Abstract
Mucopolysaccharidosis type II (MPS II or Hunter syndrome) is a rare, inherited disorder caused by deficiency of the lysosomal enzyme iduronate-2-sulfatase. As a result of this deficiency, glycosaminoglycans accumulate in lysosomes in many tissues, leading to progressive multisystemic disease. The cardiopulmonary and neurological problems associated with MPS II have received considerable attention. Orthopedic manifestations are common but not as well characterized. This study aimed to characterize the prevalence and severity of orthopedic manifestations of MPS II and to determine the relationship of these signs and symptoms with cardiovascular, pulmonary and central nervous system involvement.
Orthopedic manifestations of MPS II were studied using cross-sectional data from the Hunter Outcome Survey (HOS). The HOS is a global, physician-led, multicenter observational database that collects information on the natural history of MPS II and the long-term safety and effectiveness of enzyme replacement therapy.
As of January 2009, the HOS contained baseline data on joint range of motion in 124 males with MPS II. In total, 79% of patients had skeletal manifestations (median onset, 3.5 years) and 25% had abnormal gait (median onset, 5.4 years). Joint range of motion was restricted for all joints assessed (elbow, shoulder, hip, knee and ankle). Extension was the most severely affected movement: the exception to this was the shoulder. Surgery for orthopedic problems was rare. The presence of orthopedic manifestations was associated with the presence of central nervous system and pulmonary involvement, but not so clearly with cardiovascular involvement.
Orthopedic interventions should be considered on an individual-patient basis. Although some orthopedic manifestations associated with MPS II may be managed routinely, a good knowledge of other concurrent organ system involvement is essential. A multidisciplinary approach is required.
Key words: bone, joint, mucopolysaccharidosis, orthopedic, spine.
Introduction
Mucopolysaccharidosis type II (MIM 309900) is a rare, X-linked disorder. It occurs nearly exclusively in males, with an incidence of 1 in 77,000 live male births.1,2 Mutations causing MPS II result in deficiency of the lysosomal enzyme iduronate-2-sulfatase (I2S; EC 3.1.6.13), which catalyses the degradation of the glycosaminoglycans (GAGs) dermatan sulfate and heparan sulfate.3 In the absence of I2S, these GAGs accumulate in lysosomes within the cells of many tissues, leading to progressive multisystemic disease.
Despite being caused by a specific enzyme deficiency, the phenotype of MPS II varies greatly from patient to patient so that there is a wide spectrum of disease severity. Cardiopulmonary, musculoskeletal, gastrointestinal, dermatological and peripheral nervous system manifestations of varying severity are common features of the disease.3,4 Patients may also develop hepatosplenomegaly. Central nervous system (CNS) involvement is, however, not experienced by all patients.4,5 Patients can be broadly classified as having one of two forms of MPS II: a severe form or an attenuated form. In the severe form of the disease, signs and symptoms (including neurological impairment) develop in early childhood and progress rapidly, whereas in the attenuated form, signs and symptoms develop in adolescence or early adulthood, and patients do not experience significant cognitive impairment.3 Both forms of the disease are associated with reduced lifespan. Patients with the severe form of MPS II are expected to survive until the second decade of life. Those with the attenuated form may have a longer lifespan and some have been reported to survive into the fifth and sixth decades of life.3 Treatment of MPS II has traditionally been directed towards alleviation of the signs and symptoms of the disease. Some patients have also undergone hematopoietic stem cell transplantation, with variable degrees of success depending on the extent of neurological involvement.6–8 Recently, however, a treatment that targets the underlying cause of the disease has been developed, in the form of enzyme replacement therapy (ERT) with idursulfase (Elaprase®, Shire Human Genetic Therapies Inc., Cambridge, MA, USA). In randomized clinical trials, ERT has been shown to be successful in reducing liver and spleen volumes, improving pulmonary function and increasing distance walked in patients with MPS II.9,10 Treatment was well tolerated and most of the adverse events reported were consistent with those expected in an untreated population with MPS II.
