Abstract
Background: Irritable bowel syndrome (IBS) is a common disorder and is the largest diagnostic cohort seen by gastroenterologists. There is a bidirectional comorbidity of IBS and psychiatric illness. Ours is the first study to examine the effect of any selective serotonin reuptake inhibitor in subjects with IBS.
Method: Twenty subjects with Rome I criteria–diagnosed IBS were treated with 20 to 40 mg of paroxetine for 12 weeks. We utilized a computer-administered patient daily questionnaire taken by patients over the telephone using an interactive voice response system.
Results: Sixty-five percent of patients (13/20) reported a reduction in abdominal pain, and 55% (11/20) reported a reduction in pain frequency (total or mean number of days per week in which the patient had the symptom decreased by ≥ 50%). Constipation and diarrhea were reduced in 69% and 57% of patients (9/13 and 8/14), respectively. Similarly, a clinically significant reduction in the symptoms of feeling of incomplete emptying (53% [9/17]) and bloating/abdominal distension (55% [11/20]) was apparent at study conclusion compared with baseline. On the Clinical Global Impressions scale at week 12, 47% (8/17) of the patients were much or very much improved.
Conclusion: In our pilot open-label study, paroxetine was very effective in alleviating the abdominal pain and associated symptoms of IBS. These results warrant further examination in a placebo-controlled study.
Irritable bowel syndrome (IBS) is a functional gastrointestinal (GI) syndrome that affects an estimated 10% to 22% of the population.1 IBS may be defined as chronic abdominal discomfort with alternating symptoms of constipation and diarrhea for which no organic cause can be determined. Additional symptoms frequently include pain relieved by defecation, looser stools at onset of pain, more frequent bowel movements at onset of pain, abdominal distension, mucus per rectum, and sensation of incomplete evacuation.2
The prevalence, morbidity, and chronicity of symptoms seen in IBS contribute to a high cost of care. Patients with IBS have a 3-fold higher rate of work and school absenteeism compared with individuals without IBS.3 In addition, IBS accounts for 12% of all visits to primary care and 28% of visits to gastroenterologists, with an estimated annual cost to the health care system of $8 billion.4,5
Only 14% to 50% of IBS patients seek medical care; those who do have a high prevalence of psychiatric illness, notably mood and anxiety disorders.1 Patients seeking care for psychiatric illness also have a high prevalence of IBS. Tollefson et al.6 found that 10 (29.4%) of 34 patients with major depression (mean Hamilton Rating Scale for Depression [HAM-D] total score = 24.8) also met criteria for IBS compared with 3 (10.7%) of 28 nondepressed controls. In a study of 56 patients seeking outpatient treatment for major depression versus 40 matched controls, Masand et al.7 reported that 27% of depressed patients met criteria for IBS compared with 2.5% of controls. Similarly, in a study of patients with double depression (major depression and dysthymia), 57.7% of patients with double depression also had IBS compared with 2.5% of controls.8
While the etiology of IBS has not been determined, there is considerable evidence to suggest that a link exists between the brain and the enteric nervous system of the GI tract. Patients with mood and anxiety disorders have numerous GI symptoms, such as nausea, abdominal distress, and weight gain/loss, that may suggest a common pathophysiology between psychiatric and GI disorders.9 At a neurophysiologic level, the locus ceruleus, which is known to mediate fear and arousal states, receives afferent input from the gut.10 Distension of the bowel, as seen in IBS, results in an increased firing rate in the locus ceruleus.9 It is also noteworthy that an estimated 90% of the 5-hydroxytryptamine (5-HT) in the human body is found in the GI tract, primarily in gut enterochromaffin cells and in myenteric interneurons.11 5-HT receptors are located on afferent neurons and in the enteric and autonomic nervous systems, where they are involved in mediating sensory and reflex responses to GI stimuli and play a role in emesis, diarrhea, eating behavior, abdominal pain, and GI reflexes.11 Of the 5-HT receptor subtypes, 5-HT3 and 5-HT4 appear to be the most significant to GI function. 5-HT3 receptors are located on vagal afferent neurons and mediate visceral sensations and gut reflexes.12 5-HT4 receptors are believed to be located in the presynaptic nerve terminals of cholinergic and motor neurons and appear to influence gut motility and peristalsis.13,14
Research evidence supports the use of serotonergic agents in IBS. 5-HT3 antagonists, such as granisetron, appear to decrease smooth muscle tone, slow colonic emptying, and may decrease visceral hypersensitivity in IBS.15–17 It has further been suggested that selective serotonin reuptake inhibitors (SSRIs), which have some activity at the 5-HT3 receptor, may improve symptoms of both IBS and depression in comorbid patients.18,19
Accordingly, the present study examined the effect of the SSRI paroxetine in subjects with IBS.
