventilatory parameter between eupnoea and active breaths. A paired two-tailed t-test was used to compare the mean values of CSA/Pph relationship between active and passive breaths. A two-way repeated-measures analysis of variance (ANOVA) was used to compare each dependent variable (CSA, Pph and flow). The two factors for each dependent variable were: 1) eupnoea vs. active vs. passive and 2) respiratory cycle phases from beginning of inspiration to end expiration (five phases). A paired two-tailed t-test was used to compare the mean values of CSA at 0 flow at the beginning of inspiration (BI) of eupnoea breath and at 10th second of induced central apnea (CA). A Spearman Rank Order Correlation was used to determine the association among, age, BMI, neck circumference and CSA/Pph relationship under passive state. The overall significance level was considered at 0.05.
A two-way repeated-measures ANOVA was used to compare each dependent variable (NC and Psg). The two factors for each dependent variable were: 1) eupnoea vs. active vs. passive and 2) respiratory cycle phases from beginning of inspiration to end expiration (five phases). A paired two-tailed t-test was used to compare the mean values of EELV at 0 flow at the end of expiration (EE) of active and passive breaths during NPPV.
3. RESULTS
Figure 1 is a representative polygraph record during stable NREM sleep showing ventilation and upper airway mechanics during eupnoea, mechanical ventilation, and the post-mechanical ventilation period. Inhibition of ventilatory motor output was confirmed by the absence of negative deflection of the