Abstract
Due to an increasing life expectance, osteoarthritis (OA) is one of the most common chronic diseases. Although strong efforts have been made to regenerate degenerated joint cartilage, OA is a progressive and irreversible disease up to date. Among other factors the dysbalance between free radical burden and cellular scavenging mechanisms defined as oxidative stress is a relevant part of OA pathogenesis. Here, only little data are available about the mediation and interaction between different joint compartments. The article provides a review of the current literature regarding the influence of oxidative stress on cellular aging, senescence and apoptosis in different joint compartments (cartilage, synovial tissue and subchondral bone). Free radical exposure is known to promote cellular senescence and apoptosis. Radical oxygen species (ROS) involvement in inflammation, fibrosis control and pain nociception has been proven. The data from literature indicates a link between free radical burden and OA pathogenesis mediating local tissue reactions between the joint compartments. Hence, oxidative stress is likely not only to promote cartilage destruction but also to be involved in inflammative transformation, promoting the transition from clinically silent cartilage destruction to apparent OA. ROS induced by exogenous factors such as overload, trauma, local intraarticular lesion and consecutive synovial inflammation cause cartilage degradation. In the affected joint, free radicals mediate disease progression. The interrelationship between oxidative stress and OA etiology might provide a novel approach to the comprehension and therefore modification of disease progression and symptom control.
Key words: osteoarthritis, free radicals, oxidative damage.
Introduction
Osteoarthritis (OA) is a common and progressive chronic disease leading to impaired joint function and can result in immobility mostly in elderly people.1 The prevalence of OA increases significantly in advanced age. Radiographic evaluation of knee x-ray images taken from the Framingham osteoarthritis study showed alterations in K/L (Kellgren/ Lawrence Grade)> or =2 in 44% of parents (mean age 72 years) and 22% of offspring (mean age 54 years).2 Although several risk factors like overweight or the family history of OA are well known, the mechanisms on the cellular level leading from the presence of risk factors to cartilage degeneration are not completely understood in detail. Besides other agents, recent data focus on oxidative and nitrosative stress as one aspect involved in the pathogenesis of OA.3 In the course of the growing knowledge about the relevance of free radicals for cellular ageing, their involvement in degenerative diseases of the joint came into focus of more detailed investigations.4
Method of data selection
We present a review of the current literature over the relevance of free radicals in OA pathogenesis. Here, all relevant original publications in this topic documented in the USA National Library of Medicine and the library of the National Institutes of Health, USA were considered. Reviews and single case reports were excluded. The online search was conducted by use of the terms oxidative stress, oxidative damage and radical oxygen species, each of them combined with the search term osteoarthritis. This yielded a cumulative number of 203 studies. Of these results, 33 were identified as review or case report, leaving 170 relevant studies for this review. The relevance of the studies cited herein was considered by actuality, included sample size and methods as well as by the number of being cited evaluated by use of ISI Web of Science®. Finally, the selected studies are discussed critically and new insights were given to this innovative research field.
Lessons from actual studies
The silent way of osteoarthritis
OA is not limited to articular cartilage only, but also affects the subchondral bone, as well as the adjacent connective tissue and the synovial membrane resulting in pain, swelling, progressive deformity and instability. Although OA occurs in many joints, the knee, hip, hand and facet joints are mostly affected.5
Yet, patients suffering from OA often show a discrepancy between objective findings gathered in x-ray or magnetic resonance imaging (MRI) examinations and patient-reported pain and functional restrictions.6 The sudden onset of disabling joint pain and articular effusion is one typical clinical feature of decompensation indicating the term activated OA. On a cellular and subcellular level, proinflammatory agents such as nitric oxide, interleukin 1 (IL-1), and tumor necrosis factor (TNF) α are overexpressed in chondrocytes and joint stromal cells in OA.7,8
The influence of free radicals on osteoarthritis development
Many studies focused on damaging effects of oxidative and nitrosative agents,9–11 whereas in the following the differential role of Radical oxygen species (ROS) and radical nitric species (RNS) in osteoarthritis came into focus. A study comparing the effects of nitric oxide and oxidative dysbalance in OA and rheumatoid arthritis postulated a major role of nitric oxide (NO) to modulate chondrocyte function in OA.12 In contrast to the uncoupling effect of ROS on synovium inflammation,13 ROS have been shown to downregulate the expression of pro- inflammatory genes in chondrocytes.14 The involvement of free radicals in signal transduction highlighted that within evolution higher life forms took advantage of the ability of ROS to mediate signals over cell margins in a system consisting of discrete compartments like joint.15,16
Regarding radical nitrosative species nitric oxide is subject to extensive research. It is produced by the closely regulated nitric oxide synthase (NOS) using the substrate L-arginine. Several isoforms of NOS have been identified in OA joint tissue. Functional relevance of constitutional neuronal NOS (nNOS)17 and inducible NOS (iNOS)18 in OA pathogenesis has been shown. Furthermore, osteoarthritis-affected NOS (OA-NOS) only detectable in OA cartilage was described,19 showing properties similar to nNOS and iNOS. Evidence for NOS mediated signaling in OA was found in cartilage20 and synovium.21 Here, a relative deficit in the production of natural antagonists of the IL-1 receptor has been demonstrated, and could possibly be related to an excess production of nitric oxide in OA tissues.22 In cartilage tissue, chondrocyte interleukin-1-converting enzyme and interleukin-18 levels are shown to be mediated by nitric oxide.23,24 Another study demonstrated that proinflammatory mediators such as IL-1 give in turn rise to NO formation in inflammative joints.25 The upregulation of iNOS related NO production is a well known feature of IL-1.26 Nitric oxide was suspected to play a major role in the influence of mitochondrial function in OA (Figure 1). Furthermore, NO dependent intracellular signaling in OA nociception is subject of recent investigation.27
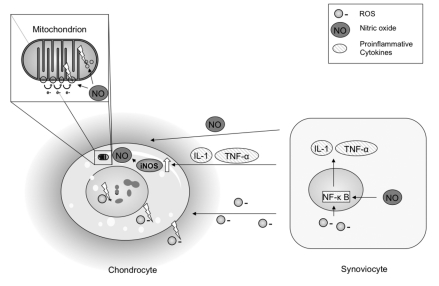
Figure 1.
