Abstract
The pathophysiological mechanisms involved in the failure of the trabecular meshwork (TM) to maintain normal levels of aqueous outflow in glaucoma are not yet understood. Aberrant activation of the transforming growth factor beta-1 (TGF-β1) pathway has been implicated in several degenerative diseases. We investigated the possibility that chronic cyclic mechanical stress that affects the TM might result in increased production of TGF-β1. Primary cultures of TM cells subjected to cyclic mechanical stress (5% stretching, 1 cycle/sec) demonstrate a significant increase in total and biologically active secreted TGF-β1 that was associated with activation of the TGF-β1 promoter, measured using a recombinant adenovirus expressing the secreted reporter gene secreted alkaline phosphatase protein (SEAP) under the TGF-β1 gene promoter (AdTGFβ1-SEAP). Associated changes in the transcription of MMP-2, TIMP-2, and CTGF were assessed by semiquantitative PCR. Immunohistochemical analysis of TGF-β1 in organ culture of human eyes revealed a generalized accumulation of this protein in the extracellular matrix (ECM) of the TM, while expression of the TGF-β1 promoter, analyzed using the LacZ reporter gene, was localized in some specific cells within the outflow pathway. Induction of the TGF-β1 promoter in organ culture was demonstrated using a novel model for cyclic mechanical stress in human perfused anterior segments infected with AdTGFβ1-SEAP. Given the relevant physiological and pathophysiological roles of TGF-β1, its induction after cyclic mechanical stress in the TM supports the hypothesis that this cytokine might play a significant role in the physiology of the TM, and contribute to the pathological changes of this tissue in certain forms of glaucoma.
The conventional outflow pathway, composed of the trabecular meshwork (TM) and Schlemm’s Canal (SC), is the tissue primarily responsible for maintaining intraocular pressure (IOP) (Bill, 1989). The functional failure of this tissue might then logically cause the elevation of IOP commonly associated with primary open angle glaucoma (POAG) (Sommer, 1989; Quigley, 1993). However, the homeostatic mechanisms responsible for IOP regulation and those associated with its alteration in glaucoma remain poorly understood.
Mechanical stress is emerging as a critical regulator of homeostasis in a number of tissues. It has been shown to initiate intracellular signaling, promote cell growth and survival, and cause morphological changes in several different cell types (Ruoslahti, 1997; Chicurel et al., 1998; Ingber, 2003). It is well described that the morphology of the outflow pathway changes dramatically under the influence of changing IOP (Johnstone and Grant, 1973; Grieson and Lee, 1975). The outflow pathway and its contained cells distend and are stretched with increasing IOP. Presumably from choroidal expansion during systole, there is continuous cycling of true IOP of small magnitudes (Johnstone, 2004).TM cells have been shown to sense and respond to mechanical stress with reorganization in the actin cytoskeleton (Tumminia et al., 1998), changes in gene expression (Mitton et al., 1997; Booth et al., 1999; Sato et al., 1999; Tamm et al., 1999), and modulation of matrix metalloproteinases (MMPs) (Okada et al., 1998; Bradley et al., 2001, 2003; WuDunn, 2001). These observations suggest the existence of regulatory feedback mechanisms for IOP homeostasis.
Transforming growth factor beta-1 (TGF-β1), a multifunctional polypeptide involved in cellular growth, differentiation, and morphogenesis, is also a potent inducer of extracellular matrix (ECM) synthesis (Roberts et al., 1992; Attisano and Wrana, 2002; Shi and Massague, 2003). This cytokine is secreted as large latent complexes, consisting of TGF-β1 covalently bound to the latent TGF binding protein (LTBP), that are stored in the pericellular space associated with the ECM. After activation, TGF-β1 is released from the ECM and interacts with the receptors located on the cell surface (Koli et al., 2001; Annes et al., 2003).The tight connection of TGF-β1 with the ECM makes this cytokine an attractive candidate as a mechanotransductor. Expression of TGF-β1 is stimulated under mechanical stress in several cell types, including glomerular mesangial cells, osteoblasts, and fibroblasts (Yasuda et al., 1996; Koli et al., 2001; Skutek et al., 2001; Annes et al., 2003; Sakata et al., 2004). Moreover, the induction of ECM in hepatic stellate and vascular smooth muscle cells under stretching seems to be mediated via TGF-β1 (Yasuda et al., 1996; Joki et al., 2000; O’Callaghan and Williams, 2000; Skutek et al., 2001; Sakata et al., 2004).
Although it has been demonstrated that cultured TM cells produce TGF-β1 and express the functional TGF-β1 receptors, possibly suggesting an autocrine action of the cytokine within the TM (Borisuth et al., 1992; Tripathi et al., 1993a,b; Li et al., 1996; Yuan and Wei, 1996), the exact functions of TGF-β1 in the outflow pathway and the mechanisms for its activation remain unknown. Here, we analyze the effect of cyclic mechanical stress on the production and secretion of TGF-β1, as well as the activation of its promoter in both TM primary cultures and anterior segment organ cultures.
MATERIALS AND METHODS
Cell cultures
Primary cultures of human TM were prepared from cadaver eyes (ages 30–60) obtained less than 48 h post-mortem from donors with no history of eye disease and as previously described (Stamer et al., 1995) and maintained at 37°C in a humidified atmosphere of 5% CO2 in low glucose Dulbecco’s Modified Eagle Medium (DMEM) with l-glutamine and 110 mg/L sodium pyruvate, supplemented with 10% fetal bovine serum (FBS), 100 µM non-essential amino acids, 100 U/ ml penicillin, 100 µg/ml streptomycin sulfate, and 0.25 µg/ml amphotericin B; all the reagents were obtained from Invitrogen Corporation (Carlsbad, CA). The protocols involving the use of human tissue were consistent with the tenets of the Declaration of Helsinki. CCL-64 cells (or mink lung epithelial cells) were obtained from the ATCC.
Mechanical stress application in cell culture
Human TM cells in passage 3 were plated on type I collagen-coated flexible silicone bottom plates (Flexcell, Hillsborough, NC). Once confluence was reached, culture medium was switched to serum-free DMEM and cells were subjected to cyclic mechanical stress (5% stretching, 1 cycle/sec) for the indicated times, using the computer-controlled, vacuum-operated FX-3000 Flexercell Strain Unit (Flexcell). Control cells were cultured under the same conditions, but no mechanical force was applied. When indicated, 2 µg of the anti-human TGF-β1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) were added to the culture medium before stress application.
Cell viability
Cell death was assayed by measuring the amount of lactate dehydrogenase (LDH) present in the culture medium as the result of damage in the plasma membrane, using the CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI).
Enzyme-linked immunosorbent assay (ELISA) for TGF-β1 protein
The level of total TGF-β1 released to the culture mediumwas assessed with a commercially available sandwich enzyme-linked immunoassay (Biosource International, Camarilla, CA), according to the manufacturer’s instructions. To accomplish the sensitivity requirement, culture medium from stretched and non-stretched human TM primary cultures were concentrated 20 times using the centrifugal filter device centricon-10 (Millipore, Billerica, MA) prior to sample extraction.
