Abstract
Lectins are a group of proteins that bind specifically and reversibly to mono- and oligosaccharide carbohydrate structures that are present on the surfaces of mammalian cells. The use of lectins as capture agents in microfluidic channels was examined with a focus on cells associated with T and B lymphocytic leukemia. In addition to examining the adhesion of Jurkat T and Raji B lymphocytes to a broad panel of lectins, this work also examined the capture of these cells from whole blood. Captured T and B lymphocytes were eluted from the microfluidic devices with a solution of the lectin’s inhibiting sugar. The capture and release steps were accomplished in under 1 h. The significance of this work lies within the realm of low-cost capture of abundant target cells with non-stimulatory elution capability.
Keywords: Microfluidics, Lectins, Cell capture, Cell adhesion, Blood, Leukemia
1 Introduction
Every year approximately 25,000 people in the United States are diagnosed with leukemia (Jemal et al. 2010). Originating in a malfunctioning bone marrow, leukemia results when abnormal red and white blood cells are produced at an amplified rate. There are several types of leukemia; the most important types include acute and chronic myelogenous leukemia and acute and chronic lymphocytic leukemia. Marked by an increased production of abnormal white blood cells, the acute type of leukemia incapacitates the body’s ability to fight infections (Peacock 2000). Current methods of diagnosing leukemia include bone marrow biopsy, bone marrow aspiration and complete blood cell count (van den Ancker et al. 2010). These existing techniques are quite intensive, invasive and require a trained technician.
The present work examines the feasibility of using lectins to capture cancerous lymphocytes from whole blood. The implementation of microfluidics technology gives the advantage of small sample volume, rapid processing time, high throughput and low cost (Toner et al. 2005). Previous work from our group and other groups has focused on effective cell capture of cells using antibodies and peptides immobilized within microfluidic channels (Plouffe et al. 2007; Nagrath et al. 2007). Lectins are a group of proteins that bind specifically and reversibly to mono- and oligosaccharide carbohydrate structures (Zheng et al. 2007; Chatterjee et al. 2008). These proteins have been isolated from viruses, bacteria, fungi, plants, and animals (Wang et al. 2000). Mammalian cell surfaces are covered with dense layers of carbohydrates that are attached to membrane glycoproteins and glycolipids (Chen et al. 2007). The carbohydrate expression patterns that exists on cell surfaces differs for each mammalian cell type, hence distinct binding affinities towards certain lectins can be expected. Altered glycosylation on cell surfaces has been associated with malignancy, its progression, and metastasis (Chatterjee et al. 2008). Lectins can therefore be utilized to differentiate between malignant and normal cells based on their agglutination patterns (Gijzen et al. 2008). Lectins can also be immobilized onto surfaces for chromatographic applications. Lectin affinity chromatography involves using columns with immobilized lectins for the capture of biological targets of interest. Here, the lectins are covalently immobilized onto beads that are packed within a column. Utilizing the interaction between the lectins and the biomolecule, effective capture is achieved (Kullolli et al. 2008). This paper describes how a similar approach can be employed to capture target cells in microfluidic devices. The use of lectins for capture of biomolecules and cells as opposed to highly specific antibodies is of interest because these molecules are considerably lower in cost as well as the reversible nature of lectin-mediated capture.
Once cells are captured with lectins they can be released easily with the use of their equivalent inhibiting sugars (Zheng et al. 2007). This approach is very attractive from the standpoint of affinity-based cell separation because these sugars are highly cyto-compatible and can be readily introduced into a separation system following cell binding. This detachment method overcomes the risk of cell damage when detachment is performed using large-magnitude fluidic forces (Lu et al. 2004; Wankhede et al. 2006) or enzymatic cleavage (Fujioka et al. 2003) and does not require any thermal or electrical interfaces.
This paper describes a technique to capture cancerous T and B lymphocytes from whole blood using lectins immobilized within microfluidic channels. These lymphocytes, specifically Jurkat T lymphocytes and Raji B lymphocytes are spiked into blood samples at concentration levels similar to those of cancerous leukocytes in the blood of leukemia patients (Mulligan et al. 2008; Hoyer et al. 1995). Capture lectins for each cell type are identified following cell adhesion studies with a broad panel of candidate molecules. The release of captured cells is also demonstrated with the use of inhibiting sugars associated with each capture lectin.
