Abstract
AKT is a serine/threonine protein kinase, also known as protein kinase B, which regulates cardiac growth, myocardial angiogenesis, glucose metabolism, and cell death in cardiac myocytes. AKT is activated by its phosphorylation at Thr 308 and ser 473 by PDK1 and mTORC2, respectively, in response to trophic stimuli such as insulin and insulin growth factor. c-Jun N-Terminal Kinases (JNKs) phosphorylate AKT at Thr 450 and potentiate its interaction with its downstream effectors. The short-term activation of AKT promotes physiological hypertrophy and protection from myocardial injury; whereas, its long-term activation causes pathological hypertrophy and heart failure. In this review we will discuss the role of AKT in regulating signalling pathways in the heart with special emphasis on the role of AKT in modulating stress induced autophagic cell death in cardiomyocytes in vitro.
Keywords: Protein kinase, AKT, Signaling pathways
Introduction
AKT is a serine/threonine protein kinase that regulates a variety of cellular functions in different tissues. AKT is the effector of PI3K and is essential during postnatal cardiac development that is achieved predominantly by hypertrophy rather than hyperplasia of individual cardiomyocytes.1 There are three isoforms of AKT, AKT1, AKT2, and AKT3. It has been shown that AKT1 KO mice have slightly diminished growth and that these mice are susceptible for spontaneous and stress-induced apoptosis.2,3 AKT2 KO mice have normal body size but are mildly insulin resistant,4 while AKT3 KO mice have reduced brain size that is attributed to decrease in cell number and cell size.5 The combined deletion of AKT1 and AKT2 or AKT1 and AKT3 genes result in perinatal lethality with multiple developmental defects, indicating a large degree of functional overlap between the three isoforms of AKT.6,7 On the other hand, increased AKT signaling in β-cells promotes hypertrophy and hyperplasia,8 whereas increased AKT function in T-lymphocytes and prostate cells promotes lymphoma9 and prostate cancer,10 respectively.
In the heart, short-term AKT1 activation promotes physiological hypertrophy,11 whereas long-term AKT1 activation induces pathological hypertrophy.12,13 Physiological and pathological hypertrophy are morphologically, functionally, and molecularly distinct from each others. Physiological hypertrophy occurs during postnatal cardiac development and in trained athletes and is characterized by normal or enhanced contractile function, normal architecture, and organization of cardiac structure without increases in interstitial fibrosis. Clinically, pathological hypertrophy is observed in patients with uncontrolled hypertension, myocardial infarction, and aortic stenosis. In its advanced stages, it is characterized by contractile dysfunction, interstitial fibrosis, and expression of foetal cardiac genes such as atrial natriuretic peptide and β-myosin heavy chain (β-MHC).14,15 Pathological hypertrophy is associated with increased morbidity and mortality and eventually the sustained pressure overload leads to myocardial contractile dysfunction, dilatation, and development of heart failure (HF) through poorly understood mechanisms.14,15 In endothelial cells, brief AKT activation attenuates damage to ischaemia, whereas prolonged AKT activation leads to increased but unorganized blood vessel formation, reminiscent of tumour vasculature.16 Hence, AKT signalling plays an important role in maintaining vascular homeostasis and that tight control of the AKT signal in endothelial cells is required for normal vascular patterning and remodelling. Growth factors such as insulin and insulin growth factor as well as exercise activate AKT through its recruitment to the cell membrane and its phosphorylation at Thr 308 and Ser 473 by PDK1 and mTORC2, respectively. On the other hand, absence of growth factors and sedentary lifestyle lead to decreased phosphorylation and hence inactivation of AKT.12,17 However, in stressed cardiac myocytes, such as in oxidative stress or in ischaemia reperfusion, AKT signalling and its interaction with its downstream effectors are altered or are overridden by other signalling pathways as discussed below. This review will address the role of AKT in promoting coronary angiogenesis and in modulating cardiac function with specific emphasis on the role of AKT in altering programmed types of cell death, such as autophagic and apoptotic cell death in cardiomyocytes and in HF.