Construction of recombinant adenovirus
For the generation of the replicant-deficient recombinant adenoviruses AdTGFβ1-LacZ and AdTGFβ1-SEAP (SEAP—secreted alkaline phosphatase protein), the −453/+11 TGF-β1 promoter region was released from the plasmid pGL3b (Kim et al., 1989) by digestion with KpnI and HindIII (New England BioLabs, Beverly, MA) and then introduced into a modified pShuttle (Stratagene, La Jolla, CA) containing either the LacZ gene or the SEAP reporter gene obtained from the commercially available plasmid pSEAP2-Basic (BD Biosciences Clontech, Palo Alto, CA). These two pShuttles containing the TGF-β1 promoter were used to generate the replicant-deficient recombinant adenoviruses AdTGFβ1-LacZ and AdTGFβ1-SEAP, respectively, using the AdEasy system (Stratagene).
SEAP reporter gene assay
Activation of TGF-β1 promoter after mechanical stress was quantified by determining the amount of the SEAP released to the culture medium using the Great EscAPe™ SEAP chemiluminescence detection kit (BD Biosciences Clontech) according to the manufacturer’s protocol.
Bioassay of TGF-β1
The concentration of active TGF-β1 in the culture medium was estimated by the growth inhibition assay of mink lung epithelial cells (Meager, 1991). Briefly, mink lung epithelial cells were cultured during 24 h in 20 times-concentrated conditioned medium from stretched or non-stretched TM cultures. Proliferation rate was assayed using the CellTiter 96® Aqueous One Solution Cell Proliferation Assay (Promega).
Semiquantitative RT-PCR
Total RNA from human TM primary cultures was isolated using RNeasy kit (Qiagen, Inc., Valencia, CA) and then treated with DNase. RNA yields were determined using the Ribo-Green® fluorescent dye (Molecular Probes, Inc., Eugene, OR). First strand cDNA was synthesized from total RNA (1 µg) by reverse transcription using random hexamer primers and Superscript II reverse transcriptase (Invitrogen Corporation) according to the manufacturer’s instructions. PCR reactions were performed in a 20 µl mixture containing 0.5 µl cDNA diluted 1:10, 400 nM of each primer, and 1 µCi dCTP-[α-32P] (MP Biomedicals, Irvine, CA) with the Advantage 2PCR kit (Clontech). The thermal parameters used were: 95°C, 30 sec; 60°C, 30 sec; and 72°C, 30 sec for 15, 18, 21, and 25 cycles to get a linear range. Half of the PCR reaction was run in a 6% acrilamide-TBE gel, and exposed, after vacuum-dried, to a PhosphoImager screen. Densitometric analysis and quantification were performed in the Molecular Imager Fx (Bio-Rad, Hercules, CA), using the Quantity One software. The sequences of the primers used for the amplifications are shown in Table 1. Identity of PCR products were confirmed by direct PCR sequencing.
TABLE 1.
Primer sequences used for the semiquantitative PCR analysis
| Gene | Primers |
|---|---|
| MMP-2 | Forward 5′-CGCGACAAGAAGTATGGCTTCTGC-3′ |
| Reverse 5′-GCCCAGGAAAGTGAAGGGGAAG-3′ | |
| TIMP-2 | Forward 5′-TCTCATTGCAGGAAAGGCCGAG-3′ |
| Reverse 5′-GGTTCAGGCTCTTCTTCTGGGTGG-3′ | |
| CTGF | Forward 5′-GCATCCGTACTCCCAAAATC-3′ |
| Reverse 5′-CTTCTTCATGACCTCGCCGT-3′ | |
| 18S RNA | Forward 5′-GTAACCCGTTGAACCCCATT-3′ |
| Reverse 5′-CCATCCAATCGGTAGTAGCG-3′ |
Immunohistochemistry
Human eye anterior segments were formalin-fixed and paraffin-embedded. Sections (5–6 µm thick) were cut using the microtome Leica RM2025, mounted on superfrost® plus slides, depariffinized in xylene, and rehydrated in a graded alcohol series. Endogenous peroxidase activity was quenched by incubation in 0.3% H2O2 in methanol. Sections were then washed in PBS, and antigen retrieval was performed by microwaving the samples in 0.01M citrate buffer for 5 min at full power, 5 min at medium power, and 5 min at low power. After three washes in PBS, non-specific sites were blocked by incubation 30 min in 5% horse serum in PBS. Slides were incubated overnight at 4°C with either rabbit anti-human TGF-β1 antibody or with rabbit non-specific IgG as a negative control (Santa Cruz Biotechnology) 1:200 in 1.5% horse serum. Primary antibody was detected with the R.T.U. Vectastain Universal Quick Kit (Vector Labs, Burlingame, CA) and developed with DAB substrate (Vector Labs), according to the manufacturer’s instructions. Sections were counterstained with hematoxylin QS (Vector Labs), dehydrated, and mounted in VectaMount permanent mounting medium (Vector Labs).
Perfusion of human eye anterior segments
Organ cultures of human anterior segments were performed using the method described by Johnson (Johnson and Tschumper, 1987). Briefly, human cadaver eyes (ages 33–74) with no history of eye disease and less than 48 h post-mortem were bisected at the equator, and the lens, iris, and vitreous were removed. The anterior segments were then clamped to a modified petri dish and perfused at a constant flow of 3 µl/min with serum-free high-glucose DMEM supplemented with 110 mg/L sodium pyruvate, 100 U/ml penicillin, 0.1 mg/ml streptomycin, 170 µg/ml gentamicin, and 250 µg/ml amphotericin using a microinfusion pump. Perfused anterior segments were incubated at 37°C in 5% CO2. IOPs were continuously monitored with a pressure transducer connected to the dish’s second cannula and recorded with an automated computerized system. Only anterior segments with stable outflow facilities between 0.09 and 0.40 µl/min/mmHg that remained unchanged after viral infection were used.
Analysis of AdTGFb1-LacZ expression in perfused anterior segments
Anterior segments of human eyes were cultured as described above. After 48 h of perfusion, the segments were inoculated with 107 pfu of AdTGFβ1-LacZ in 100 µl of perfusion media at 3 µl/min. At day 2 post-infection, anterior segments were fixed by perfusion at 15 mm Hg in 1% paraformaldehyde, 0.2% glutaraldehyde, 0.02% NP40, and 0.01% sodium deoxycholate in PBS; removed from the perfusion system; and stained overnight at 37°C in 1 mg/ml 5-bromo-4-chloro-3 β-d-galactoside, 5 mM K3Fe(CN), 5 mM K4Fe(CN)6-3H2O, and 2 mM MgCl2 in PBS for detection of β-galactosidase activity. After color development, the segments were post-fixed in 10% neutral buffered formaline, dehydrated in an ethanol and xylene series, and embedded in paraffin. Sections (5–6 µm) were counterstained with hematoxylin QS (Vector Labs). Six eyes from different donors were analyzed for the expression of the TGF-β1 promoter.
Cyclic mechanical stress application in organ culture
Human anterior segments were perfused at a constant flow rate of 3 µl/min for 24 h. Once the IOP was stable, the flow rate of one of the paired eyes was changed to a repetitive cycle of 2 µl/ min for 20 min, followed by 15 µl/min for 90 sec using the programmable syringe pump BS-8000 (Braintree Scientific, Inc., Braintree, MA).