A major source structure of reactive oxidative molecules is the mitochondrion. The energy metabolism produces in oxidative phosphorylation ROS as side product of normal cellular metabolism. Disruption of chondrocyte respiration by nitric oxide induced inhibition of electron transport has been discussed to be centrally involved in chondrocyte functional compromise.99 In contrast a very recent study showed that the mitochondrion is a target of oxidative damage, mainly by NO related TNF-α and IL-1 induction damage of mitochondrial DNA.100 Mitochondrial damage by ROS/RNS has been addressed to be an important factor for chondrocyte functional compromise and apoptosis induction. Nitric oxide is released with several proinflammative factors by synovial cells as a result of inflammative transition.101 Nuclear transcription factor κB (NF- κ B), which is involved in the upregulation of several inflammatory genes,58 is an important factor for ROS/RNS induced inflammatory transition of synovial membrane. The figure shows in addition to mitochondrial damage by nitric oxide the directly damaging effects of ROS on nuclear DNA, intracellular structures and extracellular matrix.
The role of nitrosative and oxidative stress in the three compartments of joint
There is evidence that alterations of sub-chondral bone28 and synovial cells/fluid are crucial for OA pathogenesis, which stands in contrast to the classic focus on cartilage degradation in OA development. The joint is hence to be considered as a dynamic system consisting of three discrete but permanently interacting compartments.29
Cartilage tissue is classified with conjunctive tissue, deriving from mesenchymal stem cells during embryonic development. Oxidative dysbalance in cartilage is an effect of two major mechanisms. First, it occurs when antioxidative capacity is depleted. A more oxidative intracellular state is a cause for pathologic alterations in osteoarthritic cartilage.13
Second, nutrition of articular cartilage as avascular tissue containing chondrocytes embedded into a large amount of extracellular matrix is dependent on diffusion from synovial - and subchondral bone compartment.30 Oxygen and metabolic end products have to diffuse over relative long distances, resulting in low O2 tension in cartilage tissue.31 A rise of O2 tension in cartilage tissue as is typical for inflammation in turn enhances free radical formation, an interesting finding in the context of cartilage degeneration in chronically irritated joints.
Furthermore, radical oxygen species produced by the oxidative metabolic turnover of cartilage tissue are a substantial factor for the maintenance of intracellular signaling.16,32
The hyaline cartilage of the joint provides profoundly elastic properties under pressure and shear stress. These biomechanical characteristics are based on the cartilage matrix composition which consists mostly of collagen type II and a large aggregating proteoglycan, aggrecan.33,34 Articular cartilage is not only firm under permanent movement and shear stress, a condition that would be hostile for nearly all other tissues.35 Evidence was found, that normal cartilage function is maintained only in tissue being exposed to moderate movement and pressure.36–39 A very recent study is one of the first to demonstrate that physical exercise can reduce oxidative stress in joints with experimental OA on an animal model.40
In contrast, excessive repetitive load was proven to induce chondrocyte apoptosis, a fact which points out the relevance of injurious cartilage compression for OA pathogenesis.41,42 Mechanical loading that exceeds the tolerance of the articular surface was indirectly identified to cause oxidative stress by the decrease of cell death after submission of antioxidants.43,44 A study on cartilage explants showed an increase of ROS synthesis after admission of mechanical load.45 Furthermore, some data indicate that radical nitrogen species act as mediators of cell death after mechanical overuse and injury to cartilage.46 These findings are in accordance to the fact that also excessive shear stress is able to induce chondrocyte death mediated by ROS.47
Once produced as a response to mechanical overload, free radical diffusion into all joint compartments is promoted by cyclic compression during gait. Fluctuation of synovial fluid and squeezing of fluid from cartilage tissue in static pressure phases causes a constant interchange of fluid between cartilage tissue and synovial compartment.48 It is very likely that in joint inflammation this mechanism increases the exposure of cartilage against radical oxygen species.
The synovial compartment consists of the synovial membrane and the synovial fluid. The implications of this joint compartment for OA development, also in relationship with ROS, were extensively investigated during the last decade. The synovial cells are involved in the synthesis of the synovial fluid, an ultrafiltrate of the serum enriched with products of the cells.49 It forms a capillary layer in the healthy joint and provides the minimization of friction.50 Here, a large amount of cross linked hyaluronan, an anionic non-sulfated glycosaminoglycan, contributes substantially to the rheologic properties of joint fluid.51 Oxidative stress, namely peroxynitrit, has been shown in vitro to depolymerise glycosaminoglycans,52,53 whereas hyaluronan degradation can be prevented by antioxidative agents.54 In conclusion, hyaluronan acts as a ROS scavenger in the joint by a competition with other substrates suitable for the reaction with radical oxygen molecules.
A reduced antioxidative capacity in synovial fluid of osteoarthritic joints has been found,55 investigating mainly extracellular superoxide dismutase (EC-SOD or SOD3) in irritated versus late stage OA joints. Here, the reduced antioxidative capacity was described as a disability of the joint to adapt to increased oxidative stress in late stage OA. Finally, the question if oxidative stress is an initial cause or secondary consequence of OA remains unanswered.
