Abstract
Most mammals possess stamina because their locomotor and respiratory (i.e., ventilatory) systems are mechanically coupled. These systems are decoupled, however, in bottlenose dolphins (Tursiops truncatus) as they swim on a breath-hold. Locomotion and ventilation are coupled only during their brief surfacing event, when they respire explosively (up to 90% of total lung volume in approximately 0.3s) (Ridgway et al., 1969). The predominantly slow-twitch fiber profile of their diaphragm (Dearolf, 2003) suggests that this muscle does not likely power their rapid ventilatory event. Based upon Bramble's (1989) biomechanical model of locomotor-respiratory coupling in galloping mammals, it was hypothesized that locomotor muscles function to power ventilation in bottlenose dolphins. It was further hypothesized that these muscles would be composed predominantly of fast-twitch fibers to facilitate the bottlenose dolphin's rapid ventilation. The gross morphology of cranio-cervical (scalenus, sternocephalicus, sternohyoid), thoracic (intercostals, transverse thoracis), and lumbo-pelvic (hypaxialis, rectus abdominis, abdominal obliques) muscles (n=7) and the fiber-type profiles (n=6) of selected muscles (scalenus, sternocephalicus, sternohyoid, rectus abdominis) of bottlenose dolphins were investigated. Physical manipulations of excised thoracic units were carried out to investigate potential actions of these muscles. Results suggest that the cranio-cervical muscles act to draw the sternum and associated ribs cranio-dorsally, which flares the ribs laterally, and increases the thoracic cavity volume required for inspiration. The lumbo-pelvic muscles act to draw the sternum and caudal ribs caudally, which decreases the volumes of the thoracic and abdominal cavities required for expiration. All muscles investigated were composed predominantly of fast-twitch fibers (range 61-88% by area) and appear histochemically poised for rapid contraction. These combined results suggest that dolphins utilize muscles, similar to those used by galloping mammals, to power their explosive ventilation.
Keywords: dolphin, respiration, ventilation, muscle, histochemistry, anatomy, morphology
Many terrestrial mammals mechanically couple their locomotion and respiration (i.e., ventilation) (e.g., Bramble and Carrier, 1983; Bramble, 1989; Alexander, 1993; Bramble and Jenkins, 1993). Bramble (1989) hypothesized that in galloping mammals, muscles located within the cranio-cervical and lumbo-pelvic units, which are typically viewed as locomotor muscles, create the changes in thoracic cavity shape required for inspiration and expiration, respectively. In contrast to terrestrial mammals, bottlenose dolphins (Tursiops truncatus) have decoupled locomotion and ventilation, since they swim underwater on a breath-hold. Locomotor–respiratory (i.e., ventilatory) coupling occurs only briefly at the surface during their explosive ventilatory event but to date, this mechanical coupling has not been investigated. Using Bramble's (1989) terrestrial mammal locomotor-respiratory model, this study investigated the gross morphology and histochemical profiles of the muscles within the cranio-cervical and lumbo-pelvic units of the bottlenose dolphin, which are positioned to create the thoracic volume change required for ventilation of the lungs.
Terrestrial mammals respire using a costal aspiration pump and a diaphragm muscle (reviewed in Brainerd, 1999). Lungs are ventilated by alternating changes in volume (and therefore pressure) within the thoracic cavity. This explanation of ventilation in resting mammals describes the diaphragm as the primary muscle of inspiration (reviewed in Schmidt-Nielsen, 1997). The contraction and consequent flattening of the diaphragm increases thoracic cavity volume, which decreases pressure within the lungs, to drive inspiration. Based upon gross morphological and experimental studies, other muscles that can contribute to inspiration are those that can expand the thoracic cavity by rotating the ribs cranially and laterally. These muscles include the scalenus, sternocephalicus, sternohyoid, cranial dorsal serratus, transverse thoracic, external and internal intercostals, rectus thoracis, and costal levators (e.g., Raper et al., 1966; Duron, 1973; De Troyer and Kelly, 1984; Van de Graaff et al., 1984; De Troyer et al., 1985; De Troyer and Ninane, 1986; Nickel et al., 1986; De Troyer, 1991; Whitelaw et al., 1992; Hermanson and Evans, 1993; De Troyer et al., 1994; Carrier, 1996; Fournier and Lewis, 1996; Legrand et al., 1997; De Troyer et al., 2005).
Expiration in terrestrial mammals at rest is largely controlled by passive elastic recoil of the lungs and musculoskeletal elements (reviewed in Nickel et al., 1986; Schmidt-Nielsen, 1997). When active expiration is required, muscles that can rotate the ribs caudally and medially function to decrease the volume within the thoracic and abdominal cavities (Nickel et al., 1986). These muscles include the external and internal intercostals, rectus abdominis, external and internal abdominal obliques, transverse abdominal, caudal dorsal serratus, and rectus thoracis (e.g., De Troyer et al., 1983; De Troyer et al., 1985; Nickel et al., 1986; De Troyer and Ninane, 1987; Gilmartin et al., 1987; De Troyer et al., 1989; Farkas and Schroeder, 1990; Hermanson and Evans, 1993; Deban and Carrier, 2002). The intercostal muscles can contribute to both inspiration and expiration; their function is dependent upon posture, locomotor activity, and lung volume (Carrier, 1996; Deban and Carrier 2002; DeTroyer et al., 2005).
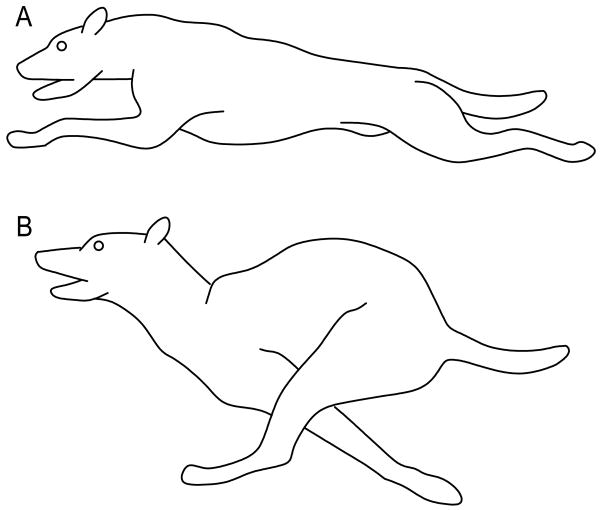
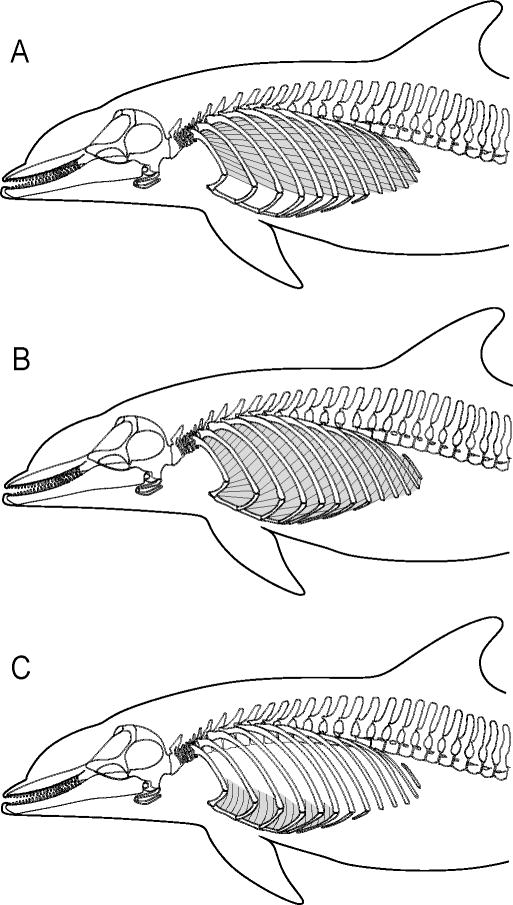
In contrast to mammals at rest, mechanical coupling occurs between the locomotor and respiratory systems in mammals that are actively moving. In galloping mammals, this coupling occurs at a 1:1 ratio (i.e., one stride per breath) (Bramble and Carrier, 1983; Bramble, 1989). This mechanical coupling occurs because locomotor movements facilitate ventilation via dorsal and ventral flexions of the body, and cranio-caudal movements of the abdominal viscera (i.e., the visceral piston) (Bramble and Carrier, 1983; Carrier, 1987). Dorsal body flexion increases the thoracic cavity volume, which decreases internal pressure and assists inspiration (Fig. 1A). During dorsal flexion, which is associated with the aerial phase of the locomotor stride, the abdominal viscera are also displaced caudally. Conversely, ventral flexion decreases the thoracic cavity volume, which increases internal pressure to drive expiration (Fig. 1B). During ventral flexion, which is associated with the ground phase of the locomotor stride, the abdominal viscera is thrusted cranially towards the diaphragm, which further decreases the volume within the thoracic cavity and aids expiration (Bramble and Carrier, 1983; Bramble, 1989; Alexander, 1993; Bramble and Jenkins, 1993). Locomotor stamina, or endurance, is achieved in many terrestrial mammals because they are able to mechanically couple locomotion and ventilation of their lungs (Carrier, 1987).
Fig. 1.
Two stages of galloping in the dog showing, (A) dorsal flexion, and (B) ventral flexion (modified from Bramble, 1989).
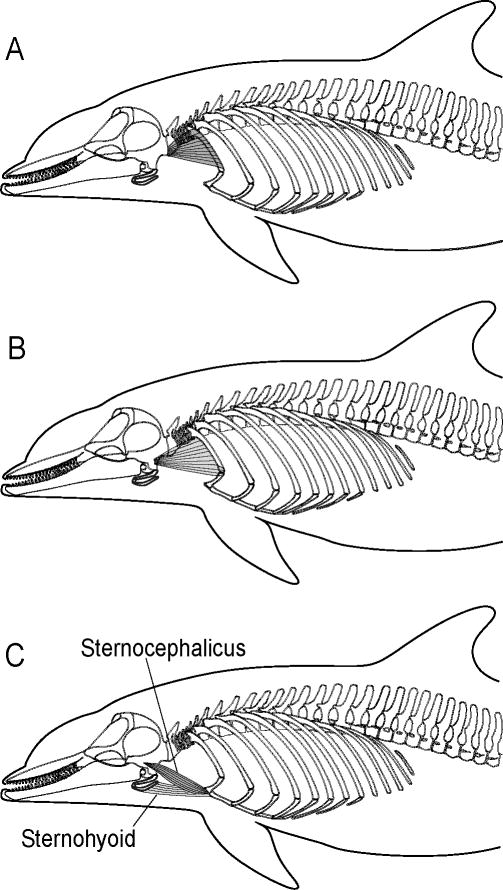
Bramble (1989) described a biomechanical model for the integration of the locomotor-respiratory systems in galloping terrestrial mammals. The model's four principal mechanical components are the thoracic unit, the cranio-cervical unit, the lumbo-pelvic unit, and the visceral piston (Fig. 2). This biomechanical model suggests that respiration (i.e., ventilation) in galloping mammals is powered by (1) alternate, rhythmic and coordinated muscle contractions of cranio-cervical and lumbo-pelvic units, which both act upon the thoracic unit, and (2) the oscillatory motions of the visceral piston (Bramble, 1989).
Fig. 2.
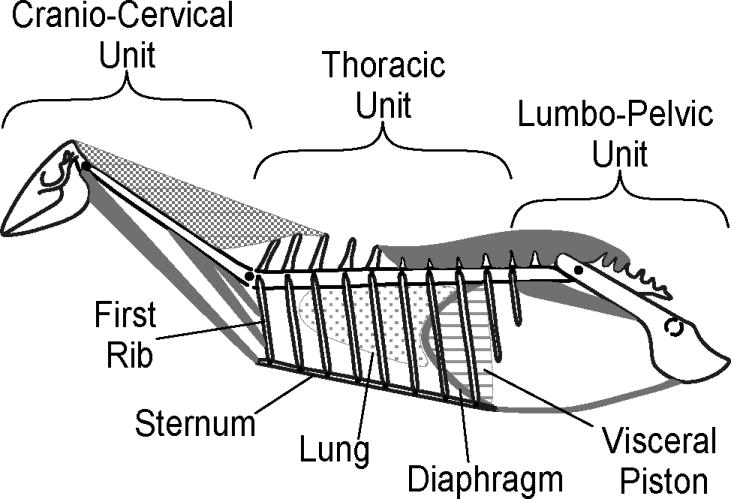
Functional units of the biomechanical model of locomotor-respiratory coupling for a galloping mammal (modified from Bramble, 1989).