pharyngeal pressure at the onset of the breath and by the development of post-hyperventilation central apnea upon termination of passive mechanical ventilation. Mechanical ventilation resulted in an increased VI (6.6±0.9 to 11.1±1.6 L/min, p<0.05), VT (0.4±0.1 to 0.6±0.1L, p<0.05) and VT/TI (0.23±0.1 to 0.50±0.1 L/sec, p<0.05).
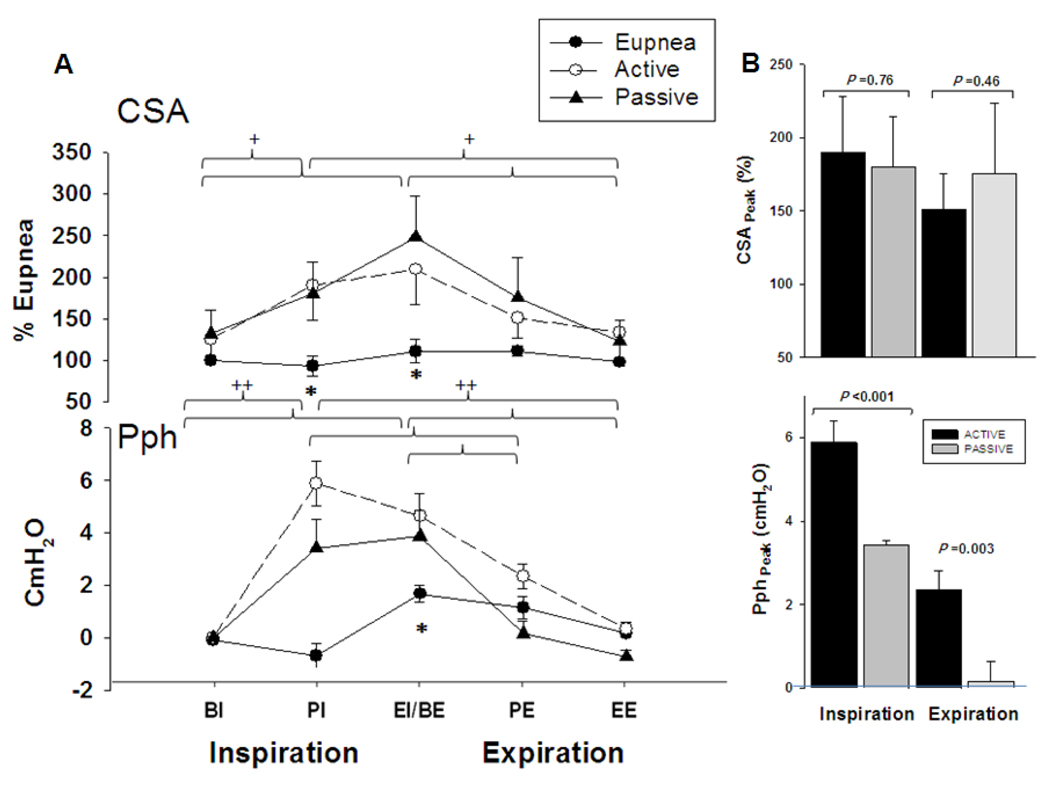
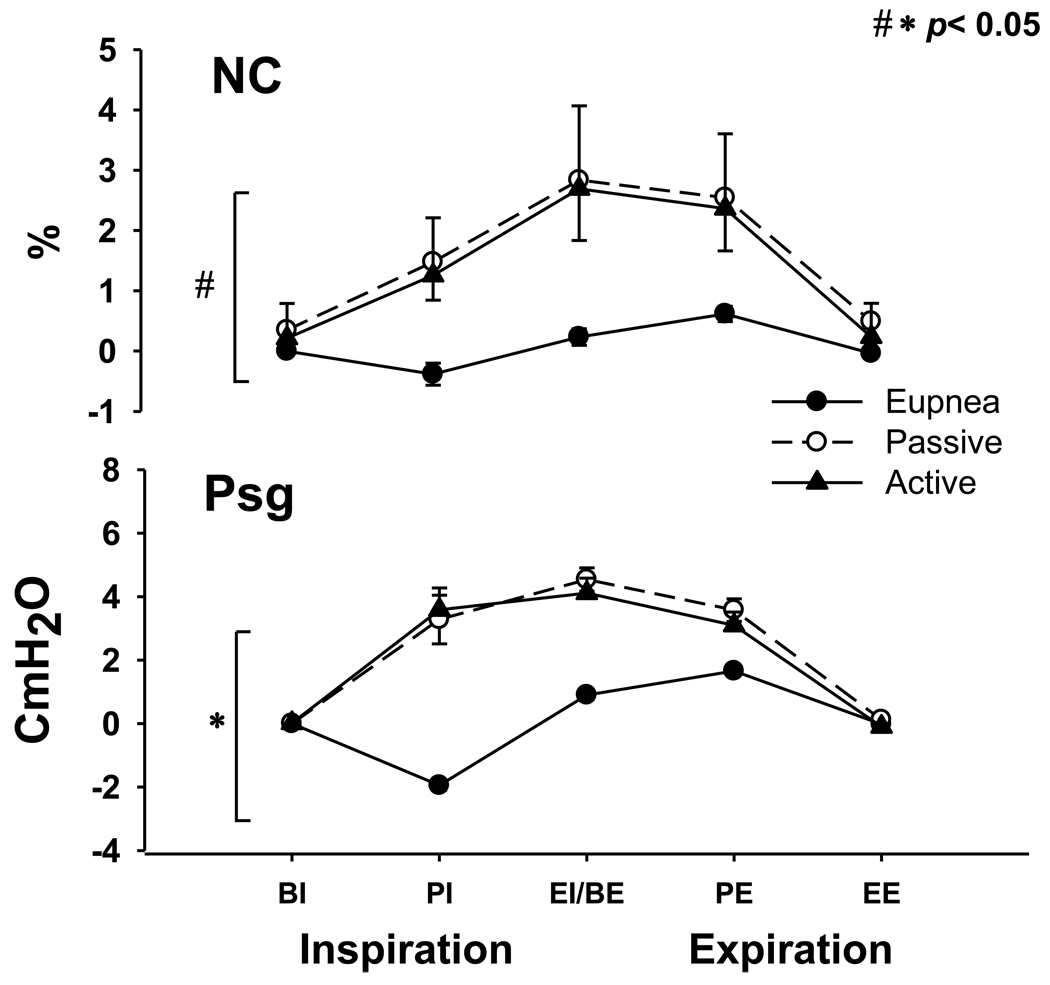
Figure 3, is a representative polygraph showing a NPPV trial during NREM sleep. End-expiratory lung volume (EELV) and end-expiratory pharyngeal pressure (EEP) did not change between active and passive breaths. Figure 4A depicts the group differences for CSA and Pph between active and passive mechanical ventilation breaths compared to eupnoea. There was no difference between active and passive breaths at any phase of the respiratory cycle (p=NS). Retro palatal CSAs of active and passive breaths, were higher than eupnoea at peak inspiration (PI) and end inspiration (EI) (190.1±38.2 and 179.9±34.5 vs. 94.6±6.8%; 209.4±36.7 and 203.7±40.3 vs.112.8±5.1%, p<0.05, for active and passive vs. eupnoea at PI and EI respectively). There was an interaction between the two factors (type: eupnoea vs. mechanical ventilation and respiratory phase: BI through EE), indicating that the changes in pharyngeal CSA during mechanical ventilation depended on the respiratory cycle phase (p<0.001). Figure 4B illustrates CSA at peak inspiration and expiration which did not change between active and passive breaths. The Pph of active breaths were higher than passive breaths at peak inspiration and expiration.
Figure 4.