The inflammatory transformation of synovial membrane in OA is observed frequently in arthroscopic examination. Distinct synovitis involving infiltration of activated B cells and T lymphocytes and overexpression of proinflammatory mediators is a common finding in histological and arthroscopic examination of patients suffering from OA.56 Increased levels of IL-1α, IL-1β, and TNF-α could be found in synovial membranes from patients with OA.57
Radical oxygen species play a major role in inflammatory intracellular signaling mechanisms of synovia.13 The activation of nuclear transcription factor (NF)-κ B that is implicated in the inflammatory response in vascular endothelium and type A synovial lining cells is a feature of synovial tissue from both RA and OA patients.58 Furthermore, synovitis is secondary to a local increase of the pro-inflammatory enzymes cyclooxygenase-2 and iNOS.56 NO mediates the effects of a number of proinflammatory cytokines, including interleukin-1 (IL-1) and tumor necrosis factor (TNF)-α.22 A canine in vivo model demonstrated a NO dependent up-regulation of IL-1-converting enzyme in chondrocytes, providing another interrelationship between synovial inflammation and cartilage degradation by paracrine free radical signaling.23
One possible mechanism leading from inflammatory transition of the synovial membrane to joint degeneration is ROS/RNS production by synovial cells59 (Figure 2). Synovial inflammation was discussed to impair the ability of chondrocytes to balance anabolic and catabolic activities in extracellular matrix formation.60 A transgenic mouse model yielded evidence that increased ROS production is a relevant cause for chondrocytic death and activation of matrix metalloproteinases in an inflammatory joint disease.61
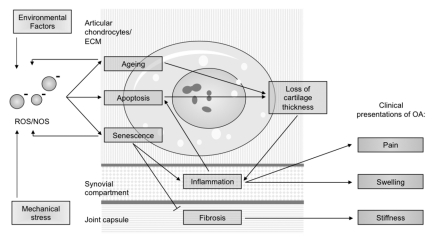
Figure 2.
Depicts the diagrammed hypothesized interrelationship between the clinical symptoms of osteoarthritis and oxidative dysbalance, attributed to the relevant compartments of the joint.
At third the subchondral bone is involved in ROS mediated OA pathogenesis. In advanced stages of OA, first hypertrophic alterations of the bone presented by subchondral sclerosis and osteophytes occur; later on osteoclast activation leads to local bone resorption (bone cysts) affecting the peri-articular bone.62 The subchondral bone together with articular cartilage is considered as a unit, playing an important role in OA disease development63 particularly on the bounding surface between bone and cartilage. Only few studies have focused on the action of radical oxygen species in the context of subchondral bone remodeling.31 One study demonstrates the effect of total fraction of avocado/soybean unsaponifiables on bone remodeling in experimental canine osteoarthritis.64 Here, after drug admission a significantly reduced expression of inducible nitric oxide synthase in cartilage together with decreased bone remodeling is reported. Yet, the exact mechanisms of bone remodeling in relationship with ROS action are only partially understood.
The implication of cellular senescence, apoptosis and ageing in osteoarthritis
The heterogeneity of OA leads to a huge variety of known etiopathogenic factors. Yet, in literature review there is a common thread regarding the uniform presentation of end stage OA.65,66 There are mainly three pathologic alterations in joint that represent OA disease development on the cellular level. Cellular senescence, apoptosis and the effects of ageing show a relationship with OA disease progression in all joint compartments. Here, ROS/RNS have been proven to be involved in the regulation of each of these factors.
Preterm cellular senescence has been addressed to play an important role in OA. The phenomenon of cellular senescence has been described for the first time by Hayflick in the 60's of the last century as the inability of matured cells to divide indefinitely.67 This Hayflick limit is mainly an effect of telomere shortening. Linear chromosomes are capped by repetitive nucleoprotein structures, called telomeres. Each cell division results in a progressive shortening of telomeres that, below a certain threshold, promotes genome instability, senescence, and apoptosis. Telomeres are highly sensitive against ROS induced instability due to their high content of guanines. Therefore, telomere length is reducing at a faster rate during oxidative stress.68 There is evidence that senescence can be induced by extrinsic factors in chondrocytes by so called free radical induced stress senescence.69 Searching a specific marker for chronic exposure to oxidative stress, irreversible telomere length shortening was discussed.70,71 These facts imply a connection between oxidative stress and OA development. Yet, to date the amount of data on the impact of telomere length shortening under in vivo conditions in tissues other than the hematopoietic system is limited.
There are two clinical presentations of OA that are possibly associated with free radical induced cellular senescence:
First, there is few data on the exact mechanisms that promote joint capsule thickening by fibrosis and consecutive stiffness. The cellular mechanism of fibrosis control is regulated by cellular senescence in at least one tissue.72 Since fibrosis is a common feature of OA, intraarticular paracrine ROS/NOS messaging might be beneficial for limitation of capsule fibrosis by promoting senescence of proliferating fibroid cells.
Second, joint inflammation is another characteristic finding in OA with often acute insert, leading to pain and swelling of the affected joint. Beside immunogenic cells a huge number of other cell types are involved in inflammation, in the context of OA pathogenesis particularily fibroblasts.73 The first study showing an inflammatory response by mesenchymal cells was performed on skin fibroblasts. The inflammation involved the transcriptional upregulation of cytokines, such as interleukins (IL-1, IL-15), their receptors and chemotactic secreted factors.74 This coordinated secretion of proinflammatory molecules is a result of cellular senescence.75 It is likely that ROS induced senescence of synovial and cartilage cells can promote the inflammatory transition of the osteoarthritic joint.