2 Materials and methods
2.1 Materials
Ethanol (200 proof), cover slips (35×60 mm, no. 1), microcentrifuge tubes, cell culture flasks, fetal bovine serum (FBS), Bovine Serum Albumin (BSA), HEPES (N-(2-Hydroxyethyl)piperazine-N'-2-ethanesulfonic Acid) and acetone were purchased from Thermo Fisher Scientific (Waltham MA). 3-Mercaptopropyl trimethoxysilane was obtained from Gelest Inc. (Morrisville, PA) and the coupling agent GMBS (N-y-maleimidobutyryloxy succini-mide ester) was obtained from Pierce Biotechnology (Rockford, IL). SU-8-50 photoresist and developer were obtained from MicroChem (Newton, MA); silicone elastomer and curing agent were obtained from Dow Corning (Midland, MI). Phosphate buffered saline (1X PBS), Penicillin-streptomycin (PS) and RPMI1640 media was purchased from Mediatech (Herndon, VA). The Jurkat and Raji T and B lymphocytes were purchased from American Type Culture Collections (Manassas, VA). Triethylamine and ethylacetate were purchased from Sigma. The lectins used include Griffonia (Bandeiraea) simplicifolia lectin (GSL), Vicia Villosa lectin (VVL), Ricinus Communis Agglutinin (RCA), Lotus Tetragonolobus lectin (LTL), Erythrina Cristagalli lectin (ECL), Maackia Amurensis lectin (MALII), Aleuria Aurantia lectin (AAL), Phaseolus vulgaris leucoagglutinin (PHA-L), Peanut Agglutinin (PNA) and Sambucus Nigra Lectin (SNA) which were all purchased from Vector Laboratories (Covington, LA). Cell tracker dyes (Green CMFDA and Orange CMTMR) were purchased from Invitrogen. L-fucose to galactose were obtained from Sigma-Aldrich (St. Louis, MO).
2.2 Microfluidic device design and fabrication
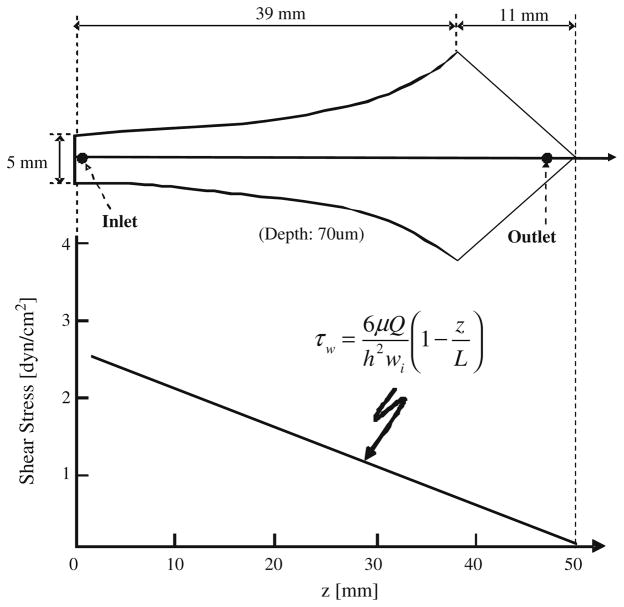
One of the microfluidic devices used in this study is designed in such a way that the shear stress along the longitudinal axis of the device decreases with device length as shown in Fig. 1. The design of this device, referred to as the Hele-Shaw device, stems from the theory of flow to design equations derived by (Usami et al. 1993). Upon obtaining an optimum capture lectin, a post array device was then used to capture T and B lymphocytes from blood. It consists of posts of 100 μm diameter and 50 μm spacing arranged hexagonally (Gleghorn et al. 2010). The design of the microfluidic device followed previously described soft lithography techniques (Plouffe et al. 2007; Xia and Whitesides 1998). Negative masters for device fabrication were manufactured at the George J. Kostas Nanoscale Technology and Manufacturing Research Center at Northeastern University. Preceding this step 2D projections of the device were drawn using AutoCAD, and the image printed at high resolution on transparency (FineLine Imaging, Colorado Springs, CO). The photomask was then used to generate a negative master. A silicon wafer was coated with SU 8–50 photoresist to a thickness of approximately 70 μm and the transparency overlaid. This was exposed to UV light (365 nm, 17.75 mW/cm2) from a Quintel 2001 mask aligner. Once curing was completed the unexposed photo-resist was removed using SU 8 developer, and the feature height verified using a Dektak surface profiler (Veeco Instruments, Santa Barbara, CA).