(A) Retro palatal cross-sectional area (CSA) during eupnoea and mechanical ventilation (active and passive, respectively). Note the larger CSA for the mechanical ventilation breaths in comparison to the eupnoea breaths at PI and BE (*p < 0.05 eupnoea vs. active and passive). No difference was found between active vs. passive breaths (p>0.05). There was a statistically significant interaction between phase and type (p <0.001); the effect of different levels of phase depends on what level of type is present. Note that the CSA was similar during eupnoea among the different phases of each respiratory cycle (from BI to EE) but different during mechanical ventilation for active and passive breaths, respectively (+p<0.01). Pharyngeal pressure (Pph) was higher during mechanical ventilation at PI and BE phases in comparison to eupnoea (p<0.05). Pph profile was higher at BE compared to PI during eupnoea (++ p <0.001) and among the different phases of each respiratory cycle of active and passive breaths (++ p<0.001). (B) The top panel illustrates the CSA at peak inspiration and expiration. Note that CSA not change between active and passive breaths (p=NS). Bottom panel illustrates the Pph which were higher during active than passive breathing at peak inspiration and expiration (P<0.05). BI: Beginning inspiration, PI: peak inspiration, EI/BE: end inspiration/beginning expiration PE: peak expiration, EE: end expiration. All presented data are mean ± SE.
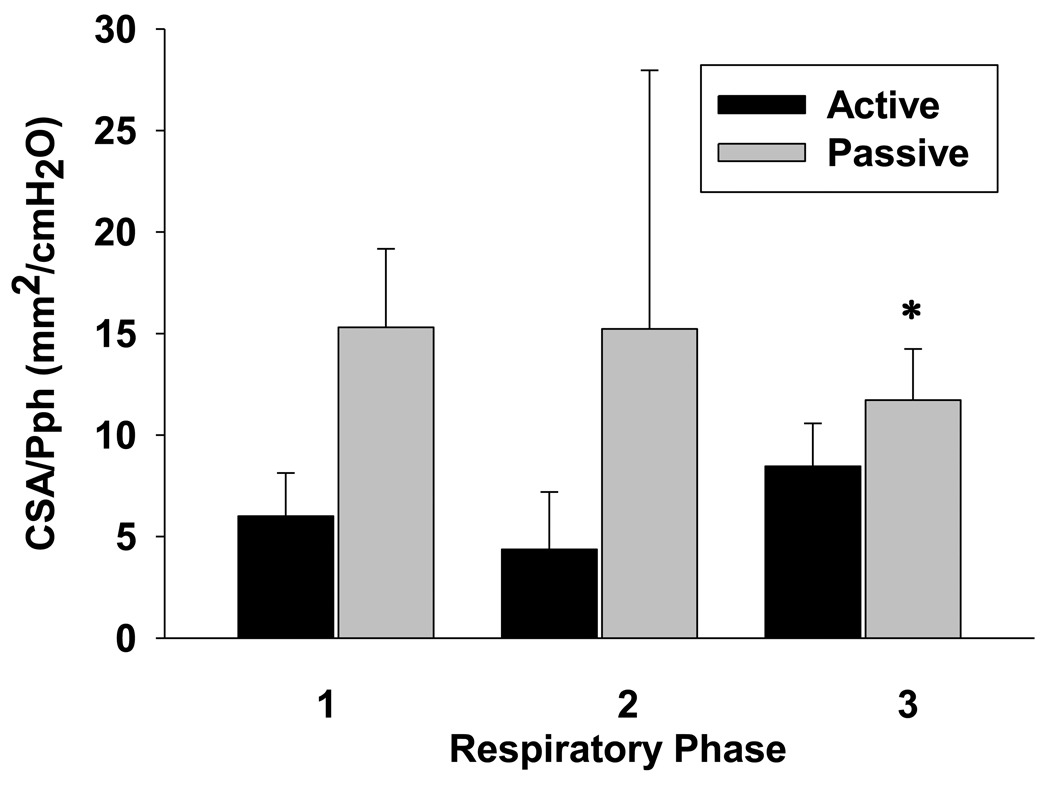
Retro palatal compliance (CUA), represented by the CSA/Pph relationship, was higher during passive compared to active mechanical ventilation breaths as shown in the individual representation (Figure 5). The CSA/Pph relationship was higher during passive mechanical ventilation breaths compared with active conditions for the expiratory phase (11.7±7.1 vs. 8.5±5.6 mm2/cmH2O, respectively; p<0.05) but not for inspiratory or post-inspiratory phases (15.3±10.9 vs. 6.0±6.0 mm2/cmH2O and 15.2±6.0 vs. 4.4±8.0 mm2/cmH2O, respectively; p=NS) (Figure 6).
Figure 6.
Grouped data for CSA/Pph relationship during active and passive breaths (Phase 1: inspiratory; phase 2: post-inspiratory; and phase 3: expiratory, respectively). Note that the expiratory CSA/Pph relationship for passive (gray bars) was higher than for active breaths (black bars) on the same airway pressure (p<0.05). All presented data are mean ± SE.
