Abstract
Objective:
Clinicians may benefit from using a joint mobility index to screen for individuals on the high end of the spectrum of joint laxity (ie, those with generalized joint laxity), which may be associated with musculoskeletal complaints. Reliability of the Beighton and Horan Joint Mobility Index (BHJMI) has not been reported in the literature. Our purpose was to determine intrarater and interrater reliability of (1) composite BHJMI scores (the overall score from 0 to 9), and (2) categorized scores, the BHJMI scores in 3 categories (0 to 2, 3 to 4, and 5 to 9)
Design and Setting:
This was an intrarater and interrater reliability study. Data were collected in an academic physical therapy department and in a high school.
Subjects:
Forty-two (intrarater) and 36 (interrater) female volunteers, aged 15 to 45 years.
Measurements:
Subjects were screened using the BHJMI. Percentage agreement and the Spearman rho were used to analyze BHJMI composite and category scores.
Results:
The percentage agreement and the Spearman rho for intrarater and interrater reliability of BHJMI composite scores were 69% and .86 and 51% and .87, respectively. The percentage agreement and the Spearman rho for intrarater and interrater reliability of the category scores were 81% and .81 and 89% and .75, respectively.
Conclusions:
Reliability of the BHJMI was good to excellent in screening for generalized joint laxity in females aged 15 to 45 years.
Keywords: generalized joint laxity, hypermobility syndrome
Generalized joint laxity (GJL), also known as systemic joint laxity, is defined as a condition in which most of an individual's synovial joints have range of motion beyond normal limits.1 Generalized joint laxity has been reported to have a familial tendency,2–10 is more common in females than males,5,6,11–15 and declines with age.15,16 The degree of joint laxity seems to vary with ethnic origin, being greatest for American Indians, followed by Africans and Caucasians.17,18 A potential consequence of GJL is the hypermobility syndrome. This syndrome, first described by Kirk et al in 1967,19 is GJL associated with musculoskeletal complaints (eg, arthralgias and joint dislocations) in an otherwise normal individual in whom all hereditary diseases are excluded. Other authors1,3–6,11,12,16,19–25 reported the association of GJL and musculoskeletal complaints such as arthralgias, joint subluxations, joint dislocations, and sprains. Early-onset osteoarthritis/osteoarthrosis has been implicated as part of the natural history of GJL.19,26
Generalized joint laxity may be a risk factor for a variety of musculoskeletal complaints and injuries. Clinical decisions regarding intervention, specifically therapeutic exercise and education, may be affected by the presence of GJL. For example, when performing manipulation, GJL must be taken into account and procedures adjusted accordingly,27,28 and athletes identified with GJL in a preseason screening may be instructed in altered training routines to lower their risk of injury. Education regarding the benign nature of the condition is recommended for individuals with GJL to ease concerns and eliminate unnecessary investigation or overuse of medication.29,30 An individual's prognosis after injury may also be affected by the presence of GJL. A slower rehabilitation course than commonly implemented may be more appropriate for an individual with GJL.30 Consequently, an effective way of screening for GJL is necessary.
Criteria for assessing GJL were first described by Carter and Wilkinson in 1964,31 modified by Beighton and Horan in 1969,2 and amended in 1973 by Beighton et al.16 The Beighton and Horan index is the most commonly employed index,13,14,16,20,22,23,33–41 is easy to use, requires no special equipment other than a goniometer, and takes less than 1 minute to complete. The index includes examination of fifth-finger extension, opposition of the thumb to the forearm with wrist flexion, elbow extension, knee extension, and trunk and hip flexion. The Beighton and Horan Joint Mobility Index (BHJMI) produces a composite, or overall, score between 0 and 9; 1 point is given if the criterion is met, 0 if it is not. The composite index scores are often placed into 1 of 3 categories: 0 to 2, 3 to 4, or 5 to 9.5,12,23 This allows the clinician to interpret the scores that are at the high end of the spectrum (ie, 5 to 9) as indicative of GJL. Although the BHJMI has been used over the past 34 years in numerous studies in a wide variety of settings, its reliability has never been described in the literature. Consequently, our purpose was to describe the intrarater and interrater reliability of the BHJMI, including the composite or overall joint-mobility index scores and the categorized joint-mobility index scores.