Fig. 1.
Flow chamber geometry and shear stress profile based on the design developed by (Murthy et al. 2004). The relationship between shear stress (τw) and axial position (z) is given by the equation derived by (Usami et al. 1993)
Polydimethysiloxane (PDMS) replicas were generated using silicone elastomer and curing agents in the ratio of 10:1 (w/w). This was poured onto the negative master and allowed to degas, then cured at 65°C for 2 h. PDMS replicas were released from the wafers prior to punching inlet to outlet holes with a 19-gauge blunt-nose needle. For some of the devices extraction of the PDMS was performed prior to bonding to glass slides as described in the following section.
For bonding, the replicas and glass slides were exposed to an oxygen plasma (100 mW with 8% oxygen for 30 s) in a PX-250 plasma chamber (March Instruments, Concord, MA) and then immediately placed in contact with each other. The irreversible bonding between PDMS and glass was completed by baking for 5 min at 65°C. Surface functionalization of the devices was performed immediately following the baking step.
2.3 PDMS extraction
The post devices alone were extracted prior to surface modification. PDMS post replicas were extracted following procedures reported by (Vickers et al. 2006). First, post device PDMS replicas were immersed in 200 mL of triethylamine solution at 25°C while being stirred for 2 h. After an hour the triethylamine solution was replaced with fresh solution. Following this the PDMS was removed from triethylamine solution and immersed in 200 mL of ethyl acetate for 2 h while being stirred. This solvent was refreshed with fresh solvent after 1 h, followed by the PDMS replicas being submerged in 200 mL of acetone for 2 h. PDMS replicas were then allowed to completely dry at 65°C for 2–6 h.
2.4 Surface modification
A 4% (v/v) solution of 3-mercaptopropyl trimethoxysilane in ethanol was prepared under a nitrogen atmosphere and injected into each device. This was left to react for 30 min undisturbed. The unreacted silane was flushed out with ethanol and a 0.28% GMBS in ethanol solution was then flowed through the devices. The GMBS was left to react for 15 min. Following this ethanol was used to flush out the unreacted GMBS and an additional flush was performed with PBS. Each lectin was diluted with 1 × PBS to a concentration of 0.01 mg/mL and this solution was injected into individual devices. Following a 30 min incubation period, the devices were flushed with PBS and either used directly in experiments or stored at 4°C. For post devices, 1% (w/v) BSA in 1× PBS was flushed into the device and left standing for 5 min (longer BSA incubation was observed to suppress lectin-mediated cell capture) after the lectin incubation step. This was followed by a rinse with PBS. These devices were then flushed with PBS and either used directly in experiments or stored at 4°C.
2.5 Cell culture
Jurkat T lymphocytes and Raji B lymphocytes were cultured in 75 cm2 tissue culture flasks at 37°C in a humidified atmosphere with 5% CO2 and 95% air. These cells were cultured in RPMI 1640 supplemented with 100 U/mL penicillin, and 100 μg/mL streptomycin, 10% fetal bovine serum, and 1% HEPES. Cells were grown to a high concentration prior to microfluidic experiments. Cell suspensions were centrifuged at 190 × g and then resuspended in PBS at a concentration of 1×105 cells/mL.
