Abstract
Objective:
To determine whether anterior cruciate ligament (ACL) laxity (as evaluated with the KT-2000 and radiographic measures) is associated with concentrations of reproductive hormones during the menstrual cycle and whether the KT-2000 knee arthrometer is a valid measurement technique, compared with radiographic techniques.
Design and Setting:
A within-subjects linear model was used. Venipuncture was conducted in an exercise science laboratory. The KT-2000 and radiographic measurements were performed in a hospital radiology laboratory.
Subjects:
Twelve females presented with a dominant right leg free of injury. They were mild to moderately active and had a 12-month history of normal menstrual cycles (28–35 days). Subjects had not used hormonal therapy for the previous 3 months.
Measurements:
Subjects were tested at the onset of menses, near ovulation, and on day 23 of the midluteal phase of the menstrual cycle. At each session, 14 mL of blood was obtained by venipuncture. Blood samples were analyzed via radioimmunoassay to determine the relative levels of each hormone. Anterior cruciate ligament laxity was simultaneously measured by KT-2000 and radiographic techniques.
Results:
Anterior cruciate ligament laxity measurements were significantly greater with the KT-2000 than with radiographic measurement. No significant differences were noted between menstrual-cycle phase and ACL laxity for either method. The concentration of luteinizing hormone was negatively correlated with laxity at the onset of menses using the radiographic technique.
Conclusions:
We found no associations between follicular-, ovulatory-, and luteal-phase hormonal concentrations and ACL laxity as measured on the KT-2000 and radiographs; no effects of menstrual-cycle phase on ACL laxity as measured by the KT-2000 and radiographs; and significant differences between KT-2000 and radiographic measures of ACL laxity.
Keywords: knee arthrometer, radioimmunoassay, estrogen, progesterone
The anterior cruciate ligament (ACL) is injured at an alarming rate in the athletic population, with more injuries occurring among females than among males.1–7 The ACL provides up to 86% of the restraint to anterior translation of the tibia on the femur; therefore, much of the research has focused on this structure.8 Several risk factors have been suggested to explain the difference in injury rates between males and females.6,9,10 One proposed risk factor for females is the fluctuating level of sex hormones throughout the menstrual cycle.6,11 Total estrogens, estradiol, progesterone, luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone are hormones normally present during the menstrual cycle, with the observed concentrations depending on the phase of the cycle.1,12–16 Changes in hormonal concentrations over the menstrual cycle may be related to the occurrence of peripheral joint laxity, possibly compounding the risk of injury.1–4,6,11,17
A few authors have examined the hormonal changes throughout the menstrual cycle in relation to the ACL,3,7,11,16–18 including ACL injury rate and menstrual-cycle phase3,7,17,18 and the relationship of laxity measurements with menstrual-cycle phase.11,16 Although selected hormones have an effect on the metabolic makeup of the ACL,19–22 it is unclear whether ligament laxity changes throughout the menstrual cycle. Replicating various aspects of the current studies, with appropriate modifications, will allow us to examine the reliability and validity of the proposed techniques and methods and examine potential relationships between other hormones and knee-joint laxity.
Our objectives were to examine the possible associations between ACL laxity and concentrations of total estrogens, estradiol, progesterone, LH, FSH, and testosterone during the 3 phases of the menstrual cycle and to compare ACL laxity using the KT-2000 arthrometer (MEDmetric Corp, San Diego, CA) and Staubli and Jakob's23 protocol for radiographic comparisons (Figure 1). We hypothesized there would be no difference among the 3 phases of the menstrual cycle in ACL laxity. Second, we proposed that more displacement would be measured on the KT-2000 than on radiographs because of soft tissue uptake but that the correlation between the values would be positive.
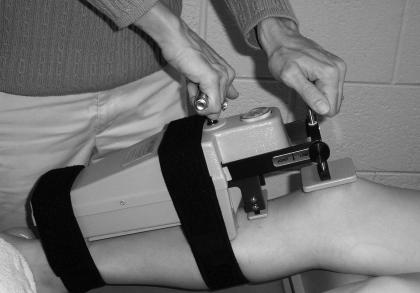
Figure 1.
Hand placement for modified KT-2000 testing.