Orthopedic involvement is common in patients with MPS II and leads to severe restrictions in motion and impaired quality of life.3,5,11 Musculoskeletal manifestations reported include arthropathy, contractures and joint stiffness, claw hands, carpal tunnel syndrome, short limb length, and deformities in the bones of the spine, chest and limbs.12–16 Spinal cord compression may also occur,17,18 either as a result of the spine deformities or due to soft tissue involvement. In patients with the attenuated form of the disease, joint contractures and skeletal alterations may be among the first reported signs of MPS II.4,19 Although joint contractures have been reported to improve in response to ERT, patients are likely to benefit from physical therapy and, in some cases, orthopedic surgery.5
MPS II is one of a group of similar disorders involving GAG accumulation called the mucopolysaccharidoses or MPS disorders. Although there are few published studies on changes to the cells of the musculoskeletal system in MPS II,20,21 a considerable amount is known about the cellular and physiological mechanisms resulting in the musculoskeletal manifestations of mucopolysaccharidoses in general. GAGs accumulate in nearly all cell types leading to enlargement of the cells and disruption of cellular processes. Progressive disturbances in cell physiology finally cause cell death. The involvement of different tissues depends on the degree and type of GAG accumulation.22 In MPS II, the GAGs stored within the tissues and excreted in urine, are heparan and dermatan sulfate. Storage is typically high within cartilage, bone and connective tissues, which may explain why the joints and bones are often severely affected in patients with MPS II.3 Inflammation also has an important role in the musculoskeletal manifestations of the mucopolysaccharidoses.23,24 In animal models of MPS disorders, GAG accumulation stimulates proliferation of synoviocytes and immune cells, and promotes infiltration of the latter into various connective tissues.25 In addition, articular chondrocytes have been found to undergo a high rate of apoptosis, which increases with age and is associated with destructive processes in the joints.23,26 Chondrocyte death is compounded by the mechanical forces placed on the deformed joints and spine, which leads to inflammatory responses and matrix abnormalities, causing further destruction of cartilage and bone.23 Osteoclasts seem to be essential mediators of inflammatory bone erosion.27 On one hand, the rate of osteoclastogenesis appears to be increased in animal models of some MPS disorders, on the other hand osteoclast morphology and function appears to be abnormal. This leads to fewer and thinner trabeculae, which are spaced further apart than normal.25,27,28 Osteoblast activity was also decreased, as are the number of osteoblast cells in the bone in other animal models of MPS disorders.25 MPS-related osteopenia has been suggested to arise from a defect in bone production within the metaphysis and diaphysis rather than at the growth plate cartilage.28 A combination of inflammation, activation of fibroblasts, disturbances in cartilage and bone, and increased skin thickness are thought to be the cause of contractures. Disturbances in the architecture of the growth plate and a decreased ability of bone cells to synthesize new bone on calcified cartilage could account for short limb length and bone deformities.23,28 The underlying causes of carpal tunnel syndrome are believed to be thickening of the ligaments and synovia, as a result of disruptions to the functioning of macrophages and fibroblasts, and radio-ulnar dissociation.29
Patients with musculoskeletal manifestations of MPS II should be referred to orthopedic specialists, neurologists and physiotherapists; however, bearing in mind the multisystemic nature of the disease, a multidisciplinary approach involving other specialist clinicians is beneficial. Furthermore, as new treatments become available, it is important to consider how best to balance and optimize patient outcomes. Using cross-sectional data from the Hunter Outcome Survey (HOS) (a global, multi-center observational database that collects information on the natural history of MPS II and the long-term safety and effectiveness of ERT) the aim of this manuscript is to characterize the natural history of orthopedic manifestations in patients with MPS II. This includes characterizing the prevalence and severity of orthopedic manifestations, and determining the relationship of these manifestations with cardiovascular, pulmonary and neurological signs and symptoms. It is hoped that the results will contribute to a framework for clinical management decisions in patients with MPS II.
Materials and Methods
The Hunter Outcome Survey (HOS) was established in 2005. By gathering data from patients with MPS II, the HOS aims to complement the information derived from clinical trials of ERT and to provide the basis for the development of clinical management guidelines. Physicians and medical centers caring for patients with MPS II are eligible to join the HOS. Before enrolling patients in the database, each participating medical center received approval for taking part in the HOS according to national regulations; typically from their local Institutional Review Board or Ethics Committee.
Patients (males and females) with a confirmed diagnosis of MPS II are eligible for enrolment in the HOS, irrespective of whether they are or are not receiving ERT. All enrolled patients, or their parents or a legal representative, have provided written informed consent for participation in the HOS. Data from patients who had died before the initiation of the HOS can also be entered into the database.