2.6 Cell capture experiments
Raji B lymphocytes and Jurkat T lymphocytes (homogeneous suspensions) were flowed through the Hele Shaw devices at 60 μL/min, which represents a shear stress range of 0.74 to 2.12 dyn/cm2, using a Harvard Apparatus PHD 2000 syringe pump (Holliston, MA). Cell adhesion was measured by placing a field finder (with 1 mm×1 mm grids) under the device and counting manually at selected points along the device axis under a Nikon Eclipse TE2000 inverted microscope. Three cell counts were taken and averaged at each location, with each location representing a 1 mm square. All flow experiments were performed at room temperature.
2.7 Capture and release experiments with blood
Raji B lymphocytes and Jurkat T lymphocytes were labeled with cell tracker dyes at a working solution of 25 mM for 30 min. Raji B lymphocytes and Jurkat T lymphocytes were dyed red and green respectively. 20 μL suspension of Raji B lymphocytes and Jurkat T lymphocytes at concentrations of 1×106 cells/mL in PBS were spiked individually into 2 mL of whole human blood. Blood was drawn from healthy volunteers upon approval from the Institutional Review Board at Northeastern University. Spiked blood samples were run into the post devices at 10 μL/min and the total volume flowed was 200 μL. Following sample injection, a rinse step was carried out with PBS flowed at 10 μL/min to a total volume of 200 μL. T and B lymphocytes were enumerated by manually raster scanning the whole device under fluorescent excitation using a Nikon TE 2000 inverted microscope with fluorescein (480± 30 nm/535±40 nm excitation/emission) and rhodamine (540±25 nm/605±50 nm) filters. Cells were then released by flowing 100 mM of L-fucose and 200 mM of galactose into LTL- and PNA-coated devices respectively at flow rates of 10 μL/min for 20 min. Undetached cells were enumerated in the entire device.
2.8 Statistics and data analysis
For each lectin, five repetitions of experiments were performed. Reported uncertainties represent standard errors of the mean (standard deviation/√n, where n=5). One-way analysis of variance (ANOVA) was performed to investigate the relationship between adhesion of the two cell types measured within Hele-Shaw devices at a shear stress level of 0.74 dyn/cm2. This analysis was executed using Kaleida-Graph 4.0. A p value ≤ 0.001 was considered significant.
3 Results and discussion
An initial set of cell adhesion experiments was carried out in order to characterize the affinity levels of the selected panel of lectins to the target B and T lymphocytes under microfluidic flow conditions. These experiments were carried out using microfluidic devices capable of generating Hele-Shaw flow (Fig. 1). The flaring channel geometry of these channels generates a linear drop in shear stress from inlet to outlet along the channel axis (Usami et al. 1993; Plouffe et al. 2007).
Cell adhesion measurements were carried out with ten different lectins: GSL, VVL, RCA, LTL, ECL, MALI, AAL, PHA-L, PNA and SNA. The lectins selected for this study have binding specificities that cover common terminal glycan structures in O- and N-linked glycproteins and are therefore useful tools to survey the repertoire of carbohydrate structures present on the surface of the Jurkat T and Raji B cells.
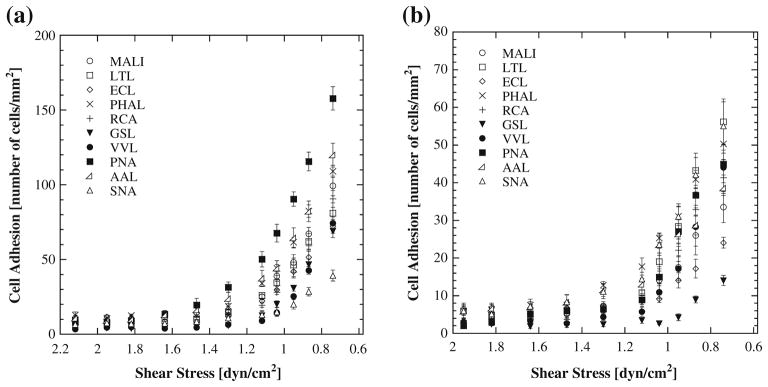
The results obtained for each cell type for shear stresses ranging from 0.6 to 2.2 dyn/cm2 are shown in Fig. 2. Note that the x-axis scale in both of these plots is reversed to be consistent with Fig. 1, where the left hand or inlet end of the channel has the highest shear stress. Both cell types show increasing adhesion as shear stress decreases. In general Jurkat cells showed a greater magnitude of adhesion relative to Raji cells for the panel of lectins examined. PNA-coated devices provided the highest level of affinity for Jurkat cells while LTL and SNA showed highest affinity for Raji cells.