METHODS
We tested 42 female students for intrarater reliability and 36 of the 42 students for interrater reliability. Students were from the Chapel Hill High School soccer team and the University of North Carolina at Chapel Hill physical therapy program. The mean age was 25.4 ± 4.2 years (range, 15 to 45 years). Relatively young females who were not injured were chosen as subjects in this investigation because we believed they represented a clinically relevant population, and their joints could be assessed at end range. Females, particularly relatively young females, are more likely to have GJL.5,6,11–16 Before participating in this investigation, all subjects read and signed an informed consent approved by the institution's internal review board for ethical treatment of human subjects, which also approved the study.
Beighton and Horan Joint Mobility Index
Two raters were trained in the appropriate use of the BHJMI. One rater had 6 years of full-time experience as a physical therapist and was trained in the BHJMI in a continuing education course. The other rater had 22 years of part-time experience and was trained in the index by the first rater before the study. All subjects examined for intrarater reliability (n = 42) were tested on 2 occasions. Thirty-six of these subjects were also tested a third time to assess interrater reliability. Both examiners were blinded to previous results. Five of the 42 subjects who completed the intrarater reliability portion of this experiment were not available for the interrater reliability portion for logistical reasons. To determine intrarater reliability, rater 1 tested subjects twice, 1 day to 2 weeks apart (mean = 6 ± 4 days). To determine interrater reliability, rater 2 tested subjects once, on the same day or within 6 days of rater 1 (mean = 1 ± 2 days). Interrater testings on the same day were spaced approximately 15 to 60 minutes apart. All tests were conducted with the subject standing, except for the knee-hyperextension test, which was measured with the subject supine. We measured fifth-finger extension using a medium-sized, plastic, 8-in (20.32-cm) goniometer with 2-degree increments and knee and elbow extension using a large, plastic, 12-in (30.48-cm) goniometer with 1-degree increments. For all procedures, goniometer placement followed the guidelines provided by Norkin and White.42 Trunk and hip flexion and thumb opposition were evaluated by the ability to complete a specific task (see below); therefore, no measurement device was needed for these tests. All measurements were performed bilaterally, except for trunk flexion. The fifth-finger extension test was demonstrated by the rater, and then the fifth finger was passively extended by the subject. The distal portion of the fifth metacarpal was stabilized with the thumb of the opposite hand, while the tip of the fifth finger was extended by the subject using the index or middle finger as far as possible without pain. Goniometric measurements were taken with the fulcrum over the center of the metacarpophalangeal joint, the distal arm along the length of the finger, and the proximal arm along the fifth metacarpal. Fifth-finger hyperextension greater than 90 degrees resulted in a score of 1. Hyperextension of 90 degrees or less resulted in a score of 0.
Wrist Flexion and Thumb Opposition
The thumb-opposition test was demonstrated by the rater and then done passively by the subject. The subject stabilized the distal portion of the forearm with the thumb of the opposite hand, and the thumb being tested was passively abducted by the fingers of the opposite hand toward the volar aspect of the forearm with the wrist in flexion. If the thumb could be abducted to touch the forearm, then the score was 1. Opposition less than this resulted in a score of 0.
Elbow Extension
The elbow-extension test was performed with the subject's shoulder abducted to approximately 80 degrees and the forearm supinated. The rater then stabilized the proximal elbow from the posterior side while applying a gentle force to the subject's palmar wrist to achieve passive end-range extension. The center of the fulcrum was placed over the lateral epicondyle of the humerus, and the distal arm of the goniometer was positioned along the lateral midline of the forearm and aligned with the radial styloid process. The proximal arm was positioned along the lateral midline of the subject's humerus. Hyperextension of the elbow greater than 10 degrees resulted in a score of 1. Hyperextension of the elbow less than 10 degrees resulted in a score of 0.
Trunk and Hip Flexion
The trunk-flexion test was demonstrated by the rater and then repeated by the subject. The subject attempted to touch the palms flat to the floor while keeping the knees either extended or hyperextended. If the subject was able to flex the trunk so that the palms were flat on the ground, then trunk flexion received a score of 1; otherwise, a score of 0 was assigned.
