Abstract
Aims
Sudden cardiac death (SCD) is a major contributor to the excess mortality of patients on maintenance dialysis. Homoarginine deficiency may lead to decreased nitric oxide availability and endothelial dysfunction. Based on this rationale we assessed whether homoarginine deficiency is a risk factor for SCD in dialysis patients.
Methods and results
This study examined the association of homoarginine with cardiovascular outcomes in 1255 diabetic haemodialysis patients from the German diabetes and dialysis study. During a median of 4 years of follow-up, hazard ratios (HR) (95% CI) for reaching the following pre-specified, adjudicated endpoints were determined: SCD, myocardial infarction, stroke, death due to heart failure, and combined cardiovascular events. There was a strong association of low homoarginine concentrations with the presence of congestive heart failure and left ventricular hypertrophy as well as increased levels of brain natriuretic peptide. Per unit decrease in homoarginine, the risk of SCD increased three-fold (HR 3.1, 95% CI 2.0–4.9), attenuating slightly in multivariate models (HR 2.4; 95% CI 1.5–3.9). Patients in the lowest homoarginine quintile experienced a more than two-fold increased risk of SCD, and more than three-fold increased risk of heart failure death than patients in the highest quintile, which accounted for the high incidence of combined cardiovascular events. Low homoarginine showed a trend towards increased risk of stroke, however, myocardial infarction was not meaningfully affected.
Conclusion
Low homoarginine is a strong risk factor for SCD and death due to heart failure in haemodialysis patients. Further studies are needed to elucidate the underlying mechanisms, offering the potential to develop new interventional strategies.
Keywords: Homoarginine, Sudden cardiac death, Heart failure, Amino acids, Haemodialysis
Introduction
Despite efforts to improve outcomes in renal replacement therapy, mortality of patients undergoing haemodialysis remains unacceptably high.1–3 Cardiac and vascular events are the predominant causes of death.1 The major cause of death is sudden cardiac death (SCD); accounting for one quarter of all deaths in dialysis patients.1,2,4 Understanding the mechanisms of SCD and identifying risk factors are essential for developing novel interventional strategies and to reduce the excess mortality of dialysis patients.
Homoarginine is a cationic amino acid, which is derived from lysine and mainly synthesized in the kidney by transamination of its precursor.5,6 Evidence suggests that homoarginine increases the availability of nitric oxide (NO),7–12 a lack of which is associated with endothelial and myocardial dysfunction.9–12 Studies have shown that homoarginine serves as a precursor of NO by increasing the intracellular concentration of L-arginine, which is the main substrate for NO synthase.9–12 Importantly, a recent study documented that low blood concentrations of homoarginine are associated with markedly increased mortality in patients referred for coronary angiography and in patients undergoing haemodialysis.13 In addition, homoarginine was inversely correlated to markers of impaired endothelial function, i.e. to the intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1). Furthermore, homoarginine was associated with the prevalence of heart failure, incrementally increasing at lower levels of homoarginine.13
We reasoned that endothelial dysfunction may play a crucial role in the arrhythmogenesis of patients with kidney failure and tested the hypothesis that low blood concentrations of homoarginine increase the risk of SCD in dialysis patients. We analysed data from the German diabetes dialysis study (4D Study: Die Deutsche Diabetes Dialyse Studie), which was a prospective study evaluating atorvastatin in 1255 patients with type 2 diabetes mellitus undergoing maintenance haemodialysis.4
Methods
Study design and participants
The methodology of the 4D study has previously been reported in detail.14 Briefly, the 4D study was a prospective randomized controlled trial of 1255 patients with type 2 diabetes mellitus, aged 18–80 years, who started haemodialysis within the last 2 years. Between March 1998 and October 2002, patients were recruited from 178 dialysis centres in Germany. After a run-in period of 4 weeks, patients were randomly assigned to double-blinded treatment with either 20 mg atorvastatin (n= 619) or placebo (n= 636) once daily. Study visits took place three times before randomization (Visit 1–3), at randomization (Visit 4), at 4 weeks (Visit 5), and then every 6 months (Visit 6, etc.) after randomization until the date of death, censoring, or the end of the study in March 2004. At each follow-up, blood samples were taken and clinical information including any adverse events, and an electrocardiogram was recorded. The study conformed to the principles outlined in the Declaration of Helsinki. It was approved by the appropriate medical ethics committee, and all patients gave their written informed consent before inclusion.
Data collection
Information on age, gender, and smoking status was obtained through patient interviews. Smoking status was classified as never, former, or current. Comorbidities, including the presence of coronary artery disease (CAD) and congestive heart failure (CHF), as well as the duration of diabetes mellitus, and dialysis treatment were reported by the patients' nephrologists. Coronary artery disease was defined as a history of myocardial infarction (MI), coronary artery bypass grafting surgery; percutaneous coronary intervention; and the presence of coronary heart disease, as documented by coronary angiography. Blood pressure was measured in sitting position. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared.
Homoarginine measurement
Homoarginine was measured in blood samples taken at baseline during study Visit 3 (1 week before randomization)., using a reverse-phase high-performance liquid chromatography method.15,16 Samples were stored at −80°C prior to analysis. Within-day coefficients of variation (CV) were 4.7% (1.21 µM) and 2.2% (3.53 µM), and between-day CV were 7.9% (1.25 µM) and 6.8% (3.66 µM), respectively. All blood samples were taken before the start of dialysis sessions and administration of drugs.