Analysis of AdTGFβ1-SEAP expression in perfused anterior segments
Human anterior segments perfused at 3 µl/min for 24 h were inoculated with 107 pfu of AdTGFβ1-SEAP. Twenty-four hours post-infection, cyclic mechanical stress was applied as described above. At the indicated times, effluent coming out from the perfusion chamber was collected, and the total amount of SEAP protein was assayed as described above.
RESULTS
Cyclic mechanical stress increases the amount of secreted TGF-β1
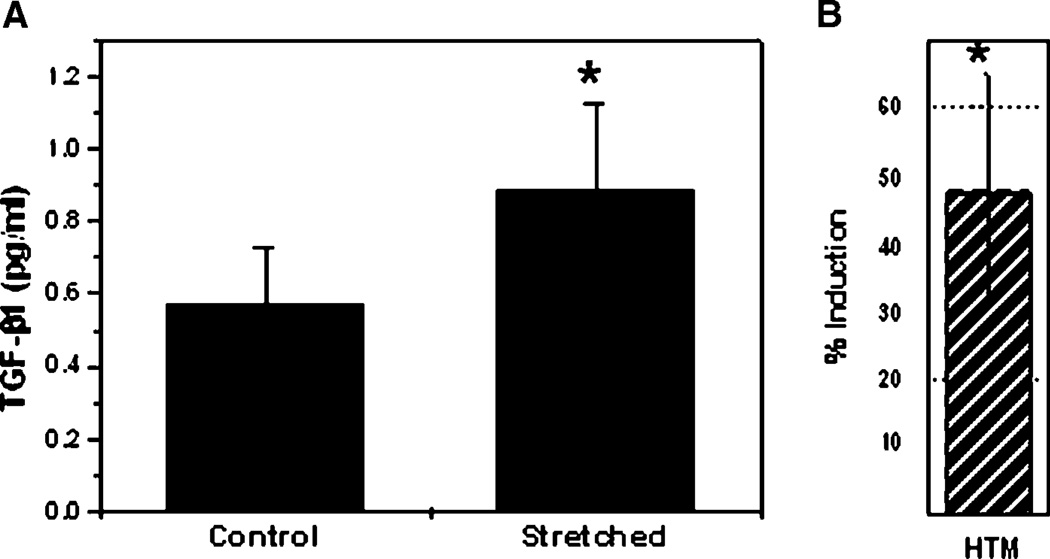
To investigate whether cyclic mechanical stress modulates the secretion of TGF-β1, a radial strain (5% elongation/sec) was applied to three different sets of human TM cells for 12 h. Total TGF-β1 present in the culture medium was quantified by ELISA. As shown in Figure 1, culture medium from all the cyclically stretched TM cultures contained significant higher amounts of total TGF-β1 than the static cultures. This increase was not due to the release of the intracellular cytokine resulting from cell death caused by mechanical stress since all cultures showed viability values higher than 95%, and no difference in viability was found between control and stretched cells (data not shown).
Fig. 1.
Effect of cyclic mechanical stretch in TGF-β1 secretion: Three different human TM primary cultures were subjected to 12 h of cyclic mechanical strain (5% elongation, 1 cycle/sec). A: Concentration of total TGF-β1 released to the culture medium assayed by ELISA. B: Percentage of TGF-β1 increase in the culture medium from stretched cells compared to the non-stretched cultures. Data represent the mean values±SD. Statistical analysis was done using t-test. *Significantly different from control (P<0.05).
TGF-β1 promoter is activated under cyclic mechanical stress in human TM cells
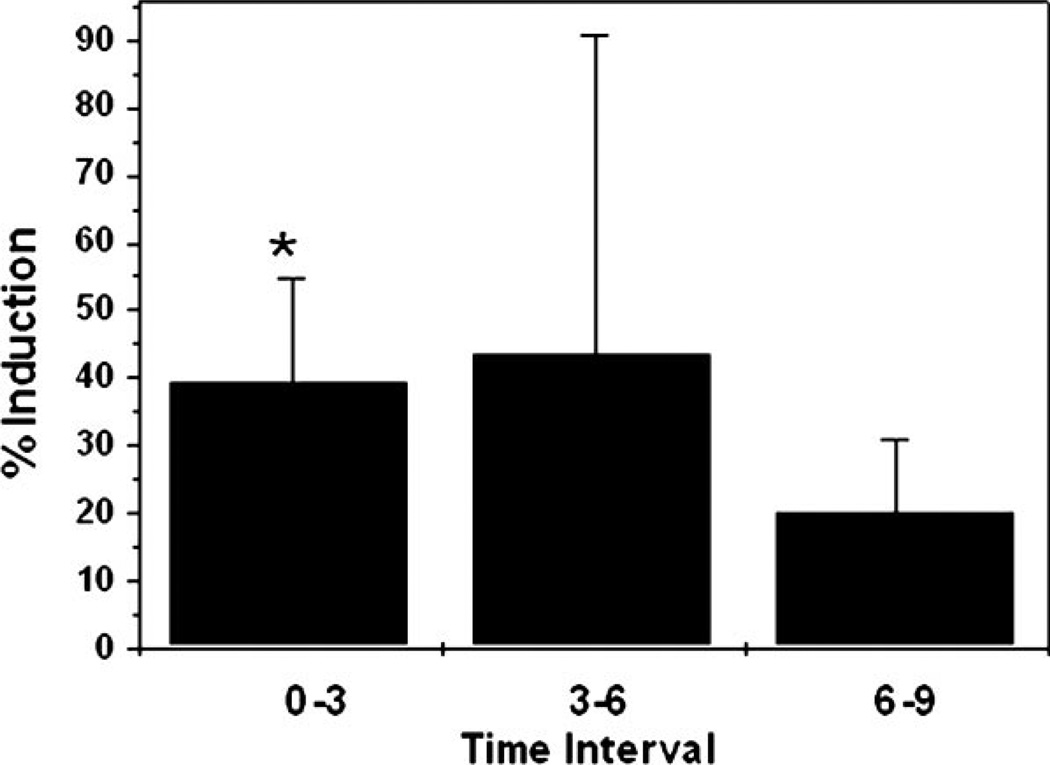
To assess whether mechanical stress can activate the transcription of TGF-β1 promoter, human TM cells were infected with 20 pfu/cell of the recombinant-deficient adenovirus AdTGFβ1-SEAP. Mechanical stress was applied at 48 h.p.i., and SEAP activity was assayed at 3, 6, and 9 h (Fig. 2). The TGF-β1 promoter was activated by mechanical stress in the three cell lines analyzed. Although mean differences were significant for the three cell lines just in the first period of time, the maximum percentage of induction was achieved at different times in the three tested cell lines: 96.85% between 3 and 6 h for the first cell line, 66.97% in the first 3 h for the second cell line, and a steady 25%–31% in the third one.
Fig. 2.
Effect of cyclic mechanical stretch in TGF-β1 promoter activity: Three different human TM primary cultures were infected with the recombinant adenovirus AdTGFβ1-SEAP (20 pfu/cell). At 48 h.p.i., cells were subjected to cyclic mechanical strain (5% elongation, 1 cycle/sec). SEAP activity in the culture medium was assayed at 3, 6, and 9 h. Differences between the amount of secreted SEAP between stretched and control cultures were calculated for each interval of time. Data represent the mean values±SD. *Significantly different from control (P < 0.05).