Knee Extension
The knee-extension test was conducted in supine with 1 or 2 towel rolls placed under the ankle. The fulcrum of the goniometer was placed over the lateral epicondyle of the femur, and the proximal arm was aligned with the lateral midline of the femur, using the greater trochanter for reference. The distal arm was aligned with the lateral malleolus. Hyperextension of the knee greater than 10 degrees resulted in a score of 1. Hyperextension of the knee less than 10 degrees resulted in a score of 0. All 5 components of the BHJMI (right and left fifth fingers, right and left wrist and thumb, right and left elbows, right and left knees and trunk and hip) were measured and assigned either a 0 or 1. The scores, either 0 or 1 for each component of the BHJMI, were totaled for the composite score. The composite scores were then placed into 1 of 3 categories (0 to 2, 3 to 4, 5 to 9) to allow us to analyze the reliability of category scores.
Statistical Analysis
We manually calculated percentage agreement for intrarater and interrater reliability of the composite index scores (0 to 9) and categorized joint-mobility index scores (0 to 2 = category 1, 3 to 4 = category 2, and 5 to 9 = category 3). A Spearman rho was calculated for the composite index scores and categorized joint-mobility scores using NCSS 2000 (Dataxiom Software Inc, Los Angeles, CA).
RESULTS
Percentage Agreement
Composite index scores were within 1 point during both intrarater and interrater testing 88% of the time (Table 1). Agreement was 81% for intrarater testing and 89% for interrater testing of categorized joint-mobility index scores (Table 2). Four cases differed between categories 1 and 2, and 4 cases differed between categories 2 and 3.
Table 1.
Reliability of the Beighton and Horan Joint Mobility Index (BHJMI) Composite Scores
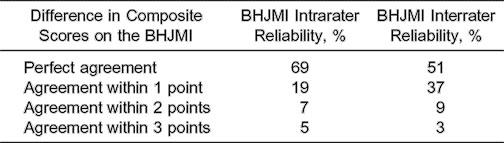
Table 2.
Percentage Agreement of the Beighton and Horan Joint Mobility Index (BHJMI) Category Scores

Spearman Rho
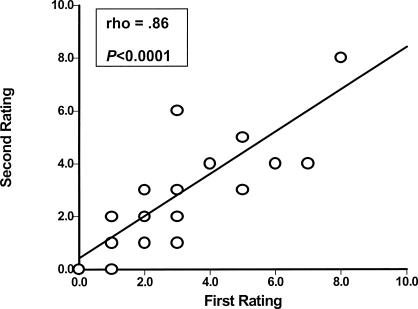
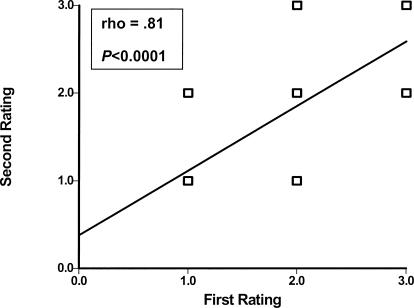
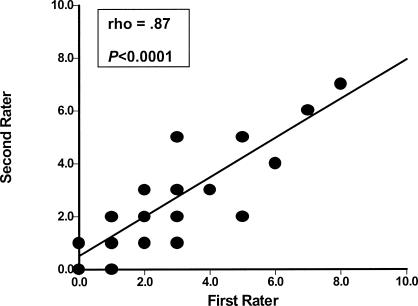
Scatter plots describing the reliability of intrarater and interrater testing of BHJMI composite scores and intrarater testing of categorized scores are provided in Figures 1 through 3. Reliability of interrater testing of BHJMI categorized scores is provided in Table 3. All P values were significant at <.0001. The intrarater and interrater reliability of BHJMI composite scores were excellent, with rho values of .86 and .87, respectively. In comparison, the intrarater and interrater reliability of BHJMI categorized scores were good to excellent, with rho values of .81 and .75, respectively.
Figure 1.
Intrarater reliability of the Beighton and Horan Joint Mobility Index composite scores (n = 42).
Figure 3.
Intrarater reliability of the Beighton and Horan Joint Mobility Index category scores (n = 42).
Table 3.