To ensure that the noted changes in compliance were not due to occult changes in luminal pressure, we measured end expiratory pharyngeal pressure (EEP; at zero flow). There was no difference in EEP between active and passive mechanical ventilation breaths (0.3±0.2 vs. 0.8±0.4 cmH2O, respectively, p=NS). In five additional subjects EELV was measured during active and passive breathing and no difference in EELV was found (EELV increased from active to passive ventilation by 60.4±40 ml, p=NS).
To ensure that the noted changes in retro palatal compliance were not due to increased neck circumference, and hence surrounding pressure, we compared NC during eupnoea and mechanical ventilation. Higher NC was noted during active mechanical ventilation breaths relative to eupnoea (p<0.05). However, there was no difference in NC between active and passive breaths as shown in figure 7.
Figure 7.
Neck circumference (NC) during eupnoea and mechanical ventilation (active and passive, respectively). Note the larger NC for the mechanical ventilation breaths in comparison to the eupnoea breaths (# p < 0.05 eupnoea vs. active). No difference was found between active vs. passive breaths (p>0.05). Supraglottic pressure (Psg) was higher during mechanical ventilation relative to eupnoea at PI, BE, PE phases in comparison to eupnoea (* p<0.05). BI: Beginning inspiration, PI: peak inspiration, EI/BE: end inspiration/beginning expiration PE: peak expiration, EE: end expiration. All presented data are mean ± SE.
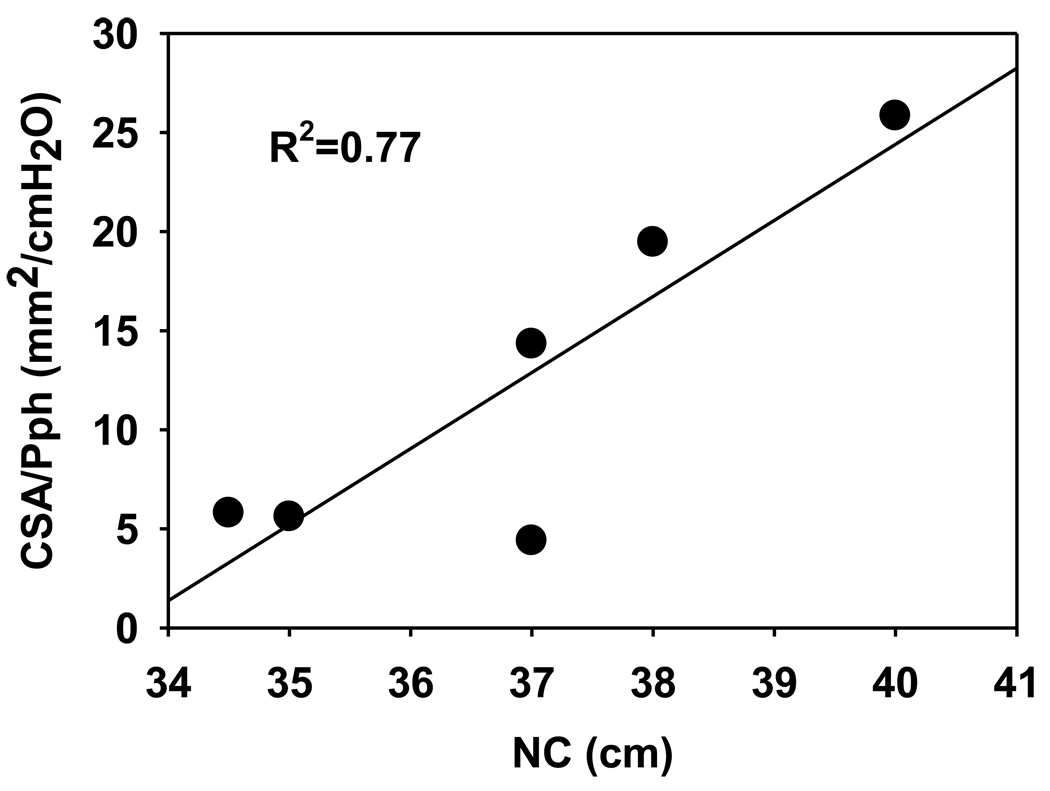
To ascertain potential determinants of increased retro palatal pharyngeal compliance, three putative variables were assessed: age, neck circumference (NC) and BMI. Neck circumference was the only variable that correlated with passive CSA/Pph during expiration (R2=0.81; p<0.05); no variable predicted changed compliance during inspiration. After an outlier was removed from the analysis, the relationship between NC and passive CSA/Pph remained strong (R2=0.77, p<0.05) as shown in figure 8.
Figure 8.