Cyclic mechanical stress activates TGF-β1
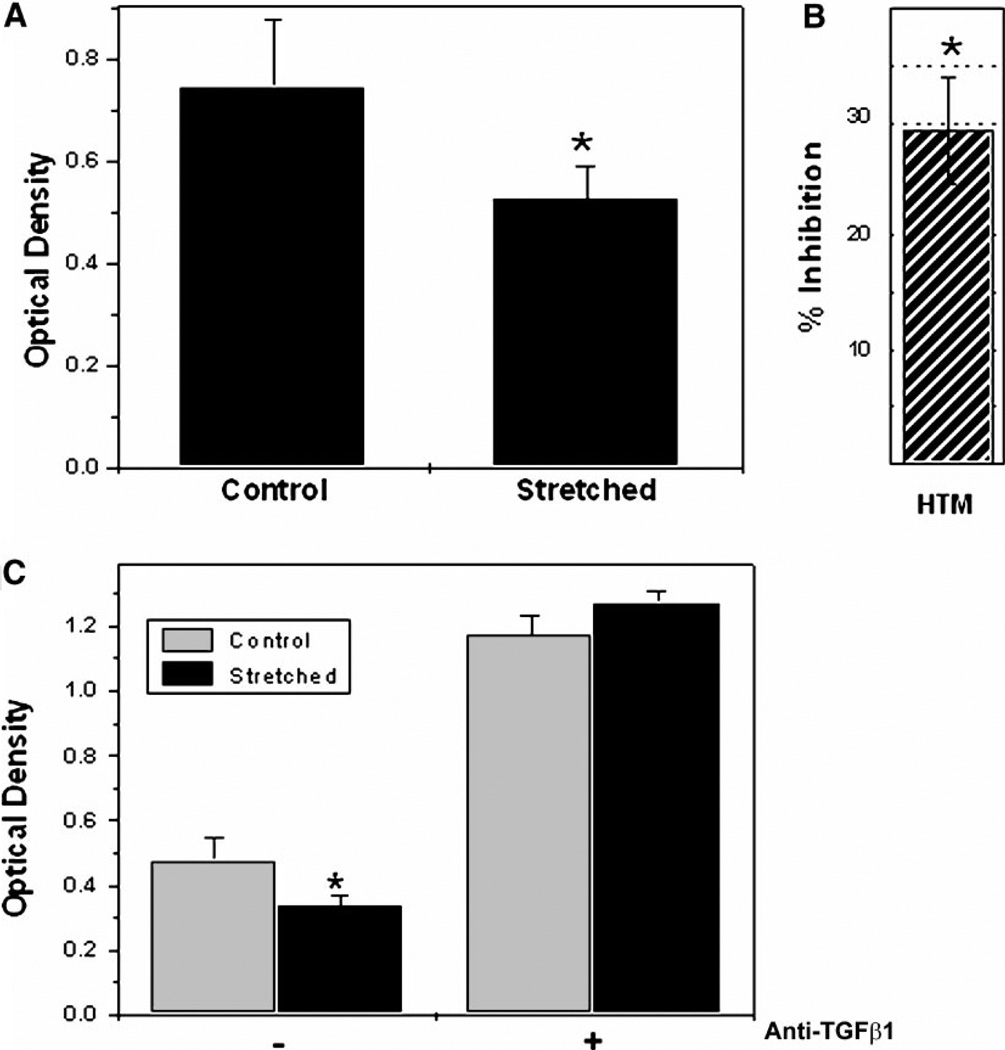
To test whether cyclic mechanical stress could induce the activation of latent TGF-β1, mink lung epithelial cells were grown in conditioned medium from human TM cells. The proliferation rate of mink lung epithelial cells was significantly inhibited when they were grown in medium from human TM cells cyclically stretched from 12 h, compared to control non-stretched cells (Fig. 3A,B). This inhibition was completely reversed when human TM cells were cultured in the presence of an anti-human TGF-β1 antibody (Fig. 3C).
Fig. 3.
Effect of cyclic mechanical stretch in the activation of latent TGF-β1: Mink lung epithelial cells were grown 24 h in conditioned medium from stretched or non-stretched (5% elongation, 1 cycle/sec, 12 h) human TM primary cultures. Proliferation rate was examined (A), and the percentage of inhibition in the proliferation was calculated and represented in (B). C: Effect of anti-TGFβ1 antibody in the inhibition of mink lung epithelial cell proliferation. Data represent the mean values ± SD. *Significantly different from control (P < 0.005, n = 3).
Cyclic mechanical stretch modulates ECM via TGF-β1
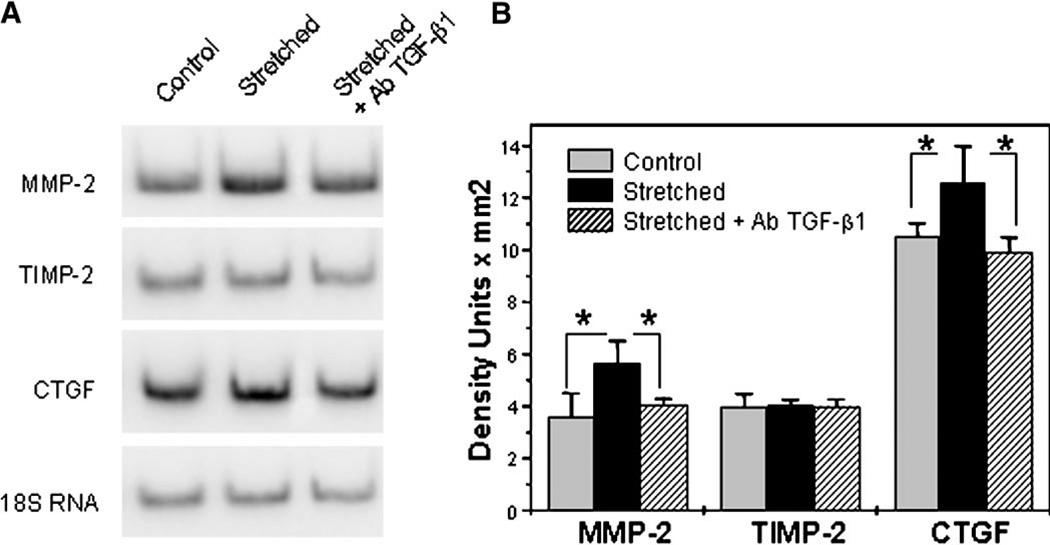
Mechanical strain has been reported to modulate ECM expression in TM cells (Okada et al., 1998; Bradley et al., 2001, 2003; WuDunn, 2001). To test whether this modulation could be the result of the activation of TGF- β1 in the stressed cultures, three different sets of human TM primary cultures were stretched 12 h per day, for 3 consecutive days. After this time, RNA was extracted, and the expression of MMP-2, TIMP-2, and CTGF was examined by semiquantitative PCR. As shown in Figure 4, the transcription of both MMP-2 and CTGF was significantly induced by cyclic mechanical stretch. This induction was inhibited when TM cells were cultured in the presence of anti-TGF-β1 antibody. In contrast, levels of TIMP-2 mRNA remained unchanged.