Interrater Reliability of the Beighton and Horan Joint Mobility Index (BHJMI) Category Scores (n = 36)*
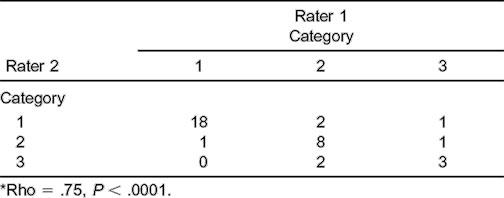
Figure 2.
Interrater reliability of the Beighton and Horan Joint Mobility Index composite scores (n = 36).
DISCUSSION
A clinical test to screen for systemic joint laxity, (ie, GJL), is needed. The presence of GJL in an individual may be associated with musculoskeletal complaints1,3–6,11,12,16,19–25 and may influence clinicians' interventions, such as therapeutic exercise and manual-therapy guidelines.26,28 Our investigation provides the first report of reliability of a joint-mobility index to screen for GJL. Intrarater and interrater reliability for both BHJMI composite and categorized scores were good to excellent. Sources of error for all testing, which may contribute to less than perfect reliability, may have included the time interval among measurements, goniometric measurement error (Rothestein et al43 reported knee and elbow intrarater and interrater reliability to have 95% agreement), experimenter error, weather changes, variability in verbal instructions to the subjects for testing technique and position (ie, the amount of pressure during the wrist-flexion and thumb-opposition test and the location of the pressure at the thumb tip or the thumb interphalangeal joint), and soft tissue surrounding the test joint having been warmed up or not before testing.
These conditions, however, are more consistent with a clinical environment rather than precisely controlled experimental conditions and, therefore, yield results with greater external validity. The 2-week interval between intrarater measurements may have allowed subjects to gain or lose flexibility for the spine test. A small goniometer in 1-degree increments would have likely been more accurate for fifth-finger measurement than the medium goniometer in 2-degree increments.
Weather changes may have affected repeat testing done on different days and at different times of the day. Joint stiffness varies throughout the day and increases with lower skin temperature and decreases with local heating.43
During the study, we noted that a few subjects could touch their forearms during the thumb test when pressing down on the tip of their thumb but could not touch if they pressed on the interphalangeal joint. Beighton and Horan did not include this level of detail in their description of the index; therefore, it was not included in our procedure. We recommend pressure be applied over the interphalangeal joint for a more rigorous criterion. Warming up the thumb before testing (perhaps through extensive writing) might have allowed the criterion to be met, when otherwise it would not have been.
Despite all of these potential sources of error, the measure has good to excellent reliability, suggesting it is a valuable clinical tool. Knowing where our athletes and patients fall in the spectrum of joint mobility (hypomobile to hypermobile) may influence our intervention and understanding of their complaints. The BHJMI is one way to obtain this information. The index is quick, taking 1 minute or less to complete, and does not require any special equipment. Our results suggest that the intrarater and interrater reliability of the BHJMI composite and category scores is good to excellent for females from 15 to 45 years of age.