Outcome assessment
The primary endpoint of the 4D study was defined as a composite of death from cardiac causes, fatal or non-fatal stroke, and non-fatal MI, which ever occurred first (composite cardiovascular endpoint; CVE). Death from cardiac causes comprised SCD, fatal MI, death due to congestive heart failure, death due to coronary heart disease during or within 28 days after an intervention, and all other deaths attributable to coronary heart disease. Sudden cardiac death was considered as: death as verified by terminal rhythm disorders in an electrocardiogram; by witnesses observed death within 1 hour after the onset of cardiac symptoms; confirmed by autopsy; unexpected death, presumably or possibly of cardiac origin and in the absence of a potassium level ≥7.5 mmol/L before the start of the three most recent sessions of haemodialysis. Myocardial infarction was diagnosed when two of the following three criteria were met: typical symptoms, elevated levels of cardiac enzymes (i.e. creatine kinase MB above 5% of the total level of creatine kinase, lactic dehydrogenase 1.5 times the upper limit of normal, or a troponin T level >2 ng/mL), or diagnostic changes on the electrocardiogram. When death occurred within 28 days after an MI as diagnosed above, it was specified as death due to MI. The classifications were made exclusively, with fatal MI only being classified as MI death, and not SCD. Stroke was defined as a neurological deficit lasting longer than 24 hours. Computed tomographic or magnetic resonance imaging was available in all but 16 cases.
The 4D study endpoints were centrally adjudicated by three members of the endpoint committee blinded to study treatment and according to pre-defined criteria.4
For the present analysis, SCD, MI (fatal and non-fatal), stroke (fatal and non-fatal), death due to CHF, and the primary endpoint of combined CVE, were all chosen as separate outcome measures. The categorization of these events was based on the primary judgement of the endpoint committee during the 4D study.
Statistical analysis
Continuous variables are expressed as mean with standard deviation or median with interquartile range (IQR) as appropriate, and categorical variables are expressed as percentages.
The study population was divided into five groups, according to quintiles of homoarginine levels at baseline: ≤0.81 µmol/L, >0.81 to ≤1.0 µmol/L, >1.0 to ≤ 1.2 µmol/L, >1.2 to ≤1.48 µmol/L, >1.48 µmol/L. First, we assessed the association of baseline homoarginine with SCD both as a continuous and as a categorical variable. For the latter, the patients in the highest homoarginine quintile were used as the reference group. Absolute (incidence) rates were calculated, and relative risks derived from Cox regression analyses, i.e. hazard ratios (HRs) and corresponding 95% confidence intervals. The Cox regression analyses were adjusted for the confounders: age, sex, atorvastatin treatment, duration of dialysis, CAD, smoking status, BMI, low-density lipoprotein-cholesterol, haemoglobin, and albumin. Second, to explore potential pathways, we performed additional analyses with inclusion of potential intermediate conditions as suggested from previous experimental and clinical research: we additionally adjusted our analyses for congestive heart failure, arrhythmia as defined by the absence of sinus rhythm, levels of brain natriuretic peptide, C-reactive protein, blood pressure, HbA1c, and calcium and phosphate as markers of mineral metabolism. Third, we investigated homoarginine and the risk of other adverse cardiac and vascular outcomes including MI, stroke, death due to heart failure, and the combined primary endpoint, to distinguish whether the potential effects of homoarginine are specific for SCD, or generally influence cardiac and vascular outcomes. Finally, to exclude potential interaction by atorvastatin treatment, we repeated our analyses stratified by medication.
All P values are reported as two sided. Analyses were performed using SPSS version 16.0.
Results
Patient characteristics
Of the 1255 patients included in the 4D study, 1244 patients had a homoarginine measurement at baseline. The mean follow-up period was 3.96 years (median 4.0 years) on atorvastatin and 3.91 years (median 4.08 years) on placebo. During follow-up, 617 patients died, including 160 patients who died of SCD, and 41 patients who died due to congestive heart failure. A total of 469 patients reached the primary endpoint of CVE with MI (fatal or non-fatal), and stroke (fatal or non-fatal) occurring in 200 and 103 patients, respectively.
The mean (standard deviation) homoarginine concentration at baseline was 1.2 (0.5) µmol/L; with no significant difference between the atorvastatin and placebo groups. The baseline patient characteristics are shown in Table 1. Homoarginine concentrations were lower at higher age, lower BMI, lower albumin, lower haemoglobin, and were lower in females as compared to males. Low homoarginine concentrations were furthermore associated with a longer history of diabetes mellitus and higher levels of C-reactive protein. Most importantly, there was a strong consistent relation of low homoarginine concentrations with indicators of impaired cardiac function, including an increased prevalence of CHF and left ventricular hypertrophy as well as incrementally increasing levels of brain natriuretic peptide at lower levels of homoarginine.
Table 1.