According to Bramble's (1989) biomechanical model, inspiration begins during the aerial phase of locomotion and is initiated by muscles within the cranio-cervical unit. These muscles simultaneously ventrally flex the cranio-cervical unit and draw the sternum and ribs cranially, which increases thoracic cavity volume. During this phase, both forelimbs are off the ground and extended cranially, and the lumbo-pelvic unit is flexed dorsally, resulting in full expansion of the thorax. The dorsal flexion of the body lowers intra-abdominal pressure and causes a caudal displacement of the abdominal viscera (i.e., visceral piston). This combination of movements causes an increase in thoracic cavity volume and a decrease in thoracic cavity pressure that draws air into the lungs for inspiration (Bramble, 1989).
Expiration is brought on by the ground reaction forces on the forelimbs, which cause ipsilateral external loading of the thorax and leads to compression of this body cavity, resulting in the reduction of thoracic volume (Bramble, 1989). Additionally, the impact of the forelimbs with the ground results in a cranial displacement of the visceral piston and the diaphragm, which further compresses the thorax and increases the intrathoracic pressure. During this phase, the lumbo-pelvic unit swings cranially due to the contraction of abdominal and hypaxial muscles. Contraction of these muscles further reduces the abdominal volume and raises the internal pressure to assist expiration (Bramble, 1989; Young et al., 1992a). Thus, locomotor movements of the body and concomitant movements of the abdominal viscera facilitate changes in thoracic cavity volume and pressure in galloping terrestrial mammals.
Like terrestrial mammals, bottlenose dolphins (Tursiops truncatus), demonstrate locomotor stamina (reviewed in Costa and Williams, 1999); however, locomotor-ventilatory coupling in a dolphin is dependent upon its position within the water column. The majority of a dolphin's life is spent swimming below the surface on a breath-hold and as a result, its locomotion is decoupled from ventilation. Only during surfacing does locomotion and ventilation appear to be mechanically coupled. At the surface, bottlenose dolphins respire explosively and can exchange up to 90% of their lung volume in approximately 0.3 seconds (Irving et al., 1941; Ridgway et al., 1969; Kooyman and Cornell, 1981; reviewed in Wartzok, 2002). In comparison, a galloping horse exchanges only 21% of its lung volume in approximately half a second (Hornicke et al., 1983; Young et al., 1992a).
Although the diaphragm is the primary muscle of inspiration in most mammals at rest (reviewed in Brainerd, 1999), its ventilatory role in running mammals is considerably diminished (Bramble, 1989; Alexander, 1993; Bramble and Jenkins, 1993). The diaphragm of bottlenose dolphins has also been hypothesized to play a minor role in ventilation (Dearolf, 2003). Histochemical analysis of the bottlenose dolphin diaphragm revealed that this muscle is composed predominantly of slow-twitch fibers (Dearolf, 2003), suggesting that this muscle may lack the contractile speed required to power explosive ventilation.
Because dolphins live in an aquatic medium they do not experience the abrupt decelerations and reaccelerations of the body experienced by running mammals due to ground reaction forces on the limbs (e.g., Bramble and Jenkins, 1993). Thus, it is unlikely that the visceral piston contributes to bottlenose dolphin ventilation as importantly as it does in terrestrial mammals. Dolphins do, however, undergo cyclical dorso-ventral body flexions during swimming, homologous to the axial body movements of galloping mammals (e.g., Fish, 1993; Pabst, 1993; Pabst et al., 1999). Bottlenose dolphins swim by alternate actions of epaxial, and hypaxial and abdominal muscles (e.g., Slijper, 1936; Arkowitz and Rommel, 1985; Bello et al., 1985; Pabst, 1990, 1993; Pabst et al., 1999). These dorso-ventral body movements could, as they do in running mammals, change thoracic and abdominal cavity volumes and pressures.
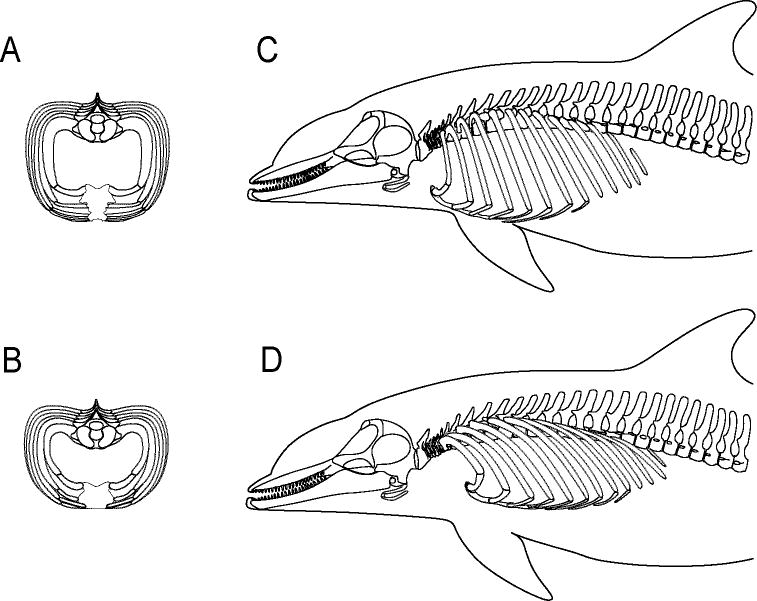
The bottlenose dolphin thoracic cavity has enhanced flexibility, relative to terrestrial mammals (reviewed by Rommel, 1990), which may contribute to the dolphin's ability to achieve a rapid ventilatory event. In contrast to most terrestrial mammals, bottlenose dolphins possess bony sternal ribs that articulate to the distal ends of vertebral ribs 1 through 7 via flexible joints (Fig. 3). This series of “extra” joints has been hypothesized to enhance flexibility to accommodate thoracic compression that occurs during diving (Ridgway et al., 1969; Hui, 1975; Rommel, 1990; Rommel and Reynolds, 2002). To date the movement of the skeletal elements within the thorax during ventilation and the muscles that drive these skeletal movements have not been investigated or described.
Fig. 3.
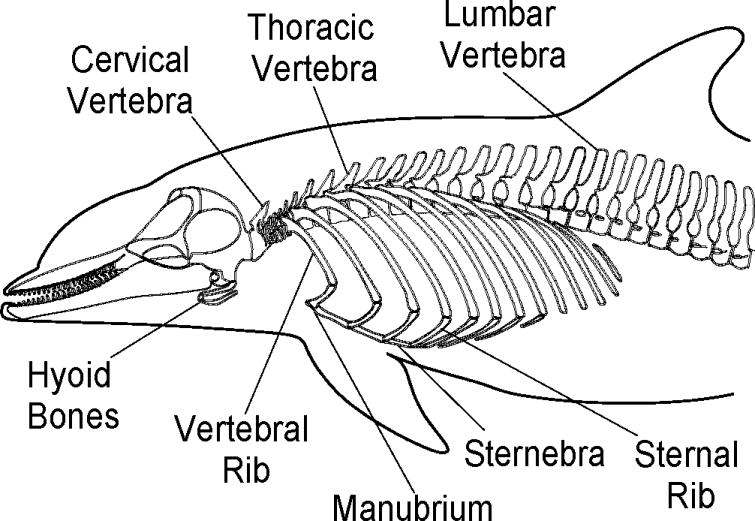
Skeletal elements of the thoracic cavity in bottlenose dolphins (Tursiops truncatus), including thoracic vertebrae, vertebral and sternal ribs, and the sternum (including its first element, the manubrium). Bones of the skull, hyoid complex, and of the cervical and lumbar vertebrae are also shown (modified from Rommel, 1990). Skeletal elements positioned by Rommel (1990) in an assumed “neutral” posture.
There exist few anatomical descriptions of post-cranial muscles in bottlenose dolphins: Pabst (1990, 1993) described the axial locomotor muscles, Reidenberg and Laitman (1994) described the anatomy and musculature of the hyoid apparatus (in Odontoceti, including Tursiops truncatus), and Dearolf (2002) described the morphology of the diaphragm muscle. Although, a small number of anatomical descriptions have been conducted on other cetaceans [e.g., Balaenoptera acutorostrata (Carte and Macalister, 1868), Grampus griseus (Murie, 1871), Kogia breviceps (Schulte and Smith, 1918), Neophocaena phocaenoides (Howell, 1927), Monodon monoceros (Howell, 1930), Phocoena phocoena (Slijper, 1936; Smith et al., 1976), Pontoporia blainvillei (Strickler, 1978)], these studies either do not mention or do not describe in detail muscles used for ventilation. To date, only Dearolf (2002, 2003) has examined a ventilatory muscle, the diaphragm, in a bottlenose dolphin.
Bramble's (1989) biomechanical model illustrates that the muscles within the cranio-cervical and lumbo-pelvic units likely facilitate ventilation in galloping mammals. Based upon this model, this study tested the hypothesis that these muscles could also function to power ventilation in bottlenose dolphins. Thus, the goals of this study were two-fold. The first was to describe the gross morphology and identify the potential actions of the muscles located within the bottlenose dolphin's cranio-cervical unit (scalenus, sternocephalicus, sternohyoid), thoracic unit (intercostals, transverse thoracis), and lumbo-pelvic unit (hypaxialis, rectus abdominis, abdominal obliques). Specifically, the muscles located within the cranio-cervical unit were hypothesized to act on the cranial ribs and sternum to increase thoracic cavity volume and create the shape change required for inspiration. The muscles located within the lumbo-pelvic unit were hypothesized to act on the caudal thoracic cavity and sternum to decrease thoracic cavity volume and create the shape change required for expiration. The second goal of this study was to determine the fiber-type profile of a subset of these muscles (scalenus, sternocephalicus, sternohyoid, and rectus abdominis). These muscles were hypothesized to be composed of predominantly fast-twitch fibers to support the bottlenose dolphin's rapid ventilatory event.
Materials and Methods
Specimens
Bottlenose dolphins (Tursiops truncatus) (Cetacea: Delphinidae) that either stranded or were incidentally killed in fishing operations were utilized in this study (Table 1). All specimens were collected by the Marine Mammal Stranding Program at the University of North Carolina Wilmington, the Cetacean and Sea Turtle Team at the National Marine Fisheries Service Laboratory in Beaufort North Carolina, and the Virginia Aquarium Stranding Response Program. Specimens were placed into life-history categories as defined by Dearolf et al. (2000) and Struntz et al. (2004) and ranged in size from 109 cm to 275 cm (Table 1). Two of the smallest specimens (i.e., WAM 606 and WAM 610) were used for preliminary, exploratory dissections; ontogenetic variation was not investigated in this study. Gross dissections were performed on specimens that were in fresh or moderate condition (Smithsonian Institution Code 2 or 3; Geraci and Lounsbury, 2005). Muscle samples used for histochemical analyses were in fresh condition.
Table 1.
Bottlenose dolphin (Tursiops truncatus) specimens utilized in this study.
| Identification Number | Total Length (cm) | Life History Category | Sex | Code |
|---|---|---|---|---|
| CALO 0173a | 109.0 | Neonate | F | 2 |
| WAM 606a,b | 109.0 | Neonate | M | 2 |
| WAM 610a,b | 124.5 | Neonate | M | 3 |
| VAQS 20061008a,b,c | 175.1 | Sub-adult | M | 3 |
| BRF 090a,b,e | 182.0 | Sub-adult | M | 3 |
| WAM 627c,e | 194.0 | Sub-adult | M | 2 |
| VMSM20041079d | 195.0 | Sub-adult | F | 2 |
| VAQS 20051086b,c | 195.4 | Sub-adult | M | 2 |
| WAM 591d | 222.8 | Adult | M | 2 |
| CJH 003d | 229.5 | Adult | F | 2 |
| VMSM 20041040d | 234.5 | Adult | M | 2 |
| WAM 579d | 237.0 | Adult | M | 2 |
| BRF 098c | 245.0 | Adult | F | 2 |
| WAM 628c,* | 246.0 | Adult | F | 3 |
| AJW 001d | 274.0 | Adult | M | 2 |
| BRF 061a,b,c,e | 275.0 | Adult | M | 2 |
| WAM 631a | 275.0 | Adult | F | 2 |
Gross morphology
Physical manipulations
Histochemistry (sternohyoid, sternocephalicus, rectus abdominis)
Histochemistry (scalenus)
Joint morphology
Regional variation in histochemical profiles
Gross Morphology and Physical Manipulations
The muscles that act upon the thorax were exposed using a routine dissection procedure (outlined in McLellan et al., 2002). The muscles of interest were carefully exposed and separated along muscle boundaries and/or fascial planes from surrounding musculature. The gross morphological description of each muscle included a description of its origin, insertion, fiber orientation, muscle shape, and relationships to other structures. Scaled, digital images (Nikon D50, JPEG image files) were taken throughout each dissection.