AKT and angiogenesis
In endothelial cells, AKT phosphorylates a wide variety of target proteins that regulate cell proliferation, survival, permeability, release of nitric oxide, and cell migration.18,19 AKT is essential in vascular endothelial growth factor (VEGF)-mediated angiogenesis and regulates the migration of endothelial cells necessary for vessel sprouting, branching, and the formation of networks during angiogenesis via the phosphorylation of Girdin, an actin binding protein, at Ser 1416 to regulate its subcellular localization and cell migration in fibroblasts.20 Kitamura et al.21 have shown that the delivery of adenovirus harbouring Girdin short interfering RNA in Matrigel embedded in mice, markedly inhibited VEGF-mediated angiogenesis. Targeted disruption of the Girdin gene in mice impaired vessel remodelling in the retina and angiogenesis from aortic rings; however, Girdin was unessential for embryonic vasculogenesis. These findings demonstrate that the AKT/Girdin signalling pathway is essential in VEGF-mediated postneonatal angiogenesis. Short-term AKT activation in inducible transgenic AKT1 mice induces physiological hypertrophy with maintained vascular density. Coronary angiogenesis is enhanced to keep pace with the growth of the myocardium. The increase in vascular density is directly related to the increase in VEGF and angiopoietin-2 during short-term AKT1 activation.18,21 On the other hand, prolonged AKT activation leads to pathological hypertrophy. Under these conditions, VEGF and angiopoietin-2 are downregulated and capillary density is reduced. The decrease in coronary angiogenesis could in part explain the decrease in cardiac function during long-term AKT activation. It has been shown that VEGF blockade in pressure overload reduces capillary density and results in an accelerated transition from compensated hypertrophy to HF.22 Thus, the balance between cardiac growth and coronary angiogenesis is critical and that the cross-talk between cardiac myocytes and coronary vasculature is essential for the maintenance of the contractile function, especially in pathological hypertrophy and in HF.
AKT and cell death
Cardiac myocyte loss in HF via the form of necrotic, apoptotic, and autophagic cell death may in part contribute to the worsening in cardiac contractile function and to left ventricular remodelling. Strong evidence in the literature suggests that short-term activation of AKT have beneficial effects via the inhibition of apoptotic cell death. Matsui et al.23 showed that adenoviral gene transfer of activated AKT1 protects cardiomyocytes from apoptosis in response to hypoxia in vitro. Cardiomyocytes were infected with either a control adenovirus (Ad.EGFP) or adenoviruses carrying constitutively active forms of PI 3-kinase (Ad.BD110) or AKT (Ad.myr-AKT-HA). Ad.BD110 significantly inhibited apoptosis of hypoxic cardiomyocytes compared with Ad.EGFP. Ad.myr-AKT-HA even more dramatically inhibited apoptosis of hypoxic cardiomyocytes. Moreover, adenovirus-mediated AKT1 gene transfer in the heart diminishes cardiomyocyte apoptosis and limits infarct size following ischaemia/reperfusion injury24 and ameliorates doxorubicin-induced contractile dysfunction.25 Beside its anti-apoptotic effect, AKT1 controls autophagic cell death by phosphorylating and thus inhibiting the translocation of forkhead box O (FOXO) family, particularly FOXO3a, from the cytoplasm into the nucleus and thus inhibits FOXO3a effector, BNIP3, from initiating mitochondrial autophagy and mitochondrial defragmentation.26 This effect of AKT is mainly seen in the presence of growth stimuli and is lost with their absence such as during starvation.27,28 However, in stressed cardiomyocytes, AKT regulates the autophagic activity, on the short term, via c-Jun N-Terminal Kinases (JNKs) signaling. Our data suggest that the expression of the mitochondrial autophagic marker (BNIP3) is significantly upregulated 2h after cardiomyocyte stress with phenylephrine (PE). We have found that JNK phosphorylation is decreased in cardiomyocytes stressed with PE for 2h. The treatment of stressed cardiomyocytes with 3-methyladenine increases JNK phosphorylation which in turn phosphorylates AKT at thr 450 and enhances its interaction with its downstream effector FOXO3a and thus inhibits autophagy. SP600125, a specific JNK inhibitor, abolished the inhibitory effect of 3-methyladenine and restored the increase in autophagic activity in PE-stressed cardiomyocytes. These findings are supported by Zhili Shao et al.29 who showed that JNKs mediate reactivation of AKT and cardiomyocyte survival after hypoxic injury in vitro and in vivo. The authors demonstrate that reactivation of AKT after resolution of hypoxia is regulated by JNKs and suggest that this is likely a central mechanism of the myocyte protective effect of JNKs.