Patient characteristics according to quintiles of homoarginine concentration at baseline; study population n= 1244
| Characteristic | Homoarginine concentration at baseline (μmol/L) |
||||
|---|---|---|---|---|---|
| Quintile 1 ≤0.81 (n=258) | Quintile 2 >0.81 to ≤1.0 (n=246) | Quintile 3 >1.0 to ≤1.2 (n=257) | Quintile 4 >1.2 to ≤1.48 (n=239) | Quintile 5 >1.48 (n=244) | |
| Age (years) | 68 (7) | 65 (9) | 66 (8) | 65 (8) | 64 (8) |
| Gender (%) male | 32 | 48 | 58 | 59 | 74 |
| BMI (kg/m2) | 26.5 (4.7) | 27.1 (4.9) | 27.7 (5.1) | 28.0 (4.6) | 28.5 (4.5) |
| Atorvastatin treatment (%) | 48 | 46 | 48 | 49 | 55 |
| Arterial hypertension (%) | 89 | 89 | 86 | 89 | 91 |
| Systolic BP (mmHg) | 144 (23) | 146 (24) | 147 (21) | 145 (21) | 146 (21) |
| Diastolic BP (mmHg) | 74 (11) | 76 (13) | 75 (11) | 76 (10) | 77 (10) |
| Smoker/ex-smoker (%) | 31 | 40 | 41 | 45 | 46 |
| Duration of diabetes (years) | 19.1 (9.1) | 18.6 (8.2) | 18.2 (8.5) | 17.8 (9.0) | 16.7 (8.9) |
| Time on dialysis (months) | 9.2 (7.2) | 8.0 (6.3) | 7.9 (6.5) | 8.4 (6.8) | 7.6 (7.3) |
| History of | |||||
| CAD (%) | 28 | 36 | 27 | 27 | 29 |
| CHF (%) | 44 | 37 | 35 | 29 | 32 |
| Absence of sinus rhythm (%) | 15 | 17 | 11 | 13 | 7 |
| Laboratory parameters | |||||
| Total cholesterol (mg/dL) | 223 (46) | 217 (44) | 221 (43) | 217 (40) | 217 (40) |
| LDL cholesterol (mg/dL) | 130 (34) | 126 (28) | 127 (30) | 121 (29) | 124 (28) |
| HDL cholesterol (mg/dL) | 37 (13) | 36 (13) | 37 (14) | 36 (13) | 35 (12) |
| Albumin g/dL | 3.7 (0.3) | 3.8 (0.3) | 3.8 (0.3) | 3.9 (0.3) | 3.9 (0.3) |
| Haemoglobin g/dL | 10.7 (1.5) | 10.9 (1.3) | 11.0 (1.3) | 11.0 (1.4) | 11.1 (1.3) |
| Calcium mmol/L | 2.3 (0.2) | 2.3 (0.3) | 2.3 (0.2) | 2.3 (0.2) | 2.3 (0.2) |
| Phosphate (mmol/L) | 6.2 (1.9) | 6.0 (1.7) | 6.0 (1.6) | 6.1 (1.5) | 5.8 (1.4) |
| NT-proBNP (pg/mL) | 6221 (2426–15728) | 4600 (2064–10608) | 3045 (1513–6832) | 2456 (1054–6400) | 2045 (896–5079) |
| C-reactive protein (mg/L) | 5.8 (2.5–16.8) | 4.9 (2.2–13.3) | 4.6 (2.1–12.3) | 5.1 (2.2–12.3) | 4.9 (2.3–10.7) |
Values are presented as means (SD) or median (interquartile range), or %.
BMI, body mass index; BP, blood pressure; HbA1c, glycated haemoglobin A1c; CAD, coronary artery disease; CHF, congestive heart failure; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Homoarginine and the risk of sudden cardiac death
Lower homoarginine levels at baseline were associated with higher incidences of SCD. Per unit decrease in homoarginine (continuous variable), the crude risk of SCD significantly increased more than three-fold (HR crude 3.1, 95% CI 2.0–4.9, Table 2). The association persisted after adjustment for confounders (HR 2.4, 95% CI 1.5–3.9). When patients were divided into quintiles according to their homoarginine level at baseline, the risk increased incrementally: patients in the lowest homoarginine quintile exhibited a >2-fold higher risk of SCD (HR adjusted 2.1 95% CI: 1.2–3.9), as compared to those in the highest quintile (Table 3 and Figure 1A).
Table 2.