Only data collected during routine medical examinations are entered in the HOS. Information reported includes vital signs, laboratory test results, signs and symptoms of organ involvement, and the results of functional analyses such as audiometric tests, echocardiograms and joint range of motion (JROM) tests. Data entry and analysis in the HOS were conducted as previously described.30 A computer-based application is used to collect data and connects via the internet to the database server using the Secure Socket Layer protocol. This allows data to be entered remotely at hospital/physician centers using locally secured connections. The database is anonymous and confidential; data that could be used to identify patients are accessible only by the team treating the patient, on their local computer. The survey is overseen by national, regional and international scientific advisory boards. Each advisory board consists of a group of participating physicians who were elected by all participating physicians. All board members (who are experienced in the management of MPS II) supervise the analysis of data collected from national, regional, and international cohorts of patients. Data collection and statistical analysis in the HOS are supported by Shire Human Genetic Therapies, Inc. (Cambridge, MA, USA), a business unit of Shire plc.
Database population
HOS data for this analysis were collected on or before 23 January 2009. At this time, 505 prospective patients (i.e. alive at HOS entry) from 97 centers in 23 countries were enrolled in the HOS. This analysis included all prospective patients (n=124; all male) who had at least one of the following JROM measurements at baseline: shoulder flexion (range of motion 0–180°), shoulder abduction (range of motion 0–180°), hip flexion with the knee bent (range of motion 0–125°), hip extension (range of motion 0–40°), and hip internal rotation (range of motion 0–60°).31 These joints were selected based on the clinical experience of the authors with patients with MPS II. Information that was available from the patient group on other joints was also included. For patients who had never received ERT, baseline was defined as the latest measurement recorded in HOS or the last visit recorded before death. In patients who had received or were currently receiving ERT, baseline was defined as treatment start for all measurements except for measurements of JROM. As JROM assessments were not routinely conducted at the start of treatment, the measurement taken closest to the date on which ERT was started (either before the start or within three months after the start of treatment) was used as baseline. Data from patients for whom treatment status was unclear were not included. For one patient, the documentation of data on hip and knee range of motion was inconsistent with the methods specified in the HOS and values could not be converted: these data points were removed from the analysis.
Descriptive statistics and data analyses
The cumulative prevalence of signs and symptoms since birth was calculated at baseline for the study population. The manifestations and disease-related variables assessed are outlined below. Variables assessed relating to orthopedic involvement included joint stiffness, spine deformities, gait, claw hand and type of orthopedic surgical procedures. The relationship between an early onset of joint stiffness and the development of scoliosis and kyphosis was also assessed. Early onset of joint stiffness was defined as onset before five years of age. Imaging data were available in the HOS for a limited number of patients (n=39 for spine; n=30 for hip). Central nervous system (CNS) manifestations included any one or combination of the following: cognitive problems, behavioral problems, seizure disorder, hyperactivity, frequent chewing and hydrocephalus. Peripheral nervous system (PNS) manifestations included any one or combination of the following: carpal tunnel syndrome, fine motor skill impairment and abnormal reflexes. Cardiovascular involvement was defined as any one or combination of valve disease, heart murmur, bradycardia, tachycardia, arrhythmia, cardiomyopathy, congestive heart failure, hypertension, angina, infarction, peripheral vascular disease, and/or an abnormal heartbeat frequency range. Patients with bronchitis, bronchospasm, lower- and/or upper-airway infection, obstructive and/or restrictive airway disease, rales/crepitations and/or sleep apnea, or those who required continuous positive airway pressure, mechanical ventilation or bi-level positive airway pressure, or who had oxygen dependency, were considered to have pulmonary involvement. For patients from whom joint range of motion measurements (JROM) for a particular joint were available on both sides of the body, the correlation between JROM measurements for the left and right sides of the same joint was assessed. As there was a high degree of correlation using Pearson's correlation coefficient between the left and right sides for all five joints (shoulder flexion [n=108]: r=0.86, P<0.001; shoulder abduction [n=117]: r=0.72, P<0.001; hip flexion [n=104]: r=0.90, P<0.001; hip extension [n=94]: r=0.93, P<0.001; hip internal rotation [n=78]: r=0.83, P<0.001), mean values were used for further analyses in patients for whom measurements were available from both sides of the body. Actual values were used when a measurement from only one side of the body was available. JROM measurements were plotted relative to age at assessment, and linear regression analyses were used to assess the relationship between these two variables. Baseline measurements for distance walked in the 6-minute walk test available in the database were correlated with age. To assess what other disease-related manifestations accompany skeletal involvement, correlations were made between the presence of different orthopedic manifestations and cardiovascular, pulmonary and CNS involvement.