Fig. 2.
Cell adhesion as a function of shear stress with (a) Jurkat T lymphocytes and (b) Raji B lymphocytes on lectin coated surfaces
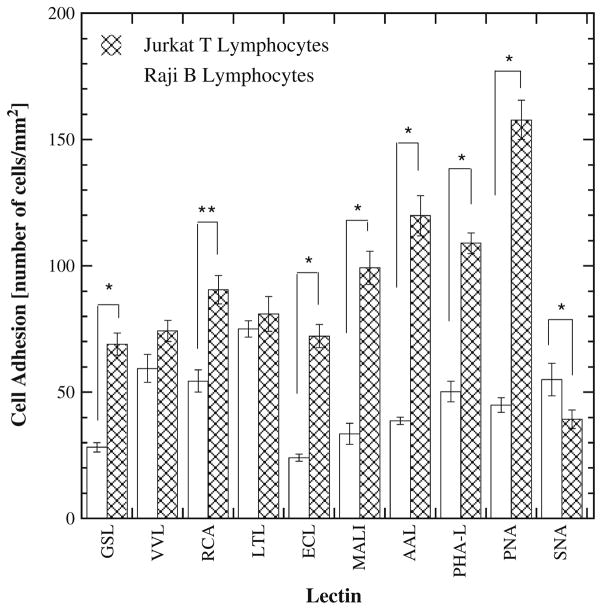
Figure 3 compares adhesion levels for each cell type and lectin pair for a shear stress level of 0.74 dyn/cm2. This value of shear stress provided the greatest adhesion levels (Fig. 2) but also corresponds to the lowest volumetric flow rate examined in the present study. Figure 3 allows an analysis of the selectivity of each lectin for one cell type relative to the other. For example, PNA shows significant preferential adhesion for Jurkat cells relative to Raji cells (p<0.0001). The differences in binding affinity between Jurkat and Raji cells on GSL, RCA, ECL, MALI, PHAL, and SNA are statistically significant but smaller in magnitude. VVL- and LTL-coated surfaces, by contrast, showed similar affinity for both cell types. It is noteworthy that of all the lectins studied only one (SNA) had a higher affinity for Raji B lymphocytes over Jurkat T lymphocytes. SNA and GSL bind with high affinity to Neu5Ac α(2,6) Gal/GalNAc (Kaku et al. 2007) and terminal α1,3-galactose (Iskratsch et al. 2009) respectively. PNA demonstrated the highest capture for T cells (Fig. 2(a)), while LTL and SNA demonstrated the highest capture for B cells (Fig. 2(b)). The PNA and LTL lectins bind with high affinity to terminal βGal, Gal β(1,3)GalNAc and α(1,2) L-fucose (terminal), respectively (Wearne et al. 2006).
Fig. 3.
Comparison of T and B cell adhesion on a lectin coated surface at a shear stress of 0.74 dyn/cm2. * denotes significant difference with p<0.0001 and ** denotes significant difference with p=0.0003
The cell adhesion data presented in Figs. 2 and 3 was utilized to identify optimal capture molecules for Jurkat and Raji cells spiked into whole human blood. Specifically, PNA was selected for Jurkat cell capture and LTL for Raji cell capture. Here, the rationale for choosing PNA and LTL was to achieve the highest level of capture rather than greatest degree of selectivity.