Muscles photographed in situ were imported into the computer engineering drafting program Easy CAD (Evolution Computing, Phoenix, Arizona) to create representative illustrations of each muscle. To maintain uniformity across all illustrations, each digital image was scaled similarly in Easy CAD and superimposed on a pre-existing, anatomically accurate illustration of a Tursiops truncatus skeleton (modified from Rommel, 1990).
Physical manipulations of the thorax were conducted in situ (n=6) and on excised thoracic cavity units (n=3). In situ manipulations were conducted on partially dissected specimens and the skeletal elements were manually moved in ways in an attempt to simulate the action of each muscle on the thoracic cavity. Excised thoracic units included skeletal elements (cervical and thoracic vertebrae, vertebral and sternal ribs, and sternum) and muscles of the thoracic wall (intercostals, transverse thoracic, and cranial portions of the hypaxialis and rectus abdominis). Muscles and connective tissues were kept moist throughout manipulations. Excised thoracic units were mounted on a stable frame, via multiple attachments to the vertebral column, thus permitting the thoracic unit to be temporarily fixed in extreme cranial and extreme caudal postures (representing inspiration and expiration, respectively). In these cases, hi-test monofilament fishing lines were attached to the cranial (first vertebral rib, first sternal rib, manubrium) and caudal (caudal sternebrae, caudal vertebral ribs) skeletal elements. These lines were manually pulled along trajectories that represented the muscle fiber orientations of the muscles being investigated. A 5 kg spring scale (Pensola®, Baar, Switzerland) was used to ensure that repeatable manipulations were achieved by imposing a constant load. Examination of these postures revealed how the muscles located within the cranio-cervical and lumbo-caudal units could create thoracic shape change. Scaled, digital images were taken during physical manipulations to be analyzed in Easy CAD to examine the changes of skeletal element positions in the two extreme postures. Postural changes of the vertebral column were not investigated because this portion of the axial skeleton was used to fix the excised thoracic unit onto the stable frame. Thoracic skeletal element movement has never been measured in a living bottlenose dolphin, thus, the postures resulting from these manipulations may underestimate or, more likely, overestimate thoracic shape change that occurs in vivo. The manipulations probably do, though, accurately illustrate the direction of skeletal element movements that would occur due to the actions of the muscles investigated here.
Muscle Histochemistry
The histochemical profiles of the scalenus (n=6), sternocephalicus (n=5), sternohyoid (n=6), and rectus abdominis (n=6) were investigated (Table 1). Myosin ATPase was used to distinguish between fast and slow contracting muscle fibers. Succinic dehydrogenase (SDH) was used to determine relative oxidative capacity of the muscle fibers. An approximately 3.0 cm thick cross-section was sampled from the mid-belly of each muscle. Because of its extreme length, three cross-sectional samples were collected from the rectus abdominis: (1) at a position midway between the origin and the umbilicus (i.e., rectus abdominis-cranial), (2) at the umbilicus (i.e., rectus abdominis-umbilicus), and (3) at a position midway between the umbilicus and its insertion (i.e., rectus abdominis-caudal). For specimens utilized for both physical manipulations and histochemical assays (see Table 1), these muscles were removed prior to physical manipulations to preserve their quality. Cross-sectional samples were wrapped in multiple layers of Saran™ Premium Wrap, placed in Ziploc® Freezer bags and frozen flat at -20°C until further analysis (following the methods of Dearolf et al., 2000; Dearolf, 2003; Etnier et al., 2004).
One small trapezoidal block (<1 cm3) was cut from the center of each frozen cross-section and permitted to thaw to room temperature (approximately 10 minutes). Regional variation in fiber-type profile across the cross-sectional face of the sternocephalicus, sternohyoid, and rectus abdominis was investigated in one adult specimen. For these muscles, samples were obtained from a site on either side of the central sample. Regional variation across the scalenus was not investigated. Once the muscle blocks were thawed, they were placed on a microtome chuck, covered with Optimal Cutting Temperature medium (OCT, Sakura Finetek, Torrance, California) and flash frozen in liquid nitrogen. These frozen muscle blocks were then stored in the microtome (Leica Cryocut 1800, Bunton Instrument Company, Mt. Airy, Maryland) at -19°C for at least two hours prior to cutting, to permit them to warm to a temperature appropriate for thin sectioning. Muscle blocks were serially sectioned (10 μm thickness) in the cryostat and mounted on “Plus” glass slides (Fisher Scientific).
Muscle sections were then stained for myosin ATPase following the methods of Brooke and Kaiser (1970) as modified by Dearolf (2003). Muscle fibers were either classified as type I (slow-twitch) or type II (fast-twitch) based upon the myosin ATPase acidic and alkaline pre-incubation protocols described in Brooke and Kaiser (1970) and Gauthier (1986).
Additional serial sections were stained for succinate dehydrogenase to determine relative oxidative potential following the methods of Dearolf et al. (2000). This method permitted the muscle fibers to be further subdivided into slow oxidative (type I), fast-twitch oxidative glycolytic (type IIa) and fast-twitch glycolytic (type IIb) based on the intensity of the color stain, which is indicative of their SDH activity.
Tissue sections were viewed using an Olympus B×60 light microscope at a magnification of 20× for image analysis. Images were captured using a RT KE SPOT camera (Diagnostic Instruments, Inc, version 3.5.6, Sterling Heights, Michigan) and stored as uncompressed images (tagged image format file – TIFF).
Muscle Fiber Analysis
Two methods were used to quantify the muscle fiber profiles. The first method was a stereological approach that determined the area percent occupied by each muscle fiber type (using a Mertz-curvilinear test system) (Bozzola and Russell, 1999; Russ and Dehoff, 2000). This method provided a more accurate fiber-type profile than the second method because it accounts for the differences in size between fibers of different type. The second method was a more traditionally reported count of the number of each fiber type as a percent of the total number of fibers within a prescribed area (Bozzola and Russell, 1999). This method permitted a broader comparison of fiber-type profiles for other species reported in the literature.
The Mertz-curvilinear test system was overlaid on a digital micrograph that was projected on a computer screen and the number of “points” residing in each fiber type was counted, following the methods of Dearolf et al. (2000). This process was repeated for each muscle until a minimum of 500 total fibers were counted. Fiber area percentages were calculated for all three treatments (acid and alkaline pre-incubation, and SDH) for each muscle. The precision of percent area values, based upon five repeated measures for each treatment, was ± 2.6%.
The number of fibers of each type, expressed as a percent of the total number of fibers within a prescribed area, was determined by overlaying a 15 cm × 15 cm grid onto a digital micrograph that was projected on a computer screen following the methods of Dearolf et al. (2000). The number of each fiber-type within the grid was counted and this process was repeated until a minimum of 150 total fibers were counted for all three treatments (acid and alkaline pre-incubation, and SDH). To compensate for partial muscle fibers that intersect the borders of the grid, only the partial muscle fibers at two of the four borders were counted (Howard and Reed, 1998). The precision of the count percent values, based upon five repeated measures for each treatment, was at most ± 1.3%.
Alkaline myosin ATPase stained tissue was used to determine the mean cross-sectional area and mean diameter of individual muscle fibers of ten fibers of each type (fast and slow-twitch) from each muscle. Fibers chosen for analysis had uniform circular cross-sections and were similar in size to surrounding fibers. These fibers were outlined in Photoshop (Adobe Systems, Inc., version 5.0.2), saved as a TIFF file, and analyzed using an imaging analysis software program, Image-Pro® Plus (Media Cybernetics, Inc., version 4.5.0.19, Silver Spring, Maryland).
Results
Gross Morphology and Physical Manipulations
Each of the following morphological descriptions includes the origin and insertion of the muscle, its relations to other muscles, its description, and its hypothesized action(s) during ventilation. Because both the origin and insertion of most muscles investigated in this study are moveable, the origin was defined as the cranial end of a muscle. For muscles that are predominantly transversely oriented (transverse thoracis and abdominal obliques), the origin was defined as either the ventral-most or dorsal-most element (sensu Schaller, 1992; Nomina Anatomica Veterinaria, 1994). The outline of each muscle, and its predominant muscle fiber orientations, are illustrated in the Easy CAD figures, which are noted after the name of each muscle.
Muscles of the Cranio-Cervical Unit
Dorsal Scalenus Muscle1 (Figs. 4A, 5A, C)
Fig. 4.
Lateral view of the (A) dorsal scalenus, (B) ventral scalenus and (C) both sternocephalicus and sternohyoid in bottlenose dolphins (Tursiops truncatus).
Fig. 5.
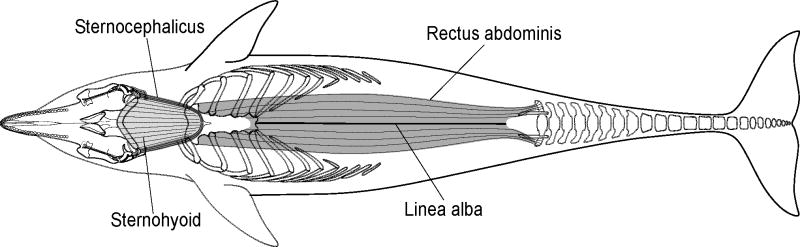
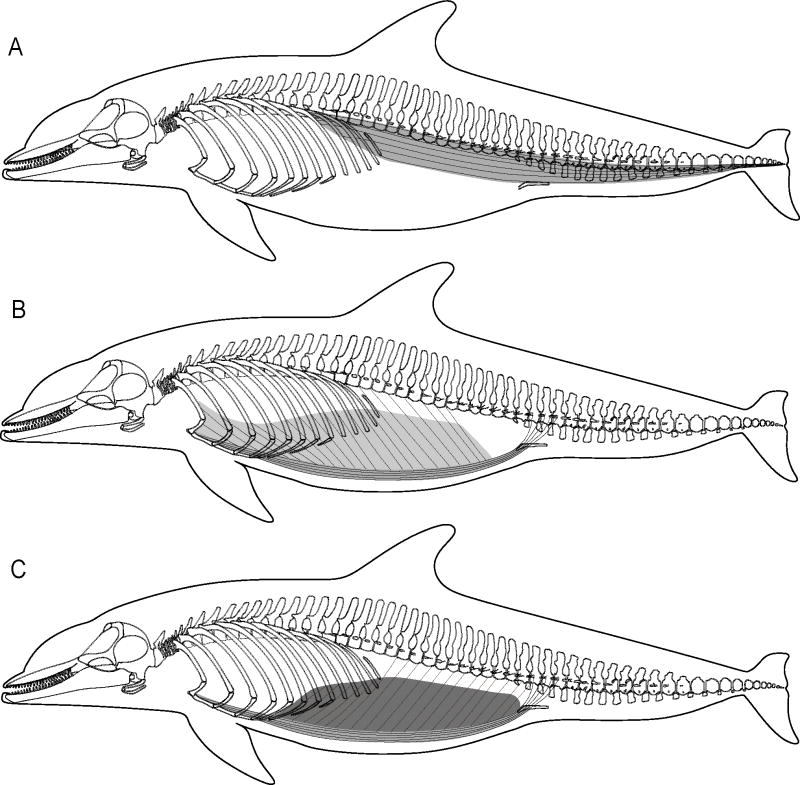
Positions of the vertebral ribs, sternal ribs, and sternum observed during physical manipulations of excised thoracic units in bottlenose dolphins (Tursiops truncatus): (A) frontal view of extreme cranial posture, (B) frontal view of extreme caudal posture, (C) lateral view of extreme cranial posture, and (D) lateral view of extreme caudal posture. Note that the outline of dolphin body does not change and extreme cranial and extreme caudal postures represent inspiration and expiration, respectively.
Origin
Fleshy, off the transverse processes of cervical vertebrae 1-7.
Insertion
Fleshy, broadly along the lateral edge of the first vertebral rib via two distinct portions. The dorsal-most portion inserts on the entire rib neck, spanning from the capitulum to the tuberculum. The ventral portion inserts more ventrally along the entire length of the rib, spanning from the angle to just dorsal to the vertebral rib–sternal rib joint.
Relations
The dorsal scalenus lies deep to the scapula and its intrinsic musculature and superficial to the dorsal portion of the ventral scalenus. At its dorsal margin, it lies just ventral to the longissimus capitis.
Description
The dorsal-most portion is trapezoidal-shaped and its fibers run predominantly longitudinally. The ventral portion is broadly fan-shaped and its fibers run laterally, and caudally to ventro-caudally to insert along the length of first vertebral rib.