Relationship between neck circumference (NC) and expiratory CSA-Pph slopes during passive state.
Post-hyperventilation was associated with pharyngeal narrowing after at least 10 seconds central apnea (78.9 12.2 % of eupnoea; p<0.05). The real time changes in retro palatal airway during the transition from eupnoea to active mechanical ventilation and from passive mechanical ventilation to induced central apnea in a representative normal subject are depicted in supplemental video recordings A and B, respectively.
4. DISCUSSION
4.1. Summary of Findings
Our study demonstrated several novel and significant findings: 1) Inhibition of ventilatory motor output resulted in increased expiratory pharyngeal compliance (CUA), independent of changes in lung volume. 2) Neck circumference (NC) was the only factor associated with increased expiratory pharyngeal compliance under passive states. However, NC did not change between active and passive breaths.
4.2. Methodological Considerations
Several considerations related to fiberoptic endoscopy (FOB) may influence the interpretation of the findings, including the inability to obtain multiple levels within the upper airway, movement of the FOB and the use of a constant reference landmark for calibration of the measurement. Our laboratory and others have utilized and validated FOB in multiple studies (Rowley et al., 1998; Rowley et al., 2002; Sankri-Tarbichi et al., 2009). To mitigate these limitations, we determined and fixed the locations of the scope and the catheter to the nasal mask after locating the retro palatal space. Moreover, patients slept in the supine position with a fixed neck position before and during each of the mechanical ventilation trials. We chose the retro palatal airway for imaging as it represented the site of maximal narrowing in many patients with sleep apnea (Badr et al., 1995; Schwab et al., 1995). Finally, our lab have validated previously the CSA measurements and showed a 10% coefficient variation (Morrell and Badr, 1998b). To insure the accuracy, the images were analyzed on each subject by same individual who is blinded to the phase of respiration.
Our study utilized the CSA/Pph relationship as a surrogate for pharyngeal retro palatal compliance. We are cognizant that this is not a true compliance measurement, which requires measurement of changes in pharyngeal volume that is independent of resistive influences. However, upper airway behavior during rhythmic breathing is not analogous to the respiratory system and is not amenable to partitioning elastic from resistive contributions in pressure-flow loops as all measurements are conducted while airflow is occurring. We believe that retro palatal CSA/Pph is an appropriate surrogate, as previously described (Isono et al., 1997; Kuna et al., 1988). Second, an accurate CUA measurement using area requires that the pressure measured be at the same level as the changes in area, as we have done in this study. Third, the measurement of CUA requires a measurement of transmural pressure rather than intra-luminal pressure alone. We recognize that the surrounding pressure may be above atmospheric, and may also change dynamically. However, the absence of a change in NC during mechanical ventilation suggests that passive mechanical ventilation did not alter the surrounding extra-luminal pressure. We recognize also that volume-pressure or area-pressure relationship is not necessarily linear throughout the respiratory cycle or during the transition from eupnea to hypopnea or apnea, this is an inevitable methodological limitation. Finally, mechanical ventilation may decrease upper airway muscle activity by a combination of factors including positive pressure, high flow, hypocapnia, and elevated lung volume. To assess the upper airway compliance under passive steady state, NPPV was used for three minutes periods until no respiratory effort is visible on the pharyngeal pressure signal and the termination of NPPV was followed by central apnea. We did not measure upper airway muscles EMG activity during sleep or the respiratory phases. It was recently found that the upper airway muscle activity (such as genioglossus) may differ during inspiration and expiration (Wilkinson et al., 2008). We cannot exclude the possibility that some expiratory motoneurons were active during hypopcapnic inhibition or even during the subsequent central apnea. In fact, expiratory motor units sustained their activity during the transition to sleep, but only 9% of the motor units were identified to be active (Wilkinson et al., 2008). In this study we aimed to assess the upper airway mechanic under passive and active states using NPPV. Relative pharyngeal hypotonia under positive pressure ventilation has been proved previously by Schwartz et al (1998).
4.3. Effect of ventilatory motor output on pharyngeal compliance
Increased pharyngeal compliance during passive mechanical ventilation relative to active mechanical breaths was not due to differences in lung volume as evidenced by the constancy of end-expiratory pharyngeal pressure and EELV. Similarly, there was no occult change in the surrounding pressure given the stability of neck circumference during passive breaths relative to active breaths. Therefore, changes in upper airway mechanics were due to changes in upper airway neuromuscular activity, per se, rather than changes in lung volume, or cervical fluid volume.