METHODS
Design
We used a 2 × 3 repeated-measures analysis of variance (ANOVA) to determine the difference between the KT-2000 and radiographic measures of laxity over time. The first independent variable (within-subjects factor) was phase, which had 3 levels: first day of menses (follicular phase), near ovulation, and day 23 (luteal phase) of menses. The second independent variable was instrument, which had 2 levels: KT-2000 and radiographic assessment. The dependent variable was displacement in millimeters. Repeated-measures ANOVAs were also used to determine the differences among hormonal concentrations over time. The independent variable was phase, with 3 levels: first day of menses, near ovulation, and day 23 of menses. The dependent variable was the concentration of each hormone.
Subjects
Twelve females (mean age = 24.3 ± 4.9 years, mean height = 157.0 ± 5.4 cm, mean mass = 65.3 ± 11.0 kg) with a dominant right leg and no history of knee-joint injury volunteered to participate in this study. The dominant leg was determined by asking the subjects which leg they would choose to kick a ball. All subjects had a minimal risk of injury during the testing period; reported no use of hormonal therapy or supplementation for the previous 3 months, no history of pregnancy, a history of eumenorrhea for the past year, and an average menstrual cycle length of 28 to 35 days; and had a Q-angle of less than 15 degrees. Procedures were approved by the university, and subjects were informed of any possible risks associated with the study before giving informed consent.
Instruments
On 3 separate occasions, 14 mL of blood was drawn from an antecubital vein of the forearm and placed in K3EDTA Vacutainer tubes (Becton Dickinson, Fairleigh, NJ). Samples were centrifuged at 1500 revolutions per minute for 10 minutes to extract the plasma. The extracted plasma was then immediately frozen and stored at −60°C for analysis via commercial radioimmunoassay (ICN Pharmaceuticals, Inc, Costa Mesa, CA). Concentrations of total estrogens, estradiol, progesterone, LH, FSH, and testosterone were determined once all the data had been collected.
We assessed ACL laxity using the KT-2000 and plain radiographic films. The KT-2000 displaces the tibia anteriorly to allow for an objective measurement of laxity at 30 lb or 133 N of force. The KT-2000 is a valid instrument for the measurement of subtle differences in the properties of the ACL.24 An intraclass correlation coefficient (ICC) (2,1) was used to determine the intratester reliability for the KT-2000 (subject n = 13). The examiner proved to be reliable over time, with an ICC of .92 (P = .0001). Reliability was established in a laboratory setting before the study began, and each subject was tested twice over an average of 3 days. Reliability testing took place over the course of approximately 1 month, and the hand-placement method was the same used for the study. Plain radiographs were taken at a local hospital radiography department by trained radiology technicians. This method of laxity measurement permitted a more precise reading of the movement of the involved bony anatomy.23 Both values were measured in millimeters.
An over-the-counter ovulation test kit (First Response, Church and Dwight, Princeton, NJ) with a sensitivity of 99% was issued to each subject for self-monitoring of the midcycle LH surge that signifies the onset of ovulation. Each subject began monitoring on day 5 of the cycle.
Procedures
All subjects were familiarized (1–3 sessions) with the KT-2000 to become acclimated to the application of the device. A subject was considered familiar with the KT-2000 when the examiner was able to obtain consistent readings on the graph that were reproducible and stable. After the familiarization screening, subjects were asked to track their menstrual cycles and contact the researchers on day 1 (follicular phase). The initial testing session was conducted between 16 and 35.5 hours (mean = 23.44 ± 7.54) after the onset of menses.
On the subject's arrival to the laboratory, a blood sample was obtained from the antecubital vein. All blood samples were drawn by 1 of 3 individuals who were all trained in phlebotomy. This was followed by measurements of the KT-2000 knee arthrometer (ICC = .92) and plain radiographs (ICC[3,1] = .84) to assess ACL laxity. Patients assumed a supine position on the examination table, and a cross-table, lateral-view radiograph of the right knee with a film-to-tube distance of 120 cm was obtained. Laxity measures were obtained according to the manufacturer's instructions with 2 modifications. The thigh support was positioned only under the right leg to allow for the placement of the radiographic film cartridge between the subject's knees. Tibial rotation was neutral and controlled with a foot support to reduce external rotation and a towel to block internal rotation. Once the subject was in position, the first radiographic picture was taken without force being exerted on the knee. Then pressure was applied to the patellar sensor pad and maintained at a constant level. Force equaling 133 N was exerted in an anterior direction and confirmed with an audible tone produced by the unit and a point on the X-Y plotter attached to the device.