Free radical damage induced cell apoptosis provides a strategy to ensure the survival of the organism by scavenge of damaged cells. The process of apoptosis is initiated by activation of caspases (cysteine-aspartic-acid-proteases), a process that what was long time considered to be is irreversible once started. Free radical species have been shown to promote apoptosis76 by activation of c-Jun N-terminal kinases (JNK) and p38 Mitogen-activated protein (MAP) kinases via apoptosis signal-regulating kinase (ASK) 1.77 Interestingly, within this pathway free radicals are formed as a second messenger amplifying ASK1 activation leading to cell death even within ROS concentrations that are not sufficient to initiate apoptosis.78
Today, chondrocytic death in OA has been examined in various studies, most of which report an increased amount of apoptotic cell death.79 Besides other stimuli, exogenous and endogenous NO has been proven to induce chondrocyte apoptosis by intracellular induction of ROS.80 The cytotoxic effect is a result of increased ROS load and formation of cytotoxic peroxynitrit by direct reaction of NO and ROS.81,82 Vice versa, chondrocyte apoptosis induced by toxins can be suppressed by hypoxia shifting chondrocytes to a less oxidative intracellular state.83 An accumulation of cartilage matrix proteins in the endoplasmic reticulum and Golgi apparatus of chondrocytes modified by oxidant stress during aging has been discussed as a cause for decreased synthesis of cartilage matrix proteins and eventual chondrocyte apoptosis.84
Most studies on apoptosis and OA are focused on cartilage tissue, only few data exist about the relevance of cell death in the other joint compartments. Furthermore, most studies are performed in advanced OA stages on cartilage explants obtained during total joint replacement surgery. In contrast, little is known about the function of apoptosis in early stage OA.
Aging leads to impaired extracellular matrix composition. A dysbalance of chondrocyte function in favor of catabolic action against extracellular matrix synthesis increases within aging. Studies measuring changes in joint cartilage using MRI gathered evidence that its thickness decreases with significant age dependency.85 Also hydration, decline of cellularity and a gradual loss of cartilage matrix are reported in aged cartilage leading to a weakening of tissue.86
Interestingly, the joint is capable of adapting to oxidative stress induced by extrinsic factors if it is not subject to pathologic alterations. Repetitive and cyclic mechanical stress, e.g. in long distance running does not induce progressive knee OA.87 The crucial biomechanical cause seems to be excessive load within overweight or joint malalignement, or the combination of such factors.88,89
As a result of the slow metabolism and low proliferation rate of cartilage tissue chondrocytes and cartilage extracellular matrix collagens lack the turnover most tissues show. A theory of aging with relevance for cartilage degeneration is the assumption that cells gather damage by free radicals produced by the electron transport chain in mitochondria.90 Cartilage tissue is highly sensitive against cumulative effects of extrinsic factors like oxidative stress.91 There is evidence of the accumulation of nonenzymatic glycated and oxidized proteins, so called advanced glycation endproducts (AGEs) in joint cartilage.92,93 The production of AGEs is increased in diabetic individuals.94 The influence of AGE accumulation on chondrocyte function is mediated by specific receptors for advanced glycation endproducts (RAGE) located on the membrane of chondrocytes. They show an increased expression in aging and during OA development.95 Stimulation of these receptors leads to an increased expression of matrix metalloproteinases followed by increased catabolic function of chondrocytes.96 The intracellular messaging of RAGE has early been proven to be ROS dependent. RAGE activation in turn induces oxidative stress,97 showing one more involvement of oxidative stress in pathologic alterations leading to joint degradation.
Resulting from the clearly demonstrated age dependency of disease development, biochemical factors influencing chondrocyte function and survival may play a central role in the pathophysiological cascade leading to OA98 and provide therefore therapeutic options on long-term.
Discussion
The progression of OA from silent cartilage destruction to painful clinical presentation is an important subject to further investigation. The novel approach to OA considers the joint as a dynamic system of three different tissues interacting by fluid diffusion and paracrine factors. Because of their chemical properties, free radicals meet the requirements to mediate and amplify the characteristic sequence of joint degeneration in all tissues affected, making them as well a crucial factor for the involvement of all three joint compartments in disease development as for the sudden breakdown of compensation leading to inflammatory transformation of osteoarthritic joints. Therapeutic modifications of these mechanisms may be promising area of further research to achieve comprehension and therefore approach to modification of disease progression.
References
- 1.Prevalence of disabilities and associated health conditions among adults--United States, 1999. MMWR Morb Mortal Wkly Rep. 2001;50:120–5. [PubMed] [Google Scholar]