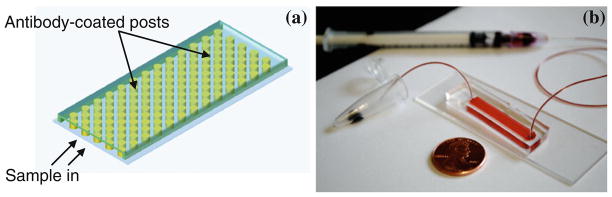
For cell capture from whole blood, a microfluidic device consisting of an array of pillars was fabricated using PDMS-based soft lithography (Fig. 4). The design of this device is analogous to that utilized by (Nagrath et al. 2007) for circulating tumor cell capture from blood. A hexagonal arrangement scheme was adopted for placement of the 100 μm-diameter posts following the optimization study performed by (Gleghorn et al. 2010) in order to maximize contact between flowing cells and posts by creation of maximum distortion streamlines. What distinguishes the present study from the work of these two other groups pursuing microfluidic capture of circulating tumor cells is the use of lectins for cell capture from blood to associated inhibiting sugars to accomplish release of captured cells in a continuous flow (no incubation) process. The use of inhibiting sugars to achieve cell detachment following capture within lectin-coated microchannels was first demonstrated by (Zheng et al. 2007). However, in this study, cell release was performed after prolonged incubation of a homogeneous suspension.
Fig 4.
Schematic diagram of the device design (a) and photograph of device (b)
Table 1 summarizes the results of capture and release studies performed with Jurkat and Raji cells spiked into whole human blood. For these experiments one of the two cell types was labeled with a cell-tracker dye and spiked into whole blood at a concentration of 104 cells/mL. The volume of spiked whole blood injected into each microfluidic device was 200 μL. Table 1 shows results for the average number of Jurkat and Raji cells captured in these experiments; representative images of captured cells are shown in Fig. 5.
Table 1.
Lectin-mediated capture and release of Jurkat and Raji from whole blood
| Cell type | Number of cells adhered in device | Capture efficiency (%) | Purity (%) | Fraction released (%) |
|---|---|---|---|---|
| Jurkat T lymphocytes | 104±18 | 5±1 | 23±2 | 50±7 |
| Raji B lymphocytes | 213±18 | 11±1 | 52±5 | 45±3 |
Fig. 5.
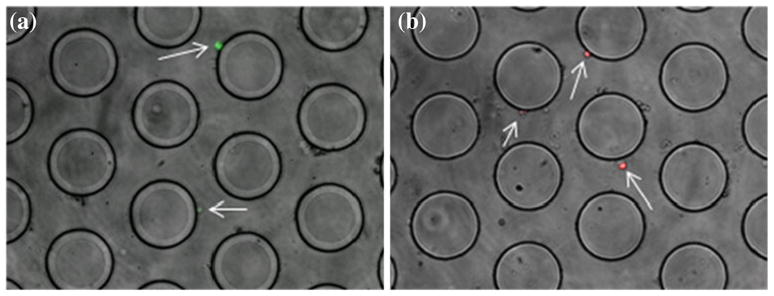
(a) Capture of Jurkat T Lymphocytes (green) on PNA coated post device, (b) Capture of Raji B Lymphocytes (red) on LTL coated post device (arrows indicate where the cells of interest are located in the device)
The capture efficiency values reported in this table represent the percentage of Jurkat or Raji cells captured relative to the number flowed through the device. The reported efficiency values for both cell types are low, as are the purity of the captured cells. The LTL coated device captured more Raji than the PNA coated device did for Jurkat cells in the post device. The Hele-Shaw data however shows the opposite trend where Jurkat cells adhered more to PNA than Raji cells did to LTL at the same shear stress. This inconsistency is due to the fact that the Hele-Shaw data was collected with homogeneous suspensions of cells where there is (1) no competitive binding with non-target species, (2) absence of physical and chemical interactions between different cell types, and (3) absence of other biological components (i.e. cell debris, serum components). Hence the Hele-Shaw is useful only so far as it provides an estimate of relative adhesion strength (to identify good capture molecules to use) and flow conditions.
