Abstract
Aims
One of the primary determinants of blood flow in regional vascular beds is perfusion pressure. Our aim was to investigate if reduction in blood pressure during the treatment of decompensated heart failure would be associated with worsening renal function (WRF). Our secondary aim was to evaluate the prognostic significance of this potentially treatment-induced form of WRF.
Methods and results
Subjects included in the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial limited data were studied (386 patients). Reduction in systolic blood pressure (SBP) was greater in patients experiencing WRF (−10.3 ± 18.5 vs. −2.8 ± 16.0 mmHg, P < 0.001) with larger reductions associated with greater odds for WRF (odds ratio = 1.3 per 10 mmHg reduction, P < 0.001). Systolic blood pressure reduction (relative change > median) was associated with greater doses of in-hospital oral vasodilators (P ≤ 0.017), thiazide diuretic use (P = 0.035), and greater weight reduction (P = 0.023). In patients with SBP-reduction, WRF was not associated with worsened survival [adjusted hazard ratio (HR) = 0.76, P = 0.58]. However, in patients without SBP-reduction, WRF was strongly associated with increased mortality (adjusted HR = 5.3, P < 0.001, P interaction = 0.001).
Conclusion
During the treatment of decompensated heart failure, significant blood pressure reduction is strongly associated with WRF. However, WRF that occurs in the setting of SBP-reduction is not associated with an adverse prognosis, whereas WRF in the absence of this provocation is strongly associated with increased mortality. These data suggest that WRF may represent the final common pathway of several mechanistically distinct processes, each with potentially different prognostic implications.
Keywords: Cardio-renal syndrome, Worsening renal function, Kidney, Decompensated heart failure, Blood pressure
Introduction
Worsening renal function (WRF) is a common complication that arises during the treatment of acute decompensated heart failure and has been associated with adverse outcomes such as decreased survival.1 Traditional teaching has held that the primary haemodynamic derangement responsible is a reduction in cardiac output that leads to reduced renal perfusion and ultimately WRF. However, these concepts have not been confirmed in recent studies.2–5 The lack of association between changes in cardiac output and WRF may be explained by the fact that regulation of renal blood flow and glomerular filtration is dependent on pressure rather than flow.6,7
Given the limited cardio-renal reserve present in patients with decompensated heart failure, it is possible that deterioration in renal function could be precipitated by relatively small reductions in blood pressure. The primary objective of this study was to determine whether WRF was associated with in-hospital blood pressure reduction. Additionally, a reduction in glomerular filtration rate (GFR) can represent a relatively normal response to treatment-induced physiological derangements such as reduction in renal perfusion and/or intravascular volume, even in health. As a result, we hypothesized that WRF in the setting of a substantial reduction in blood pressure may have limited prognostic importance as opposed to WRF associated with less clear provocation.
Methods
The Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) Trial was a National Heart, Lung and Blood Institute (NHLBI) sponsored, randomized, multicentre trial of therapy guided by pulmonary artery catheter (PAC) vs. clinical assessment in hospitalized patients with acute decompensated heart failure. Methods and results have been previously published.8,9 Briefly, 433 patients were enrolled at 26 sites from January 2000 to November 2003. Inclusion criteria were: an ejection fraction of 30% or less, systolic blood pressure of 125 mmHg or less, and at least one sign and one symptom of congestion. Exclusion criteria included an admission creatinine level >3.5 mg/dL. Patients were randomized to therapy guided by clinical assessment alone vs. PAC and clinical assessment. Treatment goals were resolution of the signs and symptoms of congestion and investigators were encouraged to ‘avoid progressive renal dysfunction or symptomatic systemic hypotension.’ The ESCAPE trial was conducted and supported by the NHLBI in collaboration with the ESCAPE study investigators. This analysis was conducted using a limited access dataset obtained from the NHLBI and does not necessarily reflect the opinions or views of the ESCAPE investigators or the NHLBI.
To account for the non-linear relationship between renal function and serum creatinine, WRF was defined as a ≥20% decrease in estimated GFR, unless otherwise specified.10,11 Changes in GFR were evaluated from baseline to discharge unless otherwise noted. Glomerular filtration rate was estimated using the four variable Modification of Diet in Renal Disease study equation.12 Haemoconcentration was defined as ≥2 of 3 of: a change in total protein, albumin, and haematocrit in the top tertile for primary analyses.5 Due to the low number of events in the haemoconcentration group as defined above (n = 4), stratified and interaction analyses utilized the component variable, change in haematocrit, due to the larger number of subjects with complete data (n = 325) and larger number of terminal events (n = 13) in patients meeting haemoconcentration by this definition. A relative change in systolic blood pressure (SBP-reduction) was calculated as the percentage change in admission to discharge systolic blood pressure using admission blood pressure as the reference. In-hospital doses of angiotensin-converting enzyme inhibitors (ACE inhibitors) and angiotensin receptor blockers (ARBs) were normalized to lisinopril equivalents and represent the maximum daily dose administered. This study was approved by the institutional review committee of the Hospital of the University of Pennsylvania.