AKT and calcium cycling proteins
AKT1 signalling may also improve contractile function by influencing myocardial calcium cycling, which plays a critical role in contractility and relaxation of cardiomyocytes. During excitation of the cardiomyocyte, small calcium influx via the L-type calcium channels (LTCC) leads to massive release of calcium from the endoplasmic reticulum via the ryanodine receptors, a phenomenon known as calcium-induced calcium release. The increase in intracellular calcium leads to contraction of the cardiomyocyte and to the activation of the sarcoendoplasmic reticulum calcium ATPase (SERCA2a), which pumps calcium from the cytoplasm into the sarcoplasmic reticulum. The activity of SERCA2a is inhibited by phospholamban (PLB). During diastole there is inhibition of PLB by its phosphorylation at two different sites; one is activated by protein kinase A (PKA) in response to β-adrenergic stimulation and the other is activated by calcium ions and calmodulin, thus promoting and enhancing the activity of SERCA2a. One of the other proteins that affects the function of SERCA2a is the protein phosphatase 1 (PP1) and its inhibitor-1 (I-1). Phosphatase 1 is a serine/threonine phosphatase that is localized to the sarcoplasmic reticulum and is inhibited by I-1, which becomes active upon phosphorylation of threonine-35 of PLB protein by PKA. This results in inhibition of PP1 and therefore enhanced PKA-mediated phosphorylation of PLB, leading to amplification of the β-adrenergic response in the heart. AKT1 appears to positively regulate contraction by increasing calcium influx through the LTCC,30 by increase in SERCA2a protein levels,31 and by augmenting PLB phosphorylation,32 possibly through down-regulation of PP1. Whether AKT1 is directly involved in the phosphorylation of LTCC, PLB, or in the activation of I-1 awaits further investigation.
AKT and metabolism
AKT1 modulates glucose and fatty acid metabolism. AKT may promote glucose oxidation by enhancing glucose uptake through glucose transporters and attenuates fatty acid oxidation through down-regulation of peroxisome proliferator-activated receptor-α (PPARα) and its coactivator, PPARγ coactivator-1(PGC-1), which transcriptionally activate the genes in fatty acid oxidation pathway. Under normal conditions, adenosine triphosphate (ATP) is produced up to 10–40% from oxidation of glucose and lactate and up to 60–90% from β-oxidation of free fatty acids. Fatty acids generate more ATP per gram of substrate than lactate or glucose, and are energy efficient, whereas glucose and lactate generate more ATP than fatty acids for each mole of oxygen and are oxygen efficient.1 Therefore, if the supply of oxygen is limited, glucose oxidation will provide more energy per equal amount of oxygen and support more work than fatty acids. Moreover, during ischaemia the accumulation of free fatty acids is toxic and induces damage to the cell membrane and death of the cell. Therefore, stimulation of glucose oxidation may be beneficial under ischaemic conditions, and the cardioprotective effects of glucose–insulin–potassium (GIK) infusion in the reperfusion phase or fatty acid oxidation inhibitors as shown in animal models of ischaemia reperfusion33 support this notion.34 Therefore, the cardioprotective and beneficial effect of short-term activation of AKT1 in the reperfusion phase may be attributed in part to the switch from fatty acid to glucose metabolism leading to the efficient myocardial consumption of oxygen.