REFERENCES
- 1.Ansell BM. Hypermobility of joints. Modern Trends Orthop. 1972;6:25–39. [Google Scholar]
- 2.Beighton P, Horan F. Orthopaedic aspects of the Ehlers-Danlos syndrome. J Bone Joint Surg Br. 1969;51:444–453. [PubMed] [Google Scholar]
- 3.Carter C, Sweetnam R. Recurrent dislocation of the patella and of the shoulder. J Bone Joint Surg Br. 1960;42:721–727. [PubMed] [Google Scholar]
- 4.Finsterbush A, Pogrund H. The hypermobility syndrome: musculoskeletal complaints in 100 consecutive cases of generalized joint hypermobility. Clin Orthop. 1982;168:124–127. [PubMed] [Google Scholar]
- 5.Gedalia A, Person DA, Brewer EJ, Jr, Giannini EH. Hypermobility of the joints in juvenile episodic arthritis/arthralgia. J Pediatr. 1985;107:873–876. doi: 10.1016/s0022-3476(85)80178-5. [DOI] [PubMed] [Google Scholar]
- 6.Howes RG, Isdale IC. The loose back: an unrecognized syndrome. Rheumatol Phys Med. 1971;11:72–77. doi: 10.1093/rheumatology/11.2.72. [DOI] [PubMed] [Google Scholar]
- 7.Jessee EF, Owen DS, Jr, Sagar KB. The benign hypermobile joint syndrome. Arthritis Rheum. 1980;23:1053–1056. doi: 10.1002/art.1780230914. [DOI] [PubMed] [Google Scholar]
- 8.Key AJ. Hypermobility of joints as a sex linked hereditary characteristic. JAMA. 1927;88:1710–1712. [Google Scholar]
- 9.Steiner ME, Grana WA, Chillag K, Schelberg-Karnes E. The effect of exercise on anterior-posterior knee laxity. Am J Sports Med. 1986;14:24–29. doi: 10.1177/036354658601400105. [DOI] [PubMed] [Google Scholar]
- 10.Sturkie P. Hypermobile joints in all descendants for two generations. J Hered. 1941;32:232–234. [Google Scholar]
- 11.Beighton P, Grahame R, Bird H. Hypermobility of Joints. New York, NY: Springer-Verlag; 1983. pp. 125–149. [Google Scholar]
- 12.Biro F, Gewanter HL, Baum J. The hypermobility syndrome. Pediatrics. 1983;72:701–706. [PubMed] [Google Scholar]
- 13.Calguneri M, Bird HA, Wright V. Changes in joint laxity occurring during pregnancy. Ann Rheum Dis. 1982;41:126–128. doi: 10.1136/ard.41.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klemp P, Stevens JE, Isaacs S. A hypermobility study in ballet dancers. J Rheumatol. 1984;11:692–696. [PubMed] [Google Scholar]
- 15.Larsson LG, Baum J, Mudholkar GS. Hypermobility: features and differential incidence between the sexes. Arthritis Rheum. 1987;30:1426–1430. doi: 10.1002/art.1780301216. [DOI] [PubMed] [Google Scholar]
- 16.Beighton P, Solomon L, Soskolne CL. Articular mobility in an African population. Ann Rheum Dis. 1973;32:413–418. doi: 10.1136/ard.32.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris H, Joseph J. Variation in extension of the metacarpophalangeal and interphalangeal joints of the thumb. J Bone Joint Surg Br. 1949;31:547–559. [PubMed] [Google Scholar]
- 18.Schweitzer G. Laxity of metacarpophalangeal joints of fingers and interphalangeal joint of the thumb: a comparative inter-racial study. S Afr Med J. 1970;28:246–249. [PubMed] [Google Scholar]
- 19.Kirk JA, Ansell BM, Bywaters EGL. The hypermobility syndrome: musculoskeletal complaints associated with generalized joint hypermobility. Ann Rheum Dis. 1967;26:419–425. doi: 10.1136/ard.26.5.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Rawi ZS, Al-Aszawi AJ, Al-Chalabi T. Joint mobility among university students in Iraq. Br J Rheumatol. 1985;24:326–331. doi: 10.1093/rheumatology/24.4.326. [DOI] [PubMed] [Google Scholar]
- 21.Arroyo IL, Brewer EJ, Giannini EH. Arthritis/arthralgia and hypermobility of the joints in schoolchildren. J Rheumatol. 1988;15:978–980. [PubMed] [Google Scholar]
- 22.Bird HA, Tribe CR, Bacon PA. Joint hypermobility leading to osteoarthrosis and chondrocalcinosis. Ann Rheum Dis. 1978;37:203–211. doi: 10.1136/ard.37.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grahame R, Edwards JC, Pitcher D, Gabell A, Harvey W. A clinical and echocardiographic study of patients with the hypermobility syndrome. Ann Rheum Dis. 1981;40:541–546. doi: 10.1136/ard.40.6.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harinstein D, Buckingham RB, Braun T, et al. Systemic joint laxity (the hypermobility syndrome) is associated with temporomandibular joint dysfunction. Arthritis Rheum. 1988;31:1259–1264. doi: 10.1002/art.1780311007. [DOI] [PubMed] [Google Scholar]