Statistical analysis
Values reported are mean±standard deviation, median (quartile1–quartile3), and percentile unless otherwise noted. Independent Student's t-test or the Mann–Whitney U test was used to compare means of independent continuous variables. Pearson's χ2 was used to evaluate categorical variables. Paired samples t-test or Wilcoxon-signed rank test was used for comparison of continuous variables over time within groups. Correlation coefficients represent Pearson's r or Spearman's rho. The primary outcome of interest was the relationship between an in-hospital reduction in blood pressure and WRF, and their associated interaction with all-cause mortality. Logistic regression modelling was used to evaluate multivariate associations between WRF and changes in blood pressure. Candidate covariates were selected by entering all variables demonstrating a difference (P≤ 0.2) between patients with a relative admission to discharge change in blood pressure above or below the median. Cox proportional modelling was used to evaluate time-to-event associations with all-cause mortality and patients alive at the end of follow-up (180 days) were censored. Candidate covariates for multivariable proportional hazards modelling were obtained by screening all baseline variables and those with a univariate association with mortality (P ≤ 0.2) were entered in the model. Stratum-specific hazard ratios (HR) were derived from proportional hazards modelling of the individual strata and the significance of the interaction was formally assessed using a model incorporating terms for the main effect of WRF, the main effect of the treatment-related variable of interest, and the interaction between these variables. In all multivariate models, covariates were removed using backward elimination (likelihood ratio) and variables with P < 0.2 were retained.13 Kaplan–Meier curves for death from any cause were plotted for the four combinations of groups between WRF and the treatment-related variable of interest, and equality of survival function was tested by means of the log-rank statistic. Significance was defined as two-tailed P < 0.05 for all analyses except for tests for interaction where P < 0.1 was considered significant. Statistical analyses were performed with SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics of the overall trial population and their interaction with PAC randomization have been previously reported.9 Additionally, the lack of association between WRF and PAC-derived haemodynamic variables at baseline, PAC removal, and the associated change has been previously described in the ESCAPE dataset.4,5 Characteristics of the 386 patients with admission and discharge data for renal function and blood pressure who were analysed in this sub-study are described in Table 1.
Table 1.
Patient characteristics
| Characteristics | Overall cohort | No SBP-reduction | Yes SBP-reduction | P |
|---|---|---|---|---|
| (n = 386) | (n = 193) | (n = 193) | ||
| Demographics | ||||
| Age (years) | 56.4 ± 13.9 | 57.1 ± 13.3 | 55.7 ± 14.5 | 0.309 |
| Males | 74.1% | 74.1% | 74.1% | 1.00 |
| White race | 60.6% | 65.8% | 55.4% | 0.037* |
| Medical history | ||||
| Ischaemic HF aetiology | 50.3% | 50.3% | 50.3% | 1.00 |
| Hypertension | 46.6% | 47.2% | 46.1% | 0.838 |
| Diabetes | 31.9% | 36.3% | 27.5% | 0.063 |
| Functional status/ejection fraction | ||||
| NYHA class (mean class) | 3.9 ± 0.4 | 3.9 ± 0.3 | 3.9 ± 0.4 | 0.773 |
| Six-minute walk (feet) | 422 ± 420 | 460 ± 445 | 384 ± 392 | 0.092 |
| Maximal oxygen consumption (mL/kg/min) | 10.1 ± 3.5 | 10.4 ± 3.9 | 9.7 ± 2.8 | 0.324 |
| Ejection fraction (%) | 19.5 ± 6.5 | 19.6 ± 6.7 | 19.3 ± 6.4 | 0.684 |
| Vital signs (baseline) | ||||
| Systolic blood pressure (mmHg) | 105.8 ± 16.0 | 98.9 ± 12.9 | 112.7 ± 15.8 | <0.001* |
| Diastolic blood pressure (mmHg) | 66.7 ± 11.4 | 61.9 ± 9.6 | 71.6 ± 11.1 | <0.001* |
| Heart rate (beats/min) | 81.6 ± 15.1 | 79.2 ± 15.2 | 84.0 ± 14.6 | 0.002* |
| Respiration rate (breaths/min) | 20.4 ± 3.9 | 19.9 ± 3.8 | 21.0 ± 3.9 | 0.008* |
| Vital signs (discharge) | ||||
| Systolic blood pressure (mmHg) | 101.5 ± 14.7 | 107.6 ± 15.0 | 95.5 ± 11.7 | <0.001* |
| Diastolic blood pressure (mmHg) | 61.3 ± 10.8 | 63.5 ± 11.5 | 59.0 ± 9.6 | <0.001* |
| Heart rate (beats/min) | 79.2 ± 13.9 | 79.0 ± 13.7 | 79.5 ± 14.3 | 0.727 |
| Respiration rate (breaths/min) | 18.5 ± 2.8 | 18.7 ± 2.9 | 18.4 ± 2.6 | 0.379 |
| Admission to discharge change in blood pressure | ||||
| Absolute (mmHg) | 4.3 ± 16.8 | 8.6 ± 10.0 | −17.2 ± 11.5 | <0.001* |
| Relative (%) | 2.8 ± 15.3 | 9.1 ± 10.8 | −14.7 ± 8.2 | <0.001* |
| Laboratory findings | ||||
| Haemoglobin (g/dL) | 12.5 ± 1.8 | 12.5 ± 1.8 | 12.6 ± 1.8 | 0.468 |
| Serum sodium (mEq/L) | 136.7 ± 4.4 | 136.2 ± 4.7 | 137.3 ± 4.0 | 0.008* |
| Glomerular filtration rate (mL/min/1.73 m2) | 56.9 ± 25.2 | 53.7 ± 25.6 | 60.1 ± 24.5 | 0.012* |
| Treatment-related parameters | ||||
| Length of stay (days) | 8.3 ± 6.3 | 7.9 ± 5.4 | 8.6 ± 7.0 | 0.298 |
| Change in weight (kg) | 3.5 ± 4.8 | 2.9 ± 4.5 | 4.0 ± 5.1 | 0.023* |
The overall cohort represents all patients with an admission and discharge pair of systolic blood pressures and creatinine levels available. The admission to discharge relative reduction in systolic blood pressure was dichotomized about the mean producing the yes and no SBP-reduction groups.