Approximately half of the captured cells in the post device can be effectively detached by the inhibiting sugars, namely galactose for PNA- and L-fucose for LTL-coated devices (Table 1). Even though the proportion of released cells is relatively low, it is worth noting that this release is accomplished by a short period of flow with materials that are highly cyto-compatible without the need for any thermal or optical stimulation (Plouffe et al. 2009; Fujioka et al. 2003; Fukumori et al. 2010). The cell-release capability of the inhibiting sugars arises from the reversible nature of the attractive binding forces between lectins and cell surface carbohydrates. Indeed, the low-magnitude of attractive forces (Kd ~ 16–200 μM; (Smith et al. 2003)) is likely the reason for the low capture efficiency and purity. The capture efficiency can, in principle, be increased by using a lower flow rate for the blood sample, using a longer device or multiple devices in series, or increasing the surface density of immobilized lectins. However, given the relatively low affinity of the lectins to the target cells, these approaches are not expected to dramatically improve performance.
The true strength of the present approach for lectin-based capture and release of target cells from blood lies within the realm of low-cost capture of abundant target cells with non-stimulatory elution capability. Furthermore, the capture and release steps in this work are accomplished in less than an hour. At its current state this technology is not capable of differentiating between healthy and cancerous lymphocytes. There is literature that suggests that lectins are able to distinguish the agglutinin pattern for cancerous cells versus healthy cells (Gijzen et al. 2008). As a cancer diagnostic tool this device serves as a proof of concept that lectins can be used as a capture molecule which may have future diagnostic potential.
4 Conclusions
Lectins and their inhibiting sugars can be utilized to achieve capture and release of target cells in microfluidic channels. While this approach has constraints with respect to captured cell efficiency and purity, the low-cost nature of the capture system and the cyto-compatibility of the inhibiting sugar make it a system of interest for blood-based diagnostic applications where target cells are relatively abundant.
Acknowledgments
We gratefully acknowledge financial support from internal research funds provided to SKM by Northeastern University. This work was also supported by the National Cancer Institute (U01-CA128427-01) and by the Korean Research WCU Grant R31-2008-000-10086-0.
Contributor Information
Dwayne A. L. Vickers, Department of Chemical Engineering, Northeastern University, 360 Huntington Ave. 342 SN, Boston, MA 02115, USA
Marina Hincapie, Barnett Institute and Department of Chemistry & Chemical, Biology, Northeastern University, Boston, MA, USA.
William S. Hancock, Barnett Institute and Department of Chemistry & Chemical, Biology, Northeastern University, Boston, MA, USA
Shashi K. Murthy, Email: smurthy@coe.neu.edu, Department of Chemical Engineering, Northeastern University, 360 Huntington Ave. 342 SN, Boston, MA 02115, USA
References
- Chatterjee U, Bose PP, Dey S, Singh TP, Chatterjee BP. Glycoconj J. 2008;25:8. doi: 10.1007/s10719-008-9134-8. [DOI] [PubMed] [Google Scholar]
- Chen SY, Zheng T, Shortreed MR, Alexander C, Smith LM. Anal Chem. 2007;79:15. doi: 10.1021/ac070423k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka N, Morimoto Y, Takeuchi K, Yoshioka M, Kikuchi M. Appl Spectrosc. 2003;57:2. doi: 10.1366/000370203321535187. [DOI] [PubMed] [Google Scholar]