Exercise and AKT signalling in heart failure
Heart failure is a growing problem in the industrialized world and has reached epidemic proportions in the USA. Although the central effects of HF are pulmonary and peripheral vascular congestion, many patients believe that exercise limitation is the most troubling feature. Traditional therapies, such as angiotensin-converting enzyme inhibitors, β-blockers, and spironolactone show impressive reductions in mortality with somewhat less significant improvement in functional capacity. Exercise training was once prohibited in HF patients out of concern for patient safety. However, it is now recognized as a therapeutic option for improving functional capacity, especially that mechanical function and functional capacity do not always have a direct correlation in HF subjects.35 Clinical trials of exercise training in HF show improvements in exercise time, functional capacity, and peak oxygen consumption and that exercise training seemed to be safe and well tolerated in HF patients.36–39 The cardiomyocyte signalling pathways driving this beneficial effect of exercise were not fully understood until recently. Miyachi et al.40 explored the signalling pathways involved in the beneficial effect of exercise on left ventricular geometry in a HF model of Dahl Salt-Sensitive hypertensive rats. The authors found that exercise training had a beneficial effect on cardiac remodelling and attenuated HF in hypertensive rats, with these effects likely being attributable to the attenuation of left ventricular concentricity and restoration of coronary angiogenesis through the activation of phosphatidylinositol 3-kinase(p110α)-AKT-mammalian target of rapamycin signaling.40 Moreover, Konhilas et al.41 have shown that there are sex differences with regard to exercise-induced cardiac adaptation in mice. The authors found that sex/gender is a dominant factor in exercise performance and found that female animals have greater capacity to increase their cardiac mass in response to similar amounts of exercise. They attributed these differences to significant increase in calcium/calmodulin-dependent protein kinase (CaMK) activity in females compared with males in response to exercise. The phosphorylation of glycogen synthase kinase-3 (GSK-3) was evident after 7 days of cage-wheel exposure in both sexes and remained elevated in females only by 21 days of exercise. However, there were no sex differences with regard to exercise-induced AKT phosphorylation.41
Exercise also has beneficial effects on the musculoskeletal system in HF subjects.42,43 Skeletal muscle abnormalities in patients with HF include atrophy of highly oxidative, fatigue-resistant type I muscle fibres, decreased mitochondrial oxidative enzyme concentration and activity, reduced mitochondrial volume and density, and reduced muscle bulk and strength.44 Exercise enhances cardiac and skeletal muscle metabolism by increasing mitochondrial biogenesis and by enhancing fatty acid β-oxidation via PGC-1α signalling.42 Recently, Toth et al.45 have shown that chronic HF reduces AKT phosphorylation in human skeletal muscle compared with patients with normal cardiac function. The decrease in AKT phosphorylation in skeletal muscle of HF subjects could partly be related to the decrease in blood and nutrient supply to the highly oxidative, fatigue-resistant type I muscle fibres. Decrease in phosphorylated AKT in skeletal muscle will lead to decrease in protein synthesis and enhances protein breakdown via the activation of FOXO transcription factors and the transcription of E3 ubiquitin ligases important for muscle proteolysis such as Atrogin-1 and MurF-1. Hence, the cardiovascular and the musculoskeletal systems are interrelated in the sense that functional deterioration in one system is negatively reflected on the other one. It will be interesting to investigate whether exercise enhances AKT phosphorylation in skeletal muscles of HF subjects and if improvement in cardiac function of HF subjects, using different therapeutic modalities, can attenuate muscle atrophy and wasting, a clinical picture often seen in patients with end-stage HF.