P values refer to the difference between these groups.
NYHA, New York Heart Association; HF, heart failure; SBP, systolic blood pressure.
*Represents a significant P value.
Despite a relatively low median admission SBP of 104 mmHg (93–117 mmHg), substantial admission to discharge reductions in blood pressure were common with 36.5% of subjects having a ≥ 10 mmHg and 17.6% having a ≥ 20 mmHg reduction in admission to discharge SBP. Significant reductions in admission to discharge diastolic blood pressure (DBP) were also common with 38.6% of the population experiencing a ≥ 10 mmHg and 15.5% a ≥ 20 mmHg reduction. Similar to previous reports from this population, in the current subset of patients' baseline to PAC removal changes in cardiac index (P = 0.50), right atrial pressure (P = 0.93), pulmonary capillary wedge pressure (P = 0.41), and systemic vascular resistance (P = 0.39) were no different between patients who did or did not develop WRF. However, the mean reduction in SBP was significantly greater in patients experiencing WRF compared to those who did not (−10.3 ± 18.5 vs. −2.8 ± 16.0 mmHg, P < 0.001). The odds for WRF increased progressively with larger reductions in SBP [odds ratio (OR) = 1.3 per 10 mmHg reduction, P < 0.001]. The change in admission to discharge DBP did not demonstrate a statistically significant difference between patients with or without WRF (−7.0 ± 13.3 vs. −5.1 ± 14.1 mmHg, P = 0.29). Changes in pulse pressure (−3.3 ± 16.8 vs. 2.3 ± 12.7 mmHg, P = 0.001) and changes in calculated mean arterial pressure (−8.1 ± 13.0 vs. −4.3 ± 13.5 mmHg, P = 0.027) were also greater in patients experiencing WRF. Change in renal perfusion pressure (mean arterial pressure – right atrial pressure, data available n = 149) was also greater in patients with WRF (−7.0 ± 14.3 vs. −0.4 ± 16.1 mmHg, P = 0.041).
The relative change in systolic blood pressure appeared to be more important than the absolute degree of discharge hypotension since discharge blood pressure was not different between patients who did or did not develop WRF (99.5 ± 13.7 vs. 102.0 ± 15.0, P = 0.17). Similarly, discharge SBP less than 70 mmHg (P = 0.98), 80 mmHg (P = 0.13), 90 mmHg (P = 0.81), 100 mmHg (P = 0.31), or 110 mmHg (P = 0.16) were not associated with WRF. On the contrary, higher admission SBP was associated with WRF (OR = 1.2 per 10 mmHg increase in SBP, P = 0.014). However, this association was no longer significant after controlling for the subsequent change in blood pressure (OR = 1.0, P = 0.80).
Given that a relative change in blood pressure appeared to be the primary driver for the association between blood pressure and WRF, the cohort was dichotomized based on the median relative admission to discharge change in systolic blood pressure. Patients with relative reductions in blood pressure above the median were defined as having had ‘SBP-reduction'. Patients with SBP-reduction were significantly more likely to have developed WRF (OR = 1.9, P = 0.017) and characteristics of these patients are presented in Tables 1 and 2. Patients who developed SBP-reduction had significantly higher baseline systolic blood pressure, lower baseline cardiac index, and demonstrated a trend towards higher baseline systemic vascular resistance (Table 2). Discharge haemodynamics were similar between groups; however, there was a greater reduction in pulmonary capillary wedge pressure and systemic vascular resistance in patients with SBP-reduction (Table 2). Both patients with and without SBP-reduction had a statistically significant improvement in baseline to PAC removal cardiac index (P < 0.001 for both groups); however, there was no difference between groups in the change in cardiac index (Table 2). Interestingly, randomization to the PAC arm of the study (P = 0.92) or treatment guided with a PAC (P = 0.68) was not associated with a lower incidence of SBP-reduction.