When the force was applied, the radiographers were instructed to take a second film of the subject's knee. To prevent radiation exposure to the examiner's hands, pressure on the patellar sensor pad was applied to the top of the handle rather than to the pad itself (see Figure 1). The 2 films were compared, according to Staubli and Jakob's23 protocol (Figure 2), to determine the tibial displacement in millimeters. However, we evaluated the displacement of the tibia at 133 N, whereas Staubli and Jakob23 used 89 N. All measurements were taken by hand with a metric ruler.
Figure 2.
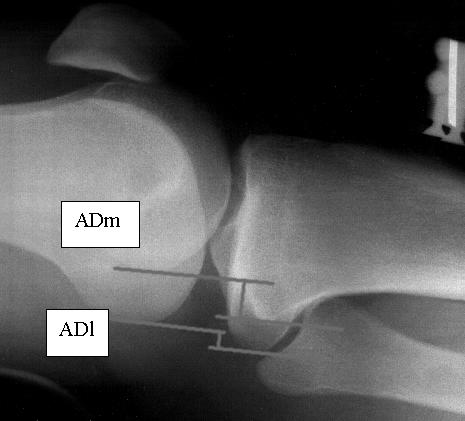
Radiographic assessment. Lateral radiograph measuring anterior displacement medially and anterior displacement laterally with applied stress. ADm indicates anterior displacement of the medial compartment; ADl, anterior displacement of the lateral compartment.
The radiographic values were obtained by first measuring the anterior position of the tibia with respect to the femur and drawing a line on the posterior tibial cortex (PTC) at the midshaft level. The most posterior aspects of the medial tibial plateau and medial femoral condyle were identified at the subchondral bone level. A tangent line was drawn to the most posterior aspect of the tibial plateau and parallel to the PTC. A second parallel line to the PTC was drawn tangent to the posterior aspect of the femoral condyle. The distance between the 2 tangent lines was considered the anterior displacement of the medial compartment (ADm). This measurement defined the anterior position of the medial tibial plateau with regard to the medial femoral condyle. Anterior displacement was also measured in the lateral compartment (ADl) using the most posterior aspects of the lateral tibial plateau and lateral femoral condyle. Then the sum of the ADm and ADl was divided by 2. The arithmetic mean of the measurements for both compartments represented the anterior knee motion by describing the anterior position of the tibia with regard to the femur at the midpoint of the knee. The amount of displacement was determined by subtracting the amount of tibial translation according to the radiograph with no exerted force from the radiograph taken during the application of 133 N.23
After the first testing session, each subject was given an at-home ovulation test kit and instructed to begin monitoring for the first day of ovulation on day 5 of the menstrual cycle. The individual was instructed to hold the test stick by the thumb grip and place the urine collection pad in the urine stream for 10 seconds. The stick was then placed on a flat surface with the result window facing up. A positive test was represented by a test line that was as dark as or darker than the reference line inside the result window. If the test line was lighter than the reference line, then the LH surge had not occurred, and the subject continued to monitor each day until the LH surge was detected. When the subject tested positive, she notified the examiners, and tests for hormonal concentrations and ACL laxity were conducted between 9.75 and 34 hours (mean = 19.9 ± 8.7 hours) after ovulation. A third series of tests was completed on day 23 of the cycle to obtain a reading from the midluteal phase.
One subject was tested by examiner 1 (B.V.L.), and the subsequent subjects (11) were examined by examiner 2 (J.R.). We intended to have 1 examiner test all subjects, but examiner 1 had to discontinue testing for health reasons. Data analyses were conducted for the 11 subjects tested by examiner 2, and the results are consistent with the reported findings of all 12 subjects. The examiners were not blinded to the phase of the menstrual cycle, but examiner 2 was blinded to measurements derived from the radiographs. Code numbers were assigned to each radiograph, and measurements were taken in random order.