Absolute rates of sudden cardiac death, myocardial infarction , stroke, primary endpoint, heart failure death, sudden or heart failure death combined, and the primary endpoint except for sudden or heart failure death; and hazard ratios (95% CI) per unit decrease in homoarginine; n= 1244
| Sudden cardiac death | Myocardial infarction | Stroke | Heart failure death | |
|---|---|---|---|---|
| Events | 160 | 200 | 103 | 41 |
| Person-years (py) | 3555 | 3368 | 3465 | 3555 |
| Incidence rate/100 py | 4.5 | 5.9 | 3.0 | 1.2 |
| HR (95% CI) | ||||
| Homoarginine unadjusted | 3.1 (2.0–4.9) | 1.0 (0.8–1.4) | 2.0 (1.1–3.0) | 1.6 (0.8–3.5) |
| Homoarginine adjusteda | 2.4 (1.5–3.9) | 1.0 (0.7–1.3) | 1.3 (0.8–2.1) | 1.9 (0.8–4.4) |
| Homoarginine adjustedb | 2.2 (1.4–3.5) | 0.9 (0.6–1.2) | 1.2 (0.7–1.9) | 1.3 (0.5–2.9) |
| Primary endpointc | Sudden or heart failure death | Primary endpoint except for sudden or heart failure death | ||
| Events | 469 | 201 | 268 | |
| Person-years (py) | 3287 | 3555 | 3287 | |
| Incidence rate/100 py | 14.3 | 5.7 | 8.2 | |
| HR (95% CI) | ||||
| Homoarginine unadjusted | 1.6 (1.3–2.0) | 2.7 (1.9–4.0) | 0.9 (0.7–1.2) | |
| Homoarginine adjusteda | 1.3 (1.1–1.7) | 2.3 (1.5–3.5) | 1.0 (0.8–1.4) | |
| Homoarginine adjustedb | 1.2 (0.9–1.5) | 2.0 (1.3–3.0) | 1.1 (0.9–1.5) | |
aAdjusted hazard ratio: Adjustments were made for age, sex, atorvastatin treatment, time on dialysis, smoking status, body mass index, levels of LDL-cholesterol, albumin, haemoglobin, and the presence of coronary artery disease (as defined by history of myocardial infarction, coronary artery bypass grafting surgery, percutaneous coronary intervention, coronary heart disease documented by coronary angiography).
bAdjusted hazard ratio: additional adjustments were made for potential intermediate conditions including congestive heart failure, arrhythmia as defined by the absence of sinus rhythm, levels of N-terminal-pro-B-type natriuretic peptide, C-reactive protein, HbA1c, calcium, phosphate, and systolic and diastolic blood pressure.
cThe primary endpoint was a composite of death from cardiac causes, fatal stroke, non-fatal myocardial infarction, or non-fatal stroke, whichever occurred first.
Table 3.
Hazard ratio and 95% confidence interval of sudden cardiac death, myocardial infarction, stroke, death due to heart failure, combined cardiovascular events, sudden and heart failure deaths combined, and cardiovascular events except for sudden and heart failure deaths, by quintiles of homoarginine at baseline; study population n= 1244
| Outcome | Homoarginine levels at baseline (μmol/L) |
||||
|---|---|---|---|---|---|
| Quintile 1 ≤0.81 (n= 258) | Quintile 2 > 0.81 to ≤1.0 (n= 246) | Quintile 3 > 1.0 to ≤1.2 (n= 257) | Quintile 4 > 1.2 to ≤1.48 (n= 239) | Quintile 5 > 1.48 (n= 244) | |
| Sudden cardiac death | |||||
| Crude HR (95% CI) | 2.9 (1.7–5.1) | 2.7 (1.6–4.8) | 2.0 (1.1–3.6) | 1.5 (0.8–2.7) | 1 |
| Adj.a HR (95% CI) | 2.1 (1.2–3.9) | 2.2 (1.2–3.9) | 1.9 (1.1–3.4) | 1.4 (0.7–2.5) | 1 |
| Myocardial infarction | |||||
| Crude HR (95% CI) | 1.1 (0.7–1.8) | 1.0 (0.7–1.6) | 1.1 (0.8–1.7) | 0.8 (0.5–1.3) | 1 |
| Adj.a HR (95% CI) | 1.1 (0.7–1.7) | 1.0 (0.6–1.5) | 1.1 (0.7–1.7) | 0.8 (0.5–1.3) | 1 |
| Stroke | |||||
| Crude HR (95% CI) | 2.6 (1.4–4.9) | 1.4 (0.7–2.9) | 1.8 (0.9–3.5) | 1.1 (0.5–2.3) | 1 |
| Adj.a HR (95% CI) | 1.7 (0.9–3.3) | 1.2 (0.6–2.5) | 1.6 (0.8–3.2) | 1.0 (0.5–2.1) | 1 |
| Death due to heart failure | |||||
| Crude HR (95% CI) | 2.6 (0.9–7.4) | 1.8 (0.6–5.4) | 1.9 (0.6–5.7) | 1.6 (0.5–4.9) | 1 |
| Adj.a HR (95% CI) | 3.3 (1.1–10.1) | 1.9 (0.6–6.0) | 1.9 (0.6–5.9) | 1.7 (0.5–5.2) | 1 |
| Cardiovascular events | |||||
| Crude HR (95% CI) | 1.8 (1.4–2.4) | 1.5 (1.1–2.1) | 1.4 (1.1–1.9) | 1.1 (0.8–1.5) | 1 |
| Adj.a HR (95% CI) | 1.5 (1.1–2.0) | 1.4 (1.0–1.8) | 1.4 (1.0–1.8) | 1.0 (0.7–1.4) | 1 |
| Sudden or heart failure death | |||||
| Crude HR (95% CI) | 2.8 (1.7–4.7) | 2.5 (1.5–4.2) | 2.0 (1.2–3.3) | 1.5 (0.9–2.6) | 1 |
| Adj.a HR (95% CI) | 2.3 (1.4–3.9) | 2.2 (1.3–3.6) | 1.9 (1.1–3.2) | 1.4 (0.8–2.5) | 1 |
| Cardiovascular events except sudden or heart failure death | |||||
| Crude HR (95% CI) | 1.4 (0.9–2.0) | 1.1 (0.7–1.6) | 1.2 (0.8–1.7) | 0.9 (0.6–1.3) | 1 |
| Adj.a HR (95% CI) | 1.1 (0.8–1.7) | 1.0 (0.7–1.4) | 1.1 (0.8–1.7) | 0.8 (0.6–1.3) | 1 |
aModel 1: age, sex, atorvastatin treatment, time on dialysis, smoking status, body mass index, levels of LDL-cholesterol, albumin, haemoglobin, and the presence of coronary artery disease.