Action
Contraction of the dorsal scalenus would draw the first vertebral rib cranially. Physical manipulations of the thorax reveal that this movement causes the first vertebral rib to pivot cranially relative to the thoracic vertebrae, in two body planes (Fig. 5A,C). The first vertebral rib swings cranially along its length and becomes more perpendicularly oriented, relative to the long axis of its body. It also pivots cranio-laterally, which causes it to flare laterally within the transverse body plane. These movements of the first vertebral rib increase thoracic cavity height and width and therefore thoracic cavity volume, which is required for inspiration.
Ventral Scalenus Muscle (Figs. 4B, 5A, C)
Origin
Mostly fleshy, with short tendons at its ventro-lateral margin, off the ventro-lateral border of the exoccipital bone.
Insertion
As long, thin tendons along the medial edge of the first vertebral rib spanning from the angle to just dorsal to the vertebral rib–sternal rib joint; fleshy, onto a narrow region that spans across the vertebral rib–sternal rib joint and just onto the sternal rib.
Relations
At its origin, the ventral scalenus lies caudal to the tympanic bulla and dorsal to the stylohyal articulation with the paroccipital process (Rommel, 1990) of the exoccipital of the skull. The entire muscle lies deep to the scapula and its associated musculature. Its dorsal portion lies deep to the dorsal scalenus. Deep to the caudal end of the ventral scalenus lies the cranial-most portion of the lung.
Description
The ventral scalenus is a broad, fan-shaped muscle, whose fibers radiate from the origin and run dorso-caudally to ventro-caudally along its insertion.
Action
The action of the ventral scalenus depends upon which skeletal element is stabilized because both its origin and insertion are moveable. If the first rib were stabilized, bilateral contraction of the ventral scalenus would draw the skull ventrally relative to the atlanto-occipital joint; unilateral contraction would draw the skull laterally. Alternatively, if the skull were stabilized, contraction of the ventral scalenus would cause movements of the first vertebral ribs similar to that of the dorsal scalenus (Fig. 5A, C). Because its insertion crosses the first vertebral rib–sternal rib joint, contraction of the ventral scalenus would also directly cause the first sternal rib to pivot cranially. These movements would increase thoracic volume.
Sternocephalicus Muscle2 (Figs. 4C, 5A, C, 6)
Fig. 6.
Ventral view of the left and right sternocephalicus, sternohyoid, and the rectus abdominis in bottlenose dolphins (Tursiops truncatus). Thin black lines represent tendons of the sternocephalicus, that insert onto second sternebra; tendons of the rectus abdominis that insert onto caudal vertebrae; and the linea alba.
Origin
As a discrete, cylindrically-shaped tendon off the lateral border of the squamosal, just rostral to the tympano-periotic process.
Insertion
As a fan-shaped tendon along entire lateral edge of the ventral face of manubrium. The tendon also extends caudally to the midline of the second sternebra.
Relations
At its origin, the sternocephalicus lies just ventral and deep to the origins of the brachiocephalicus3 and longissimus capitis. The muscle belly lies just ventral and deep to the thinner brachiocephalicus and just superficial to the ventral scalenus.
Description
The sternocephalicus is a strap-like muscle, with an elliptical cross-section that tapers at both ends. Its fibers run ventro-caudally from its origin.
Action
The action of the sternocephalicus depends upon which skeletal element is stabilized. If the sternum were stabilized, bilateral contraction of the sternocephalicus would draw the skull ventrally relative to the atlanto-occipital joint; unilateral contraction would draw the skull laterally. If the skull were stabilized, bilateral contraction of the sternocephalicus would cause movements similar to the sternohyoid (see full description below) and therefore aid in inspiration (Fig. 5A, C).
Sternohyoid Muscle (Figs. 4C, 5A, C, 6)
Origin
Fleshy, off the ventral surfaces of basihyal and thyrohyals.
Insertion
Fleshy, onto entire ventral face of manubrium; lateral-most fibers blend with those of the sternocephalicus.
Relations
Just deep to blubber.
Description
The sternohyoid is a trapezoidal-shaped muscle that originates broadly and tapers to its more narrow insertion. Its fibers run caudally from its origin and, near its insertion, blend with those of the sternocephalicus.
Action
The action of the sternohyoid depends upon which skeletal element is stabilized. If the sternum were stabilized, the sternohyoid would draw the hyoid apparatus ventro-caudally. This movement has been hypothesized to function during suction feeding by drawing the tongue ventrally and caudally (e.g., Reidenberg and Laitman, 1994; Heyning and Mead, 1996; Werth, 2000). Alternatively, if the hyoid apparatus were stabilized, the sternohyoid would draw the sternum dorso-cranially. Physical manipulations of the thorax reveal that this movement draws the articulated sternal and vertebral ribs cranially, and causes the vertebral ribs to pivot cranially, relative to the thoracic vertebrae, in two body planes (Fig. 5A, C). The vertebral ribs swing cranially along their length and become more perpendicularly oriented, relative to the long axis of the body. These ribs also pivot cranio-laterally, which causes a lateral flaring of the ribs, within the transverse body plane. These combined movements of the sternum and the vertebral and sternal ribs, increase thoracic cavity volume.
Muscles of the Thoracic Unit
External Intercostal Muscle (Fig. 7A)
Fig. 7.
Lateral view of the (A) external intercostals, (B) internal intercostals, and (C) transverse thoracis in bottlenose dolphins (Tursiops truncatus).
Origin
Fleshy, off the entire caudo-lateral border of each vertebral rib.
Insertion
Fleshy, and with thin superficial tendons, onto the cranio-lateral margin of each caudally adjacent vertebral rib.
Relations
The external intercostals lie superficial to the internal intercostals along the length of intercostal space. The ventral portions of the external intercostals lie deep to the external abdominal oblique muscle.
Description
The external intercostals, which connect all serially adjacent vertebral ribs, form the outer muscular layer of the thoracic wall. Between each pair of vertebral ribs, its muscle fibers extend from the tuberculum to the vertebral rib–sternal rib joint. At the dorsal-most portion of the external intercostals (i.e., spanning from the tuberculum to the angle of the rib) muscle fibers run longitudinally from their origin. The muscle fibers spanning from the angle of the vertebral rib to its ventral margin run caudo-ventrally from their origin.
Action
The action of the external intercostals depends upon which skeletal elements are stabilized. The muscle fiber orientation suggests that if the cranial ribs were stabilized, contraction of the external intercostals would draw the caudal ribs dorso-cranially. In contrast, if the caudal ribs were stabilized, contraction of this muscle would draw the cranial ribs ventro-caudally. If these muscles contract synchronously with the internal intercostals (see below) then they could decrease the intercostal space. Physical manipulations suggested the importance of the external intercostals in mechanically linking the ribs together to create a single functional unit.
Internal Intercostal Muscle (Fig. 7B)
Origin
As thin, flat tendons along the entire length of the caudo-medial margin of all vertebral and sternal ribs.
Insertion
Fleshy, onto the cranio-medial margin of each caudally adjacent vertebral and sternal rib.
Relations
The internal intercostals lie deep to the external intercostals along the length of the vertebral ribs, and superficial to the transverse thoracic muscle in the ventral thoracic cavity. The cranio-ventral portion of the internal intercostals (between sternal ribs 1-6) lies deep to the external abdominal obliques. Caudal to rib 6, the ventral portion of the internal intercostals lies deep to both the internal and external abdominal obliques.
Description
The internal intercostals, which connect all serially adjacent vertebral and sternal ribs, form the deep muscular layer of the lateral thoracic wall and the superficial layer of the ventral wall of the thorax. Between each pair of ribs, muscle fibers extend from the angle of the vertebral rib to the distal end of all vertebral and sternal ribs. Muscle fibers run dorso-caudally from their origin. The tendons of origin extend approximately halfway across the intercostal space before the muscle becomes fleshy and inserts onto the caudal adjacent rib.
Action
The action of the internal intercostals depends upon which skeletal elements are stabilized. The muscle fiber orientation suggests that if the cranial ribs were stabilized, contraction of the internal intercostals would draw the caudal ribs ventro-cranially. In contrast, if the caudal ribs were stabilized, contraction of this muscle would draw the cranial ribs dorso-caudally. If these muscles contract synchronously with the external intercostals (see above) then they could decrease the intercostal space. Similar to the external intercostals, physical manipulations suggested the importance of the internal intercostals in mechanically linking the ribs together.
Transverse Thoracis Muscle (Fig. 7C)
Origin
As thin tendons off the entire lateral border of the sternum that span dorsally to a position half-way along the length of each sternal rib before the muscle becomes fleshy. Its cranial-most margin is along the caudal margin of the first sternal rib and its caudal-most margin is along the cranial margin of the sixth sternal rib.
Insertion
Fleshy, onto the caudo-medial surface of vertebral ribs 1-6 dorsal to vertebral rib-sternal rib joint. Between these ribs, the muscle fibers also insert into the deep surface of the internal intercostal muscle.
Relations
Deep to the internal intercostals.
Description
The transverse thoracis is a thin muscle that forms the deepest muscular layer of the ventral thoracic wall. The tendon and muscle fibers run predominantly in the transverse plane, but near the insertion, the fibers turn to run slightly dorso-cranially.
Action
The muscle fiber orientation suggests that if the transverse thoracis contracts it would decrease the angle of the vertebral-sternal rib joints and aid in dorso-ventral compression of the thoracic cavity for expiration.
Muscles of the Lumbo-Pelvic Unit4
Hypaxialis Muscle (Figs. 8A, 5B, D)
Fig. 8.
Lateral view of the (A) hypaxialis, (B) external abdominal obliques and rectus abdominis, and (C) internal abdominal obliques and rectus abdominis in bottlenose dolphins (Tursiops truncatus). Thin black lines represent the tendons of origin of the abdominal oblique muscles and the tendons of the rectus abdominis that insert onto caudal vertebrae.
Origin
Fleshy, off the medial surface of vertebral ribs 9-13 and the ventral surface of vertebral bodies from thoracic vertebra 9 to caudal vertebra 18.
Insertion
As flattened tendons into the subdermal connective tissue sheath (Pabst, 1990) and onto the ventral aspects of vertebrae from mid-lumbar to caudal vertebra 25, including all caudal chevrons.
Relations
The hypaxialis lies deep to ribs 9-13 and ventral to the transverse processes of thoracic 9-13, lumbar, and caudal vertebrae.
Description
The long and robust hypaxialis originates as a dorso-ventrally flattened muscle and increases in thickness along its length. At its cranial-most origin (thoracic vertebrae 9 and 10), the hypaxialis spans from the ventral surface of the vertebral body to the vertebra–vertebral rib joints. Between vertebral ribs 11-12, the hypaxialis widens laterally and spans across the proximal third of the caudal-most vertebral ribs. Its fibers run longitudinally from its origin.
Action
The action of the hypaxialis depends upon which skeletal element is stabilized. If the caudal tailstock were stabilized, contraction of the hypaxialis would draw vertebral ribs 9-13 dorso-caudally (Fig. 5D). Physical manipulations of the thorax revealed that these movements would decrease the volume of the thoracic cavity and, thus, aid expiration. If vertebral ribs 9-13 were stabilized, contraction of the hypaxialis would draw the tailstock ventro-cranially (Pabst, 1990). This movement would compress the abdominal cavity and therefore could assist expiration.
Rectus Abdominis Muscle (Figs. 8B, C, 6, 5D)
Origin
As thin, flat tendons spanning from the distal half of sternal ribs 1 through 4 to their respective sternal rib–sternum joint and onto the lateral border of the sternum. Caudal to sternal rib 4, it becomes fleshy and also originates off the ventral surface of the caudal-most sternebra.
Insertion
Deeply, as a cylindrical tendon onto the cranial margin of the pelvic bone. Superficially as a fan-shaped tendon that crosses the lateral surface of the pelvic bone to join the subdermal connective tissue sheath before ultimately inserting broadly onto the transverse processes of caudal vertebrae 2 through 7/8.
Relations
The rectus abdominis lies just deep to the blubber layer. At its cranial margin, this muscle lies superficial to the sternum, sternal ribs, and internal intercostals. At its cranio-lateral border, the rectus abdominis lies deep to the pectoralis muscle. Along its length, its lateral edge abuts the ventral margins of the external and internal abdominal obliques. Its superficial and deep surfaces are covered respectively by the external and internal laminae of the rectus sheath. These laminae are formed by the ventral aponeurotic tendons of the abdominal muscles (see descriptions below).