Increased expiratory compliance during passive breaths was associated with hysteresis in the pressure-CSA curve, analogous to the hysteresis of the pressure-volume loop of the respiratory system. Hysteresis is characterized by wider and curvilinear changes between inspiration and expiration as the elastic properties of the passive upper airway determine its behavior during inflation and deflation. Accordingly, tissue friction and the compression of elastic structures may impair dilatation of the upper airway during inspiration and enhance deflation immediately upon release of the inflating pressure. Thus, hysteresis indicates that the upper airway becomes more susceptible to deformation and collapse during expiration. This finding corroborates studies demonstrating hysteresis in upper airway in sleep apnea patients, manifesting by higher critical opening pressure during expiration relative to inspiratory critical collapsing pressure (Pcrit) under relative hypotonia (Morrison et al., 1993). There is evidence that upper airway obstruction is influenced by intra-breath hysteresis during positive pressure ventilation (Isono et al., 1993). The role of hysteresis on airway patency is important especially in apneic patients, whom CSA is at its nadir at end expiration (Condos et al., 1994; Morrell et al., 1998a).
Increased pharyngeal compliance during expiratory phase explains a potential mechanism for the retro palatal airway expiratory pharyngeal narrowing during central hypopnea (Sankri-Tarbichi et al., 2009) and apnea (Badr et al., 1995). Thus, increased pharyngeal compliance during expiration renders the upper airway vulnerable to collapsing extra luminal pressure.
The correlation between increased expiratory compliance and neck circumference provides a mechanistic explanation for the strong correlation between neck circumference and indices of sleep apnea severity. For example, neck circumference is an independent predictor of sleep apnea (Davies and Stradling., 1990) and a determinant of optimal CPAP levels in patients with sleep apnea (Miljeteig and Hoffstein., 1993). Likewise, neck circumference accounts for the reported gender difference in upper airway compliance during NREM sleep (Rowley et al., 2002). This is not surprising if we consider that neck circumference represents an aggregate of non-neuromuscular structures of the upper airway including blood vessels, connective and adipose tissue. The NC did not change however between passive and active respiration indicating that increased pharyngeal compliance was not dependent on dynamic changes in the surrounding pressure or on a fluid shift during sleep. Thus, non-neuromuscular structures that do not change dynamically, such as cervical adipose tissue, may play important role in the increased expiratory pharyngeal compliance under passive conditions. Likewise, neuromuscular properties of upper airway play an important role in the upper airway patency during sleep especially in susceptible individuals who have large neck circumference.
4.4. Implications to the pathogenesis of obstructive apnea
Increased expiratory pharyngeal compliance under passive conditions has significant implications regarding the pathogenesis of upper airway obstruction/narrowing during sleep. First, increased expiratory compliance was observed, followed by pharyngeal narrowing during induced central apnea, providing a mechanistic explanation of the adverse effects of reduced ventilatory drive on upper airway patency. Accordingly, inhibited upper airway muscle activity renders the upper airway vulnerable to collapsing extra luminal pressure generated by cervical soft tissue, blood vessels or fat deposits, with subsequent expiratory narrowing. Second, the association between neck circumference and the magnitude of passive expiratory pharyngeal compliance explains the importance of non-neuromuscular properties surrounding the upper airway in determining upper airway patency during sleep. This is particularly seen when the neuromuscular activity is reduced.
Increased expiratory compliance during passive breaths suggests that the retro palatal pharynx is more susceptible to extrinsic collapse during expiration via collapsing extra luminal pressure. This finding provides possible mechanism for the pharyngeal narrowing during central apnea (Badr et al., 1995) or hypopnea (Sankri-Tarbichi et al., 2009). Accordingly, obstructive sleep apnea may be an expiratory rather than an inspiratory phenomenon. There is substantial evidence to support this notion; Sanders et al. (1983) showed that low expiratory flow persisted during the effort-free portion of a mixed apnea, suggesting that the upper narrowing began during the expiratory phase of the respiratory cycle. Stanescu et al (1996) found significant expiratory flow limitations in heavy snorers and OSA patients. Borowiecki et al (1978) utilized fiberoptic upper airway visualization and found that pharyngeal occlusion began before inspiration. Using a similar fiberoptic methodology, Morrell el al (1998) demonstrated that the retro palatal airway narrowed progressively, during expiration, prior to obstructive apnea. Once upper airway obstruction occurs; tissue adhesion forces and cervical structures would impede opening despite resumption of respiratory effort.
In summary, we have shown an increase in upper airway compliance during passive versus active conditions. Significant intra-breath hysteresis occurred after abolishing neuromuscular activity. The increased pharyngeal compliance during expiration correlated with the neck size indicating that neuromuscular factors are important determinants of the upper airway patency during sleep in susceptible individuals.
Supplementary Material
Supplemental Video: Fiberoptic video recording of the retro palatal airway from a representative subject for the transition from eupnoea to active ventilation (A) and from passive ventilation to central apnea (B). Note the progressive airway narrowing during central apnea compared to eupnoea and the resumption of airway patency following this apnea.