Table 2.
Pulmonary artery catheter-derived variables
| Characteristics | No SBP-reduction | Yes SBP-reduction | P |
|---|---|---|---|
| Haemodynamics (baseline) | (n = 87) | (n = 89) | |
| Right atrial pressure (mmHg) | 13.1 ± 10.5 | 13.4 ± 7.4 | 0.839 |
| Pulmonary artery systolic pressure (mmHg) | 55.2 ± 15.6 | 55.8 ± 13.7 | 0.773 |
| Pulmonary capillary wedge pressure (mmHg) | 23.9 ± 9.7 | 26.2 ± 8.9 | 0.196 |
| Cardiac index (L/min/m²) | 2.09 ± 0.68 | 1.86 ± 0.53 | 0.010* |
| Systemic vascular resistance (dyn-s/cm5) | 1365 ± 601 | 1624 ± 1034 | 0.051 |
| Haemodynamics (PAC removal) | (n = 80) | (n = 77) | |
| Right atrial pressure (mmHg) | 8.8 ± 5.8 | 9.2 ± 5.0 | 0.676 |
| Pulmonary artery systolic pressure (mmHg) | 46.9 ± 12.8 | 44.8 ± 12.4 | 0.294 |
| Pulmonary capillary wedge pressure (mmHg) | 18.1 ± 8.1 | 16.0 ± 7.4 | 0.204 |
| Cardiac index (L/min/m²) | 2.41 ± 0.69 | 2.30 ± 0.58 | 0.268 |
| Systemic vascular resistance (dyn-s/cm5) | 1154 ± 501 | 1079 ± 461 | 0.355 |
| Haemodynamics (change) | (n = 75) | (n = 76) | |
| Right atrial pressure (mmHg) | −4.3 ± 11.2 | −4.5 ± 6.8 | 0.927 |
| Pulmonary artery systolic pressure (mmHg) | −8.6 ± 12.6 | −11.0 ± 12.3 | 0.246 |
| Pulmonary capillary wedge pressure (mmHg) | −5.9 ± 9.1 | −9.1 ± 8.5 | 0.039* |
| Cardiac index (L/min/m²) | 0.35 ± 0.68 | 0.45 ± 0.65 | 0.379 |
| Systemic vascular resistance (dyn-s/cm5) | −220 ± 472 | −551 ± 1026 | 0.020* |
Values represent mean±standard deviation or %. The admission to discharge relative reduction in systolic blood pressure was dichotomized about the mean producing the yes and no SBP-reduction groups.
P values refer to the difference between these groups.
PAC, pulmonary artery catheter; SBP, systolic blood pressure.
*Represents a significant P value.
Controlling for baseline differences between groups (serum sodium, heart rate, SBP, DBP, respiratory rate, GFR, race, 6 min walk distance, and diabetes) did not alter the relationship between the degree of reduction in SBP and WRF (OR = 1.4 per 10 mmHg decrease in SBP, P < 0.001). Likewise, the association with WRF was not significantly altered by controlling for baseline cardiac index and systemic vascular resistance (OR = 1.3 per 10 mmHg decrease in SBP, P = 0.015) (data available n = 167). The dose of intravenous inotropes (milrinone P = 0.27, dobutamine P = 0.48), and intravenous vasodilators (nitroprusside P = 0.36, nitroglycerine P = 0.81, nesiritide P = 0.43) were not significantly different between patients with or without SBP-reduction. Given that blood pressure change was assessed from admission to discharge, this analysis is limited by the short half-life of these agents. However, the mean doses of in-hospital oral hydralazine (46.3 ± 116.6 vs. 21.4 ± 58.4 mg, P = 0.008), nitrates (42.0 ± 61.0 vs. 27.9 ± 43.9 mg, P = 0.010), and ACE inhibitors or ARBs (19.6 ± 20.4 vs. 15.2 ± 14.7 mg, P = 0.017) were significantly greater in the group with SBP-reduction. Additionally, the use of in-hospital adjuvant thiazide diuretics (OR = 1.6, P = 0.035) and the amount of weight lost during hospitalization (4.0 ± 5.1 vs. 2.9 ± 4.5 kg, P = 0.023) were greater in the group with SBP-reduction. Data on the in-hospital administration of other oral antihypertensives such as beta-blockers and calcium channel blockers were not available for analysis. Discharge life prolonging medication use (ACE inhibitors or ARBs, beta-blockers, hydralazine, nitrates, and spironolactone) was similar between patients with or without WRF (P ≥ 0.21 for all) or between patients with or without SBP-reduction (P ≥ 0.09 for all).