- 2.Felson DT, Couropmitree NN, Chaisson CE, et al. Evidence for a Mendelian gene in a segregation analysis of generalized radiographic osteoarthritis: the Framingham Study. Arthritis Rheum. 1998;41:1064–71. doi: 10.1002/1529-0131(199806)41:6<1064::AID-ART13>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 3.Alcaraz MJ, Megias J, Garcia-Arnandis I, et al. New molecular targets for the treatment of osteoarthritis. Biochem Pharmacol. 2010;80:13–21. doi: 10.1016/j.bcp.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Henrotin Y, Kurz B, Aigner T. Oxygen and reactive oxygen species in cartilage degradation: friends or foes? Osteoarthritis Cartilage. 2005;13:643–54. doi: 10.1016/j.joca.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Riede U, Schaefer H. Allgemeine und Spezielle Pathologie Thieme Verlag. 2001 [Google Scholar]
- 6.Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27:1513–7. [PubMed] [Google Scholar]
- 7.Kaneko S, Satoh T, Chiba J, et al. Interleukin-6 and interleukin-8 levels in serum and synovial fluid of patients with osteoarthritis. Cytokines Cell Mol Ther. 2000;6:71–9. doi: 10.1080/13684730050515796. [DOI] [PubMed] [Google Scholar]
- 8.Venn G, Nietfeld JJ, Duits AJ, et al. Elevated synovial fluid levels of interleukin-6 and tumor necrosis factor associated with early experimental canine osteoarthritis. Arthritis Rheum. 1993;36:819–26. doi: 10.1002/art.1780360613. [DOI] [PubMed] [Google Scholar]
- 9.Galleron S, Borderie D, Ponteziere C, et al. Reactive oxygen species induce apoptosis of synoviocytes in vitro. Alpha-Tocopherol provides no protection. Cell Biol Int. 1999;23:637–42. doi: 10.1006/cbir.1999.0424. [DOI] [PubMed] [Google Scholar]
- 10.Mapp PI, Grootveld MC, Blake DR. Hypoxia, oxidative stress and rheumatoid arthritis. Br Med Bull. 1995;51:419–36. doi: 10.1093/oxfordjournals.bmb.a072970. [DOI] [PubMed] [Google Scholar]
- 11.Tiku ML, Shah R, Allison GT. Evidence linking chondrocyte lipid peroxidation to cartilage matrix protein degradation - Possible role in cartilage aging and the pathogenesis of osteoarthritis. J Biol Chem. 2000;275:20069–76. doi: 10.1074/jbc.M907604199. [DOI] [PubMed] [Google Scholar]
- 12.Mazzetti I, Grigolo B, Pulsatelli L, et al. Differential roles of nitric oxide and oxygen radicals in chondrocytes affected by osteoarthritis and rheumatoid arthritis. Clin Sci. 2001;101:593–9. [PubMed] [Google Scholar]
- 13.Henrotin YE, Bruckner P, Pujol JP. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage. 2003;11:747–55. doi: 10.1016/s1063-4584(03)00150-x. [DOI] [PubMed] [Google Scholar]
- 14.Mathy-Hartert M, Martin G, Devel P, et al. Reactive oxygen species downregulate the expression of pro-inflammatory genes by human chondrocytes. Inflamm Res. 2003;52:111–8. doi: 10.1007/s000110300023. [DOI] [PubMed] [Google Scholar]
- 15.Farrell AJ, Blake DR, Palmer RM, Moncada S. Increased concentrations of nitrite in synovial fluid and serum samples suggest increased nitric oxide synthesis in rheumatic diseases. Ann Rheum Dis. 1992;51:1219–22. doi: 10.1136/ard.51.11.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibson JS, Milner PI, White R, et al. Oxygen and reactive oxygen species in articular cartilage: modulators of ionic homeostasis. Pflugers Arch. 2008;455:563–73. doi: 10.1007/s00424-007-0310-7. [DOI] [PubMed] [Google Scholar]
- 17.Amin AR, Dicesare PE, Vyas P, et al. The expression and regulation of nitric oxide synthase in human osteoarthritis-affected chondrocytes: evidence for up-regulated neuronal nitric oxide synthase. J Exp Med. 1995;182:2097–102. doi: 10.1084/jem.182.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vuolteenaho K, Moilanen T, Al-Saffar N, et al. Regulation of the nitric oxide production resulting from the glucocorticoidinsensitive expression of iNOS in human osteoarthritic cartilage. Osteoarthritis and Cartilage. 2001;9:597–605. doi: 10.1053/joca.2001.0431. [DOI] [PubMed] [Google Scholar]
- 19.Amin AR, Attur MG, Thakker GD, et al. A novel mechanism of action of tetracyclines: Effects on nitric oxide synthases. Proc Natl Acad Sci USA. 1996;93:14014–9. doi: 10.1073/pnas.93.24.14014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Studer R, Jaffurs D, Stefanovic-Racic M, et al. Nitric oxide in osteoarthritis. Osteoarthritis Cartilage. 1999;7:377–9. doi: 10.1053/joca.1998.0216. [DOI] [PubMed] [Google Scholar]
- 21.Jovanovic DV, Mineau F, Notoya K, et al. Nitric oxide induced cell death in human osteoarthritic synoviocytes is mediated by tyrosine kinase activation and hydrogen peroxide and/or superoxide formation. J Rheumatol. 2002;29:2165–75. [PubMed] [Google Scholar]
- 22.Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39:237–46. [PubMed] [Google Scholar]
- 23.Boileau C, Martel-Pelletier J, Moldovan F, et al. The in situ up-regulation of chondrocyte interleukin-1-converting enzyme and interleukin-18 levels in experimental osteoarthritis is mediated by nitric oxide. Arthritis Rheum. 2002;46:2637–47. doi: 10.1002/art.10518. [DOI] [PubMed] [Google Scholar]
- 24.Davies CM, Guilak F, Weinberg JB, Fermor B. Reactive nitrogen and oxygen species in interleukin-1-mediated DNA damage associated with osteoarthritis. Osteoarthritis Cartilage. 2008;16:624–30. doi: 10.1016/j.joca.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hauselmann HJ, Stefanovic-Racic M, Michel BA, Evans CH. Differences in nitric oxide production by superficial and deep human articular chondrocytes: implications for proteoglycan turnover in inflammatory joint diseases. J Immunol. 1998;160:1444–8. [PubMed] [Google Scholar]
- 26.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–147. [PubMed] [Google Scholar]