Acknowledgements
The authors would like to thank Alexandria D. Leonardi, Maryelsa Anita D’Souza, Sukanya Pranathiageswaran and Nicole Nickert for their technical assistance.
Funded By: Department of Veterans Affairs, NHLBI, and the Medical Staff Trust Fund -Harper University Hospital
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alex CG, Aronson RM, Onal E, et al. Effects of continuous positive airway pressure on upper airway and respiratory muscle activity. J.Appl.Physiol. 1987;62(5):2026–2030. doi: 10.1152/jappl.1987.62.5.2026. [DOI] [PubMed] [Google Scholar]
- Badr MS, Toiber F, Skatrud JB, et al. Pharyngeal narrowing/occlusion during central sleep apnea. J.Appl.Physiol. 1995;78(5):1806–1815. doi: 10.1152/jappl.1995.78.5.1806. [DOI] [PubMed] [Google Scholar]
- Badr MS. Effect of ventilatory drive on upper airway patency in humans during NREM sleep. Respir.Physiol. 1996;103(1):1–10. doi: 10.1016/0034-5687(95)00079-8. [DOI] [PubMed] [Google Scholar]
- Borowiecki B, Pollak CP, Weitzman ED, et al. Fibro-optic study of pharyngeal airway during sleep in patients with hypersomnia obstructive sleep-apnea syndrome. Laryngoscope. 1978;88(8 Pt 1):1310–1313. doi: 10.1288/00005537-197808000-00012. [DOI] [PubMed] [Google Scholar]
- Condos R, Norman RG, Krishnasamy I, et al. Flow limitation as a noninvasive assessment of residual upper-airway resistance during continuous positive airway pressure therapy of obstructive sleep apnea. Am.J.Respir.Crit Care Med. 1994;150(2):475–480. doi: 10.1164/ajrccm.150.2.8049832. [DOI] [PubMed] [Google Scholar]
- Davies RJ, Stradling JR. The relationship between neck circumference, radiographic pharyngeal anatomy, and the obstructive sleep apnoea syndrome. Eur.Respir.J. 1990;3(5):509–514. [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson AL, et al. The AASM Manual for the Scoring of Sleep and Associated Events : Rules, Terminology, and Technical Specifications. Westchester, Ill: American Academy of Sleep Medicine. 2007 [Google Scholar]
- Isono S, Morrison DL, Launois SH, et al. Static mechanics of the velopharynx of patients with obstructive sleep apnea. J.Appl.Physiol. 1993;75(1):148–154. doi: 10.1152/jappl.1993.75.1.148. [DOI] [PubMed] [Google Scholar]
- Isono S, Tanaka A, Sho Y, et al. Advancement of the mandible improves velopharyngeal airway patency. J.Appl.Physiol. 1995;79(6):2132–2138. doi: 10.1152/jappl.1995.79.6.2132. [DOI] [PubMed] [Google Scholar]
- Isono S, Remmers JE, Tanaka A, et al. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J.Appl.Physiol. 1997;82(4):1319–1326. doi: 10.1152/jappl.1997.82.4.1319. [DOI] [PubMed] [Google Scholar]
- Kuna ST, Bedi DG, Ryckman C. Effect of nasal airway positive pressure on upper airway size and configuration. Am.Rev.Respir.Dis. 1988;138(4):969–975. doi: 10.1164/ajrccm/138.4.969. [DOI] [PubMed] [Google Scholar]
- Miljeteig H, Hoffstein V. Determinants of continuous positive airway pressure level for treatment of obstructive sleep apnea. Am.Rev.Respir.Dis. 1993;147:1526–1530. doi: 10.1164/ajrccm/147.6_Pt_1.1526. [DOI] [PubMed] [Google Scholar]
- Morrell MJ, Arabi Y, Zahn B, et al. Progressive retro palatal narrowing preceding obstructive apnea. Am.J.Respir.Crit Care Med. 1998a;158(6):1974–1981. doi: 10.1164/ajrccm.158.6.9712107. [DOI] [PubMed] [Google Scholar]
- Morrell MJ, Badr MS. Effects of NREM sleep on dynamic within-breath changes in upper airway patency in humans. J.Appl.Physiol. 1998b;84(1):190–199. doi: 10.1152/jappl.1998.84.1.190. [DOI] [PubMed] [Google Scholar]
- Morrison DL, Launois SH, Isono S, et al. Pharyngeal narrowing and closing pressures in patients with obstructive sleep apnea. Am.Rev.Respir.Dis. 1993;148(3):606–611. doi: 10.1164/ajrccm/148.3.606. [DOI] [PubMed] [Google Scholar]
- Mortimore IL, Marshall I, Wraith PK, et al. Neck and total body fat deposition in nonobese and obese patients with sleep apnea compared with that in control subjects. Am.J.Respir.Crit Care Med. 1998;157(1):280–283. doi: 10.1164/ajrccm.157.1.9703018. [DOI] [PubMed] [Google Scholar]