Concentrations of each hormone (total estrogens, estradiol, progesterone, LH, FSH, and testosterone) in plasma samples were assayed in a batch using a single kit. The intra-assay coefficients of variation on duplicate measures of 36 samples agreed with the manufacturer's specifications regarding assay reliability (total estrogens = 9.5 ± 2.8%, estradiol = 2.6 ± 0.3%, progesterone = 1.6 ± 0.2%, LH = 5.4 ± 1.0%, FSH = 3.9 ± 0.5%, testosterone = 1.6 ± 0.3%).
Statistical Analysis
We used repeated-measures ANOVAs to determine the differences between the KT-2000 and radiographic measures of laxity over time and the changes in hormonal levels over time with a Bonferroni adjustment for post hoc comparisons. With a Pearson product moment correlation coefficient (95% confidence interval [CI] around Pearson r based on the Fisher z transformation), we measured the relationship of ACL laxity to hormonal levels throughout the 3 phases of the menstrual cycle. An α level of .05 was the criterion for statistical significance. Intraclass correlation coefficients (2,1) (3,1) were used to evaluate intratester reliability for the KT-2000 and the radiographic readings, respectively. We analyzed the data with SPSS for Windows (version 10.01; SPSS Inc, Chicago, IL).
RESULTS
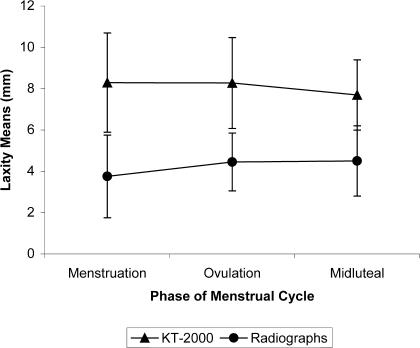
Differences in each hormone concentration over time were as expected (Table). A main effect was noted for testing method (F1,11 = 74.57, P = .0001) with greater laxity measurements reported using the KT-2000 (8.08 ± 0.52 mm) than on radiographs (4.24 ± 0.36 mm). Anterior cruciate ligament laxity did not change among follicular (6.02 ± 0.54 mm), near-ovulation (6.36 ± 0.38 mm), and midluteal (6.1 ± 0.39 mm) phases of the menstrual cycle (F2,22 = 0.469, P = .632), nor was there a significant interaction between method (KT-2000 or radiograph) and time (F2,22 = 1.202, P = .32, Figure 3).
Mean Reproductive Hormone Concentration at the Onset of Menses (Day 1), Near Ovulation, and on Cycle Day 23
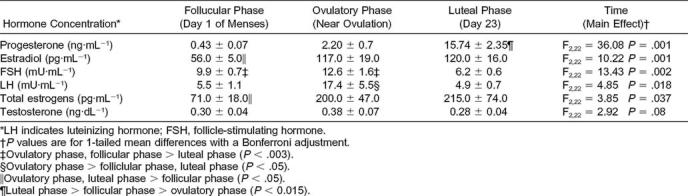
Figure 3.
Laxity means as measured on KT-2000 and radiographs at each phase of the menstrual cycle.
A Pearson product moment correlation coefficient revealed no statistically significant relationships between laxity measurements of the KT-2000 or radiographic comparisons at menstruation (r = .461, 95% CI = −0.16, ± 0.91, P = .131), near ovulation (r = .02, 95% CI = −0.56, ± 0.58, P = .951), or the midluteal phase (r = .236, 95% CI = −0.39 ± 0.72, P = .461) and concentrations of hormones. However, LH in the follicular phase, at the onset of menses, was significantly negatively correlated with the radiographic readings (r = −.628, 95% CI = −0.88, −0.09, P = .029). This was not observed in the coinciding reading from the KT-2000. No other correlations were found.