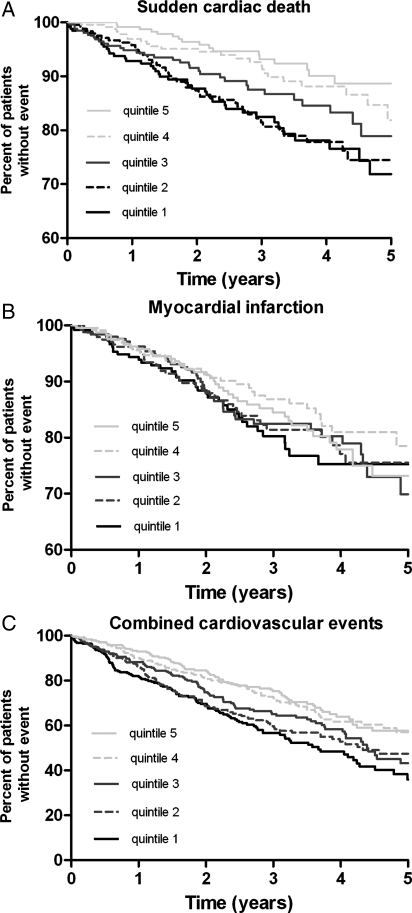
Figure 1.
Kaplan−Meier curves for the time to (A) sudden cardiac death, (B) myocardial infarction, (C) combined cardiovascular events in subgroups of patients according to the homoarginine concentrations at baseline (quintiles).
We investigated potential intermediate pathways and additionally adjusted our analyses for congestive heart failure, arrhythmia, brain natriuretic peptide levels, C-reactive protein, blood pressure, HbA1c, and markers of mineral metabolism. As expected, the effect estimates decreased, supporting the existence of the suggested mechanisms of homoarginine action.
Homoarginine and the risk of myocardial infarction, stroke, death due to heart failure, and combined cardiovascular events
In contrast to the results seen for SCD, homoarginine did not meaningfully affect the risk of MI. Both in continuous (HR adjusted 1.0, 95% CI 0.7–1.3) and in categorical analyses, the incidence of MI did not increase over varying concentrations of homoarginine (Tables 2 and 3, Figure 1B). When non-fatal and fatal MIs were analysed separately, the results were similar showing no association with homoarginine.
An increased risk of stroke was observed at low levels of homoarginine in unadjusted analyses. This association was partly attenuated after adjustment for confounders, leaving a trend for low homoarginine concentrations to increase the risk of stroke. Importantly, the adjusted analyses revealed a strong relation of low homoarginine with the risk of death due to heart failure. Patients with lowest levels in the first homoarginine quintile were three times more likely to die of heart failure than patients in the highest homoarginine quintile.(Table 3).
When investigating cardiovascular events combined as reflected by the primary endpoint of the 4D study, the incidence of the latter was markedly higher at lower concentrations of homoarginine (Figure 1C). Patients in the lowest homoarginine quintile had an 80% increased risk of developing a cardiovascular event, which remained increased by 50% after adjustment for confounders (Table 3). Based on our analyses investigating the single endpoints, we were interested to know whether this increased risk of the combined primary endpoint was mainly explained by the effect of homoarginine on sudden cardiac and heart failure deaths. When excluding these, no association was seen for the primary endpoint any longer (Tables 2 and 3).
In order to strengthen our results, and to eliminate any potential influence of atorvastatin treatment, we repeated all analyses in the placebo group only. The results were similar, indicating no interaction and supporting the use of the complete data.
Discussion
We analysed data from 1255 haemodialysis patients with type 2 diabetes mellitus, who took part in the 4D study and experienced a high incidence of pre-specified and centrally adjudicated endpoints. In the present analysis, low baseline homoarginine concentration was a strong risk factor for SCD and death due to heart failure during 4 years of follow-up. In patients of the lowest homoarginine quintile, the risk of dying suddenly was more than twice as high, and the risk of death due to heart failure three times as high compared to those of the highest homoarginine quintile. Furthermore, there was a trend for low homoarginine concentrations to increase the risk of stroke; however, MI was not affected. The incidence of the combined primary endpoint was significantly higher at lower concentrations of homoarginine, and this was mainly explained by the effect of homoarginine on sudden cardiac and heart failure deaths.