METHOD
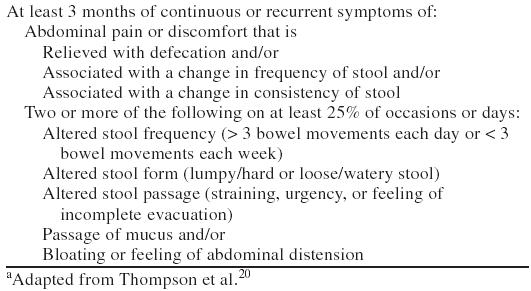
Twenty subjects (age range, 18–65 years) were enrolled in the study. Entry criteria were GI symptoms for ≥ 2 days/week for > 6 months, a diagnosis of IBS according to the Rome I criteria (Table 1),20 no evidence of lactose intolerance explaining the GI symptoms, absence of systemic or GI diseases or GI surgery that would interfere with the interpretation of symptoms, use of birth control, and access to a touch-tone telephone. Exclusion criteria included recent (prior 2 weeks) or current monoamine oxidase inhibitor use, active history of alcohol/substance use or abuse in the preceding 6 months, presence of DSM-IV bipolar disorder or schizophrenia, and active suicidal or homicidal ideation or intent.
Table 1.
Rome I Diagnostic Criteria for Irritable Bowel Syndromea
The study was approved by the institutional review board at the participating institutions. All patients provided informed consent and underwent a physical examination, laboratory evaluations including complete blood count, chemistry, fecal occult blood, and flexible sigmoidoscopy to confirm the diagnosis of IBS. At baseline, all patients were administered a clinician-version Structured Clinical Interview for DSM-IV (SCID-CV).21 In addition, the 21-item HAM-D,22 Clinical Global Impressions scale (CGI),23 and Udvalg for Kliniske Undersogelser (UKU) Side Effect Rating Scale24 were administered at each follow-up visit.
All IBS patients were treated for 12 weeks with paroxetine, 20 to 40 mg, in the morning. The dose was increased to 40 mg/day after 4 weeks in patients with a partial response on paroxetine, 20 mg/day.
Patient self-rated symptom improvement was monitored using a telephone-based interactive voice response system (IVRS). All subjects were required to complete daily diary entries of their GI symptoms for a baseline week and for 12 weeks on treatment with study medication. Patients were instructed to call a toll-free number and enter a password and identification number. Patients recorded their diary entries by pressing the appropriate key on their telephone keypad in response to prerecorded questions (e.g., “Did you experience abdominal pain or discomfort today? If yes, press 1; if no, press 2.”). Patients were instructed to call in daily before bedtime. The psychometric validity of IVRS in administering diagnostic and symptom rating scales by telephone has been evaluated in several studies. IVRS has been compared with the clinician-administered SCID.25 IVRS has also been used to administer the HAM-D, Hamilton Rating Scale for Anxiety,26,27 and Zung Depression Scale.28
Clinical response was measured in several ways. Abdominal pain severity was measured with an ordinal scale rated from 1 to 9 (1 = mild pain/discomfort, 9 = very severe pain/discomfort). Abdominal pain frequency was measured on a 4-point scale (1 = pain or discomfort present only occasionally, 2 = pain or discomfort present less than half the day, 3 = pain or discomfort present more than half of the day, 4 = pain or discomfort almost all day). The same ordinal scale was used to quantify the distress or discomfort caused by a feeling of incomplete evacuation, bloating, or abdominal pain and general level of stress or tension.
For dichotomous variables (i.e., abdominal pain, constipation, diarrhea, feeling of incomplete emptying, and bloating), response was defined as a ≥ 50% reduction from baseline to the last study week in the total or mean number of days having the symptom. For continuous variables (i.e., severity and/or frequency of symptoms and general level of stress), improvement was defined as ≥ 50% reduction from baseline week to the last study week in the total or mean severity score. Remission was defined as a reduction ≥ 70% from baseline week.
The proportion of patients who experienced response or remission (≥ 50% or ≥ 70% reduction in symptoms, respectively) was compared among anxiety groups using the Fisher exact test, and the mean change in the number of bowel movements between the first and last study week was assessed using the t test.