DISCUSSION
Anterior cruciate ligament laxity, regardless of measurement technique, and follicular, near-ovulation, and midluteal concentrations of total estrogens, estradiol, progesterone, LH, FSH, and testosterone were not associated. The changes in these hormone concentrations over the cycle were consistent with previous reports for women with regular menstrual cycles.11–14,25 Moreover, the mean radiographic measure was significantly different from that of the KT-2000, but we detected no differences for either method over time. Although the relationships between ACL laxity and menstrual-cycle phase11,16,17 and ACL laxity and estrogen and progesterone levels11 have been the focus of previous studies, we are unaware of any research examining knee laxity and varying levels of estradiol (the principal estrogen in the plasma of females),25 LH, FSH, or testosterone. Another finding of this study was the negative relationship between LH during the follicular phase and the radiographic findings. We were unable to determine why this occurred other than the fact that it may be due to chance.
Heitz et al11 reported a significant increase in ACL laxity occurring in conjunction with the approximate time of ovulation and preovulation (days 10–13) and an increase in laxity during the midluteal peak in estrogens and progesterone. This finding does not agree with Karageanes et al,16 who reported no significant difference in right- or left-knee ACL laxity in adolescent females over the menstrual cycle. This discrepancy may be because they examined the laxity difference at 89 N, and Heitz et al11 tested the laxity difference at 133 N. We chose to use 133 N of force as well because it produced more tension on the knee joint, thus taking into account the soft tissue involvement and therefore evaluating ACL involvement.26 In addition, measurement reliability at 133 N is comparable with that at 89 N of force.27 Our results do not support those of Heitz et al11 and may be due to the fact that we spent additional time familiarizing the subjects with the KT-2000 equipment. All subjects had to have a stable printout before they could be included in the study (1–3 familiarization sessions), which allowed us to address the validity concerns of instrumentation.28 Although our hand placement for the KT-2000 was adjusted to accommodate for radiation exposure, we were able to obtain high values of reliability before testing, and we used this same technique in the familiarization session and produced consistent results. We also used the dominant leg for all testing. When using the KT-2000, the examiner is generally more comfortable testing a particular limb, and differences when testing the right versus the left leg have been documented.16
The difference between the radiographic measures and the KT-2000 measures can possibly be explained by several theories. Staubli and Jakob23 used this technique in evaluating both knees of 16 patients who had ACL disruption with a displacement force of 89 N. The average displacements for the ACL-deficient knee were 11.9 and 11.2 for the KT-1000 and radiographic measures, respectively. The values for the ACL-intact knee were 6.2 for the KT-1000 and 4.9 for the radiographic measures. Both techniques measure displacement, but many factors must be considered when they are compared. Neither technique may be able to detect the amount of rotation occurring during a single test, and both use anatomic landmarks as part of the measurement technique. The degree of soft tissue involvement in the calculation of each technique cannot be determined. The radiographic technique measures the changes in bony alignments, whereas the KT-2000 measures total displacement of the knee. In our study, the average value of the radiographic measures was lower for all tests compared with the KT-2000. We postulate that this may be because the KT-2000 must also take up the soft tissue involvement before the tibia is actually displaced, thereby accounting for higher displacement values.24 We also took displacement measurements at 133 N, and the 2 techniques have not been compared.
Because we chose to examine the relationship between the values from the KT-2000 and the radiographic techniques, we had to limit the number of times data were collected throughout the cycle. We chose to evaluate a point within each phase of the menstrual cycle based on the past findings of significance in other studies.11,17,18,21,22,29 Testing near ovulation using the ovulation test kits may prove problematic for the researcher. Our average time from a positive test reading was 19.9 ± 8.7 hours; therefore, the measurements took place as the estrogen levels should have been rapidly decreasing.25 It is also problematic to assume that all women will ovulate between days 10 and 13. Some authors have chosen to examine the follicular phase of the menstrual cycle by collecting data in the window of time ovulation is estimated to occur.11,16 We had to begin monitoring for LH increases at day 5 because we initially missed 3 subjects' ovulation times (positive tests ranged from days 8 to 17). Only 1 of those subjects completed the study, and data had to again be collected for all 3 phases of the menstrual cycle.