In advanced chronic kidney disease and end-stage renal disease, the pattern and composition of cardiovascular risk is changing. It is determined by various components such as SCD, stroke, and MI, and may vary with changing proportions of these components. In the general population, cardiovascular risk is mainly determined by the incidence of MI, representing the most frequent cause of death. In contrast, dialysis patients pre-dominantly die of SCD, which as a single cause accounts for one quarter of all deaths.1,2 Various causes may account for SCD in dialysis patients, including microvascular and macrovascular disease, sympathetic over-activity, structural heart disease, cardiac fibrosis, and electrolyte and volume shifts due to the haemodialysis procedure.17,18 Furthermore, we have recently shown that poor glycaemic control and vitamin D deficiency were strongly associated with the incidence of SCD in dialysis patients.19,20 Despite these advances in the evaluation of risk factors, current knowledge is still limited, i.e. the known risk factors do not sufficiently explain the excess rate of SCD in dialysis patients.
The present work is the first study to identify low homoarginine concentrations as a novel risk factor for SCD in dialysis patients. Evidence suggests that homoarginine may increase NO availability, the lack of which is associated with endothelial, and myocardial dysfunction.9–12 In a previous study, we found an inverse association between homoarginine and markers of impaired endothelial function (ICAM-1 and VCAM-1).13 Furthermore, we found an association between indicators of inflammation including albumin and CRP, and low homoarginine concentration. Endothelial dysfunction and inflammation may increase the risk of SCD via the development of premature atherosclerosis and cytokine-induced plaque instability, or by direct effects on the myocardium, and electrical conduction system.21 Cytokines are also involved in the modulation of ion channel function and the generation of arrhythmias,22,23 as well as in the aggravation of sympathetic tone, leading to tachycardia and cardiac electrical instability. In our study, patients with low homoarginine concentrations more frequently had arrhythmias, particularly absence of sinus rhythm. Furthermore, we observed a high burden of CHF and increased levels of brain natriuretic peptide in patients in the lowest quintile of homoarginine concentration. These factors are known to be strong predictors of SCD and may, at least in part, reflect the effect of homoarginine on structural changes in the heart.17,18,24
The role of homoarginine affecting structural changes in the heart may also account for its association with heart failure. Besides the effect on endothelial dysfunction and the release of cytokines, low homoarginine may also affect blood pressure and insulin secretion, thus contributing to cardiac hypertrophy, and fibrosis as major predisposing conditions for heart failure. Furthermore, it is of high interest that the key enzyme for homoarginine synthesis, arginine:glycine amidinotransferase (AGAT), is upregulated in heart failure.25 One study found that myocardial AGAT mRNA expression, and enzyme activity were elevated in heart failure compared with controls and returned to normal after functional normalization and recovery. The data indicated that the expression of AGAT enabled the myocardium to synthesize creatine locally. This upregulation of myocardial AGAT expression was suggested to be an adaptive process, counteracting the decreased intracellular creatine availability in heart failure by local creatine production.25 The notion of AGAT being involved in cellular energy metabolism is supported by further data. An experimental study showed that AGAT mRNA was upregulated in the skeletal muscles of mice with Duchenne muscular dystrophy compared to mature mice.26 It has been argued that this process may help maintain muscle creatine levels and limit cellular energy failure in the leaky skeletal muscles of affected mice. Extending these data, our study adds important new knowledge by identifying low homoarginine as an important risk factor for death due to heart failure and also provides a new target for interventions.
In this context, it is not surprising that the increased risk of combined cardiovascular events associated with low homoarginine concentrations in our study was mainly explained by the impact of homoarginine concentration on sudden cardiac and heart failure deaths. Other studies have suggested that homoarginine plays a role in the pathogenesis of diabetes mellitus and arterial hypertension.8,27 Homoarginine has been shown to stimulate insulin secretion,27,28 with glycaemic state meaningfully increasing the incidence of SCD, but not of myocardial infarction.19 The risk of MI as a major macrovascular complication was not affected by low homoarginine in our study. There was a moderate effect on stroke, which is thought to result from both micro- and macrovascular complications. Therefore, the potential influence of homoarginine in glucose metabolism and the importance of microvascular complications may help to explain the effect on stroke in contrast to myocardial infarction. Furthermore, previous studies have found that administration of L-homoarginine increased urinary excretion of nitrate, the degradation product of NO, and reduced blood pressure in salt-sensitive hypertensive rats.8 This may explain why we observed in our study a trend for an association between low homoarginine concentrations and the incidence of stroke, which is in line with results from a previous study, identifying low homoarginine as a novel risk factor for fatal strokes in patients undergoing coronary angiography.29 As mentioned above, homoarginine plays a role in the metabolism of NO, which is—apart from the cardiac effects—critically involved in the regulation of cerebral blood flow and cell viability.30
Potential limitations of our study need to be acknowledged. It was a post-hoc analysis within a selected cohort of German patients with type 2 diabetes mellitus undergoing haemodialysis. Therefore, the relationship between low homoarginine and adverse outcomes may not be generalizable to other patient populations. In this context, potential effects of homoarginine on MI may not have been seen because of competing risks in diabetic dialysis patients. Despite careful adjustments for possible confounders, we cannot rule out residual confounding. However, since the known important confounders were considered, the effect of potential residual confounding is likely to be small. Furthermore, we cannot draw conclusions regarding causality from our data but our data indicate that low homoarginine levels reflect a novel pathophysiological process related to a poor cardiovascular outcome. Given that single-nucleotide polymorphisms (SNPs) or activity of the key enzyme for homoarginine synthesis have been previously related to renal and myocardial diseases supports a causal association between homoarginine metabolism and adverse health outcomes.The main strengths of this study are that we were able to analyse specific outcomes including SCD. Further strengths include the long-term follow-up, adequate sample size, and high incidence of pre-specified and centrally adjudicated endpoints.