RESULTS
Twenty subjects (7 men, 13 women, mean age = 42.8 years) with IBS, 10 with a lifetime history of comorbid anxiety disorder and 10 without anxiety disorder, were enrolled. Mean duration of IBS was 7.15 years (range, 0.5–30 years). The anxiety disorders present in the anxiety group were specific phobia (N = 5), panic disorder without agoraphobia (N = 3) and with agoraphobia (N = 1), social anxiety disorder (N = 3), and posttraumatic stress disorder (N = 1). In addition, a history of major depressive disorder was present in 1 patient; dysthymia, in 1 patient; and adjustment disorder, in 1 patient.
Thirteen (65%) of 20 subjects reported a reduction of ≥ 50% in abdominal pain; 14 (70%), in pain severity; and 11 (55%), in pain frequency (Table 2). For constipation, 9 (69%) of 13 patients experienced ≥ 50% relative change and 8 (62%) had a reduction in severity. For diarrhea, 8 (57%) of 14 patients experienced a reduction in the symptom and a reduction in severity. For feeling of incomplete emptying, 9 (53%) of 17 patients experienced a reduction in the symptom and 10 (59%) had a reduction in severity. For bloating/abdominal distension, 11 (55%) of 20 patients had a reduction in the symptom and 12 (60%) experienced a reduction in severity.
Table 2.
Change in Irritable Bowel Syndrome Symptoms During a 12-Week Study of Paroxetine, 20 to 40 mg/daya
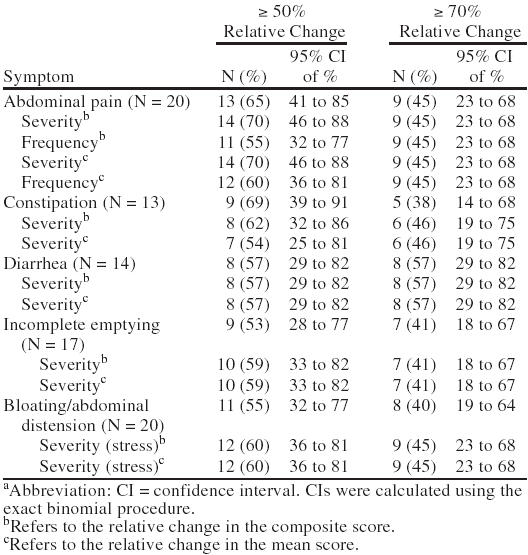
For the more stringent criterion of remission (≥ 70% reduction), 9 (45%) of 20 patients experienced a reduction in abdominal pain, pain severity, and frequency; 5 (38%) of 13 experienced a reduction in constipation and 6 (46%) of 13 experienced a reduction in constipation severity; 8 (57%) of 14 had a reduction in diarrhea and diarrhea severity; 7 (41%) of 17 had a reduction in feeling of incomplete emptying and severity of feeling of incomplete emptying; 8 (40%) of 20 experienced a reduction in bloating/abdominal distension; and 9 (45%) of 20 had a reduction in bloating/abdominal distension severity. Improvement on the CGI is seen in Figure 1.
Figure 1.
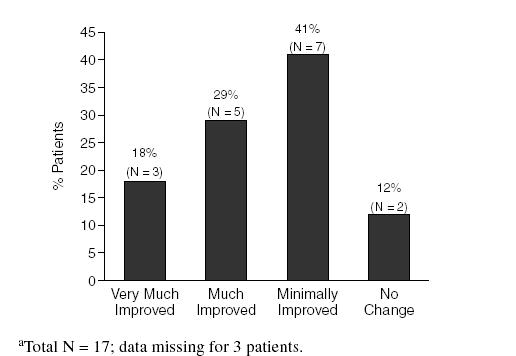
Improvement Based on the Clinical Global Impressions Scale at Week 12a
On the Patient Global Impression of Improvement (available from the authors on request), 13 (65%) of 20 patients reported feeling at least a little better compared with study entry for at least 3 or 4 consecutive days, 6 (30%) felt much better, and 2 (10%) felt very much better. Paroxetine was generally well tolerated by the patients in this study. The most commonly reported adverse events were sedation, asthenia, dry mouth, somnolence, sweating, and increased dream activity. There were no unexpected or severe adverse events. Adverse events were mild to moderate in severity and did not result in study discontinuation.