Description
The rectus abdominis is a long and robust muscle (with no tendinous intersections), and with right and left bellies separated by the linea alba. Each belly is deep, broad, and tear-drop shaped in cross-section. From its origin, the muscle progressively deepens and broadens to the level of the umbilicus and then slowly tapers towards its insertion. At its terminal insertion, the bellies separate, and each tapers to a cone before becoming tendinous.
Action
The action of the rectus abdominis depends upon which skeletal elements are stabilized. If the sternum and sternal ribs were stabilized, contraction of the rectus abdominis would draw the pelvic bone and caudal vertebrae (via the subdermal sheath) ventro-cranially (Arkowitz and Rommel, 1985; Pabst et al., 1999). This movement would compress the abdominal cavity and therefore could assist expiration. If the caudal vertebrae were stabilized, contraction of the rectus abdominis would draw the sternal ribs and sternum caudally (Fig. 5D). Physical manipulations of the thorax reveal that this movement would also decrease the volume within the thoracic cavity to assist with expiration.
External Abdominal Oblique Muscle (Fig. 8B)
Origin
Serially, as separate slips off the caudo-lateral border of all vertebral ribs, and occasionally off the superficial surface of the external intercostals. Its caudal-most origin is off the last vertebral rib and the subdermal connective tissue sheath just caudal to it. The external abdominal oblique arises broadly off the distal-half of the first vertebral rib via robust, flat tendons and via fleshy slips off the proximal first sternal rib. For vertebral ribs 2-6, slips originate via broad flat tendons off the distal one-third of the rib. From vertebral rib 7 caudally, the muscle arises as fleshy slips off the distal portions of the ribs.
Insertion
As an aponeurotic tendon contributing to the external lamina of the rectus sheath. This aponeurosis extends over the superficial surface of the rectus abdominis and joins with the aponeurosis from the opposite side at the linea alba. These tendons span from the second sternal rib to the level of the caudal-most lumbar vertebrae.
Relations
Across the thoracic wall, the cranio-dorsal most portion of the external abdominal oblique lies superficial to the external intercostals; the cranio-ventral most portions are superficial to the internal intercostals. Caudal to vertebral rib 7, it lies superficial to the internal abdominal oblique muscle (see below). At vertebral ribs 2 through 4, the slips of the external abdominal oblique interdigitate with those of the serratus ventralis.
Description
The external abdominal oblique is an expansive sheet that covers the ventral portion of the thoracic wall and forms the outer layer of the abdominal wall. Its fibers run ventro-caudally from each slip of its origin. Spanning from ribs 1 through 5, a fascial plane exists between the deep surface of this muscle and the superficial surfaces of the sternal ribs and the internal intercostals; this fascial plane appears to permit separate movement of these layers. Caudal to vertebral rib 5, superficial tendons span across the surface of the muscle and the deep surface is tightly adherent to the underlying internal abdominal oblique (i.e., no fascial plane exists). Caudal to vertebral rib 10, the superficial tendon fibers replace muscle fibers for much of the length of the external abdominal oblique from its origin to insertion. The muscle originates as fleshy slips but grades into intervening superficial tendons. Just lateral to the rectus abdominis, the muscle becomes fleshy again before its tendons form the external lamina of the rectus sheath. Thus, much of the external layer of the abdominal wall is formed by aponeurosis rather than muscle belly.
Action
If the rectus sheath were stabilized, then the fiber orientation suggests that this muscle would draw the vertebral and sternal ribs ventro-caudally. The distinct fascial plane that exists between the external abdominal oblique and the surface of sternal ribs 1 though 5 suggest that contraction of the external abdominal oblique muscle would decrease the angle of these vertebral rib–sternal rib joints. Physical manipulations of the thorax reveal that the external abdominal oblique can decrease the angle of the vertebral rib–sternal rib joints and aid in rotating the vertebral and sternal ribs caudally. These movements would decrease thoracic and abdominal cavity volume and assist expiration. The tightly adherent connections between the external and internal obliques caudal to rib 6 suggest that no such independent movement of the underlying skeletal elements likely occurs. Simultaneous contractions of both the external and internal abdominal obliques are hypothesized to aid in compression of the thoracic and abdominal cavities.
Internal Abdominal Oblique Muscle (Fig. 8C)
Origin
Fleshy, off the cranio-lateral surface of the distal one-third of vertebral ribs 7-12/13 and the subdermal connective tissue sheath just caudal to the caudal-most rib.
Insertion
Serially, as long, discrete, flattened tendons into the external and internal laminae of the rectus sheath. These aponeurotic tendons extend over the superficial and deep surface of the rectus abdominis and join with the aponeurosis from the opposite side at the linea alba. These aponeurotic tendons span from the level of the sixth sternal rib to the cranial pole of the pelvic bone and to the level of cranial-most caudal vertebrae.
Relations
Across the thoracic wall, the internal abdominal oblique lies superficial to the external intercostals between the vertebral ribs, and internal intercostals between the caudal-most sternal ribs. Across the abdominal wall, it lies deep to the external abdominal oblique.
Description
The internal abdominal oblique is an expansive sheet that is completely covered, except at its caudal-most margin, by the external abdominal oblique. Its fibers run ventro-cranially from their origins and cross under those of the external abdominal oblique at approximately right angles. Unlike the external abdominal oblique, muscle fibers traverse most of the distance between its origin and insertion. The superficial surface of this muscle is tightly adherent to the deep surface of the external abdominal obliques.
Action
If the rectus sheath is stabilized, the fiber orientation suggests that the cranial portion of this muscle could draw the vertebral and sternal ribs ventro-cranially. Alternatively, if the subdermal connective tissue sheath is stabilized, the caudal portion of this muscle would draw the vertebral ribs dorso-caudally. The tightly adherent connections between the external and internal abdominal obliques caudal to rib 6 suggest that these muscles act simultaneously to form a functional unit. Because of the approximate perpendicular fiber orientations of the external and internal abdominal obliques, simultaneous contractions of these muscles are hypothesized to aid in the compression of thoracic and abdominal cavities. Physical manipulations of the thorax revealed that the internal abdominal obliques could decrease the angle of the vertebral rib–sternal ribs joints of the caudal-most ribs. These combined movements would decrease thoracic and abdominal cavity volume to aid expiration.
Muscle Histochemistry
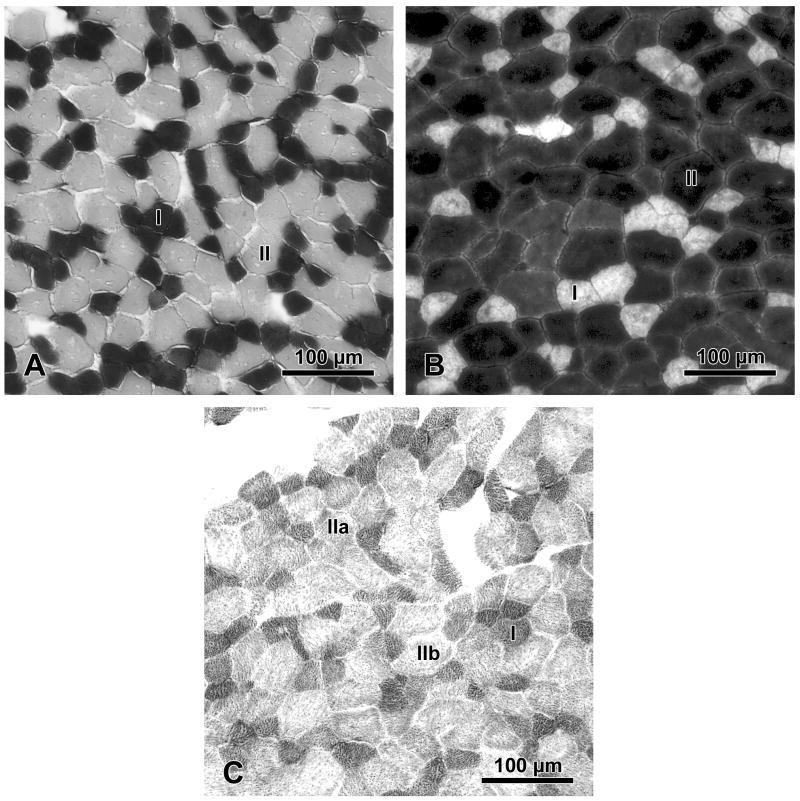
The alkaline myosin ATPase assay demonstrated that the scalenus, sternocephalicus, sternohyoid, and rectus abdominis each possess a predominantly fast fiber-type profile (range 61.2-88.5% by area) (Table 2; Fig. 9A, B). For the sternocephalicus, sternohyoid, and rectus abdominis, the succinic dehydrogenase (SDH) assay demonstrated three fiber-type populations based upon the staining intensity of the fibers: dark staining fibers type I (slow oxidative), intermediate staining fibers type IIa (fast oxidative glycolytic), and light staining fibers type IIb (fast glycolytic) (Fig. 9C). The SDH assay demonstrated that these muscles each possess a predominantly fast-glycolytic profile (Table 3). In contrast, the scalenus muscle fibers could not be classified into the same three distinct fiber-type populations because they displayed highly variable staining intensities. For this reason, the oxidative profile of the scalenus is not reported here.
Table 2.
Mean (± S.E.) percent type II fibers as determined by stereological methods (area) and by direct count, and ratio of type II to type I mean fiber diameters and cross-sectional areas for respiratory muscles of bottlenose dolphins (Tursiops truncatus). Values are reported for alkaline myosin ATPase.
| Muscle | % Type II Fibers by Area | % Type II Fibers by Count | Diameter II:I | Cross-Sectional Area II:I |
|---|---|---|---|---|
| Scalenus | 61.2 ± 2.1 | 57.6 ± 1.6 | 1.1 | 1.3 |
| Sternohyoid | 88.5 ± 1.3 | 78.9 ± 2.0 | 1.4 | 1.8 |
| Sternocephalicus | 72.8 ± 2.2 | 57.5 ± 1.7 | 1.4 | 2.0 |
| Rectus abdominis - cranial | 77.4 ± 3.7 | 63.0 ± 4.9 | 1.5 | 2.1 |
| Rectus abdominis - umbilicus | 75.6 ± 1.9 | 58.2 ± 3.5 | 1.5 | 2.3 |
| Rectus abdominis - caudal | 74.3 ± 2.0 | 60.8 ± 2.2 | 1.3 | 1.7 |
Fig. 9.
Micrographs of representative cross-sections of the rectus abdominis muscle of the bottlenose dolphin (Tursiops truncatus) after histochemical staining: (A) myosin ATPase in acidic pre-incubation solution, (B) myosin ATPase in alkaline pre-incubation solution, and (C) succinic dehydrogenase. Muscle fiber-types are categorized as: I, slow-twitch; II, fast-twitch; IIa, fast-twitch oxidative-glycolytic; IIb, fast-twitch glycolytic.
Table 3.
Mean (± S.E.) percent fiber-types determined by stereological methods (area) and direct count, using the succinic dehydrogenase assay for respiratory muscles of bottlenose dolphins (Tursiops truncatus).
| % Type I | % Type IIa | % Type IIb | ||||
|---|---|---|---|---|---|---|
| Muscle | Area | Count | Area | Count | Area | Count |
| Sternohyoid | 28.7 ± 3.0 | 34.1 ± 1.8 | 19.9 ± 2.0 | 20.7 ± 2.0 | 51.4 ± 2.0 | 45.2 ± 1.3 |
| Sternocephalicus | 30.8 ± 4.7 | 37.5 ± 3.9 | 17.6 ± 2.8 | 23.0 ± 2.8 | 51.6 ± 2.3 | 39.5 ± 1.8 |
| Rectus abdominis - cranial | 29.2 ± 1.3 | 35.1 ± 1.3 | 16.1 ± 1.8 | 19.7 ± 1.8 | 54.7 ± 1.5 | 45.2 ± 1.5 |
| Rectus abdominis - umbilicus | 24.3 ± 4.4 | 32.5 ± 4.4 | 16.7 ± 2.2 | 19.7 ± 2.2 | 59.1 ± 2.9 | 47.8 ± 2.9 |
| Rectus abdominis - caudal | 28.7 ± 3.3 | 40.2 ± 3.3 | 13.2 ± 2.1 | 13.2 ± 2.1 | 58.1 ± 3.7 | 46.6 ± 3.7 |
For the myosin ATPase assays, the percent fast-twitch (type II) fibers resulting from the stereological approach (i.e., Mertz-curvilinear test system) was always higher (by 4-17%) than that resulting from direct counts (Table 2). Similarly, for the succinic dehydrogenase assay, the percent type IIb fibers resulting from the stereological approach was higher (by 6-12%) than that resulting from direct counts (Table 3). The Mertz-curvilinear test system was a more accurate method than that of the direct fiber counts because it accounts for the differences in size between different fiber-types (Bozzola and Russell, 1999; Russ and Dehoff, 2000). Type II fibers were approximately 1.5 times larger in diameter and two times larger in cross-sectional area than type I fibers (Table 2).