Changes in blood pressure did not appear to be solely a reflection of aggressive diuresis since there was minimal correlation between changes in blood pressure and variables reflecting haemoconcentration such as the change in total protein (r = 0.01, P = 0.92), albumin (r = 0.05, P = 0.57), or haematocrit (r = 0.14, P = 0.015). Additionally, the change in blood pressure was not different between patients with or without haemoconcentration (3.1 ± 17.0 vs. 3.1 ± 15.0 mmHg, P = 1.0) and haemoconcentration was not related to SBP-reduction (OR = 1.1, P = 0.82). Additionally, controlling for variables directly related to the aggressiveness of diuresis (change in weight, loop diuretic dose, adjuvant thiazide diuretic use) did not alter the odds for WRF in patients with SBP-reduction (OR = 1.9, P = 0.026).
Associations with mortality
Similar to prior reports from the ESCAPE trial, WRF did not demonstrate a statistically significant univariate association with all-cause mortality in this subpopulation (HR = 1.4, P = 0.28). There was also a lack of association between mortality and SBP-reduction (HR = 0.88, P = 0.59). This lack of association remained after adjusting for differences in baseline characteristics (serum sodium, heart rate, SBP, DBP, respiratory rate, GFR, race, 6 min walk distance, and diabetes) (HR = 1.1, P = 0.67) and discharge medication use (ACE inhibitors or ARBs, beta-blockers, hydralazine, nitrates, and spironolactone) (HR = 1.0, P = 0.97).
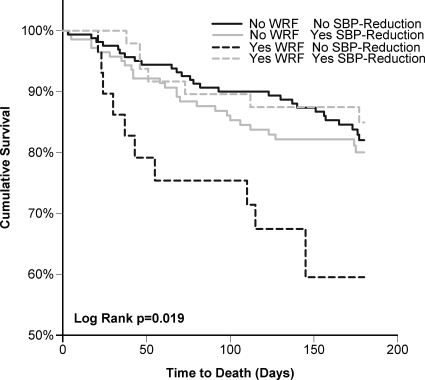
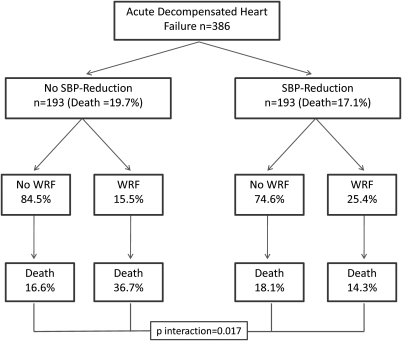
In patients who experienced SBP-reduction, WRF had no statistically significant association with mortality (HR = 0.72, P = 0.45) (Figure 1). After controlling for baseline variables associated with mortality (age, 6 min walk distance, SBP, DBP, sodium, GFR, ischaemic heart failure aetiology, and hypertension) (Table 3) or discharge medications (ACE inhibitors or ARBs, beta blockers, hydralazine, nitrates, and spironolactone) (HR = 0.74, P = 0.49) this lack of association again persisted. However, in patients who did not have SBP-reduction, WRF was strongly associated with increased mortality (HR = 2.7, P = 0.005, P interaction = 0.017) (Figure 1), an association which was strengthened by adjusting for baseline factors associated with mortality (age, 6 min walk distance, SBP, DBP, sodium, GFR, ischaemic aetiology, and hypertension) (Table 3). Adjusting for discharge medication use (ACE inhibitors or ARBs, beta-blockers, hydralazine, nitrates, and spironolactone) did not substantially alter the association (HR = 3.0, P = 0.004, P interaction = 0.015). The incidence of WRF and 6-month mortality in patients with and without SBP-reduction is illustrated in Figure 2.
Figure 1.
Kaplan–Meier plots for total survival grouped by degree of admission to discharge systolic blood pressure reduction and worsening renal function status. Systolic blood pressure reduction dichotomized about the median.
Table 3.
Association between various definitions of worsening renal function and reduction in blood pressure or all-cause mortality
| Risk of death associated with WRF in patients with: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WRF definition | Frequency | Association with reduction in SBPa | P | Risk of death in overall populationb | P | No SBP-reductionb | P | Yes SBP-Reductionb | P | P interactionb |
| ≥20% decrease in GFR (MDRD) | 20.5% | 1.3 | <0.001 | 1.8 | 0.060 | 5.3 | <0.001 | 0.76 | 0.577 | 0.001 |
| ≥20% decrease in GFR (CKD-EPI) | 18.7% | 1.3 | 0.004 | 1.7 | 0.104 | 4.8 | <0.001 | 0.54 | 0.259 | 0.002 |
| ≥25% increase in serum creatinine | 16.6% | 1.2 | 0.013 | 1.6 | 0.142 | 5.4 | <0.001 | 0.33 | 0.130 | 0.001 |
| ≥0.3 mg/dL increase in serum creatinine | 18.1% | 1.3 | 0.001 | 1.7 | 0.076 | 4.9 | <0.001 | 0.63 | 0.392 | 0.002 |
| ≥25% and ≥0.3 mg/dL increase in serum creatinine | 15.3% | 1.2 | 0.008 | 1.5 | 0.256 | 4.5 | 0.001 | 0.323 | 0.133 | 0.001 |
WRF, worsening renal function; SBP, systolic blood pressure; MDRD, modified diet and renal disease equation; CKD-EPI, chronic kidney disease epidemiology collaboration.
aPer 10 mmHg reduction in admission to discharge reduction in SBP.
bAdjusted for serum sodium, heart rate, SBP, diastolic blood pressure, respiratory rate, glomerular filtration rate, race, 6 min walk distance, and diabetes.