Conclusion
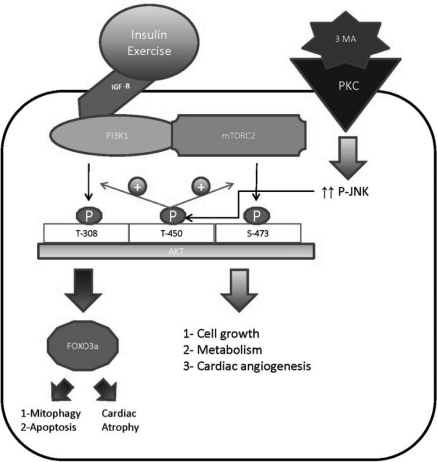
In conclusion, the beneficial effects of short-term AKT activation, in HF or in stressed cardiomyocyte in vitro, could be explained through enhancing angiogenesis and the delivery of trophic substances to the myocardium. Also, short-term AKT activation promotes the utilization of glucose, instead of free fatty acids, and the efficient consumption of oxygen by the myocardium. Besides that, short-term AKT may directly improve the contractile function of the myocardium by increasing SERCA2a levels or by enhancing its activity through the phosphorylation and thus the inhibition of PLB. These effects of AKT are short lived and are overridden and altered by other signalling pathways if the cardiomyocyte stressor persists. It seems that JNKs are the key regulators of AKT and modulate its interaction with its downstream effectors. The short-term activation of JNK is protective and attenuates apoptotic and autophagic cell death via potentiating the inhibitory effect of AKT on FOXO3a as stated above. Although short-term activation of AKT proves to be beneficial in HF, there still exist major concerns regarding the long-term benefit of AKT signalling and potential risks that may arise with its prolonged activity. Prolonged AKT signalling in HF will lead to worsening in cardiac function which, in part, is caused by decrease in myocardial angiogenesis. Furthermore, prolonged AKT activity may promote tumourigenesis and contributes to the proliferation of cancerous cells. Hence, AKT activity should be balanced in a way so asto maintain its protective effect on the myocardium. This might be achieved through the pulsatile activation of AKT. Whether this may prove beneficial or not needs to be investigated in the correct future (Figure 1).
Figure 1.
AKT signalling in the heart. The short-term activation of AKT protects the cardiomyocyte against environmental stressors via the inhibition of cell death and the promotion of cell growth and cardiac angiogenesis. The short-term activation of JNK is cardioprotective as well. JNK phosphorylates AKT at threonine 450 and potentiates its interaction with its downstream effectors. It remains unclear as to why these favourable effects of AKT are lost with its prolonged activation. One hypothesis is that other signalling pathways dominate and override the interaction of AKT with its downstream effectors. This hypothesis clearly needs to be investigated in the future. IGF-R, insulin-like growth factor receptor; PI3K1, phosphatidylinositol 3-kinase 1; mTORC2, mammalian target of rapamycin 2; 3 MA, 3-methyladenine; PKC, protein kinase C; JNK, c-Jun N-terminal kinases; green arrows, promote signalling pathway; red arrows, inhibit signalling pathway.
Funding
This work is supported by NIH R01 HL093183, HL088434, HL071763, HL080498, HL083156, and P20HL100396 (RJH).
Conflict of interest: none declared.