- 27.Abramson SB. Nitric oxide in inflammation and pain associated with osteoarthritis. Arthritis Res Ther. 2008;10:S2–S2. doi: 10.1186/ar2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayami T, Pickarski M, Wesolowski GA, et al. The role of subchondral bone remodeling in osteoarthritis - Reduction of cartilage degeneration and prevention of osteophyte formation by alendronate in the rat anterior cruciate ligament transection model. Arthritis and Rheumatism. 2004;50:1193–206. doi: 10.1002/art.20124. [DOI] [PubMed] [Google Scholar]
- 29.Samuels J, Krasnokutsky S, Abramson SB. Osteoarthritis: a tale of three tissues. Bull NYU Hosp Jt Dis. 2008;66:244–50. [PubMed] [Google Scholar]
- 30.Levick JR. Microvascular architecture and exchange in synovial joints. Microcirculation. 1995;2:217–33. doi: 10.3109/10739689509146768. [DOI] [PubMed] [Google Scholar]
- 31.Zhou S, Cui Z, Urban JP. Factors influencing the oxygen concentration gradient from the synovial surface of articular cartilage to the cartilage-bone interface: a modeling study. Arthritis Rheum. 2004;50:3915–24. doi: 10.1002/art.20675. [DOI] [PubMed] [Google Scholar]
- 32.Lo YY, Conquer JA, Grinstein S, Cruz TF. Interleukin-1 beta induction of c-fos and collagenase expression in articular chondrocytes: involvement of reactive oxygen species. J Cell Biochem. 1998;69:19–29. doi: 10.1002/(sici)1097-4644(19980401)69:1<19::aid-jcb3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 33.Aigner T, McKenna L. Molecular pathology and pathobiology of osteoarthritic cartilage. Cell Mol Life Sci. 2002;59:5–18. doi: 10.1007/s00018-002-8400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roughley PJ, Lee ER. Cartilage proteoglycans: structure and potential functions. Microsc Res Tech. 1994;28:385–97. doi: 10.1002/jemt.1070280505. [DOI] [PubMed] [Google Scholar]
- 35.Basser PJ, Schneiderman R, Bank RA, et al. Mechanical properties of the collagen network in human articular cartilage as measured by osmotic stress technique. Arch Biochem Biophys. 1998;351:207–19. doi: 10.1006/abbi.1997.0507. [DOI] [PubMed] [Google Scholar]
- 36.Jortikka MO, Inkinen RI, Tammi MI, et al. Immobilisation causes longlasting matrix changes both in the immobilised and contralateral joint cartilage. Ann Rheum Dis. 1997;56:255–61. doi: 10.1136/ard.56.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hinterwimmer S, Krammer M, Krotz M, et al. Cartilage atrophy in the knees of patients after seven weeks of partial load bearing. Arthritis Rheum. 2004;50:2516–20. doi: 10.1002/art.20378. [DOI] [PubMed] [Google Scholar]
- 38.Vanwanseele B, Eckstein F, Knecht H, et al. Knee cartilage of spinal cord-injured patients displays progressive thinning in the absence of normal joint loading and movement. Arthritis Rheum. 2002;46:2073–8. doi: 10.1002/art.10462. [DOI] [PubMed] [Google Scholar]
- 39.Wilkins RJ, Browning JA, Urban JP. Chondrocyte regulation by mechanical load. Biorheology. 2000;37:67–74. [PubMed] [Google Scholar]
- 40.Cifuentes DJ, Rocha LG, Silva LA, et al. Decrease in oxidative stress and histological changes induced by physical exercise calibrated in rats with osteoarthritis induced by monosodium iodoacetate. Osteoarthritis Cartilage. 2010 Apr 21; doi: 10.1016/j.joca.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Ewers BJ, Dvoracek-Driksna D, Orth MW, Haut RC. The extent of matrix damage and chondrocyte death in mechanically traumatized articular cartilage explants depends on rate of loading. J Orthop Res. 2001;19:779–84. doi: 10.1016/S0736-0266(01)00006-7. [DOI] [PubMed] [Google Scholar]
- 42.Loening AM, James IE, Levenston ME, et al. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch Biochem Biophys. 2000;381:205–12. doi: 10.1006/abbi.2000.1988. [DOI] [PubMed] [Google Scholar]
- 43.Martin JA, Buckwalter JA. Post-traumatic osteoarthritis: the role of stress induced chondrocyte damage. Biorheology. 2006;43:517–21. [PubMed] [Google Scholar]
- 44.Martin JA, Brown T, Heiner A, Buckwalter JA. Post-traumatic osteoarthritis: the role of accelerated chondrocyte senescence. Biorheology. 2004;41:479–91. [PubMed] [Google Scholar]
- 45.Tomiyama T, Fukuda K, Yamazaki K, et al. Cyclic compression loaded on cartilage explants enhances the production of reactive oxygen species. J Rheumatol. 2007;34:556–62. [PubMed] [Google Scholar]
- 46.Green DM, Noble PC, Ahuero JS, Birdsall HH. Cellular events leading to chondrocyte death after cartilage impact injury. Arthritis Rheum. 2006;54:1509–17. doi: 10.1002/art.21812. [DOI] [PubMed] [Google Scholar]
- 47.Healy ZR, Lee NH, Gao X, et al. Divergent responses of chondrocytes and endothelial cells to shear stress: cross-talk among COX-2, the phase 2 response, and apoptosis. Proc Natl Acad Sci USA. 2005;102:14010–5. doi: 10.1073/pnas.0506620102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong M, Carter DR. Articular cartilage functional histomorphology and mechanobiology: a research perspective. Bone. 2003;33:1–13. doi: 10.1016/s8756-3282(03)00083-8. [DOI] [PubMed] [Google Scholar]
- 49.Fam H, Bryant JT, Kontopoulou M. Rheological properties of synovial fluids. Biorheology. 2007;44:59–74. [PubMed] [Google Scholar]
- 50.Rhee DK, Marcelino J, Baker M, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115:622–31. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knudson CB, Knudson W. Cartilage proteoglycans. Semin Cell Dev Biol. 2001;12:69–78. doi: 10.1006/scdb.2000.0243. [DOI] [PubMed] [Google Scholar]
- 52.Moseley R, Waddington R, Evans P, et al. The chemical modification of glycosaminoglycan structure by oxygen-derived species in vitro. Biochim Biophys Acta-Gen Subj. 1995;1244:245–52. doi: 10.1016/0304-4165(95)00010-9. [DOI] [PubMed] [Google Scholar]