Fig. 4.
Cyclic mechanical stress modulates ECM via TGF-β1. A: Human TM primary cultures were stretched 12 h/day for 3 consecutive days. Expression of MMP-2, TIMP-2, and CTGF was analyzed by semiquantitative PCR in three different cell lines. Levels of 18S RNA were used for normalization. B: Normalized mean values of the densitometric analysis of the bands. *Significantly different from control (P < 0.05, n = 3).
Expression of TGF-β1 in the outflow pathway
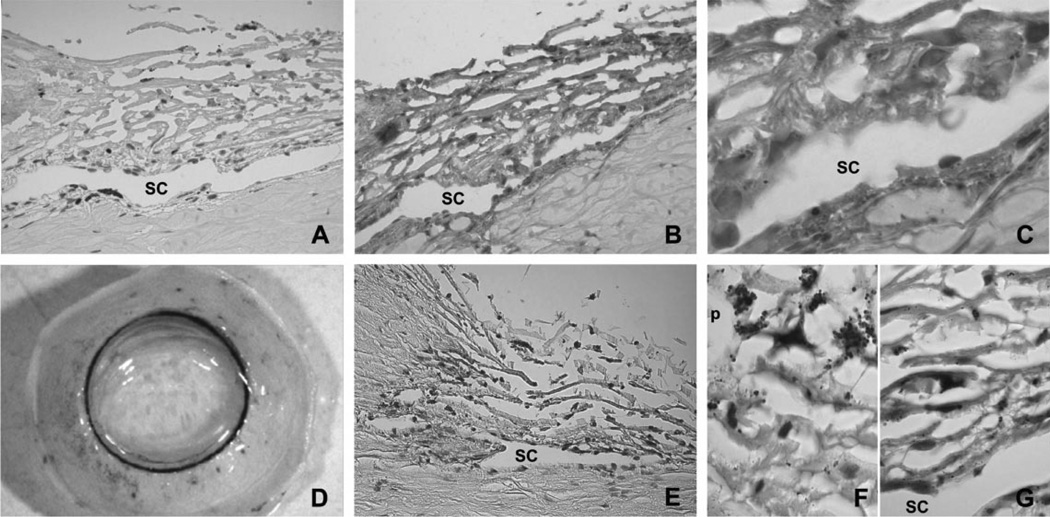
Although other previous studies have demonstrated the expression of TGF-β1 in cultured TM cells, little is known about the expression of this cytokine in the outflow pathway (Tripathi et al., 1993; Yuan and Wei, 1996). Immunohistochemistry in human paraffin sections showed positive staining for TGF-β1 in the ECM surrounding the cells of the TM, including those from the uveal meshwork, corneoscleral meshwork, and juxtacanalicular tissue (JCT). Strong positive staining for TGF-β1 was also found in the ECM surrounding the endothelial cells from both the inner and outer walls of the SC (Fig. 5B,C). In order to identify the specific cells responsible for the production of the TGF-β1 detected in the immunohistochemical analysis, human anterior segments were infected with 107 pfu of AdTGFβ1-LacZ. Macroscopic observation showed that positive β-galactosidase staining associated with the expression of TGF-β1 promoter was localized in the outflow pathway (Fig. 5D). Microscopic analysis of histological sections showed that the expression of the TGF-β1 promoter was restricted to some specific cells within the outflow pathway (Fig. 5E–G). An important level of variability in the distribution of positively stained cells was observed among the six pairs of eyes analyzed.
Fig. 5.
Immunohistochemical localization of TGF-β1 in the outflow pathway in human paraffin sections: (A) Negative control using a rabbit non-specific IgG as primary antibody; (B) low magnification of the outflow pathway stained with anti-human TGF-β1 antibody (brown precipitate); and (C) high magnification of the same section showing the presence of TGF-β1 in the ECM surrounding the TM and SC cells. Analysis of the TGF-β1 gene promoter expression in the outflow pathway: (D) Macroscopic observation of a human perfused anterior segment transduced with 107 pfu of AdTGFb1-LacZ showing that positive β-galactosidase staining associated with the expression of TGF-β1 promoter was localized in the outflow pathway (blue staining); (E) paraffin section from the same eye showing that the expression of the TGF-β1 promoter was restricted to some specific cells within the outflow pathway; and (F and G) higher magnification of the TM and SC showing variable levels of LacZ expression in independent cells. Similar results were obtained in six independent experiments. Pigment, p.
Cyclic mechanical stress activates TGF-β1 in perfused anterior segments
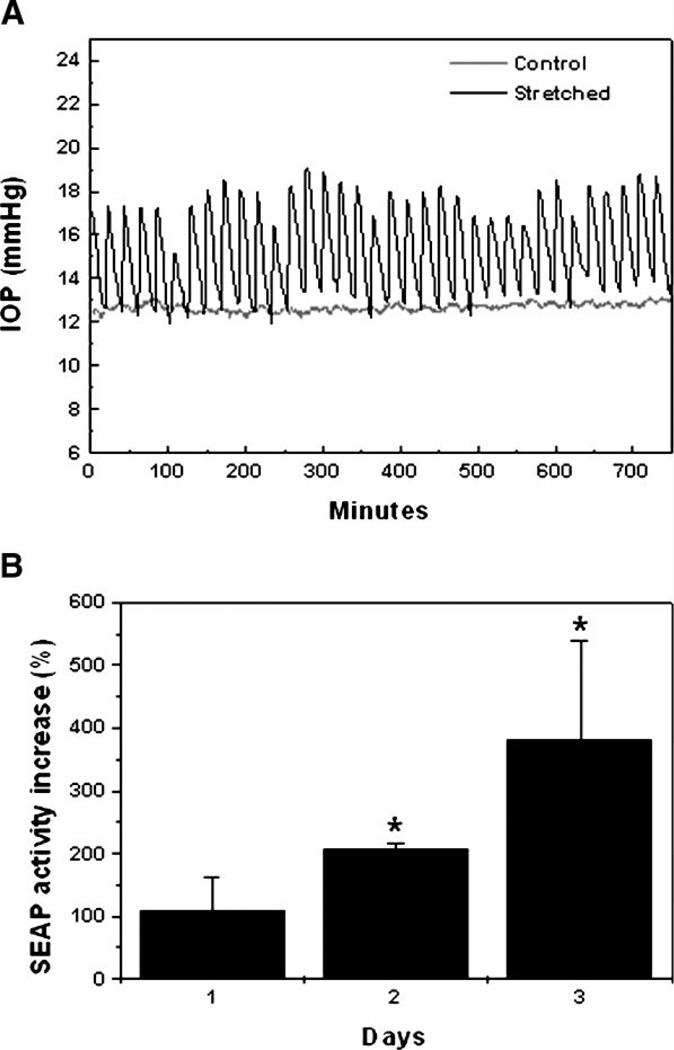
An in vitro model for cyclic mechanical stress in human perfused anterior segments was designed by generating cyclic infusion flow rate changes as described in section “Materials and Methods.” Under this regime of cyclic flow, the IOP in the experimental eye oscillated cyclically from 12–18 mm Hg, while the IOP in the paired-control eye was maintained at 12 mm Hg (Fig. 6A). To study if cyclic mechanical stress could also activate the TGF-β1 promoter in organ culture, human perfused anterior segments were infected with AdTGFβ1-SEAP (107 pfu). Twenty-four hours postinfection, cyclic mechanical stress was applied, and the total amount of SEAP in the effluent was examined. As shown in Figure 6B, cyclic mechanical stress significantly induced the activity of TGF-β1 promoter when compared to the paired-control eyes.