References
- 1.Shiojima I, Walsh K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006;20:3347–3365. doi: 10.1101/gad.1492806. [DOI] [PubMed] [Google Scholar]
- 2.Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, Kadowaki T, Hay N. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 4.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 5.Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, Lee VM, Szabolcs M, de Jong R, Oltersdorf T, Ludwig T, Efstratiadis A, Birnbaum MJ. Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Mol Cell Biol. 2005;25:1869–1878. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bae SS, Cho H, Mu J, Birnbaum MJ. Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. J Biol Chem. 2003;278:49530–49536. doi: 10.1074/jbc.M306782200. [DOI] [PubMed] [Google Scholar]
- 7.Peng XD, Xu PZ, Chen ML, Hahn-Windgassen A, Skeen J, Jacobs J, Sundararajan D, Chen WS, Crawford SE, Coleman KG, Hay N. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003;17:1352–1365. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jetton TL, Lausier J, LaRock K, Trotman WE, Larmie B, Habibovic A, Peshavaria M, Leahy JL. Mechanisms of compensatory beta-cell growth in insulin-resistant rats: roles of Akt kinase. Diabetes. 2005;54:2294–2304. doi: 10.2337/diabetes.54.8.2294. [DOI] [PubMed] [Google Scholar]
- 9.Rathmell JC, Elstrom RL, Cinalli RM, Thompson CB. Activated Akt promotes increased resting T cell size, CD28-independent T cell growth, and development of autoimmunity and lymphoma. Eur J Immunol. 2003;33:2223–2232. doi: 10.1002/eji.200324048. [DOI] [PubMed] [Google Scholar]
- 10.Majumder PK, Sellers WR. Akt-regulated pathways in prostate cancer. Oncogene. 2005;24:7465–7474. doi: 10.1038/sj.onc.1209096. [DOI] [PubMed] [Google Scholar]
- 11.Shiojima I, Yefremashvili M, Luo Z, Kureishi Y, Takahashi A, Tao J, Rosenzweig A, Kahn CR, Abel ED, Walsh K. Akt signaling mediates postnatal heart growth in response to insulin and nutritional status. J Biol Chem. 2002;277:37670–37677. doi: 10.1074/jbc.M204572200. [DOI] [PubMed] [Google Scholar]
- 12.Kemi OJ, Ceci M, Wisloff U, Grimaldi S, Gallo P, Smith GL, Condorelli G, Ellingsen O. Activation or inactivation of cardiac Akt/mTOR signaling diverges physiological from pathological hypertrophy. J Cell Physiol. 2008;214:316–321. doi: 10.1002/jcp.21197. [DOI] [PubMed] [Google Scholar]
- 13.Matsui T, Nagoshi T, Rosenzweig A. Akt and PI 3-kinase signaling in cardiomyocyte hypertrophy and survival. Cell Cycle. 2003;2:220–223. [PubMed] [Google Scholar]
- 14.Mann DL, Bogaev R, Buckberg GD. Cardiac remodelling and myocardial recovery: lost in translation? Eur J Heart Fail. 2010;12:789–796. doi: 10.1093/eurjhf/hfq113. [DOI] [PubMed] [Google Scholar]
- 15.Shah AM, Solomon SD. A unified view of ventricular remodelling. Eur J Heart Fail. 2010;12:779–781. doi: 10.1093/eurjhf/hfq131. [DOI] [PubMed] [Google Scholar]
- 16.Phung TL, Ziv K, Dabydeen D, Eyiah-Mensah G, Riveros M, Perruzzi C, Sun J, Monahan-Earley RA, Shiojima I, Nagy JA, Lin MI, Walsh K, Dvorak AM, Briscoe DM, Neeman M, Sessa WC, Dvorak HF, Benjamin LE. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell. 2006;10:159–170. doi: 10.1016/j.ccr.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longnus SL, Segalen C, Giudicelli J, Sajan MP, Farese RV, Van Obberghen E. Insulin signalling downstream of protein kinase B is potentiated by 5′AMP-activated protein kinase in rat hearts in vivo. Diabetologia. 2005;48:2591–2601. doi: 10.1007/s00125-005-0016-3. [DOI] [PubMed] [Google Scholar]