Figure 2.
Incidence of worsening renal function and 6-month mortality grouped in patients with and without a systolic blood pressure reduction below the median. SBP, systolic blood pressure; WRF, worsening renal function. Interaction P value derived from Cox proportional hazards modelling.
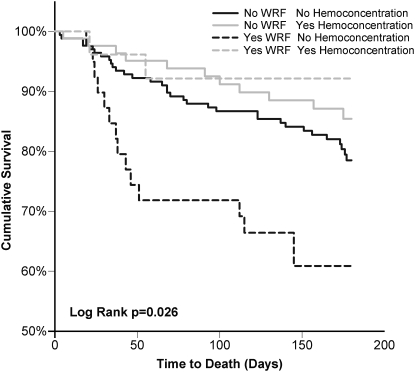
Haemoconcentration (defined as an admission to discharge change in haematocrit in the top tertile) also demonstrated a significant interaction with WRF-associated mortality (P = 0.075) (Figure 3). In patients who experienced haemoconcentration, WRF was not associated with mortality (HR = 0.57, P = 0.429). However, there was a significantly higher risk of death associated with WRF in patients who did not experience haemoconcentration (HR = 2.2, P = 0.019). Notably, patients with both characteristics indicative of aggressive treatment (haemoconcentration and SBP-reduction, n = 58) actually had improved survival associated with WRF (P = 0.024, P interaction = 0.004). A reliable hazard ratio cannot be reported due to the absence of deaths in the group with haemoconcentration/SBP-reduction and WRF. Of note, in patients experiencing WRF but without haemoconcentration/SBP-reduction, 26.9% of these patients died.
Figure 3.
Kaplan–Meier plots for total survival grouped by haemoconcentration and worsening renal function status. Haemoconcentration is defined as admission to discharge increase in haematocrit in the top tertile.
Discussion
The principal findings of this analysis are the strong relationship between WRF and blood pressure reduction during the treatment of acute decompensated heart failure and the differential relationship between WRF and mortality depending on change in blood pressure. In the subgroup of patients with a significant admission to discharge reduction in blood pressure, WRF was free from adverse prognostic implications. On the contrary, in patients without significant blood pressure reduction, WRF was associated with substantially increased mortality. Similar findings were noted after dichotomizing the cohort by the presence or absence of haemoconcentration, a parameter previously demonstrated to reflect aggressive diuresis.5 These data suggest that WRF may represent the final common pathway of several mechanistically distinct processes and, as such, its negative impact on survival may be contingent on the mechanism by which renal function is impaired.
The finding that changes in cardiac index, or interventions that increase cardiac index, have repeatedly failed to correlate with the development of WRF in contemporary studies is not surprising given what is known about the physiology governing flow in regional vascular beds.2–5 Blood flow in any vascular bed may be described using Ohm's law (Q = ΔP/R) where Q = regional blood flow, ΔP = the change in pressure, and R = resistance.14 In a series circuit total flow is directly coupled to the flow in all resistance beds. However, the kidney is configured in a parallel rather than series relationship allowing a disconnect between total flow in the overall circuit (e.g. cardiac output) and blood flow in individual resistance beds (i.e. renal blood flow), as long as ΔP is maintained. In health, renal autoregulation provides substantial stabilization in the setting of changes in blood pressure, largely by varying R in the above equation. However, vasodilators such as calcium channel blockers, common comorbidities such as chronic kidney disease, diabetes, and hypertension, in addition to heart failure itself, can lead to substantial impairment of renal autoregulation.15–18
A large body of literature has established that WRF is associated with significantly worsened outcomes.1 However, recent reports suggest that there may be multiple pathophysiological subtypes of cardio-renal dysfunction. One such factor appears to be venous congestion, as it has been demonstrated that baseline measures of venous congestion correlate with renal function.4,19–22 Notably, in the latter cited studies by Damman et al.21,22 not only was venous congestion correlated with a lower baseline GFR, but it was also strongly associated with subsequent mortality. In line with these observations, we have recently reported that patients with decompensated heart failure with evidence of venous congestion at baseline frequently experience an improvement in renal function with aggressive diuresis.23 We subsequently demonstrated that patients with improved renal function frequently have a significant post-discharge deterioration in renal function and a significantly increased risk for subsequent mortality.24 The increased mortality in patients with probable venous congestion induced renal dysfunction, described in the above studies, is in contrast to data from the current study where WRF in the setting of a reduction in blood pressure or haemoconcentration was not associated with adverse outcomes. Overall, these data argue that the cardio-renal syndrome may be a heterogeneous group of pathophysiological entities and the associated prognosis may be largely dependent on the mechanism by which the renal function is reduced.