- 53.Soltes L, Mendichi R, Kogan G, et al. Degradative action of reactive oxygen species on hyaluronan. Biomacromolecules. 2006;7:659–68. doi: 10.1021/bm050867v. [DOI] [PubMed] [Google Scholar]
- 54.Mendoza G, Alvarez AI, Pulido MM, et al. Inhibitory effects of different antioxidants on hyaluronan depolymerization. Carbohydr Res. 2007;342:96–102. doi: 10.1016/j.carres.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 55.Regan EA, Bowler RP, Crapo JD. Joint fluid antioxidants are decreased in osteoarthritic joints compared to joints with macroscopically intact cartilage and subacute injury. Osteoarthritis Cartilage. 2008;16:515–21. doi: 10.1016/j.joca.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 56.Benito MJ, Veale DJ, FitzGerald O, et al. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64:1263–7. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith MD, Triantafillou S, Parker A, et al. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol. 1997;24:365–71. [PubMed] [Google Scholar]
- 58.Marok R, Winyard PG, Coumbe A, et al. Activation of the transcription factor nuclear factor-kappa B in human inflamed synovial tissue. Arthritis Rheum. 1996;39:583–91. doi: 10.1002/art.1780390407. [DOI] [PubMed] [Google Scholar]
- 59.Schneider N, Mouithys-Mickalad AL, Lejeune JP, et al. Synoviocytes, not chondrocytes, release free radicals after cycles of anoxia/re-oxygenation. Biochem Biophys Res Commun. 2005;334:669–73. doi: 10.1016/j.bbrc.2005.06.147. [DOI] [PubMed] [Google Scholar]
- 60.Loeser RF. Molecular mechanisms of cartilage destruction: mechanics, inflammatory mediators, and aging collide. Arthritis Rheum. 2006;54:1357–60. doi: 10.1002/art.21813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Lent PL, Nabbe KC, Blom AB, et al. NADPH-oxidase-driven oxygen radical production determines chondrocyte death and partly regulates metalloproteinase-mediated cartilage matrix degradation during interferon-gamma-stimulated immune complex arthritis. Arthritis Res Ther. 2005;7:R885–95. doi: 10.1186/ar1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burr DB. Anatomy and physiology of the mineralized tissues: role in the pathogenesis of osteoarthrosis. Osteoarthritis Cartilage. 2004;12:S20–30. doi: 10.1016/j.joca.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 63.Goldring SR. Role of bone in osteoarthritis pathogenesis. Med Clin North Am. 2009;93:25–35. doi: 10.1016/j.mcna.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 64.Boileau C, Martel-Pelletier J, Caron J, et al. Protective effects of total fraction of avocado/soybean unsaponifiables on the structural changes in experimental dog osteoarthritis: inhibition of nitric oxide synthase and matrix metalloproteinase-13. Arthritis Res Ther. 2009;11:R41–R41. doi: 10.1186/ar2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Felson DT. The epidemiology of knee osteoarthritis: results from the Framingham Osteoarthritis Study. Semin Arthritis Rheum. 1990;20:42–50. doi: 10.1016/0049-0172(90)90046-i. [DOI] [PubMed] [Google Scholar]
- 66.D'Ambrosia RD. Epidemiology of osteoarthritis. Orthopedics. 2005;28:S201–5. doi: 10.3928/0147-7447-20050202-04. [DOI] [PubMed] [Google Scholar]
- 67.Evan GI, d'Adda di Fagagna F. Cellular senescence: hot or what? Curr Opin Genet Dev. 2009;19:25–31. doi: 10.1016/j.gde.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 68.Kawanishi S, Oikawa S. Mechanism of telomere shortening by oxidative stress. Ann N Y Acad Sci. 2004;1019:278–84. doi: 10.1196/annals.1297.047. [DOI] [PubMed] [Google Scholar]
- 69.Yudoh K, van Trieu N, Nakamura H, et al. Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Res Ther. 2005;7:R380–R91. doi: 10.1186/ar1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Balasubramanyam M, Adaikalakoteswari A, Sameermahmood Z, Mohan V. Biomarkers of oxidative stress: methods and measures of oxidative DNA damage (COMET assay) and telomere shortening. Methods Mol Biol. 2010;610:245–61. doi: 10.1007/978-1-60327-029-8_15. [DOI] [PubMed] [Google Scholar]
- 71.Houben JM, Moonen HJ, van Schooten FJ, Hageman GJ. Telomere length assessment: biomarker of chronic oxidative stress? Free Radic Biol Med. 2008;44:235–46. doi: 10.1016/j.freeradbiomed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 72.Krizhanovsky V, Yon M, Dickins RA, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–67. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krtolica A, Parrinello S, Lockett S, et al. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci USA. 2001;98:12072–7. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shelton DN, Chang E, Whittier PS, et al. Microarray analysis of replicative senescence. Curr Biol. 1999;9:939–45. doi: 10.1016/s0960-9822(99)80420-5. [DOI] [PubMed] [Google Scholar]
- 75.Coppe JP, Kauser K, Campisi J, Beausejour CM. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J Biol Chem. 2006;281:29568–74. doi: 10.1074/jbc.M603307200. [DOI] [PubMed] [Google Scholar]
- 76.Bertram C, Hass R. Cellular responses to reactive oxygen species-induced DNA damage and aging. Biol Chem. 2008;389:211–20. doi: 10.1515/BC.2008.031. [DOI] [PubMed] [Google Scholar]
- 77.Kim MH, Kim MO, Heo JS, et al. Acetylcholine inhibits long-term hypoxia-induced apoptosis by suppressing the oxidative stress-mediated MAPKs activation as well as regulation of Bcl-2, c-IAPs, and caspase-3 in mouse embryonic stem cells. Apoptosis. 2008;13:295–304. doi: 10.1007/s10495-007-0160-y. [DOI] [PubMed] [Google Scholar]