In conclusion, low blood homoarginine concentrations were strongly associated with SCD and death due to heart failure in haemodialysis patients. Furthermore, there was a trend for low homoarginine concentrations to be associated with a higher risk of stroke, but not of myocardial infarction. The risk of combined cardiovascular events increased significantly at lower concentrations of homoarginine, and was mainly explained by the impact of homoarginine on sudden cardiac and heart failure deaths. Longitudinal assessment of homoarginine may be useful for risk monitoring of dialysis patients. Beyond this, homoarginine may become a promising novel target for therapeutic interventions in populations with high incidences of heart failure and SCD.
Funding
C.D. is grateful to the Deutsche Forschungsgemeinschaft and to the Medical Faculty of the University of Wuerzburg for the support with a research fellowship.
Conflict of interest: none declared.
Acknowledgements
We thank all patients who participated in the 4D study. We are grateful to all investigators, study nurses, and collaborators involved in patient recruitment, sample and data handling, and the laboratory staff at the Universities of Freiburg, Würzburg, and Graz.
References
- 1. US Renal Data System: USRDS 2009 Annual Data Report. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2009. [Google Scholar]
- 2.Collins AJ, Foley RN, Herzog C, Chavers BM, Gilbertson D, Ishani A, Kasiske BL, Liu J, Mau LW, McBean M, Murray A, St PW, Guo H, Li Q, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers PW, Agodoa L. Excerpts from the US Renal Data System 2009 Annual Data Report. Am J Kidney Dis. 2010;55:S1–S7. doi: 10.1053/j.ajkd.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Dijk PC, Jager KJ, de CF, Collart F, Cornet R, Dekker FW, Gronhagen-Riska C, Kramar R, Leivestad T, Simpson K, Briggs JD. Renal replacement therapy in Europe: the results of a collaborative effort by the ERA-EDTA registry and six national or regional registries. Nephrol Dial Transplant. 2001;16:1120–1129. doi: 10.1093/ndt/16.6.1120. [DOI] [PubMed] [Google Scholar]
- 4.Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 5.Ryan WL, Wells IC. Homocitrulline and homoarginine synthesis from lysine. Science. 1964;144:1122–1127. doi: 10.1126/science.144.3622.1122. [DOI] [PubMed] [Google Scholar]
- 6.Ryan WL, Johnson RJ, Dimari S. Homoarginine synthesis by rat kidney. Arch Biochem Biophys. 1969;131:521–526. doi: 10.1016/0003-9861(69)90425-1. [DOI] [PubMed] [Google Scholar]
- 7.Bauersachs J, Widder JD. Endothelial dysfunction in heart failure. Pharmacol Rep. 2008;60:119–126. [PubMed] [Google Scholar]
- 8.Chen PY, Sanders PW. Role of nitric oxide synthesis in salt-sensitive hypertension in Dahl/Rapp rats. Hypertension. 1993;22:812–818. doi: 10.1161/01.hyp.22.6.812. [DOI] [PubMed] [Google Scholar]
- 9.Hrabak A, Bajor T, Temesi A. Comparison of substrate and inhibitor specificity of arginase and nitric oxide (NO) synthase for arginine analogues and related compounds in murine and rat macrophages. Biochem Biophys Res Commun. 1994;198:206–212. doi: 10.1006/bbrc.1994.1029. [DOI] [PubMed] [Google Scholar]
- 10.Knowles RG, Palacios M, Palmer RM, Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci USA. 1989;86:5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valtonen P, Laitinen T, Lyyra-Laitinen T, Raitakari OT, Juonala M, Viikari JS, Heiskanen N, Vanninen E, Punnonen K, Heinonen S. Serum L-homoarginine concentration is elevated during normal pregnancy and is related to flow-mediated vasodilatation. Circ J. 2008;72:1879–1884. doi: 10.1253/circj.cj-08-0240. [DOI] [PubMed] [Google Scholar]
- 12.Yang Z, Ming XF. Endothelial arginase: a new target in atherosclerosis. Curr Hypertens Rep. 2006;8:54–59. doi: 10.1007/s11906-006-0041-8. [DOI] [PubMed] [Google Scholar]
- 13.Marz W, Meinitzer A, Drechsler C, Pilz S, Krane V, Kleber ME, Fischer J, Winkelmann BR, Bohm BO, Ritz E, Wanner C. Homoarginine, cardiovascular risk, and mortality. Circulation. 2010;122:967–975. doi: 10.1161/CIRCULATIONAHA.109.908988. [DOI] [PubMed] [Google Scholar]
- 14.Wanner C, Krane V, Marz W, Olschewski M, Asmus HG, Kramer W, Kuhn KW, Kutemeyer H, Mann JF, Ruf G, Ritz E. Randomized controlled trial on the efficacy and safety of atorvastatin in patients with type 2 diabetes on hemodialysis (4D study): demographic and baseline characteristics. Kidney Blood Press Res. 2004;27:259–266. doi: 10.1159/000080241. [DOI] [PubMed] [Google Scholar]