DISCUSSION
The potential effectiveness of antidepressants in managing IBS symptoms was first examined 20 years ago. Two early studies29,30 of trimipramine reported that this agent was efficacious in alleviating IBS-associated abdom pain, nausea, sleeplessness, and depression. A recent meta-analysis examined data obtained from 12 randomized, placebo-controlled trials of antidepressants in IBS.31 Study medications included tricyclic antidepressants (amitriptyline, clomipramine, and trimipramine), a heterocyclic (doxepin), desipramine, and an antiserotonin agent, mianserin. The summary odds ratio for improvement in GI symptoms with antidepressant therapy was 4.2 (95% confidence interval [CI] = 2.3 to 7.9). The standardized mean improvement in pain was equal to 0.9 standard deviation units (95% CI = 0.6 to 1.2), which is considered to be a large treatment effect. In reviewing their 5-year clinical experience with antidepressants in outpatients with IBS (N = 138), Clouse et al.32 reported improvement in 89% and complete remission of bowel symptoms in 61% of patients during antidepressant therapy with tricyclic, newer, or anxiolytic antidepressants. Median doses to achieve remission were lower than those used to obtain an antidepressant effect. The presence/absence of psychological symptoms was not predictive of treatment remission. However, a pain-predominant IBS symptom pattern was more commonly associated with symptom remission.
In a recent review of randomized controlled treatment trials in IBS, Jailwala et al.33 reported that evidence supports the efficacy of smooth-muscle relaxants (e.g., cimetropium, trimebutine) but not that of bulking agents (e.g., psyllium, bran, coarse fiber). Loperamide appeared to be effective for diarrhea but not for abdominal pain. Psychotropic agents (e.g., amitriptyline, nortriptyline, desipramine) were shown to produce global improvement, but the evidence was based on a small number of trials and requires further study. Three recent case reports have suggested that serotonergic antidepressants (fluvoxamine,34 paroxetine,35 mirtazapine36) are efficacious in alleviating IBS symptoms.
In the present open-label study, paroxetine was effective compared with baseline in improving the cardinal symptoms of IBS, including abdominal pain, constipation, diarrhea, feeling of incomplete emptying, and bloating/abdominal distension. The underlying mechanisms of these effects are unknown but may be attributable, in part, to the activity of paroxetine at the 5-HT3 receptor. Some investigators have suggested that the beneficial effect of serotonergic and noradrenergic antidepressants in IBS may be partially due to the antinociceptive (analgesic) effect of these agents independent of their antidepressant effect.37 This hypothesis requires further study to determine the mechanisms of action of antidepressants in general, and paroxetine in specific, in alleviating IBS symptoms.
The strengths of our study included the use of Rome I criteria to diagnose IBS plus a detailed medical workup that included flexible sigmoidoscopy to confirm the diagnosis of IBS. In addition, the psychiatric diagnoses were made using a structured psychiatric interview and depressive symptoms were monitored using the HAM-D to control depression as a confounding variable. In addition, the daily automated telephone IVRS avoided the recall biases associated with retrospective reports of symptoms as was the case in patient-rated symptom diaries brought in weekly or biweekly in previous studies of IBS. In our study, patients completed 86% of all required daily calls. Limitations of our study included the absence of a placebo control, small sample size, and inclusion of patients with comorbid psychiatric diagnosis. The results should be interpreted cautiously, as ours was a preliminary, uncontrolled, open trial with a small number of participants.
CONCLUSION
Data from this open-label pilot study suggest that paroxetine is effective in alleviating the pain and associated symptoms of IBS. These results warrant further examination in a randomized, double-blind, placebo-controlled study.
Drug names: amitriptyline (Elavil and others), desipramine (Norpramin and others), doxepin (Sinequan and others), fluvoxamine (Luvox and others), granisetron (Kytril), loperamide (Imodium and others), mirtazapine (Remeron), nortriptyline (Aventyl and others), paroxetine (Paxil), trimipramine (Surmontil).
Footnotes
This study was supported in part by GlaxoSmithKline.
Presented in part at the 41st annual meeting of the New Clinical Drug Evaluation Unit, May 28–31, 2001; Phoenix, Ariz.