Fig. 6.
Analysis of TGF-β1 promoter in cyclically stretched human perfused anterior segments: (A) Graphic representation of cyclic IOP fluctuation as the result of the flow rate change. (B) Human perfused anterior segments were infected with 107 pfu of AdTGFβ1-SEAP. At 24 h.p.i., eyes were subjected to cyclic mechanical strain, and SEAP activity in effluent was assayed at 24, 48, and 72 h. Data represent the mean values ± SD. *Significantly different from control (P < 0.05, n = 3).
DISCUSSION
As result of the cyclic fluctuation of IOP with each heart beat, the conventional outflow pathway is subjected to continuous cycles of stretching and relaxation that might play an important role in tissue homeostasis and, consequently, in IOP regulation. To investigate if this cyclic mechanical stress could activate TGF-β1 in the outflow pathway, a cyclic stretch regimen of 5% elongation every second was selected as an in vitro model to mimic the mechanical forces to which TM cells are normally exposed (Johnstone, 2004). Under these conditions, we observed both a higher concentration of biologically active TGF-β1 released to the culture medium and activation of the TGF-β1 promoter in cyclically stretched human TM primary cultures compared to non-stretched ones.
TGF-β1 synthesis and, most importantly, its activation are processes that are tightly regulated (Taipale et al., 1998; Piek et al., 1999; Attisano and Wrana, 2002; Shi and Massague, 2003). Activation of latent TGF-β1 has been described to be mediated by a number of factors that can be activated by ECM perturbations (Piek et al., 1999; Koli et al., 2001; Annes et al., 2003). Indeed, it has been postulated that alterations in the ECM may be the primary change detected by the TGF-β1 “sensor” (Annes et al., 2003).
Previous studies from other groups have demonstrated that mechanical stress of the TM is followed by changes in MMPs, including the activation of MMP-2 (Okada et al., 1998; Bradley et al., 2001, 2003; WuDunn, 2001). We observed that this upregulation of MMP-2 was partially inhibited by anti-TGFβ1 antibodies, consistent with other reports indicating that TGF-β1 could mediate MMP-2 synthesis (Coletta et al., 1999; Wilson et al., 2002; Phillips et al., 2003).
Based on our findings, we hypothesize that mechanical stress promotes changes in the ECM that lead to the activation of latent TGF-β1. Among possible multiple responses, TGF-β1 can induce the synthesis of MMP-2, which, in turn, may activate newly synthesized latent TGF-β1. Treatment of TM cells with TGF-β1 has been demonstrated to activate TGF-β1 synthesis (Li et al., 1996). Since TIMP-2 levels do not seem to be altered on a short-term basis by mechanical stress, this positive loop might be sustained until others stop signals are activated. The expression of TGF-β1 associated with this loop could reduce outflow resistance by increasing ECM turnover.
While TGF-β1 could increase outflow facility in the short term, the permanent activation of this cytokine with chronic mechanical stress might lead to undesirable secondary effects. Likely because TGF-β1 is a powerful inducer of ECM synthesis, dysregulation of TGF-β1 expression has been associated with a large number of diseases involving abnormal ECM production (Flanders et al., 1998; Blobe et al., 2000; Cheng and Grande, 2002; Krein and Winston, 2002). CTGF, a protein strongly induced by TGF-β1, has been reported to play a crucial role in mediating the pro-fibrotic effects of TGF-β1 (Gore-Hyer et al., 2002; Ihn, 2002). As demonstrated by Chudgar et al. (2004) and our own results, CTGF expression is upregulated by mechanical stretch in TM cells, an effect that was blocked by an anti-TGFβ1 antibody. The continuous presence of TGF-β1 and CTGF might be responsible for the observed ECM accumulation in the outflow pathway with age (McMenamin and Lee, 1980; Miyazaki et al., 1987; Gong et al., 1992; Tripathi et al., 1997), and in glaucoma (Lutjen-Drecoll et al., 1981; Babizhayev and Brodskaya, 1989; Gong et al., 1992; Schlotzer-Schrehardt and Naumann, 1995; Ritch et al., 2003), subsequent to the transdifferentiation of TM cells towards a myofibroblast-like cell type (Tamm et al., 1996).
Analysis of the TGF-β1 pattern of expression in the TM provided some surprising results. While TGF-β1 was immunolocalized in the ECM of the outflow pathway, expression of LacZ gene driven by the TGF-β1 promoter did not seem to produce a consistent pattern of expression, and some variability was observed in the six pairs of eyes that were analyzed. This heterogeneity was not probably due to lack of uniformity in cellular infection efficiency since a uniform distribution has been observed after infecting human anterior segments with adenoviruses containing either the CMV promoter or the Matrix Gla Protein promoter using the same m.o.i. (Gonzalez et al., 2004). Therefore, these results may reflect the complex pattern of regulation of TGF-β1 gene expression (Kim et al., 1989).
Finally, it is worth emphasizing that the expression analysis of the SEAP reporter gene driven by the TGF-β1 promoter in organ culture demonstrates the potential usefulness of this reporter gene for monitoring changes in gene expression in the outflow pathway of perfused anterior segments under different experimental conditions, as well as the potential utility of the TGF-β1 promoter for gene transfer to the cells of the TM. The new feature offered by the use of this promoter, compared to those previously published (Gonzalez et al., 2004; Liton et al., 2005), is that transgene expression can be modulated by mechanical stress.
In summary, the data presented here demonstrates that cyclic mechanical stretch induces the expression of TGF-β1 in the outflow pathway. While such activation might play an important role in the homeostatic regulation of mechanical stress-induced matrix synthesis by human TM cells, chronic mechanical stress might contrarily lead to pathological effects consequent to increased production of TGF-β1.
ACKNOWLEDGMENTS
The authors thank Dr. Seon-Jin Kim for supplying the plasmid pGL3b encoding the TGF-β1 promoter region.
Contract grant sponsor: Research to Prevent Blindness Foundation; Contract grant sponsor: National Institute of Health; Contract grant numbers: EY05722, EY01894, 1K23EY014019-01A1.