For the sternocephalicus, sternohyoid and rectus abdominis, the myosin ATPase assay using the alkaline pre-incubation medium resulted in a slightly faster muscle fiber profile (by 1-8%) than the acidic pre-incubation medium. For the scalenus muscle, the alkaline pre-incubation medium resulted in a slightly slower muscle fiber profile (by 6%) than the acidic pre-incubation medium (Table 4). Samples taken at multiple positions across the muscle's cross-sectional face yielded fiber-type profiles that were similar (differences ranged between 1-7%) to those at the mid-belly (Table 5).
Table 4.
Mean (± S.E.) percent type II fibers as determined by stereological methods using alkaline and acidic pre-incubation media for respiratory muscles of bottlenose dolphins (Tursiops truncatus).
| % Type II Fibers by Area | ||
|---|---|---|
| Muscle | Alkaline | Acid |
| Scalenus | 61.2 ± 2.1 | 67.4 ± 1.0 |
| Sternohyoid | 88.5 ± 1.3 | 87.5 ± 2.0 |
| Sternocephalicus | 72.8 ± 2.2 | 68.6 ± 2.4 |
| Rectus abdominis - cranial | 77.4 ± 3.7 | 69.5 ± 4.7 |
| Rectus abdominis - umbilicus | 75.6 ± 1.9 | 69.2 ± 3.5 |
| Rectus abdominis - caudal | 74.3 ± 2.0 | 66.2 ± 2.4 |
Table 5.
Mean (± S.E.) percent type II fibers as determined by stereological methods (area) for variation across the respiratory muscle's cross-sectional face of bottlenose dolphins (Tursiops truncatus). For rectus abdominis samples, peripheral site A was a lateral position, and peripheral site B was a medial position. Values are reported for alkaline myosin ATPase.
| % Type II Fibers by Area | |||
|---|---|---|---|
| Sample Site | |||
| Muscle | Peripheral A | Central | Peripheral B |
| Sternohyoid | 87.7 ± 0.6 | 88.5 ± 1.3 | 90.7 ± 0.1 |
| Sternocephalicus | 73.5 ± 0.1 | 72.8 ± 2.2 | 70.3 ± 0.1 |
| Rectus abdominis - cranial | 78.3 ± 0.1 | 77.4 ± 3.7 | 80.4 ± 1.0 |
| Rectus abdominis - umbilicus | 82.7 ± 0.8 | 75.6 ± 2.0 | 77.7 ± 0.8 |
| Rectus abdominis - caudal | 73.7 ± 0.8 | 74.3 ± 2.0 | 75.5 ± 1.0 |
Discussion
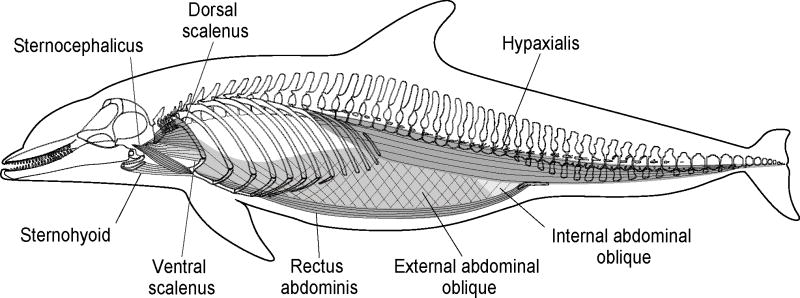
Many terrestrial mammals mechanically couple their locomotion and respiration (i.e., ventilation), which occurs in part because locomotor muscles within the cranio-cervical and lumbo-pelvic units act upon the thoracic unit to facilitate ventilation (Bramble and Carrier, 1983; Bramble, 1989; Bramble and Jenkins, 1993). Using Bramble's (1989) biomechanical model of the integration of locomotor and ventilatory systems in galloping terrestrial mammals, this study investigated the gross morphology and histochemistry of muscles within the cranio-cervical and lumbo-pelvic units in bottlenose dolphins (Tursiops truncatus) (Fig. 10). The hypotheses that these muscles (1) act upon the thorax to change its internal volume, and thus pressure, to assist with ventilation and (2) are composed of predominantly fast-twitch fibers, to support the dolphin's rapid ventilation, were tested.
Fig. 10.
Lateral view of the respiratory muscles within the cranio-cervical and lumbo-pelvic units in bottlenose dolphins (Tursiops truncatus).
Gross Morphology and Physical Manipulations
Physical manipulations revealed that the bottlenose dolphin thorax can undergo dramatic shape changes (Fig. 5). The enhanced flexibility of the dolphin thorax has been attributed to the presence of mobile joints between the vertebrae and vertebral ribs and the vertebral and sternal ribs (Rommel, 1990; Rommel and Reynolds, 2002). This study demonstrates that the sternal rib-sternum joints are also mobile, which likely further enhances thoracic cavity shape change.
The muscles investigated within the cranio-cervical unit of the bottlenose dolphin are in a position to increase thoracic cavity volume, which is required for inspiration (Figs. 5A, C, 10). Physical manipulations revealed that the sternocephalicus and sternohyoid can draw the sternum dorso-cranially, causing the articulated sternal and vertebral ribs to swing cranially and become more perpendicular relative to the long axis of the body. Contractions of these muscles, and of the scalenus, may pivot the first vertebral and sternal rib cranio-laterally, which also causes lateral flaring of the ribs. Therefore, muscles within the cranio-cervical unit can likely increase thoracic cavity volume by expanding both its dorso-ventral height and lateral width.
The muscles investigated within the lumbo-pelvic unit of bottlenose dolphins are in a position to decrease thoracic cavity volume, which is required for expiration (Figs. 5B, D, 10). Physical manipulations revealed that the hypaxialis draws the ribs caudally, decreasing the dorso-ventral height, and therefore the volume, of the thoracic cavity. The rectus abdominis draws the sternum, sternal ribs, and the vertebral ribs – via their bony articulations with the sternal ribs – caudally, also decreasing thoracic cavity dorso-ventral height and volume.
Thus, the results of this study support the hypothesis that muscles within the cranio-cervical and lumbo-pelvic units of the bottlenose dolphin can facilitate inspiration and expiration, respectively. The explosive ventilation of a bottlenose dolphin occurs so rapidly, though, that it is unlikely that a complete locomotor sequence can be mechanically coupled to this event. The tail-beat frequency of a dolphin swimming at approximately 2 m/s, a typical swimming speed for this species (Williams, 1999), is approximately 1 Hz (Videler and Kamermans, 1985). A full locomotor sequence (downstroke and upstroke) therefore requires at least 1s, but a ventilatory event can be completed in only 0.3s (Irving et al., 1941; Ridgway et al., 1969; Kooyman and Cornell, 1981; reviewed in Wartzok, 2002). Therefore, unlike galloping mammals with a 1:1 ratio of breaths per stride, bottlenose dolphins do not require a full locomotor sequence to complete their ventilatory event.
A typical bottlenose dolphin locomotor sequence entails a series of downstrokes and upstrokes (Arkowitz and Rommel, 1985; Pabst, 1990; Fish, 1993; Pabst, 1993; Pabst et al., 1999). The downstroke sequence has two portions, (1) the head flexes ventrally via muscles within the cranio-cervical unit (Reidenberg, 2006) and (2) the tail-stock flexes ventrally via the muscles within the lumbo-pelvic unit. The upstroke sequence also has two portions, (1) the head flexes dorsally via cranial epaxial muscles and (2) the tail-stock flexes dorsally via caudal epaxial muscles.
The locomotor muscles within the lumbo-pelvic unit that power the downstroke (Arkowitz and Rommel, 1985; Pabst, 1990; Pabst et al., 1999) also function to decrease thoracic and abdominal cavity volumes (Figs. 5B, D, 10). Thus, expiration is likely mechanically coupled to the downstroke. The morphological results of this study suggest that the ensuing rapid inspiration likely occurs due to simultaneous contractions of the cranial epaxial muscles (the muscles that initiate the upstroke) and the cranio-cervical muscles. The muscles in the cranio-cervical unit can either (1) draw the sternum and ribs dorso-cranially to increase thoracic cavity volume required for inspiration or (2) ventrally flex the head to initiate the downstroke. To create the movements of the thoracic cavity required for inspiration, the dolphin head must be stabilized. The cranial epaxial muscles could function to stabilize the head so that simultaneous contractions of the cranio-cervical unit muscles could act to increase thoracic cavity volume for inspiration. Therefore, it is hypothesized that in a bottlenose dolphin, a ventilatory event begins during the terminal portion of the downstroke via lumbo-pelvic muscles (expiration), and the subsequent initial portion of the upstroke, via cranial epaxial muscles. This typical locomotor sequence has superimposed upon it contractions of the cranio-cervical unit (inspiration) simultaneously to those of the cranial epaxial muscles (head stabilization).
In the only published kinematic analysis of surfacing behavior in any cetacean, a wild harbor porpoise (Phocoena phocoena) was observed to approach the surface at an oblique angle and simultaneously ventrally flex its caudal tailstock and dorsally flex its head prior to expiration (Smith et al., 1976). Once the head was above the surface of the water, it was immediately flexed ventrally and inspiration occurred (Smith et al., 1976). Although these rare kinematic data lend partial support for the sequence of muscle activity hypothesized above, they are insufficient to address the ventilatory mechanisms used by cetaceans. Kinematic studies of swimming and respiring bottlenose dolphins are required to provide insight into the distinct portions of their locomotor sequence that may be mechanically coupled to their ventilatory event.
When swimming, bottlenose dolphins undergo dorso-ventral flexions of the body, (similar to galloping terrestrial mammals) which must affect thoracic cavity volume and pressure, yet they do so on an extended breath-hold. The mechanism that permits bottlenose dolphins to selectively couple and uncouple their locomotion and ventilation remains unknown. However, the results of this study suggest that bottlenose dolphins must be able to differentially stabilize their thoracic unit during swimming versus surfacing. While swimming on a breath-hold, the thoracic unit must be relatively more stable, or immoveable, to resist shape changes imposed upon it by contractions of muscles within the cranio-cervical and lumbo-pelvic units. In contrast, when a dolphin surfaces for a ventilatory event, the thoracic unit must be dynamic to undergo shape changes required for ventilation.
Results from the physical manipulations of the thoracic unit support the hypothesis that the variable stability of the thoracic cavity can be achieved by differential actions of the intercostal muscles. When swimming, the intercostal muscles may act as antagonists to the muscles within the cranio-cervical and lumbo-pelvic units. For example, if the intercostal muscles contract to draw the ribs caudally, the thoracic unit would resist deformation imposed upon it by muscles in the cranio-cervical unit. In contrast, when surfacing, the intercostal muscles may act as synergists to the muscles within the cranio-cervical and lumbo-pelvic units. That is, if the intercostal muscles contract to draw the ribs cranially, they could facilitate the change in thoracic cavity shape caused by the cranio-cervical muscles. Consequently, the action of the intercostal muscles could be three-fold: (1) aid in cranial movement of the thorax during contractions of the cranio-cervical muscles, to assist inspiration, (2) aid in caudal movement of the thorax during contractions of the lumbo-pelvic muscles, to assist expiration, and (3) enhance the stability of the whole thoracic unit, when the dolphin is swimming on a breath-hold.
There exist experimental data from terrestrial mammals that support the hypothesis that the intercostal muscles may function differently during ventilation and locomotion (Carrier, 1996, Deban and Carrier, 2002). In trotting dogs, when locomotion and ventilation were mechanically coupled, the intercostal muscles were active during inspiration and expiration (Carrier, 1996). In contrast, during brief episodes of uncoupled locomotion, intercostal muscle activity was coupled to footfall patterns, not to ventilatory events. Thus, during these periods of decoupled locomotion and ventilation, the intercostal muscles functioned primarily as locomotor muscles that stabilized the thoracic cavity (Carrier, 1996). These comparative results lend support to the hypothesis that the intercostal muscles of bottlenose dolphins may function differently during swimming and breathing
The bottlenose dolphin's diaphragm muscle may also contribute to its ability to selectively uncouple locomotion and ventilation. Dearolf (2002) hypothesized that connections between the dolphin diaphragm and the robust ventral connective tissue sheath on the ventral surface of the hypaxialis muscle (Pabst, 1990) prevent the diaphragm from being displaced too far cranially. By limiting the cranial movement of the diaphragm, the cranial movement of the abdominal organs, which likely would occur during ventral flexions of the body, would also be prevented (Dearolf, 2002). Thus, the diaphragm may play an active postural role during locomotion. Dearolf (2002) also hypothesized that during a ventilatory event, the bottlenose dolphin diaphragm may function as an elastic strain energy storage system. The cranial face of the diaphragm is covered by long tendons that if stretched during expiration, may be able to recoil elastically to assist with the inspiratory phase. Future studies that investigate the mechanical properties of the tendons within diaphragm of bottlenose dolphins may provide insight into its potential role as an inspiratory spring (Dearolf, 2002).