Dr. Masand has received grant/research support from Astra-Zeneca, Forest Laboratories, GlaxoSmithKline, Janssen Pharmaceutica, and Wyeth Ayerst; has been a consultant for Bristol-Myers Squibb, Forest Laboratories, GlaxoSmithKline, Health Care Technology, Janssen Pharmaceutica, Organon, Pfizer, and Wyeth Ayerst; and has been on the speakers bureau for Abbott, Astra-Zeneca, Bristol-Myers Squibb, Eli Lilly, Forest Laboratories, GlaxoSmithKline, Janssen Pharmaceutica, Novartis, Pfizer, and Wyeth Ayerst. Dr. Gupta has received grant/research support from Abbott, Eli Lilly, and Janssen Pharmaceutica; has been a consultant for Eli Lilly, Forest Laboratories, and Pfizer; has received honoraria from Eli Lilly, Forest Laboratories, GlaxoSmithKline, and Pfizer; and has been on the speakers bureau for Eli Lilly, Forest Laboratories, and Pfizer.
REFERENCES
- Lynn RB, Friedman LS. Irritable bowel syndrome. N Engl J Med. 1993;329:1940–1945. doi: 10.1056/NEJM199312233292608. [DOI] [PubMed] [Google Scholar]
- Manning AP, Thompson WG, Heaton KW, et al. Towards positive diagnosis of the irritable bowel. Br Med J. 1978;2:653–654. doi: 10.1136/bmj.2.6138.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drossman DA, Li Z, Andruzzi E, et al. US householder survey of functional gastrointestinal disorders: prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–1580. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- Mitchell CM, Drossman DA. Survey of the AGA membership relating to patients with functional gastrointestinal disorders. Gastroenterology. 1987;92(5, pt 1):1282–1284. doi: 10.1016/s0016-5085(87)91099-7. [DOI] [PubMed] [Google Scholar]
- Norton N. Functional bowel disorders survey. Participate. 1997;6:1–3. [Google Scholar]
- Tollefson GD, Tollefson SL, Pederson M, et al. Comorbid irritable bowel syndrome in patients with generalized anxiety and major depression. Ann Clin Psychiatry. 1991;3:215–222. [Google Scholar]
- Masand PS, Kaplan DS, Gupta S, et al. Major depression and irritable bowel syndrome: is there a relationship? J Clin Psychiatry. 1995;56:363–367. [PubMed] [Google Scholar]
- Masand P, Kaplan D, Gupta S, et al. Relationship between irritable bowel syndrome (IBS) and double depression (dysthymia plus major depression) Depression. 1995/1996;3:303–306. [Google Scholar]
- Lydiard RB, Falsetti SA. Experience with anxiety and depression treatment studies: implications for designing irritable bowel syndrome clinical trials. Am J Med. 1999;107(5A):65S–73S. doi: 10.1016/s0002-9343(99)00082-0. [DOI] [PubMed] [Google Scholar]
- Elam M, Thorén P, Svensson TH. Locus ceruleus neurons and sympathetic nerves: activation by visceral afferents. Brain Res. 1986;375:117–125. doi: 10.1016/0006-8993(86)90964-9. [DOI] [PubMed] [Google Scholar]
- Read NW, Gwee K-A. The importance of 5-hydroxytryptamine receptors in the gut. Pharmacol Ther. 1994;62:159–173. doi: 10.1016/0163-7258(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Richardson BP, Engel G. The pharmacology and function of 5-HT3 receptors. Trends Neurosci. 1986;9:424–429. [Google Scholar]
- Gershon MD. Review article: roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol Ther. 1999;13(suppl 2):15–30. [PubMed] [Google Scholar]
- Talley NJ. Review article: 5-hydroxytryptamine agonists and antagonists in the modulation of gastrointestinal motility and sensation: clinical implications. Aliment Pharmacol Ther. 1992;6:273–289. doi: 10.1111/j.1365-2036.1992.tb00050.x. [DOI] [PubMed] [Google Scholar]
- Farthing MJG. New drugs in the management of the irritable bowel syndrome. Drugs. 1998;56:11–21. doi: 10.2165/00003495-199856010-00002. [DOI] [PubMed] [Google Scholar]
- Ohta M, Suzuki T, Ohmori J, et al. Novel 5-hydroxytryptamine (5-HT3) receptor antagonists, 2: synthesis and structure-activity relationships of 4,5,6,7-tetrahydro-1H-benzimidazole derivatives. Chem Pharm Bull. 1996;44:1000–1008. doi: 10.1248/cpb.44.1000. [DOI] [PubMed] [Google Scholar]