The rate of occurrence of ACL injury and the relationship to the menstrual cycle has recently been documented.9,17 A retrospective survey by Wojyts et al17 reported that 13% of ACL injuries occurred between days 1 and 9, whereas 29% occurred between days 10 and 14. Initially, these data were reported to support a significant association between menstrual-cycle phase and ACL injury, but they were later reported as statistically insignificant.30 The proportion of ACL injuries that occurred between day 15 and the end of the menstrual cycle (58%) was not statistically significant. A similar study by Wojyts et al18 helped to clarify previous findings and provided a more in-depth evaluation of the research question. Women were asked to provide a urine sample within 24 hours of tearing their ACLs and again within 24 hours of the onset of menses to validate menstrual-cycle phase by measurement of estrogen, progesterone, LH metabolites, and creatinine levels. Information was gathered concerning the mechanism and nature of the injury and frequency of athletic participation. The subjects were also asked to provide a detailed history of their menstrual cycles, the date of the last menstrual period, the average length of the cycle, and oral-contraceptive or hormone-replacement use. The observed number of ACL tears during ovulation was more than 2.5 times the expected number in women not taking oral contraceptives. This finding coincides with the increase in estrogen production and may be linked to the physiologic changes affecting the ligament throughout the menstrual cycle.
The proper function of a ligament depends on the appropriate type, synthesis, assembly, cross-linking, and remodeling of collagen.31 Type I collagen imparts greater mechanical strength to connective tissue, whereas type 3 collagen has been correlated directly with tissue elasticity.32 The relative decrease in type I procollagen synthesis with increasing estradiol concentrations may translate to ligament weakening,21,22 but as progesterone increases and estrogen is held constant, fibroblast proliferation and type I procollagen synthesis are increased significantly.22 Overall, these fluctuations through the menstrual cycle seem to induce changes in the metabolism of the ACL fibroblasts and may, therefore, result in reduced strength of the ACL and predispose more female athletes to injury.19,22 This relationship is consistent with the findings of Slauterbeck et al,20 who examined the ACLs of 2 groups of ovariectomized white rabbits. One group was given an estrogen supplement composed of estradiol crystals, and the other served as a control. The ligament's load to failure was tested, and the estrogen supplement group had a significantly lower load to failure compared with the controls. Exposure to higher levels of estrogen may be linked with a decreased load to failure, suggesting that estrogen plays a role in the tensile strength of the ligament. Previous authors noted increased laxity coinciding with increased levels of estrogen.11 We may need to consider evaluating other aspects of the ligament, such as receptor expression and hormone-binding proteins, as related to load to failure to determine what is truly happening with the ACL ligament over time. Researchers have identified estrogen receptors in humans33 and estrogen and progesterone receptors in humans and New Zealand White rabbits,34 respectively. Also, the expression of estrogen receptors in females was different from males.34 These findings34 support the concept that hormones may be a factor in the rate of ACL injuries in the female population.
In conclusion, we demonstrated no differences between laxity measures and the phase of the menstrual cycle and no overall relationships among all measures, except for the LH level during the follicular phase and the value for radiographic assessment. It is evident that we are just beginning to understand the complex role hormones play in the ACL injury epidemic in females, and the investigation of a larger sample size throughout the entire menstrual cycle may be beneficial. We must first thoroughly investigate the physiologic effects of the hormones on the ACL and then apply this knowledge to the incidence of ACL injury. Future researchers should investigate the effects of the menstrual-associated hormones on the metabolic changes occurring to the ACL. We should also evaluate what these changes do to the tensile strength of the ACL and find ways to better measure the laxity or stiffness of minute changes occurring throughout menstruation. Lastly, we need to determine how the ligament is affected by oral contraceptives. All of these avenues should be investigated; however, we should also keep in mind the many other factors (structural, neuromuscular, etc) that could contribute to an increased ACL injury rate in the athletic female.