- 15.Meinitzer A, Puchinger M, Winklhofer-Roob BM, Rock E, Ribalta J, Roob JM, Sundl I, Halwachs-Baumann G, Marz W. Reference values for plasma concentrations of asymmetrical dimethylarginine (ADMA) and other arginine metabolites in men after validation of a chromatographic method. Clin Chim Acta. 2007;384:141–148. doi: 10.1016/j.cca.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Teerlink T, Nijveldt RJ, de Jong S, van Leeuwen PA. Determination of arginine, asymmetric dimethylarginine, and symmetric dimethylarginine in human plasma and other biological samples by high-performance liquid chromatography. Anal Biochem. 2002;303:131–137. doi: 10.1006/abio.2001.5575. [DOI] [PubMed] [Google Scholar]
- 17.Herzog CA, Mangrum JM, Passman R. Sudden cardiac death and dialysis patients. Semin Dial. 2008;21:300–307. doi: 10.1111/j.1525-139X.2008.00455.x. [DOI] [PubMed] [Google Scholar]
- 18.Ritz E, Wanner C. The challenge of sudden death in dialysis patients. Clin J Am Soc Nephrol. 2008;3:920–929. doi: 10.2215/CJN.04571007. [DOI] [PubMed] [Google Scholar]
- 19.Drechsler C, Krane V, Ritz E, Marz W, Wanner C. Glycemic control and cardiovascular events in diabetic hemodialysis patients. Circulation. 2009;120:2421–2428. doi: 10.1161/CIRCULATIONAHA.109.857268. [DOI] [PubMed] [Google Scholar]
- 20.Drechsler C, Pilz S, Obermayer-Pietsch B, Verduijn M, Tomaschitz A, Krane V, Espe K, Dekker F, Brandenburg V, Marz W, Ritz E, Wanner C. Vitamin D deficiency is associated with sudden cardiac death, combined cardiovascular events, and mortality in haemodialysis patients. Eur Heart J. 2010;31:2253–2261. doi: 10.1093/eurheartj/ehq246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dernellis J, Panaretou M. C-reactive protein and paroxysmal atrial fibrillation: evidence of the implication of an inflammatory process in paroxysmal atrial fibrillation. Acta Cardiol. 2001;56:375–380. doi: 10.2143/AC.56.6.2005701. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman BF, Guo SD, Feinmark SJ. Arrhythmias caused by platelet activating factor. J Cardiovasc Electrophysiol. 1996;7:120–133. doi: 10.1111/j.1540-8167.1996.tb00507.x. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman BF, Feinmark SJ, Guo SD. Electrophysiologic effects of interactions between activated canine neutrophils and cardiac myocytes. J Cardiovasc Electrophysiol. 1997;8:679–687. doi: 10.1111/j.1540-8167.1997.tb01831.x. [DOI] [PubMed] [Google Scholar]
- 24.Winkler K, Wanner C, Drechsler C, Lilienthal J, Marz W, Krane V. Change in N-terminal-pro-B-type-natriuretic-peptide and the risk of sudden death, stroke, myocardial infarction, and all-cause mortality in diabetic dialysis patients. Eur Heart J. 2008;29:2092–2099. doi: 10.1093/eurheartj/ehn278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cullen ME, Yuen AH, Felkin LE, Smolenski RT, Hall JL, Grindle S, Miller LW, Birks EJ, Yacoub MH, Barton PJ. Myocardial expression of the arginine:glycine amidinotransferase gene is elevated in heart failure and normalized after recovery: potential implications for local creatine synthesis. Circulation. 2006;114:I16–I20. doi: 10.1161/CIRCULATIONAHA.105.000448. [DOI] [PubMed] [Google Scholar]
- 26.McClure WC, Rabon RE, Ogawa H, Tseng BS. Upregulation of the creatine synthetic pathway in skeletal muscles of mature mdx mice. Neuromuscul Disord. 2007;17:639–650. doi: 10.1016/j.nmd.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henningsson R, Lundquist I. Arginine-induced insulin release is decreased and glucagon increased in parallel with islet NO production. Am J Physiol. 1998;275:E500–E506. doi: 10.1152/ajpendo.1998.275.3.E500. [DOI] [PubMed] [Google Scholar]
- 28.Blachier F, Mourtada A, Sener A, Malaisse WJ. Stimulus-secretion coupling of arginine-induced insulin release. Uptake of metabolized and nonmetabolized cationic amino acids by pancreatic islets. Endocrinology. 1989;124:134–141. doi: 10.1210/endo-124-1-134. [DOI] [PubMed] [Google Scholar]
- 29.Pilz S, Tomaschitz A, Meinitzer A, Drechsler C, Ritz E, Krane V, Wanner C, Böhm BO, März W. Low serum homoarginine Is a novel risk factor for fatal strokes in patients undergoing coronary angiography. Stroke. 2011;42:1132–1134. doi: 10.1161/STROKEAHA.110.603035. [DOI] [PubMed] [Google Scholar]
- 30.Toda N, Ayajiki K, Okamura T. Cerebral blood flow regulation by nitric oxide: recent advances. Pharmacol Rev. 2009;61:62–97. doi: 10.1124/pr.108.000547. [DOI] [PubMed] [Google Scholar]