- Sato Y, Yamada M, Yoshida S, et al. Benzoxazole derivatives as novel 5-HT3 receptor partial agonists in the gut. J Med Chem. 1998;41:3015–3021. doi: 10.1021/jm9801004. [DOI] [PubMed] [Google Scholar]
- Clouse RE. Antidepressants for functional gastrointestinal syndromes. Dig Dis Sci. 1994;39:2352–2363. doi: 10.1007/BF02087651. [DOI] [PubMed] [Google Scholar]
- Lucchelli A, Santagostino-Barbone MG, Barbieri A, et al. The interaction of antidepressant drugs with central and peripheral (enteric) 5-HT3 and 5-HT4 receptors. Br J Pharmacol. 1995;114:1017–1025. doi: 10.1111/j.1476-5381.1995.tb13307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WG, Creed F, Drossman DA, et al. Functional bowel disorders and functional abdominal pain. Gastroenterol Int. 1992;5:75–91. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, and et al. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), Clinical Version. Washington, DC: American Psychiatric Press. 1996 [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology. US Dept Health, Education, and Welfare publication (ADM) 76-338. Rockville, Md: National Institute of Mental Health. 1976 218–222. [Google Scholar]
- Scandinavian Society of Psychopharmacology Committee of Clinical Investigations (UKU) The UKU Side Effects Rating Scale: scale for the registration of unwanted effects of psychotropics. Acta Psychiatr Scand. 1987;76(334, suppl):81–94. [Google Scholar]
- Kobak KA, Taylor LH, Dottl SL, et al. A computer-administered telephone interview to identify mental disorders. JAMA. 1997;278:905–910. [PubMed] [Google Scholar]
- Mundt JC, Kobak KA, Taylor LV, et al. Administration of the Hamilton Depression Rating Scale using interactive voice response technology. MD Comput. 1998;15:31–39. [PubMed] [Google Scholar]
- Kobak KA, Greist JH, Jefferson JW, et al. Computerized assessment of depression and anxiety over the telephone using interactive voice response. MD Comput. 1999;16:64–68. [PubMed] [Google Scholar]
- Baer L, Jacobs DG, Cukor P, et al. Automated telephone screening survey for depression. JAMA. 1995;273:1943–1944. [PubMed] [Google Scholar]
- Myren J, Groth H, Larssen SE, et al. The effect of trimipramine in patients with the irritable bowel syndrome: a double-blind study. Scand J Gastroenterol. 1982;17:871–875. doi: 10.3109/00365528209181108. [DOI] [PubMed] [Google Scholar]
- Myren J, Lovland B, Larssen SB, et al. Psychopharmacologic drugs in the treatment of the irritable bowel syndrome: a double blind study of the effect of trimipramine. Ann Gastroenterol Hepatol. 1984;20:117–123. [PubMed] [Google Scholar]
- Jackson JL, O'Malley PG, Tomkins G, et al. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med. 2000;108:65–72. doi: 10.1016/s0002-9343(99)00299-5. [DOI] [PubMed] [Google Scholar]
- Clouse RE, Lustman PJ, Geisman RA, et al. Antidepressant therapy in 138 patients with irritable bowel syndrome: a five-year clinical experience. Aliment Pharmacol Ther. 1994;8:409–416. doi: 10.1111/j.1365-2036.1994.tb00308.x. [DOI] [PubMed] [Google Scholar]
- Jailwala J, Imperiale TF, Kroenke K. Pharmacologic treatment of the irritable bowel syndrome: a systematic review of randomized, controlled trials. Ann Intern Med. 2000;133:136–147. doi: 10.7326/0003-4819-133-2-200007180-00013. [DOI] [PubMed] [Google Scholar]
- Emmanuel NP, Lydiard RB, Crawford M. Treatment of irritable bowel syndrome with fluvoxamine [letter] Am J Psychiatry. 1997;154:711–712. doi: 10.1176/ajp.154.5.711b. [DOI] [PubMed] [Google Scholar]
- Kirsch MA, Louie AK. Paroxetine and irritable bowel syndrome. Am J Psychiatry. 2000;157:1523–1524. doi: 10.1176/appi.ajp.157.9.1523-a. [DOI] [PubMed] [Google Scholar]
- Thomas SG. Irritable bowel syndrome and mirtazapine. Am J Psychiatry. 2000;157:1341–1342. doi: 10.1176/appi.ajp.157.8.1341-a. [DOI] [PubMed] [Google Scholar]
- Fishbain D. Evidence-based data on pain relief with antidepressants. Ann Med. 2000;32:305–316. doi: 10.3109/07853890008995932. [DOI] [PubMed] [Google Scholar]