The finding that SBP demonstrated a stronger association with WRF than either mean or DBP may have several potential explanations. It has recently been determined that systolic blood pressure provides the primary stimulus for afferent arteriolar myogenic autoregulatory response, and the afferent arteriole is the proposed location for the renal baroreceptor sensor controlling renin release in the kidney.6,17,25,26 As a result, it is possible that reductions in SBP could lead to exaggerated renin release further compounding the effects of the reduced blood pressure in a kidney with impaired autoregulation. Additionally, admission and discharge blood pressures in this study were determined by auscultation. It has been well described that DBP determination by the auscultatory method may be particularly prone to errors.27–29 These errors may have been compounded by the low blood pressure and stroke volume in this advanced heart failure population.
Limitations
Given the post-hoc nature of this study, the limitations of retrospective analyses apply, residual confounding cannot be excluded, and causality is impossible to conclusively determine. The ESCAPE trial was not designed to investigate WRF and given that treating physicians were not blinded to renal, PAC, or blood pressure data, it is likely that treatment strategies were modified in response to these variables. Additionally, the availability of complete data for only baseline and discharge serum creatinine and blood pressure limits the ability to determine temporal relationships between changes in blood pressure and WRF and could potentially introduce selection bias. While the ESCAPE trial is one of the largest contemporary datasets with detailed haemodynamic information regarding decompensated heart failure, by nature of the trial design, PAC data are only available in slightly more than half the patients, limiting power. Analysis of the effects of renal perfusion pressure is particularly limited by this factor. Additionally, the ESCAPE trial had stringent inclusion/exclusion criteria demanding a very high degree of heart failure disease severity. As a result, the characteristics of these patients are significantly different than that found in heart failure registries, limiting generalizability of these findings. Specifically, patients with advanced renal insufficiency (creatinine >3.5 mg/dL) and those with a systolic blood pressure ≥ 125 mmHg further limits generalization to other populations. Additionally, the small number of patients and events available for the analysis of haemoconcentration and haemoconcentration/SBP-reduction is a significant limitation and these analyses should be regarded as hypothesis generating only.
Conclusion
Significant admission to discharge blood pressure reductions, which appear to be at least partially treatment related, are strongly associated with WRF during the treatment of acute decompensated heart failure. Worsening renal function occurring in the absence of a reduction in blood pressure is associated with substantially increased mortality; however, WRF in the setting of in-hospital lowering of blood pressure is free from adverse prognostic implications. These findings support the concept that WRF is the result of more than one prognostically relevant cardio-renal phenotype. Further research is necessary to confirm these findings in alternative populations and investigate risk factors for WRF that are unrelated to aggressive diuresis or reductions in blood pressure.
Funding
National Institutes of Health Grant (grant number: 5T32HL007843-15).
Conflict of interest: none declared.
References
- 1.Damman K, Navis G, Voors AA, Asselbergs FW, Smilde TD, Cleland JG, van Veldhuisen DJ, Hillege HL. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail. 2007;13:599–608. doi: 10.1016/j.cardfail.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Klein L, Massie BM, Leimberger JD, O'Connor CM, Pina IL, Adams KF, Jr., Califf RM, Gheorghiade M. Admission or changes in renal function during hospitalization for worsening heart failure predict postdischarge survival: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) Circ Heart Fail. 2008;1:25–33. doi: 10.1161/CIRCHEARTFAILURE.107.746933. [DOI] [PubMed] [Google Scholar]
- 3.Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, Yancy CW, Califf RM, Stevenson LW, Hill JA. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol. 2008;51:1268–1274. doi: 10.1016/j.jacc.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 5.Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122:265–272. doi: 10.1161/CIRCULATIONAHA.109.933275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cupples WA. Interactions contributing to kidney blood flow autoregulation. Curr Opin Nephrol Hypertens. 2007;16:39–45. doi: 10.1097/MNH.0b013e3280117fc7. [DOI] [PubMed] [Google Scholar]
- 7.Brenner BM, Rector FC. Brenner & Rector's The Kidney. Philadelphia: Saunders Elsevier; 2008. [Google Scholar]