- Onal E, Burrows DL, Hart RH, Lopata M. Induction of periodic breathing during sleep causes upper airway obstruction in humans. J Appl Physiol. 1986;61:1438–1443. doi: 10.1152/jappl.1986.61.4.1438. [DOI] [PubMed] [Google Scholar]
- Rowley JA, Williams BC, Smith PL, et al. Neuromuscular activity and upper airway collapsibility. Mechanisms of action in the decerebrate cat. Am.J.Respir.Crit Care Med. 1997;156(2 Pt 1):515–521. doi: 10.1164/ajrccm.156.2.9607115. [DOI] [PubMed] [Google Scholar]
- Rowley JA, Zahn BR, Babcock MA, et al. The effect of rapid eye movement (REM) sleep on upper airway mechanics in normal human subjects. J.Physiol. 1998;510(Pt 3):963–976. doi: 10.1111/j.1469-7793.1998.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley JA, Sanders CS, Zahn BR, et al. Gender differences in upper airway compliance during NREM sleep: role of neck circumference. J.Appl.Physiol. 2002;92(6):2535–2541. doi: 10.1152/japplphysiol.00553.2001. [DOI] [PubMed] [Google Scholar]
- Sanders MH, Moore SE. Inspiratory and expiratory partitioning of airway resistance during sleep in patients with sleep apnea. Am.Rev.Respir.Dis. 1983;127(5):554–558. doi: 10.1164/arrd.1983.127.5.554. [DOI] [PubMed] [Google Scholar]
- Sankri-Tarbichi AG, Rowley JA, Badr MS. Expiratory pharyngeal narrowing during central hypocapnic hypopnea. Am.J.Respir.Crit Care Med. 2009;179(4):313–319. doi: 10.1164/rccm.200805-741OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H, Boudewyns A, Smith PL, et al. Modulation of upper airway collapsibility during sleep: influence of respiratory phase and flow regimen. J Appl Physiol. 2002;93:1365–1376. doi: 10.1152/japplphysiol.00942.2001. [DOI] [PubMed] [Google Scholar]
- Schwab RJ, Gupta KB, Gefter WB, et al. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am.J.Respir.Crit Care Med. 1995;152(5 Pt 1):1673–1689. doi: 10.1164/ajrccm.152.5.7582313. [DOI] [PubMed] [Google Scholar]
- Schwartz AR, O'Donnell CP, Baron J, et al. The hypotonic upper airway in obstructive sleep apnea: role of structures and neuromuscular activity. Am.J.Respir.Crit Care Med. 1998;157(4 Pt 1):1051–1057. doi: 10.1164/ajrccm.157.4.9706067. [DOI] [PubMed] [Google Scholar]
- Series F, Marc I. Effects of inspiratory and expiratory positive pressure difference on airflow dynamics during sleep. J.Appl.Physiol. 1998;85(5):1855–1862. doi: 10.1152/jappl.1998.85.5.1855. [DOI] [PubMed] [Google Scholar]
- Shiota S, Ryan CM, Chiu KL, et al. Alterations in upper airway cross-sectional area in response to lower body positive pressure in healthy subjects. Thorax. 2007;62(10):868–872. doi: 10.1136/thx.2006.071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanescu D, Kostianev S, Sanna A, et al. Expiratory flow limitation during sleep in heavy snorers and obstructive sleep apnoea patients. Eur.Respir.J. 1996;9(10):2116–2121. doi: 10.1183/09031936.96.09102116. [DOI] [PubMed] [Google Scholar]
- Whittle AT, Marshall I, Mortimore IL, et al. Neck soft tissue and fat distribution: comparison between normal men and women by magnetic resonance imaging. Thorax. 1999;54(4):323–328. doi: 10.1136/thx.54.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson V, Malhotra A, Nicholas CL, Worsnop C, Jordan AS, et al. Discharge patterns of human genioglossus motor units during sleep onset. Sleep. 2008;31(4):525–533. doi: 10.1093/sleep/31.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XS, Rowley JA, Demirovic F, et al. Effect of testosterone on the apneic threshold in women during NREM sleep. J.Appl.Physiol. 2003;94(1):101–107. doi: 10.1152/japplphysiol.00264.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Video: Fiberoptic video recording of the retro palatal airway from a representative subject for the transition from eupnoea to active ventilation (A) and from passive ventilation to central apnea (B). Note the progressive airway narrowing during central apnea compared to eupnoea and the resumption of airway patency following this apnea.