REFERENCES
- 1.Moller-Nielsen J, Hammar M. Sports injuries and oral contraceptive use: is there a relationship? Sports Med. 1991;12:152–160. doi: 10.2165/00007256-199112030-00002. [DOI] [PubMed] [Google Scholar]
- 2.Arendt EA, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer: NCAA data and review of literature. Am J Sports Med. 1995;23:694–701. doi: 10.1177/036354659502300611. [DOI] [PubMed] [Google Scholar]
- 3.Arendt EA, Agel J, Dick R. Anterior cruciate ligament injury patterns among collegiate men and women. J Athl Train. 1999;34:86–92. [PMC free article] [PubMed] [Google Scholar]
- 4.Gray J, Taunton JE, McKenzie DC, Clement DB, McConkey JP, Davidson RG. A survey of injuries to the anterior cruciate ligament of the knee in female basketball players. Int J Sports Med. 1985;6:314–316. doi: 10.1055/s-2008-1025861. [DOI] [PubMed] [Google Scholar]
- 5.Gwinn DE, Wilckens JH, McDevitt ER, Ross G, Kao TC. The relative incidence of anterior cruciate ligament injury in men and women at the United States Naval Academy. Am J Sports Med. 2000;28:98–102. doi: 10.1177/03635465000280012901. [DOI] [PubMed] [Google Scholar]
- 6.Ireland ML. Anterior cruciate ligament injury in female athletes: epidemiology. J Athl Train. 1999;34:150–154. [PMC free article] [PubMed] [Google Scholar]
- 7.Moller-Nielsen J, Hammar M. Women's soccer injuries in relation to the menstrual cycle and oral contraceptive use. Med Sci Sports Exerc. 1988;21:126–129. [PubMed] [Google Scholar]
- 8.Butler DL, Noyes FR, Grood ES. Ligamentous restraints to anterior-posterior drawer in the human knee: a biomechanical study. J Bone Joint Surg Am. 1980;62:259–270. [PubMed] [Google Scholar]
- 9.Bonci CM. Assessment and evaluation of predisposing factors to anterior cruciate ligament injury. J Athl Train. 1999;34:155–164. [PMC free article] [PubMed] [Google Scholar]
- 10.Loudon JK, Jenkins W, Loudon KL. The relationship between static posture and ACL injury in female athletes. J Orthop Sports Phys Ther. 1996;24:91–97. doi: 10.2519/jospt.1996.24.2.91. [DOI] [PubMed] [Google Scholar]
- 11.Heitz NA, Eisenman PA, Beck CL, Walker JA. Hormonal changes throughout the menstrual cycle and increased anterior cruciate ligament laxity in females. J Athl Train. 1999;34:144–149. [PMC free article] [PubMed] [Google Scholar]
- 12.Speroff L, Glass RH, Kase NG. Clinical Gynecologic Endocrinology and Infertility. 2nd ed. Baltimore, MD: Williams & Wilkins Co; 1978. pp. 38–39.pp. 49–60. [Google Scholar]
- 13.Yen SSC, Jaffe RB. Reproductive Endocrinology: Physiology, Pathophysiology and Clinical Management. 2nd ed. Philadelphia, PA: WB Saunders Co; 1986. pp. 201–230. [Google Scholar]
- 14.Owen JA., Jr Physiology of the menstrual cycle. Am J Clin Nutr. 1975;28:333–338. doi: 10.1093/ajcn/28.4.333. [DOI] [PubMed] [Google Scholar]
- 15.Hadley ME. Endocrinology. 2nd ed. Englewood Cliffs, NJ: Prentice Hall; 1992. pp. 481–485. [Google Scholar]
- 16.Karageanes SJ, Blackburn K, Vangelos ZA. The association of the menstrual cycle with the laxity of the anterior cruciate ligament in adolescent female athletes. Clin J Sport Med. 2000;10:162–168. doi: 10.1097/00042752-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Wojyts EM, Huston LJ, Lindenfeld TN, Hewett TE, Greenfield ML. Association between the menstrual cycle and anterior cruciate ligament injuries in female athletes. Am J Sports Med. 1998;26:614–619. doi: 10.1177/03635465980260050301. [DOI] [PubMed] [Google Scholar]
- 18.Wojyts EM, Huston LJ, Boynton MD, Spindler KP, Lindenfeld TN. The effect of the menstrual cycle on anterior cruciate ligament injuries in women as determined by hormone levels. Am J Sports Med. 2002;30:182–188. doi: 10.1177/03635465020300020601. [DOI] [PubMed] [Google Scholar]