LITERATURE CITED
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116(Pt 2):217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- Attisano L, Wrana JL. Signal transduction by the TGF-beta superfamily. Science. 2002;296(5573):1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- Babizhayev MA, Brodskaya MW. Fibronectin detection in drainage outflow system of human eyes in ageing and progression of open-angle glaucoma. Mech Ageing Dev. 1989;47(2):145–157. doi: 10.1016/0047-6374(89)90017-1. [DOI] [PubMed] [Google Scholar]
- Bill A. Uveoscleral drainage of aqueous humor: Physiology and pharmacology. Prog Clin Biol Res. 1989;312:417–427. [PubMed] [Google Scholar]
- Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342(18):1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- Booth A, Nguyen T, Polansky J. TIGR and stretch in the trabecular meshwork. Invest Ophthalmol Vis Sci. 1999;40(8):1888–1889. [PubMed] [Google Scholar]
- Borisuth NS, Tripathi BJ, Tripathi RC. Identification and partial characterization of TGF-beta 1 receptors on trabecular cells. Invest Ophthalmol Vis Sci. 1992;33(3):596–603. [PubMed] [Google Scholar]
- Bradley JM, Kelley MJ, Zhu X, Anderssohn AM, Alexander JP, Acott TS. Effects of mechanical stretching on trabecular matrix metalloproteinases. Invest Ophthalmol Vis Sci. 2001;42(7):1505–1513. [PubMed] [Google Scholar]
- Bradley JM, Kelley MJ, Rose A, Acott TS. Signaling pathways used in trabecular matrix metalloproteinase response to mechanical stretch. Invest Ophthalmol Vis Sci. 2003;44(12):5174–5181. doi: 10.1167/iovs.03-0213. [DOI] [PubMed] [Google Scholar]
- Cheng J, Grande JP. Transforming growth factor-beta signal transduction and progressive renal disease. Exp Biol Med (Maywood) 2002;227(11):943–956. doi: 10.1177/153537020222701102. [DOI] [PubMed] [Google Scholar]
- Chicurel ME, Chen CS, Ingber DE. Cellular control lies in the balance of forces. Curr Opin Cell Biol. 1998;10(2):232–239. doi: 10.1016/s0955-0674(98)80145-2. [DOI] [PubMed] [Google Scholar]
- Chudgar SM, Epstein DL, Rao PV. Elevated intraocular pressure and mechanical stress increase connective tissue growth factor expression in the trabecular meshwork. ARVO meeting. 2004 [Google Scholar]
- Coletta RD, Almeida OP, Reynolds MA, Sauk JJ. Alteration in expression of MMP-1 and MMP-2 but not TIMP-1 and TIMP-2 in hereditary gingival fibromatosis is mediated by TGF-beta 1 autocrine stimulation. J Periodontal Res. 1999;34(8):457–463. doi: 10.1111/j.1600-0765.1999.tb02281.x. [DOI] [PubMed] [Google Scholar]
- Flanders KC, Ren RF, Lippa CF. Transforming growth factor-betas in neurodegenerative disease. Prog Neurobiol. 1998;54(1):71–85. doi: 10.1016/s0301-0082(97)00066-x. [DOI] [PubMed] [Google Scholar]
- Gong H, Freddo TF, Johnson M. Age-related changes of sulfated proteoglycans in the normal human trabecular meshwork. Exp Eye Res. 1992;55(5):691–709. doi: 10.1016/0014-4835(92)90174-q. [DOI] [PubMed] [Google Scholar]
- Gonzalez P, Caballero M, Liton PB, Stamer WD, Epstein DL. Expression analysis of the matrix GLA protein and VE-cadherin gene promoters in the outflow pathway. Invest Ophthalmol Vis Sci. 2004;45(5):1389–1395. doi: 10.1167/iovs.03-0537. [DOI] [PubMed] [Google Scholar]
- Gore-Hyer E, Shegogue D, Markiewicz M, Lo S, Hazen-Martin D, Greene EL, Grotendorst G, Trojanowska M. TGF-beta and CTGF have overlapping and distinct fibrogenic effects on human renal cells. Am J Physiol Renal Physiol. 2002;283(4):F707–F716. doi: 10.1152/ajprenal.00007.2002. [DOI] [PubMed] [Google Scholar]
- Grieson I, Lee WR. The fine structure of the trabecular meshwork at graded levels of intraocular pressure. Exp Eye Res. 1975;20:505. doi: 10.1016/0014-4835(75)90218-3. [DOI] [PubMed] [Google Scholar]
- Ihn H. Pathogenesis of fibrosis: Role of TGF-beta and CTGF. Curr Opin Rheumatol. 2002;14(6):681–685. doi: 10.1097/00002281-200211000-00009. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Mechanobiology and diseases of mechanotransduction. Ann Med. 2003;35(8):564–577. doi: 10.1080/07853890310016333. [DOI] [PubMed] [Google Scholar]
- Johnson DH, Tschumper RC. Human trabecular meshwork organ culture. A new method. Invest Ophthalmol Vis Sci. 1987;28(6):945–953. [PubMed] [Google Scholar]
- Johnstone MA. The aqueous system as a mechanical pump: Evidence from examination of tissue & aqueous movement in human and non-human primates. J Glaucoma. 2004 doi: 10.1097/01.ijg.0000131757.63542.24. (in press) [DOI] [PubMed] [Google Scholar]
- Johnstone MA, Grant WM. Pressure-dependent changes in structures of the aqueous outflow system of human and monkey eyes. Am J Ophthalmol. 1973;75:365. doi: 10.1016/0002-9394(73)91145-8. [DOI] [PubMed] [Google Scholar]
- Joki N, Kaname S, Hirakata M, Hori Y, Yamaguchi T, Fujita T, Katoh T, Kurokawa K. Tyrosine-kinase dependent TGF-beta and extracellular matrix expression by mechanical stretch in vascular smooth muscle cells. Hypertens Res. 2000;23(2):91–99. doi: 10.1291/hypres.23.91. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Glick A, Sporn MB, Roberts AB. Characterization of the promoter region of the human transforming growth factor-beta 1 gene. J Biol Chem. 1989;264(1):402–408. [PubMed] [Google Scholar]
- Koli K, Saharinen J, Hyytiainen M, Penttinen C, Keski-Oja J. Latency, activation, and binding proteins of TGF-beta. Microsc Res Tech. 2001;52(4):354–362. doi: 10.1002/1097-0029(20010215)52:4<354::AID-JEMT1020>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Krein PM, Winston BW. Roles for insulin-like growth factor I and transforming growth factor-beta in fibrotic lung disease. Chest. 2002;122 Suppl 6:289S–293S. doi: 10.1378/chest.122.6_suppl.289s. [DOI] [PubMed] [Google Scholar]
- Li J, Tripathi BJ, Chalam KV, Tripathi RC. Transforming growth factor-beta 1 and -beta 2 positively regulate TGF-beta 1 mRNA expression in trabecular cells. Invest Ophthalmol Vis Sci. 1996;37(13):2778–2782. [PubMed] [Google Scholar]
- Liton PB, Liu X, Stamer WD, Challa P, Epstein DL, Gonzalez P. Specific targeting of gene expression to a subset of human trabecular meshwork cells using the chitinase 3-like 1 promoter. Invest Ophthalmol Vis Sci. 2005;46(1):183–190. doi: 10.1167/iovs.04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutjen-Drecoll E, Futa R, Rohen JW. Ultrahistochemical studies on tangential sections of the trabecular meshwork in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 1981;21(4):563–573. [PubMed] [Google Scholar]