- 8.Shah MR, O'Connor CM, Sopko G, Hasselblad V, Califf RM, Stevenson LW. Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE): design and rationale. Am Heart J. 2001;141:528–535. doi: 10.1067/mhj.2001.113995. [DOI] [PubMed] [Google Scholar]
- 9.Binanay C, Califf RM, Hasselblad V, O'Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW, Investigators E, Coordinators ES. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294:1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 10.Testani JM, McCauley BD, Chen J, Shumski M, Shannon RP. Worsening renal function defined as an absolute increase in serum creatinine is a biased metric for the study of cardio-renal interactions. Cardiology. 2010;116:206–212. doi: 10.1159/000316038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damman K, Jaarsma T, Voors AA, Navis G, Hillege HL, van Veldhuisen DJ investigators C. Both in- and out-hospital worsening of renal function predict outcome in patients with heart failure: results from the Coordinating Study Evaluating Outcome of Advising and Counseling in Heart Failure (COACH) Eur J Heart Fail. 2009;11:847–854. doi: 10.1093/eurjhf/hfp108. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F Chronic Kidney Disease Epidemiology C. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 13.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 14.Guyton AC, Hall JE. Textbook of Medical Physiology. Philadelphia: Saunders; 2000. [Google Scholar]
- 15.Johnson RJ, Segal MS, Srinivas T, Ejaz A, Mu W, Roncal C, Sanchez-Lozada LG, Gersch M, Rodriguez-Iturbe B, Kang DH, Acosta JH. Essential hypertension, progressive renal disease, and uric acid: a pathogenetic link? J Am Soc Nephrol. 2005;16:1909–1919. doi: 10.1681/ASN.2005010063. [DOI] [PubMed] [Google Scholar]
- 16.Carmines PK. The renal vascular response to diabetes. Curr Opin Nephrol Hypertens. 2010;19:85–90. doi: 10.1097/MNH.0b013e32833240fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loutzenhiser R, Griffin K, Williamson G, Bidani A. Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1153–R1167. doi: 10.1152/ajpregu.00402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rea ME, Dunlap ME. Renal hemodynamics in heart failure: implications for treatment. Curr Opin Nephrol Hypertens. 2008;17:87–92. doi: 10.1097/MNH.0b013e3282f357da. [DOI] [PubMed] [Google Scholar]
- 19.Uthoff H, Breidthardt T, Klima T, Aschwanden M, Arenja N, Socrates T, Heinisch C, Noveanu M, Frischknecht B, Baumann U, Jaeger KA, Mueller C. Central venous pressure and impaired renal function in patients with acute heart failure. Eur J Heart Fail. 2011;13:432–439. doi: 10.1093/eurjhf/hfq195. [DOI] [PubMed] [Google Scholar]
- 20.Damman K, Navis G, Smilde TD, Voors AA, van der Bij W, van Veldhuisen DJ, Hillege HL. Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail. 2007;9:872–878. doi: 10.1016/j.ejheart.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–588. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 22.Damman K, Voors AA, Hillege HL, Navis G, Lechat P, van Veldhuisen DJ, Dargie HJ. Congestion in chronic systolic heart failure is related to renal dysfunction and increased mortality. Eur J Heart Fail. 2010;12:974–982. doi: 10.1093/eurjhf/hfq118. [DOI] [PubMed] [Google Scholar]
- 23.Testani JM, Khera AV, St John Sutton MG, Keane MG, Wiegers SE, Shannon RP, Kirkpatrick JN. Effect of right ventricular function and venous congestion on cardiorenal interactions during the treatment of decompensated heart failure. Am J Cardiol. 2010;105:511–516. doi: 10.1016/j.amjcard.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Testani JM, McCauley BD, Kimmel SE, Shannon RP. Characteristics of Patients With Improvement or Worsening in Renal Function During Treatment of Acute Decompensated Heart Failure. Am J Cardiol. 2010;106:1763–1769. doi: 10.1016/j.amjcard.2010.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cupples WA, Braam B. Assessment of renal autoregulation. Am J Physiol Renal Physiol. 2007;292:F1105–F1123. doi: 10.1152/ajprenal.00194.2006. [DOI] [PubMed] [Google Scholar]
- 26.Loutzenhiser R, Griffin KA, Bidani AK. Systolic blood pressure as the trigger for the renal myogenic response: protective or autoregulatory? Curr Opin Nephrol Hypertens. 2006;15:41–49. doi: 10.1097/01.mnh.0000199011.41552.de. [DOI] [PubMed] [Google Scholar]
- 27.Van Bergen FH, Weatherhead DS, Treloar AE, Dobkin AB, Buckley JJ. Comparison of indirect and direct methods of measuring arterial blood pressure. Circulation. 1954;10:481–490. doi: 10.1161/01.cir.10.4.481. [DOI] [PubMed] [Google Scholar]
- 28.Rebenson-Piano M, Holm K, Powers M. An examination of the differences that occur between direct and indirect blood pressure measurement. Heart Lung. 1987;16:285–294. [PubMed] [Google Scholar]
- 29.Tochikubo O, Kawano Y, Miyajima E, Ishii M. A new photo-oscillometric method employing the delta-algorithm for accurate blood pressure measurement. J Hypertens. 1997;15:147–156. doi: 10.1097/00004872-199715020-00005. [DOI] [PubMed] [Google Scholar]