- 18.Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90:1243–1250. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- 19.Somanath PR, Razorenova OV, Chen J, Byzova TV. Akt1 in endothelial cell and angiogenesis. Cell Cycle. 2006;5:512–518. doi: 10.4161/cc.5.5.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enomoto A, Murakami H, Asai N, Morone N, Watanabe T, Kawai K, Murakumo Y, Usukura J, Kaibuchi K, Takahashi M. Akt/PKB regulates actin organization and cell motility via Girdin/APE. Dev Cell. 2005;9:389–402. doi: 10.1016/j.devcel.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Kitamura T, Asai N, Enomoto A, Maeda K, Kato T, Ishida M, Jiang P, Watanabe T, Usukura J, Kondo T, Costantini F, Murohara T, Takahashi M. Regulation of VEGF-mediated angiogenesis by the Akt/PKB substrate Girdin. Nat Cell Biol. 2008;10:329–337. doi: 10.1038/ncb1695. [DOI] [PubMed] [Google Scholar]
- 22.Izumiya Y, Shiojima I, Sato K, Sawyer DB, Colucci WS, Walsh K. Vascular endothelial growth factor blockade promotes the transition from compensatory cardiac hypertrophy to failure in response to pressure overload. Hypertension. 2006;47:887–893. doi: 10.1161/01.HYP.0000215207.54689.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsui T, Li L, del Monte F, Fukui Y, Franke TF, Hajjar RJ, Rosenzweig A. Adenoviral gene transfer of activated phosphatidylinositol 3′-kinase and Akt inhibits apoptosis of hypoxic cardiomyocytes in vitro. Circulation. 1999;100:2373–2379. doi: 10.1161/01.cir.100.23.2373. [DOI] [PubMed] [Google Scholar]
- 24.Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–335. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- 25.Taniyama Y, Walsh K. Elevated myocardial Akt signaling ameliorates doxorubicin-induced congestive heart failure and promotes heart growth. J Mol Cell Cardiol. 2002;34:1241–1247. doi: 10.1006/jmcc.2002.2068. [DOI] [PubMed] [Google Scholar]
- 26.Mammucari C, Schiaffino S, Sandri M. Downstream of Akt: FoxO3 and mTOR in the regulation of autophagy in skeletal muscle. Autophagy. 2008;4:524–526. doi: 10.4161/auto.5905. [DOI] [PubMed] [Google Scholar]
- 27.Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6:458–471. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 2008;23:160–170. doi: 10.1152/physiol.00041.2007. [DOI] [PubMed] [Google Scholar]
- 29.Shao Z, Bhattacharya K, Hsich E, Park L, Walters B, Germann U, Wang YM, Kyriakis J, Mohanlal R, Kuida K, Namchuk M, Salituro F, Yao YM, Hou WM, Chen X, Aronovitz M, Tsichlis PN, Bhattacharya S, Force T, Kilter H. c-Jun N-terminal kinases mediate reactivation of Akt and cardiomyocyte survival after hypoxic injury in vitro and in vivo. Circ Res. 2006;98:111–118. doi: 10.1161/01.RES.0000197781.20524.b9. [DOI] [PubMed] [Google Scholar]
- 30.Catalucci D, Zhang DH, DeSantiago J, Aimond F, Barbara G, Chemin J, Bonci D, Picht E, Rusconi F, Dalton ND, Peterson KL, Richard S, Bers DM, Brown JH, Condorelli G. Akt regulates L-type Ca2+ channel activity by modulating Cavalpha1 protein stability. J Cell Biol. 2009;184:923–933. doi: 10.1083/jcb.200805063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SJ, Abdellatif M, Koul S, Crystal GJ. Chronic treatment with insulin-like growth factor I enhances myocyte contraction by upregulation of Akt-SERCA2a signaling pathway. Am J Physiol Heart Circ Physiol. 2008;295:H130–H135. doi: 10.1152/ajpheart.00298.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Catalucci D, Latronico MV, Ceci M, Rusconi F, Young HS, Gallo P, Santonastasi M, Bellacosa A, Brown JH, Condorelli G. Akt increases sarcoplasmic reticulum Ca2+ cycling by direct phosphorylation of phospholamban at Thr17. J Biol Chem. 2009;284:28180–28187. doi: 10.1074/jbc.M109.036566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang HX, Zang YM, Huo JH, Liang SJ, Zhang HF, Wang YM, Fan Q, Guo WY, Wang HC, Gao F. Physiologically tolerable insulin reduces myocardial injury and improves cardiac functional recovery in myocardial ischemic/reperfused dogs. J Cardiovasc Pharmacol. 2006;48:306–313. doi: 10.1097/01.fjc.0000249873.73197.c3. [DOI] [PubMed] [Google Scholar]