- 19.Charlton WP, Coslett-Charlton LM, Ciccotti MG. Correlation of estradiol in pregnancy and anterior cruciate ligament laxity. Clin Orthop. 2001;387:165–170. doi: 10.1097/00003086-200106000-00022. [DOI] [PubMed] [Google Scholar]
- 20.Slauterbeck J, Clevenger C, Lundberg W, Burchfield D. Estrogen level alters the failure load of the rabbit anterior cruciate ligament. J Orthop Res. 1999;17:405–408. doi: 10.1002/jor.1100170316. [DOI] [PubMed] [Google Scholar]
- 21.Yu WD, Liu SH, Hatch JD, Panossian V, Finerman GA. Effect of estrogen on cellular metabolism of the human anterior cruciate ligament. Clin Orthop. 1999;366:229–238. doi: 10.1097/00003086-199909000-00030. [DOI] [PubMed] [Google Scholar]
- 22.Yu WD, Panossian V, Hatch JD, Liu SH, Finerman GA. Combined effects of estrogen and progesterone on the anterior cruciate ligament. Clin Orthop. 2001;383:268–281. doi: 10.1097/00003086-200102000-00031. [DOI] [PubMed] [Google Scholar]
- 23.Staubli HU, Jakob RP. Anterior knee motion analysis: measurement and simultaneous radiography. Am J Sports Med. 1991;19:172–177. doi: 10.1177/036354659101900213. [DOI] [PubMed] [Google Scholar]
- 24.Rijke AM, Perrin DH, Goitz HT, McCue FC., 3rd Instrumented arthrometry for diagnosing partial versus complete anterior cruciate ligament tears. Am J Sports Med. 1994;22:294–298. doi: 10.1177/036354659402200223. [DOI] [PubMed] [Google Scholar]
- 25.Guyton AC, Hall JE. Textbook of Medical Physiology. 9th ed. Philadelphia, PA: WB Saunders Co; 1996. pp. 1037–1039. [Google Scholar]
- 26.Highgenboten CL, Jackson AW, Jansson KA, Meske NB. KT-1000 arthrometer: conscious and unconscious test results using 15, 20, and 30 pounds of force. Am J Sports Med. 1992;20:450–454. doi: 10.1177/036354659202000415. [DOI] [PubMed] [Google Scholar]
- 27.Robnett NJ, Riddle DL, Kues JM. Intertester reliability of measurements obtained with the KT-1000 on patients with reconstructed anterior cruciate ligaments. J Orthop Sports Phys Ther. 1995;21:113–119. doi: 10.2519/jospt.1995.21.2.113. [DOI] [PubMed] [Google Scholar]
- 28.Wroble RR, Van Ginkel LA, Grood ES, Noyes FR, Shaffer BL. Repeatability of the KT-1000 arthrometer in a normal population. Am J Sports Med. 1990;18:396–399. doi: 10.1177/036354659001800411. [DOI] [PubMed] [Google Scholar]
- 29.Liu SH, Al-Shaikh RA, Panossian V, Finerman GAM, Lane JM. Estrogen affects the cellular metabolism of the anterior cruciate ligament: a potential explanation for female athletic injury. Am J Sports Med. 1997;25:704–709. doi: 10.1177/036354659702500521. [DOI] [PubMed] [Google Scholar]
- 30.Wojyts EM. Letter to the editor: author's response. Am J Sports Med. 2000;28:131. [Google Scholar]
- 31.Cooper RR, Misol S. Tendon and ligament insertion: a light and electron microscopic study. J Bone Joint Surg Am. 1992;52:1–20. [PubMed] [Google Scholar]
- 32.Liu SH, Yang RS, Al-Shaikh R, Lane JM. Collagen in tendon, ligament, and bone healing: a current review. Clin Orthop. 1995;318:265–278. [PubMed] [Google Scholar]
- 33.Liu SH, Al-Shaikh R, Panossian V, Yang RS, Nelson SD, Soleiman N, Finerman GA, Lane JM. Primary immunolocalization of estrogen and progesterone target cells in the human anterior cruciate ligament. J Orthop Res. 1996;14:526–533. doi: 10.1002/jor.1100140405. [DOI] [PubMed] [Google Scholar]
- 34.Sciore P, Frank CB, Hart DA. Identification of sex hormone receptors in human and rabbit ligaments of the knee by reverse transcription-polymerase chain reaction: evidence that receptors are present in tissue from both male and female subjects. J Orthop Res. 1998;16:604–610. doi: 10.1002/jor.1100160513. [DOI] [PubMed] [Google Scholar]