- McMenamin PG, Lee WR. Age related changes in extracellular materials in the inner wall of Schlemm’s canal. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1980;212(3–4):159–172. doi: 10.1007/BF00410512. [DOI] [PubMed] [Google Scholar]
- Meager A. Assays for transforming growth factor beta. J Immunol Methods. 1991;141(1):1–14. doi: 10.1016/0022-1759(91)90204-s. [DOI] [PubMed] [Google Scholar]
- Mitton KP, Tumminia SJ, Arora J, Zelenka P, Epstein DL, Russell P. Transient loss of alphaB-crystallin: An early cellular response to mechanical stretch. Biochem Biophys Res Commun. 1997;235(1):69–73. doi: 10.1006/bbrc.1997.6737. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Segawa K, Urakawa Y. Age-related changes in the trabecular meshwork of the normal human eye. Jpn J Ophthalmol. 1987;31(4):558–569. [PubMed] [Google Scholar]
- O’Callaghan CJ, Williams B. Mechanical strain-induced extracellular matrix production by human vascular smooth muscle cells: Role of TGFbeta(1) Hypertension. 2000;36(3):319–324. doi: 10.1161/01.hyp.36.3.319. [DOI] [PubMed] [Google Scholar]
- Okada Y, Matsuo T, Ohtsuki H. Bovine trabecular cells produce TIMP-1 and MMP-2 in response to mechanical stretching. Jpn J Ophthalmol. 1998;42(2):90–94. doi: 10.1016/s0021-5155(97)00129-9. [DOI] [PubMed] [Google Scholar]
- Phillips PA, McCarroll JA, Park S, Wu MJ, Pirola R, Korsten M, Wilson JS, Apte MV. Rat pancreatic stellate cells secrete matrix metalloproteinases: Implications for extracellular matrix turnover. Gut. 2003;52(2):275–282. doi: 10.1136/gut.52.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piek E, Heldin CH, Ten Dijke P. Specificity, diversity, and regulation in TGF-beta superfamily signaling. FASEB J. 1999;13(15):2105–2124. [PubMed] [Google Scholar]
- Quigley HA. Open-angle glaucoma. N Engl J Med. 1993;328(15):1097–1106. doi: 10.1056/NEJM199304153281507. [DOI] [PubMed] [Google Scholar]
- Ritch R, Schlotzer-Schrehardt U, Konstas AG. Why is glaucoma associated with exfoliation syndrome? Prog Retin Eye Res. 2003;22(3):253–275. doi: 10.1016/s1350-9462(02)00014-9. [DOI] [PubMed] [Google Scholar]
- Roberts AB, McCune BK, Sporn MB. TGF-beta: Regulation of extracellular matrix. Kidney Int. 1992;41(3):557–559. doi: 10.1038/ki.1992.81. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Stretching is good for a cell. Science. 1997;276(5317):1345–1346. doi: 10.1126/science.276.5317.1345. [DOI] [PubMed] [Google Scholar]
- Sakata R, Ueno T, Nakamura T, Ueno H, Sata M. Mechanical stretch induces TGF-beta synthesis in hepatic stellate cells. Eur J Clin Invest. 2004;34(2):129–136. doi: 10.1111/j.1365-2362.2004.01302.x. [DOI] [PubMed] [Google Scholar]
- Sato Y, Matsuo T, Ohtsuki H. A novel gene (oculomedin) induced by mechanical stretching in human trabecular cells of the eye. Biochem Biophys Res Commun. 1999;259(2):349–351. doi: 10.1006/bbrc.1999.0797. [DOI] [PubMed] [Google Scholar]
- Schlotzer-Schrehardt U, Naumann GO. Trabecular meshwork in pseudoexfoliation syndrome with and without open-angle glaucoma. A morphometric, ultrastructural study. Invest Ophthalmol Vis Sci. 1995;36(9):1750–1764. [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Skutek M, van Griensven M, Zeichen J, Brauer N, Bosch U. Cyclic mechanical stretching modulates secretion pattern of growth factors in human tendon fibroblasts. Eur J Appl Physiol. 2001;86(1):48–52. doi: 10.1007/s004210100502. [DOI] [PubMed] [Google Scholar]
- Sommer A. Intraocular pressure and glaucoma. Am J Ophthalmol. 1989;107(2):186–188. doi: 10.1016/0002-9394(89)90221-3. [DOI] [PubMed] [Google Scholar]
- Stamer WD, Seftor RE, Williams SK, Samaha HA, Snyder RW. Isolation and culture of human trabecular meshwork cells by extracellular matrix digestion. Curr Eye Res. 1995;14(7):611–617. doi: 10.3109/02713689508998409. [DOI] [PubMed] [Google Scholar]
- Taipale J, Saharinen J, Keski-Oja J. Extracellular matrix-associated transforming growth factor-beta: Role in cancer cell growth and invasion. Adv Cancer Res. 1998;75:87–134. doi: 10.1016/s0065-230x(08)60740-x. [DOI] [PubMed] [Google Scholar]
- Tamm ER, Siegner A, Baur A, Lutjen-Drecoll E. Transforming growth factor-beta 1 induces alpha-smooth muscle-actin expression in cultured human and monkey trabecular meshwork. Exp Eye Res. 1996;62(4):389–397. doi: 10.1006/exer.1996.0044. [DOI] [PubMed] [Google Scholar]
- Tamm ER, Russell P, Epstein DL, Johnson DH, Piatigorsky J. Modulation of myocilin/TIGR expression in human trabecular meshwork. Invest Ophthalmol Vis Sci. 1999;40(11):2577–2582. [PubMed] [Google Scholar]
- Tripathi RC, Borisuth NS, Kolli SP, Tripathi BJ. Trabecular cells express receptors that bind TGF-beta 1 and TGF-beta 2: A qualitative and quantitative characterization. Invest Ophthalmol Vis Sci. 1993a;34(1):260–263. [PubMed] [Google Scholar]
- Tripathi RC, Li J, Borisuth NS, Tripathi BJ. Trabecular cells of the eye express messenger RNA for transforming growth factor-beta 1 and secrete this cytokine. Invest Ophthalmol Vis Sci. 1993b;34(8):2562–2569. [PubMed] [Google Scholar]
- Tripathi BJ, Li T, Li J, Tran L, Tripathi RC. Age-related changes in trabecular cells in vitro. Exp Eye Res. 1997;64(1):57–66. doi: 10.1006/exer.1996.0178. [DOI] [PubMed] [Google Scholar]
- Tumminia SJ, Mitton KP, Arora J, Zelenka P, Epstein DL, Russell P. Mechanical stretch alters the actin cytoskeletal network and signal transduction in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1998;39(8):1361–1371. [PubMed] [Google Scholar]
- Wilson MJ, Sellers RG, Wiehr C, Melamud O, Pei D, Peehl DM. Expression of matrix metalloproteinase-2 and -9 and their inhibitors, tissue inhibitor of metalloproteinase-1 and -2, in primary cultures of human prostatic stromal and epithelial cells. J Cell Physiol. 2002;191(2):208–216. doi: 10.1002/jcp.10092. [DOI] [PubMed] [Google Scholar]
- WuDunn D. The effect of mechanical strain on matrix metalloproteinase production by bovine trabecular meshwork cells. Curr Eye Res. 2001;22(5):394–397. doi: 10.1076/ceyr.22.5.394.5500. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Kondo S, Homma T, Harris RC. Regulation of extracellular matrix by mechanical stress in rat glomerular mesangial cells. J Clin Invest. 1996;98(9):1991–2000. doi: 10.1172/JCI119003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Wei H. Expression of mesenger RNA for transforming growth factor-beta 1 in bovine trabecular meshwork. Yan Ke Xue Bao. 1996;12(1):1–4. [PubMed] [Google Scholar]