- 78.Pan JS, Hong MZ, Ren JL. Reactive oxygen species: a double-edged sword in oncogenesis. World J Gastroenterol. 2009;15:1702–7. doi: 10.3748/wjg.15.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Horton WE, Jr., Yagi R, Laverty D, Weiner S. Overview of studies comparing human normal cartilage with minimal and advanced osteoarthritic cartilage. Clin Exp Rheumatol. 2005;23:103–12. [PubMed] [Google Scholar]
- 80.Blanco FJ, Guitian R, Vazquez-Martul E, et al. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998;41:284–9. doi: 10.1002/1529-0131(199802)41:2<284::AID-ART12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 81.Pfeiffer S, Gorren AC, Schmidt K, et al. Metabolic fate of peroxynitrite in aqueous solution. Reaction with nitric oxide and pH-dependent decomposition to nitrite and oxygen in a 2:1 stoichiometry. J Biol Chem. 1997;272:3465–70. doi: 10.1074/jbc.272.6.3465. [DOI] [PubMed] [Google Scholar]
- 82.Wu GJ, Chen TG, Chang HC, et al. Nitric oxide from both exogenous and endogenous sources activates mitochondria-dependent events and induces insults to human chondrocytes. J Cell Biochem. 2007;101:1520–31. doi: 10.1002/jcb.21268. [DOI] [PubMed] [Google Scholar]
- 83.Chaudhari AA, Seol JW, Lee YJ, et al. Hypoxia protects articular chondrocytes from thapsigargin-induced apoptosis. Biochem Biophys Res Commun. 2009;17(381):513–7. doi: 10.1016/j.bbrc.2009.02.073. [DOI] [PubMed] [Google Scholar]
- 84.Yang L, Carlson SG, McBurney D, Horton WE., Jr. Multiple signals induce endoplasmic reticulum stress in both primary and immortalized chondrocytes resulting in loss of differentiation, impaired cell growth, and apoptosis. J Biol Chem. 2005;280:31156–65. doi: 10.1074/jbc.M501069200. [DOI] [PubMed] [Google Scholar]
- 85.Hudelmaier M, Glaser C, Hohe J, et al. Age-related changes in the morphology and deformational behavior of knee joint cartilage. Arthritis Rheum. 2001;44:2556–61. doi: 10.1002/1529-0131(200111)44:11<2556::aid-art436>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 86.Temple MM, Bae WC, Chen MQ, et al. Age-and site-associated biomechanical weakening of human articular cartilage of the femoral condyle. Osteoarthritis Cartilage. 2007;15:1042–52. doi: 10.1016/j.joca.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 87.Lane NE, Michel B, Bjorkengren A, et al. The risk of osteoarthritis with running and aging: a 5-year longitudinal study. J Rheumatol. 1993;20:461–8. [PubMed] [Google Scholar]
- 88.Felson DT, Goggins J, Niu J, et al. The effect of body weight on progression of knee osteoarthritis is dependent on alignment. Arthritis Rheum. 2004;50:3904–9. doi: 10.1002/art.20726. [DOI] [PubMed] [Google Scholar]
- 89.Sharma L, Song J, Felson DT, et al. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. Jama. 2001;286:188–95. doi: 10.1001/jama.286.2.188. [DOI] [PubMed] [Google Scholar]
- 90.Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20:145–7. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 91.Parsch D, Brummendorf TH, Richter W, Fellenberg J. Replicative aging of human articular chondrocytes during ex vivo expansion. Arthritis Rheum. 2002;46:2911–6. doi: 10.1002/art.10626. [DOI] [PubMed] [Google Scholar]
- 92.Verzijl N, Bank RA, TeKoppele JM, DeGroot J. AGEing and osteoarthritis: a different perspective. Curr Opin Rheumatol. 2003;15:616–22. doi: 10.1097/00002281-200309000-00016. [DOI] [PubMed] [Google Scholar]
- 93.Verzijl N, DeGroot J, Thorpe SR, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275:39027–31. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- 94.Dettoraki A, Gil AP, Spiliotis BE. Association between serum levels of the soluble receptor (sRAGE) for advanced glycation endproducts (AGEs) and their receptor (RAGE) in peripheral blood mononuclear cells of children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2009;22:895–904. doi: 10.1515/jpem.2009.22.10.895. [DOI] [PubMed] [Google Scholar]
- 95.Loeser RF, Yammani RR, Carlson CS, et al. Articular chondrocytes express the receptor for advanced glycation end products: Potential role in osteoarthritis. Arthritis Rheum. 2005;52:2376–85. doi: 10.1002/art.21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yammani RR, Carlson CS, Bresnick AR, Loeser RF. Increase in production of matrix metalloproteinase 13 by human articular chondrocytes due to stimulation with S100A4: Role of the receptor for advanced glycation end products. Arthritis Rheum. 2006;54:2901–11. doi: 10.1002/art.22042. [DOI] [PubMed] [Google Scholar]
- 97.Yan SD, Schmidt AM, Anderson GM, et al. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994;269:9889–97. [PubMed] [Google Scholar]
- 98.Martin JA, Brown TD, Heiner AD, Buckwalter JA. Chondrocyte senescence, joint loading and osteoarthritis. Clin Orthop Relat Res. 2004:S96–103. doi: 10.1097/01.blo.0000143818.74887.b1. [DOI] [PubMed] [Google Scholar]
- 99.Terkeltaub R, Johnson K, Murphy A, Ghosh S. Invited review: the mitochondrion in osteoarthritis. Mitochondrion. 2002;1:301–19. doi: 10.1016/s1567-7249(01)00037-x. [DOI] [PubMed] [Google Scholar]
- 100.Kim J, Xu M, Xo R, et al. Mitochondrial DNA damage is involved in apoptosis caused by pro-inflammatory cytokines in human OA chondrocytes. Osteoarthritis and Cartilage. 2010;18:424–32. doi: 10.1016/j.joca.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 101.Sutton S, Clutterbuck A, Harris P, et al. The contribution of the synovium, synovial derived inflammatory cytokines and neuropeptides to the pathogenesis of osteoarthritis. Vet J. 2009;179:10–24. doi: 10.1016/j.tvjl.2007.08.013. [DOI] [PubMed] [Google Scholar]