- Fukumori K, Akiyama Y, Kumashiro Y, Kobayashi J, Yamato M, Sakai K, Okano T. Macromol Biosci. 2010;10:10. doi: 10.1002/mabi.201000043. [DOI] [PubMed] [Google Scholar]
- Gijzen K, Raymakers RAP, Broers KM, Figdor CG, Torensma R. Exp Hematol. 2008;36:7. doi: 10.1016/j.exphem.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Gleghorn JP, Pratt ED, Denning D, Liu H, Bander NH, Tagawa ST, Nanus DM, Giannakakou PA, Kirby BJ. Lab Chip. 2010;10:1. doi: 10.1039/b917959c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer JD, Ross CW, Li CY, Witzig TE, Gascoyne RD, Dewald GW, Hanson CA. Blood. 1995;86:3. [PubMed] [Google Scholar]
- Iskratsch T, Braun A, Paschinger K, Wilson IBH. Anal Biochem. 2009;386:2. doi: 10.1016/j.ab.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Xu J, Ward E. CA Cancer J Clin. 2010 doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Kaku H, Kaneko H, Minamihara N, Iwata K, Jordan ET, Rojo MA, Minami-Ishii N, Minami E, Hisajima S, Shibuya N. J Biochem. 2007;142:3. doi: 10.1093/jb/mvm146. [DOI] [PubMed] [Google Scholar]
- Kullolli M, Hancock WS, Hincapie M. J Sep Sci. 2008;31:14. doi: 10.1002/jssc.200800233. [DOI] [PubMed] [Google Scholar]
- Lu H, Koo LY, Wang WCM, Lauffenburger DA, Griffith LG, Jensen KF. Anal Chem. 2004;76:18. doi: 10.1021/ac049837t. [DOI] [PubMed] [Google Scholar]
- Mulligan CS, Thomas ME, Mulligan SP. N Engl J Med. 2008;359:19. doi: 10.1056/NEJMc086211. [DOI] [PubMed] [Google Scholar]
- Murthy SK, Sin A, Tompkins RG, Toner M. Langmuir. 2004;20:26. doi: 10.1021/la048047b. [DOI] [PubMed] [Google Scholar]
- Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A, Ryan P, Balis UJ, Tompkins RG, Haber DA, Toner M. Nature. 2007;450:7173. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock J. Leukemia: perspectives on disease and illness. Capstone Press; Mankato: 2000. pp. 5–18. [Google Scholar]
- Plouffe BD, Njoka DN, Harris J, Liao JH, Horick NK, Radisic M, Murthy SK. Langmuir. 2007;23:9. doi: 10.1021/la0700220. [DOI] [PubMed] [Google Scholar]
- Plouffe BD, Brown MA, Iyer RK, Radisic M, Murthy SK. Lab Chip. 2009;9:11. doi: 10.1039/b823523f. [DOI] [PubMed] [Google Scholar]
- Smith EA, Thomas WD, Kiessling LL, Corn RM. J Am Chem Soc. 2003;125:20. doi: 10.1021/ja034165u. [DOI] [PubMed] [Google Scholar]
- Toner M, Irimia D. Annu Rev Biomed Eng. 2005:7. doi: 10.1146/annurev.bioeng.7.011205.135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usami S, Chen HH, Zhao YH, Chien S, Skalak R. Ann Biomed Eng. 1993;21:1. doi: 10.1007/BF02368167. [DOI] [PubMed] [Google Scholar]
- van den Ancker W, Terwijn M, Westers TM, Merle PA, van Beckhoven E, Drager AM, Ossenkoppele GJ, van de Loosdrecht AA. Leukemia. 2010;24:7. doi: 10.1038/leu.2010.119. [DOI] [PubMed] [Google Scholar]
- Vickers JA, Caulum MM, Henry CS. Anal Chem. 2006;78:21. doi: 10.1021/ac0609632. [DOI] [PubMed] [Google Scholar]
- Wang HX, Ng TB, Ooi VEC, Liu WK. Int J Biochem Cell Biol. 2000;32:3. doi: 10.1016/s1357-2725(99)00130-2. [DOI] [PubMed] [Google Scholar]
- Wankhede SP, Du ZQ, Berg JM, Vaughn MW, Dallas T, Cheng KH, Gollahon L. Biotechnol Prog. 2006;22:5. doi: 10.1021/bp060127d. [DOI] [PubMed] [Google Scholar]
- Wearne KA, Winter HC, O’Shea K, Goldstein IJ. Glycobiology. 2006;16:10. doi: 10.1093/glycob/cwl019. [DOI] [PubMed] [Google Scholar]
- Xia YN, Whitesides GM. Annu Rev Mater Sci. 1998;28 [Google Scholar]
- Zheng T, Yu HM, Alexander CM, Beebe DJ, Smith LM. Biomed Microdevices. 2007;9:4. doi: 10.1007/s10544-007-9074-2. [DOI] [PubMed] [Google Scholar]