- 25.Nicholas JA. Injuries to knee ligaments: relationship to looseness and tightness in football players. JAMA. 1970;212:2236–2239. doi: 10.1001/jama.212.13.2236. [DOI] [PubMed] [Google Scholar]
- 26.Kisner C, Colby LA. Therapeutic Exercise: Foundations and Techniques. 3rd ed. Philadelphia, PA: FA Davis Co; 1985. pp. 191–192. [Google Scholar]
- 27.Maitland GD. Vertebral Manipulation. 5th ed. Oxford, England: Butterworth Heinemann; 1986. pp. 109–112. [Google Scholar]
- 28.Lewkonia RM. Hypermobility of joints. Arch Dis Child. 1987;62:1–2. doi: 10.1136/adc.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewkonia RM, Ansell BM. Articular hypermobility simulating chronic rheumatic disease. Arch Dis Child. 1983;58:988–992. doi: 10.1136/adc.58.12.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardin JA, Voight ML, Blackburn TA, Canner GC, Soffer SR. The effects of “decelerated” rehabilitation following anterior cruciate ligament reconstruction on a hyperelastic female adolescent: a case study. J Orthop Sports Phys Ther. 1997;26:29–34. doi: 10.2519/jospt.1997.26.1.29. [DOI] [PubMed] [Google Scholar]
- 31.Carter CO, Wilkinson JA. Persistent joint laxity and congenital dislocation of the hip. J Bone Joint Surg Br. 1964;46:40–45. [PubMed] [Google Scholar]
- 32.Fairbank JCT, Pynsent PB, Philips H. Quantitative measurements of joint mobility in adolescents. Ann Rheum Dis. 1984;43:288–294. doi: 10.1136/ard.43.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bird HA, Brodie DA, Wright V. Quantification of joint laxity. Rheumatol Rehabil. 1979;18:161–166. doi: 10.1093/rheumatology/18.3.161. [DOI] [PubMed] [Google Scholar]
- 34.Dijkstra PU, de Bont LG, Stegenga B, Boering G. Temporomandibular joint osteoarthrosis and generalized joint hypermobility. J Craniomandibular Pract. 1992;10:221–227. doi: 10.1080/08869634.1992.11677913. [DOI] [PubMed] [Google Scholar]
- 35.Grahame R, Jenkins JM. Joint hypermobility–asset or liability? A study of joint mobility in ballet dancers. Ann Rheum Dis. 1972;31:109–111. doi: 10.1136/ard.31.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ondrasik M, Rybar I, Rus V, Bosak V. Joint hypermobility in primary mitral valve prolapse patients. Clin Rheumatol. 1988;7:69–73. doi: 10.1007/BF02284059. [DOI] [PubMed] [Google Scholar]
- 37.Pitcher D, Grahame R. Mitral valve prolapse and joint hypermobility: evidence for a systemic connective tissue abnormality? Ann Rheum Dis. 1982;41:352–354. doi: 10.1136/ard.41.4.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plunkett GAJ, West VC. Systemic joint laxity and mandibular range of movement. J Craniomandibular Pract. 1988;6:320–326. doi: 10.1080/08869634.1988.11678255. [DOI] [PubMed] [Google Scholar]
- 39.Scott D, Bird H, Wright V. Joint laxity leading to osteoarthrosis. Rheumatol Rehabil. 1979;18:167–169. doi: 10.1093/rheumatology/18.3.167. [DOI] [PubMed] [Google Scholar]
- 40.Westling L. Craniomandibular disorders and general joint mobility. Acta Odontol Scand. 1989;47:293–299. doi: 10.3109/00016358909007715. [DOI] [PubMed] [Google Scholar]
- 41.Norkin CC, White DJ. Measurement of Joint Motion: A Guide to Goniometry. Philadelphia, PA: FA Davis Co; 1985. pp. 38–39.pp. 88–89. [Google Scholar]
- 42.Rothestein JM, Miller PT, Roettger RF. Goniometric reliability in a clinical setting: elbow and knee measurements. Phys Ther. 1983;63:1611–1615. doi: 10.1093/ptj/63.10.1611. [DOI] [PubMed] [Google Scholar]
- 43.Wright V, Dowson D, Longfied MD. Joint stiffness–its characterisation and significance. Biomed Eng. 1969;4:8–14. [PubMed] [Google Scholar]