No study to date has investigated the activity of the cranio-cervical or lumbo-pelvic muscles in any cetacean to determine which muscles may be active during ventilation. Kinematic and electromyographic studies, coupled with studies that measure intrathoracic pressure pulses in swimming and respiring dolphins, would be of value to address these hypotheses.
Muscle Histochemistry
The myosin ATPase assay demonstrated that all muscles investigated within the cranio-cervical and lumbo-pelvic units of bottlenose dolphins were composed predominantly of fast-twitch fibers (Table 2). These fiber-type profiles suggest that each of these muscles is poised for the rapid contractions likely required for their explosive ventilatory event. Interestingly, these dolphin fiber-type profiles are also within the range reported for several terrestrial mammal species that differ in size and locomotor style (Table 6). This shared pattern in fiber-type profiles is likely influenced by a number of factors.
Table 6.
Mean percent type II fibers of selected muscles in several terrestrial mammal species. Methods used to quantify fiber-types are noted.
| % Type II Fibers | ||||||
|---|---|---|---|---|---|---|
| Animal | Scalenes | Sternohyoid | Sternomastoid | Rectus Abdominis | Quantification Method | Reference |
| Dog | ||||||
| (Canis familiaris) | 62 | Count | Bracher et al., 1997 | |||
| Dog | ||||||
| (Canis familiaris) | 69 | 66 | 79 | 52 | Count | Armstrong et al., 1982 |
| Cat | ||||||
| (Felis catus) | 84.6 | Unreported | Dick and van Lunteren, 1990 | |||
| Cat | ||||||
| (Felis catus) | 91 | Count | Richmond et al., 1999 | |||
| Hamster | ||||||
| (Mesocricetus auratus) | 91 | 94 | Unreported | Mattson et al., 2002 | ||
| Rat | ||||||
| (Rattus norvegicus) | 95 | Count | Bracher et al., 1997 | |||
| Rat | ||||||
| (Rattus norvegicus) | 80 | Count | Hijikata et al., 1992 | |||
| Tufted Capuchun Monkey | ||||||
| (Cebus apella) | 73 | Unreported | Simionato et al., 2006 | |||
| Rhesus Monkey | ||||||
| (Macaca mulatta) | 71 | 77 | Count | Richmond et al., 2001 | ||
| Bull | ||||||
| (Bos taurus) | 68 | Area | Totland and Kryvi, 1991 | |||
For example, the fast muscle fiber profiles observed in smaller species may support their relatively high ventilatory frequencies (Young et al., 1992b). These muscles also play functional roles in multiple body systems. In several families of odontocetes (Kogiidae, Physeteridae, and Ziphiidae), the sternohyoid is hypothesized to be the main contributor to suction feeding (Reidenberg and Laitman, 1994; Heyning and Mead, 1996) and would likely have a fast fiber-type profile to support this rapid kinematic event (Werth, 2000; Bloodworth and Marshall, 2005). However, bottlenose dolphins are primarily ram-based feeders (Bloodworth and Marshall, 2005). This observation suggests that the fast fiber-type profile of the sternohyoid in bottlenose dolphins may more likely contribute to their rapid ventilation than to their feeding strategy.
In addition, muscles located within the cranio-cervical and lumbo-pelvic units have traditionally been categorized as locomotor muscles. Fast fiber-type profiles of these muscles may reflect their locomotor as well as ventilatory roles. The dual roles of muscles within the cranio-cervical and lumbo-pelvic units suggest that their profiles should be similar to those of other locomotor muscles. In bottlenose dolphins, two epaxial locomotor muscles, the dorsal intertransversarius and the extensor caudal lateralis, are also composed predominantly of fast-twitch fibers (73.3 and 74.1% by area, respectively) (Dearolf et al., 2000; Etnier et al., 2004). Thus, dolphin locomotor and ventilatory muscles have similar fiber-type profiles.
Conclusion
This study revealed that muscles located within the bottlenose dolphin's cranio-cervical unit could create shape changes of the thoracic unit that facilitate inspiration and that muscles within the lumbo-pelvic unit could create shape changes of the thoracic unit that facilitate expiration. The fast fiber-type profile of these muscles suggests they are poised to support the dolphin's explosive ventilatory cycle. This study also suggests that the typical dolphin locomotor sequence is likely altered during their brief ventilatory event. Expiration is hypothesized to be mechanically coupled to the terminal phase of the downstroke, because locomotor muscles within the lumbo-pelvic unit power the downstroke and function to decrease thoracic and abdominal cavity volumes. The ensuing rapid inspiration is hypothesized to occur due to simultaneous contractions of the cranial epaxial muscles, which initiate the upstroke, and the cranio-cervical muscles, which function to increase the volume of the thoracic cavity. It is further hypothesized that the intercostal muscles provide variable stability to the thorax during locomotion versus ventilation.
Acknowledgments
We thank the Virginia Aquarium Stranding Response Program (especially Susan Barco), the National Marine Fisheries Service Beaufort Laboratory (especially Gretchen Lovewell and Bruce Ferrier), and the University of North Carolina Wilmington Marine Mammal Stranding Program for access to specimens. Special thanks to the VAB LAB, Mark Gay, UNCW Marine Mammal Stranding Program volunteers, Evan Brickell, Erin Guy, Brandon Thurow, Eric Traister, and Kara Weigand. This manuscript was improved by comments from Drs. Heather Koopman, Steve Kinsey, Richard Dillaman, and Timothy Ballard. This work was supported with funding from NOAA Prescott Stranding Grants #NA03NMF4390411 and #NA05NMF4391181 (UNCW), Arkansas IDeA Networks of Biomedical Research Excellence (INBRE) NIH Grant #P20 RR-16460, Program of the National Center for Research Resources, Hendrix College Odyssey Program, and Arkansas Department of Higher Education SURF Program (Hendrix College). Specimens used in this study were collected under a National Marine Fisheries Service Letter of Authorization and UNCW IACUC permits (#2003-13 and #2006-015).
Footnotes
Bottlenose dolphins possess only two scalenus muscles, named here as dorsal and ventral (sensu Howell, 1928). The brachial plexus emerges between the dorsal and ventral scalenus.
This muscle is identified as the sternomastoid by Howell (1928, 1931) and Schulte and Smith (1918).
This muscle is identified as the mastohumeralis by Howell (1928, 1931) and Schulte and Smith (1918).
Unlike terrestrial mammals, in bottlenose dolphins the muscles described below insert not only upon the reduced pelvic element (Rommel, 1990) but also upon the caudal vertebrae. To maintain consistency with Bramble (1989), the term lumbo-pelvic unit is maintained.
Literature Cited
- Alexander RM. Breathing while trotting. Science. 1993;262:196–197. doi: 10.1126/science.8211137. [DOI] [PubMed] [Google Scholar]
- Arkowitz R, Rommel S. Force and bending moment of the caudal muscles in the shortfin pilot whale. Mar Mam Sci. 1985;1:203–209. [Google Scholar]
- Armstrong R, Saubert C, Seeherman H, Taylor C. Distribution of fiber types in locomotory muscles of dogs. Am J Anat. 1982;163:87–98. doi: 10.1002/aja.1001630107. [DOI] [PubMed] [Google Scholar]
- Bello M, Roy R, Martin T, Goforth H, Edgerton V. Axial musculature in the dolphin (Tursiops truncatus): some architectural and histochemical characteristics. Mar Mam Sci. 1985;1:324–336. [Google Scholar]
- Bloodworth B, Marshall CD. Feeding kinematics of Kogia and Tursiops (Odontoceti: Cetacea): characterization of suction and ram feeding. J Exp Biol. 2005;208:3721–3730. doi: 10.1242/jeb.01807. [DOI] [PubMed] [Google Scholar]
- Bozzola JJ, Russell LD. Electron microscopy: principles and techniques for biologists. Sudbury, MA: Jones and Bartlett Publishers; 1999. p. 670. [Google Scholar]
- Bracher A, Coleman R, Schnall R, Oliven A. Histochemical properties of upper airway muscles: comparison of dilator and nondilator muscles. Eur Respir J. 1997;10:990–993. doi: 10.1183/09031936.97.10050990. [DOI] [PubMed] [Google Scholar]
- Brainerd EL. New perspectives on the evolution of lung ventilation mechanisms in vertebrates. Exp Biol Online. 1999;4:1–28. [Google Scholar]
- Bramble DM. Axial-appendicular dynamics and the integration of breathing and gait in mammals. Amer Zool. 1989;29:171–186. [Google Scholar]
- Bramble DM, Carrier DR. Running and breathing in mammals. Science. 1983;219:251–256. doi: 10.1126/science.6849136. [DOI] [PubMed] [Google Scholar]
- Bramble DM, Jenkins F. Mammalian locomotor-respiratory integration: implications for diaphragmatic and pulmonary design. Science. 1993;262:235–240. doi: 10.1126/science.8211141. [DOI] [PubMed] [Google Scholar]
- Brooke M, Kaiser K. Muscle fiber types: how many and what kind? Arch Neurol. 1970;23:369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Carrier DR. The evolution of locomotor stamina in tetrapods: circumventing a mechanical constraint. Paleobiology. 1987;13:326–341. [Google Scholar]
- Carrier DR. Function of the intercostal muscles in trotting dogs: ventilation or locomotion? J Exp Biol. 1996;199:1455–1465. doi: 10.1242/jeb.199.7.1455. [DOI] [PubMed] [Google Scholar]
- Carte A, Macalister A. On the anatomy of Balaenoptera rostrata. Philos Trans R Soc London B. 1868;158:201–261. [Google Scholar]
- Costa DP, Williams TM. Marine mammal energetics. In: Reynolds JE, Rommel SA, editors. Biology of marine mammals. Washington: Smithsonian Institution Press; 1999. pp. 176–217. [Google Scholar]
- Dearolf JL. PhD thesis. Ithaca: Cornell University; 2002. Morphology and development of the diaphragm of bottlenose dolphins (Tursiops truncatus) [Google Scholar]
- Dearolf JL. Diaphragm muscle development in bottlenose dolphins Tursiops truncatus. J Morphol. 2003;256:79–88. doi: 10.1002/jmor.10077. [DOI] [PubMed] [Google Scholar]
- Dearolf JL, McLellan WA, Dillaman RM, Frierson DJ, Pabst D. Precocial development of axial locomotor muscle in bottlenose dolphins Tursiops truncatus. J Morphol. 2000;244:203–215. doi: 10.1002/(SICI)1097-4687(200006)244:3<203::AID-JMOR5>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Deban SM, Carrier DR. Hypaxial muscle activity during running and breathing in dogs. J Exp Biol. 2002;205:1953–1967. doi: 10.1242/jeb.205.13.1953. [DOI] [PubMed] [Google Scholar]