- 34.Diaz R, Goyal A, Mehta SR, Afzal R, Xavier D, Pais P, Chrolavicius S, Zhu J, Kazmi K, Liu L, Budaj A, Zubaid M, Avezum A, Ruda M, Yusuf S. Glucose-insulin-potassium therapy in patients with ST-segment elevation myocardial infarction. JAMA. 2007;298:2399–2405. doi: 10.1001/jama.298.20.2399. [DOI] [PubMed] [Google Scholar]
- 35.Davies EJ, Moxham T, Rees K, Singh S, Coats AJ, Ebrahim S, Lough F, Taylor RS. Exercise training for systolic heart failure: Cochrane systematic review and meta-analysis. Eur J Heart Fail. 2010;12:706–715. doi: 10.1093/eurjhf/hfq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pina IL. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whellan DJ, O'Connor CM, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Houston-Miller N, Fleg JL, Schulman KA, Pina IL. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J. 2007;153:201–211. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Giannuzzi P, Temporelli PL, Corra U, Gattone M, Giordano A, Tavazzi L. Attenuation of unfavorable remodeling by exercise training in postinfarction patients with left ventricular dysfunction: results of the Exercise in Left Ventricular Dysfunction (ELVD) trial. Circulation. 1997;96:1790–1797. doi: 10.1161/01.cir.96.6.1790. [DOI] [PubMed] [Google Scholar]
- 39.McKelvie RS, Teo KK, McCartney N, Humen D, Montague T, Yusuf S. Effects of exercise training in patients with congestive heart failure: a critical review. J Am Coll Cardiol. 1995;25:789–796. doi: 10.1016/0735-1097(94)00428-S. [DOI] [PubMed] [Google Scholar]
- 40.Miyachi M, Yazawa H, Furukawa M, Tsuboi K, Ohtake M, Nishizawa T, Hashimoto K, Yokoi T, Kojima T, Murate T, Yokota M, Murohara T, Koike Y, Nagata K. Exercise training alters left ventricular geometry and attenuates heart failure in dahl salt-sensitive hypertensive rats. Hypertension. 2009;53:701–707. doi: 10.1161/HYPERTENSIONAHA.108.127290. [DOI] [PubMed] [Google Scholar]
- 41.Konhilas JP, Maass AH, Luckey SW, Stauffer BL, Olson EN, Leinwand LA. Sex modifies exercise and cardiac adaptation in mice. Am J Physiol Heart Circ Physiol. 2004;287:H2768–H2776. doi: 10.1152/ajpheart.00292.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ventura-Clapier R, Mettauer B, Bigard X. Beneficial effects of endurance training on cardiac and skeletal muscle energy metabolism in heart failure. Cardiovasc Res. 2007;73:10–18. doi: 10.1016/j.cardiores.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 43.You Fang Z, Marwick TH. Mechanisms of exercise training in patients with heart failure. Am Heart J. 2003;145:904–911. doi: 10.1016/S0002-8703(02)94822-2. [DOI] [PubMed] [Google Scholar]
- 44.Ventura-Clapier R, De Sousa E, Veksler V. Metabolic myopathy in heart failure. News Physiol Sci. 2002;17:191–196. doi: 10.1152/nips.01392.2002. [DOI] [PubMed] [Google Scholar]
- 45.Toth MJ, Ward K, van der Velden J, Miller MS, Vanburen P, Lewinter MM, Ades PA. Chronic heart failure reduces Akt phosphorylation in human skeletal muscle: relationship to muscle size and function. J Appl Physiol. 2011;110:892–900. doi: 10.1152/japplphysiol.00545.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]