- De Troyer A. Inspiratory elevation of the ribs in the dog: primary role of the parasternals. J Appl Physiol. 1991;70:1447–1455. doi: 10.1152/jappl.1991.70.4.1447. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Sampson M, Sigrist S, Kelly S. How the abdominal muscles act on the rib cage. J Appl Physiol. 1983;54:465–469. doi: 10.1152/jappl.1983.54.2.465. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Kelly S. Action of neck accessory muscles on rib cage in dogs. J Appl Physiol. 1984;56:326–332. doi: 10.1152/jappl.1984.56.2.326. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Kelly S, Macklem PT, Zin WA. Mechanics of intercostal space and actions of external and internal intercostal muscles. J Clin Invest. 1985;75:850–857. doi: 10.1172/JCI111782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A, Ninane V. Triangularis sterni: a primary muscle of breathing in the dog. J Appl Physiol. 1986;60:14–21. doi: 10.1152/jappl.1986.60.1.14. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Ninane V. Effect of posture on expiratory muscle use during breathing in the dog. Respir Physiol. 1987;67:311–322. doi: 10.1016/0034-5687(87)90061-2. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Gilmartin JJ, Ninane V. Abdominal muscle use during breathing in unanesthetized dogs. J Appl Physiol. 1989;66:20–27. doi: 10.1152/jappl.1989.66.1.20. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Cappello M, Brichant JF. Do canine scalene and sternomastoid muscles play a role in breathing? J Appl Physiol. 1994;76:242–252. doi: 10.1152/jappl.1994.76.1.242. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Kirkwood P, Wilson T. Respiratory action of the intercostal muscles. Physiol Rev. 2005;85:717–756. doi: 10.1152/physrev.00007.2004. [DOI] [PubMed] [Google Scholar]
- Dick TE, van Lunteren E. Fiber subtype distribution of pharyngeal dilator muscles and diaphragm in the cat. J Appl Physiol. 1990;68:2237–2240. doi: 10.1152/jappl.1990.68.5.2237. [DOI] [PubMed] [Google Scholar]
- Duron B. Postural and ventilatory functions of intercostal muscles. Acta Neurobiol. 1973;33:355–380. [PubMed] [Google Scholar]
- Etnier SA, Dearolf JL, McLellan WA, Pabst DA. Postural role of lateral axial muscles in developing bottlenose dolphins (Tursiops truncatus) Proc R Soc London B Biol Sci. 2004;271:909–918. doi: 10.1098/rspb.2004.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas GA, Schroeder MA. Mechanical role of expiratory muscles during breathing in prone anesthetized dogs. J Appl Physiol. 1990;69:2137–2142. doi: 10.1152/jappl.1990.69.6.2137. [DOI] [PubMed] [Google Scholar]
- Fish FE. Power output and propulsive efficiency of swimming bottlenose dolphins (Tursiops truncatus) J Exp Biol. 1993;185:179–193. [Google Scholar]
- Fournier M, Lewis MI. Functional role and structure of the scalenus: an accessory inspiratory muscle in hamster. J Appl Physiol. 1996;81:2436–2444. doi: 10.1152/jappl.1996.81.6.2436. [DOI] [PubMed] [Google Scholar]
- Gauthier GF. Skeletal muscle fiber types. In: Engel A, Banker B, editors. Myology. New York: McGraw-Hill; 1986. pp. 255–283. [Google Scholar]
- Geraci JR, Lounsbury VJ. Marine mammals ashore: A field guide for strandings. Baltimore, MD: National Aquarium in Baltimore; 2005. p. 371. [Google Scholar]
- Gilmartin JJ, Ninane V, De Troyer A. Abdominal muscle use during breathing in the anesthetized dog. Respir Physiol. 1987;70:159–171. doi: 10.1016/0034-5687(87)90047-8. [DOI] [PubMed] [Google Scholar]
- Hermanson J, Evans H. The muscular system Miller's anatomy of the dog. Philadelphia: W.B. Saunders; 1993. pp. 258–384. [Google Scholar]
- Heyning JE, Mead JG. Suction feeding in beaked whales: morphological and observational evidence. Contributions to Science. 1996;464:1–12. [Google Scholar]
- Hijikata T, Wakisaka H, Yohro T. Architectural design, fiber-type composition, and innervation of the rat rectus abdominis muscle. Anat Rec. 1992;234:500–512. doi: 10.1002/ar.1092340406. [DOI] [PubMed] [Google Scholar]
- Hörnicke H, Meixner R, Pollmann U. Respiration in exercising horses. In: Snow D, Persson S, Rose R, editors. Equine exercise physiology. Cambridge: Granta Editions; 1982. pp. 7–16. [Google Scholar]
- Howard CV, Reed MG. Unbiased stereology: Three dimensional measurement in microscopy. New York: Springer-Verlag; 1998. p. 246. [Google Scholar]
- Howell AB. Contribution to the anatomy of the chinese finless porpoise, Neomeris phocaenoides. Proceedings US National Museum. 1927;70:1–43. [Google Scholar]
- Howell AB. Myology of the narwhal (Monodon monoceros) Am J Anat. 1930;46:187–215. [Google Scholar]
- Hui CA. Thoracic collapse as affected by the retia thoracica in the dolphin. Respir Physiol. 1975;25:63–70. doi: 10.1016/0034-5687(75)90051-1. [DOI] [PubMed] [Google Scholar]
- Irving L, Scholander P, Grinnell S. The respiration of the porpoise, Tursiops truncatus. J Cell Comp Physiol. 1941;17:145–168. [Google Scholar]
- Kooyman G, Cornell L. Flow properties of expiration and inspiration in a trained bottle-nosed porpoise. Physiol Zool. 1981;54:55–61. [Google Scholar]
- Legrand A, Ninane V, De Troyer A. Mechanical advantage of sternomastoid and scalene muscles in dogs. J Appl Physiol. 1997;82:1517–1522. doi: 10.1152/jappl.1997.82.5.1517. [DOI] [PubMed] [Google Scholar]
- Mattson J, Miller T, Poole D, Delp M. Fiber composition and oxidative capacity of hamster skeletal muscle. J Histochem Cytochem. 2002;50:1685–1692. doi: 10.1177/002215540205001214. [DOI] [PubMed] [Google Scholar]
- McLellan WA, Koopman HN, Rommel SA, Read AJ, Potter CW, Nicolas JR, Westgate AJ, Pabst DA. Ontogenetic allometry and body composition of harbour porpoises (Phocoena phocoena, L.) from the western North Atlantic. J Zool. 2002;257:457–471. [Google Scholar]
- Murie J. On Risso's grampus, G. rissoanus (Desm.) J Anat Physiol. 1871;5:118–138. [PMC free article] [PubMed] [Google Scholar]
- Nickel R, Schummer A, Seiferle E, Wilkens H, Wille K, Frewein J. The anatomy of the domestic animals. Vol. 1. New York: Springer-Verlag; 1986. p. 449. [Google Scholar]
- Nomina Anatomica Veterinaria. Ithaca: The World Assn of Veterinary Anatomists. (4th) 1994:198. [Google Scholar]
- Pabst DA. Axial muscles and connective tissues of the bottlenose dolphin. In: Leatherwood S, Reeves RR, editors. The Bottlenose dolphin. San Diego: Academic Press; 1990. pp. 51–67. [Google Scholar]
- Pabst DA. Intramuscular morphology and tendon geometry of the epaxial swimming muscles of dolphins. J Zool. 1993;230:159–176. [Google Scholar]
- Pabst DA, Rommel S, McLellan WA. The functional morphology of marine mammals. In: Reynolds J, Rommel S, editors. Biology of marine mammals. Washington: Smithsonian Institution Press; 1999. pp. 35–37. [Google Scholar]
- Raper AJ, Thompson WT, Shapiro W, Patterson JL. Scalene and sternomastoid muscle function. J Appl Physiol. 1966;21:497–502. doi: 10.1152/jappl.1966.21.2.497. [DOI] [PubMed] [Google Scholar]
- Reidenberg JS. Motor mouth moby: is the hyoid a locomotor bone in whales, dolphins, and porpoises? FASEB J Experimental Biology Conference: American Association of Anatomist. 2006:A845–d. [Google Scholar]
- Reidenberg JS, Laitman JT. Anatomy of the hyoid apparatus in odontoceti (toothed whales): specializations of their skeleton and musculature compared with those of terrestrial mammals. Anat Rec. 1994;240:598–624. doi: 10.1002/ar.1092400417. [DOI] [PubMed] [Google Scholar]
- Richmond F, Liinamaa T, Keane J, Thomson D. Morphometry, histochemistry, and innervation of cervical shoulder muscles in the cat. J Morphol. 1999;239:255–269. doi: 10.1002/(SICI)1097-4687(199903)239:3<255::AID-JMOR3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Richmond F, Singh K, Corneil B. Neck muscles in the rhesus monkey. I. Muscle morphometry and histochemistry. J Neurophysiol. 2001;86:1717–1728. doi: 10.1152/jn.2001.86.4.1717. [DOI] [PubMed] [Google Scholar]
- Ridgway SH, Scronce BL, Kanwisher J. Respiration and deep diving in the bottlenose porpoise. Science. 1969;166:1651–1654. doi: 10.1126/science.166.3913.1651. [DOI] [PubMed] [Google Scholar]
- Rommel S. Osteology of the bottlenose dolphin. In: Leatherwood S, Reeves RR, editors. The Bottlenose dolphin. San Diego: Academic Press; 1990. pp. 29–49. [Google Scholar]
- Rommel SA, Reynolds JE. Skeletal anatomy. In: Perrin W, Wursig B, Thewissen J, editors. Encyclopedia of marine mammals. San Diego: Academic Press; 2002. pp. 1089–1103. [Google Scholar]
- Russ JC, Dehoff RT. Practical stereology. New York: Plenum Press; 2000. p. 185. [Google Scholar]
- Schaller O, editor. Illustrated veterinary anatomical nomenclature. Stuttgart: Ferdinand Enke Verlag; 1992. p. 614. [Google Scholar]
- Schulte von HW, Smith de FM. The external characters, skeletal muscles, and peripheral nerves of Kogia breviceps (Blainville) Bull Am Mus Nat Hist. 1918;38:7–72. [Google Scholar]
- Schmidt-Nielsen K. Animal physiology: adaptation and environment. Cambridge: Cambridge University Press; 1997. Respiration; pp. 5–64. [Google Scholar]
- Simionato LH, Andreo JC, de Oliveira JA, Bortoluci CH, dos Santos NB, Moraes LH, Rodrigues AC, Andreo MB. Morphometry and histochemistry of the rectus abdominis muscle fibers of tufted capuchin monkeys (Cebus apella Linnaeus, 1758) Int J Morphol. 2006;24:53–60. [Google Scholar]
- Slijper EJ. Die Cetaceen, vergleichend-anatomisch und systematisch. Capita Zool Bd VI and VII. 1936;7:1–600. [Google Scholar]
- Smith G, Browne K, Gaskin D. Functional myology of the harbour porpoise, Phocoena phocoena. Can J Zool. 1976;54:716–729. doi: 10.1139/z76-083. [DOI] [PubMed] [Google Scholar]
- Strickler T. Myology of the shoulder of Pontoporia blainvillei, including a review of the literature on shoulder morphology in the Cetacea. Am J Anat. 1978;152:419–431. doi: 10.1002/aja.1001520310. [DOI] [PubMed] [Google Scholar]
- Struntz DJ, McLellan WA, Dillaman RM, Blum JE, Kucklick JR, Pabst DA. Blubber development in bottlenose dolphins (Tursiops truncatus) J Morphol. 2004;259:7–20. doi: 10.1002/jmor.10154. [DOI] [PubMed] [Google Scholar]
- Totland G, Kryvi H. Distribution patterns of muscle fiber types in major muscles of the bull (Bos taurus) Anat Embryol. 1991;184:441–450. doi: 10.1007/BF01236050. [DOI] [PubMed] [Google Scholar]
- Van de Graaff WB, Gottfried SB, Mitra J, van Lunteren E, Cherniack NS, Strohl KP. Respiratory function of hyoid muscles and hyoid arch. J Appl Physiol. 1984;57:197–204. doi: 10.1152/jappl.1984.57.1.197. [DOI] [PubMed] [Google Scholar]
- Videler J, Kamermans P. Differences between upstroke and downstroke in swimming dolphins. J Exp Biol. 1985;119:265–274. doi: 10.1242/jeb.119.1.265. [DOI] [PubMed] [Google Scholar]
- Wartzok D. Breathing. In: Perrin W, Würsig B, Thewissen H, editors. Encyclopedia of marine mammals. San Diego: Academic Press; 2002. pp. 164–168. [Google Scholar]
- Werth A. A kinematic study of suction feeding and associated behavior in the long-finned pilot whale, Globicephala melas (Traill) Mar Mam Sci. 2000;16:299–314. [Google Scholar]
- Whitelaw WA, Ford GT, Rimmer KP, De Troyer A. Intercostal muscles are used during rotation of the thorax in humans. J Appl Physiol. 1992;72:1940–1944. doi: 10.1152/jappl.1992.72.5.1940. [DOI] [PubMed] [Google Scholar]
- Williams TM. The evolution of cost-efficient swimming in marine mammals: limits to energetic optimization. Phil Trans R Soc Lond B. 1999;354:193–201. [Google Scholar]
- Young I, Alexander R, Woakes A, Butler P, Anderson L. The synchronization of ventilation and locomotion in horses (Equus caballus) J Exp Biol. 1992a;166:19–31. doi: 10.1242/jeb.166.1.19. [DOI] [PubMed] [Google Scholar]
- Young I, Warren R, Altringham J. Some properties of the mammalian locomotory and respiratory systems in relation to body mass. J Exp Biol. 1992b;164:283–294. doi: 10.1242/jeb.164.1.283. [DOI] [PubMed] [Google Scholar]