Results
Baseline demographics
The population analyzed included 124 out of 505 prospective patients with MPS II enrolled in the HOS. Baseline characteristics of this population are shown in Table 1. There was a delay between the age at onset of symptoms (median 2.0 years) and the age at diagnosis (median 4.0 years), and between the age at diagnosis and the age at which patients started ERT (median 10.2 years). The majority of patients (n=113) were receiving ERT. Baseline data were collected at a median age of 10.3 years.
Table 1. Baseline characteristics of patients with mucopolysaccharidosis type II (MPS II) included in the study (n=124)a.
| Characteristic | nb | |
|---|---|---|
| Median age (10th–90th percentile) at onset of symptoms, years | 2.0(0.3–4.5) | 105 |
| Median age (10th–90th percentile) at diagnosis, years | 4.0(1.5–8.0) | 121 |
| Receiving enzyme replacement therapy (ERT), n (%) | ||
| Yes | 113 (91%) | |
| No | 11 (9%) | |
| Started ERT before HOS entry, n (%) | 74 (65%) | 113 |
| Median age (10th–90th percentile) at start of ERT, years | 10.2 (5.1–24.9) | 113 |
| Median age (10th–90th percentile) at baselinec, years | 10.3 (4.6–24.1) | 124 |
| Sex, n (%) | ||
| Male | 124 (100%) | |
| Other family member known to have MPS II, n (%) | 117 | |
| Yes | 34 (27%) | |
| No | 83 (67%) | |
| Region, n (%) | ||
| North America | 12 (10%) | |
| Latin America | 25 (20%) | |
| Europe | 87 (70%) |
Total number of patients in the population studied;
Number of patients for whom data were available on a given variable;
Baseline was defined as the last measurement recorded in HOS for patients who had never received ERT and as treatment start for patients receiving ERT.
Of the 124 patients included in the analysis, 27% had a family member with MPS II (67% did not have a family member with MPS II, and for 6% this information was missing). Patients attended centers in Europe (70%), Latin America (20%) and North America (10%).
Signs and symptoms in the patient cohort
Table 2 shows the prevalence of CNS, PNS, cardiovascular, pulmonary and skeletal involvement in the 124 patients with MPS II included in this study. Data show that cardiovascular signs and symptoms were the most prevalent manifestations, affecting 87.1% of patients. Skeletal manifestations (excluding abnormal gait) affected 79.0% of the patient group with a median age at onset of 3.5 years. Of interest, abnormal gait was reported in 25.0% of the patients, with a median age at onset of 5.4 years (10th–90th percentile, 1.7–11.6 years). Pulmonary involvement was also very common (affecting 70%) and was on average the earliest manifestation to develop (median age at onset, 2.7 years). Almost half of all patients (46.8%) had CNS involvement, with the first signs and symptoms being reported at a median of 3.3 years of age. PNS involvement also affected 48.4% of the patient group, although the onset of these manifestations was slightly later than CNS manifestations (median age at onset, 5.7 years). Carpal tunnel syndrome affected 27.4% of patients and began at a median age of 7.0 years (10th–90th percentile, 3.6–17.3 years).
Table 2. Prevalence of central nervous system (CNS), peripheral nervous system (PNS), cardiovascular, pulmonary and skeletal involvement in patients with MPS II (n=124).
| Sign or symptom | N. patientsa | Median age (10th–90th percentile) at onset (years) |
|---|---|---|
| CNS | 58 (46.8%) | 3.3 (0.1–11.5) |
| PNS | 60 (48.4%) | 5.7 (1.9–15.7) |
| Cardiovascular | 108 (87.1%) | 5.6 (1.7–14.2) |
| Pulmonaryb | 86 (70.0%) | 2.7 (0.1–13.4) |
| Skeletalc | 98 (79.0%) | 3.5 (0.9–8.6) |
Prevalences are calculated from birth to last visit for untreated patients and from birth to treatment start for treated patients.
Data on Chest and lung signs and symptoms were missing for one patient (n=123).
Any skeletal manifestations defined in Table 3A).
Figure 1 shows the typical skeletal manifestations of MPS II. The prevalence of the individual skeletal manifestations for which data are available in the HOS ranges from 21.0–75.0% (Table 3A). Median onset of these manifestations was generally between four and seven years of age. Orthopedic surgery was reported in some patients (Table 3B). The most common operation was carpal tunnel decompression which was performed in 24 patients. All other orthopedic surgeries (spinal decompression, spine fusion, femoral osteotomy and trigger finger release surgery) were carried out in 2.4% or fewer of the 124 patients. Pelvic osteotomy, hip replacement and arthroscopy were not reported to have been performed in any patient.
Figure 1.
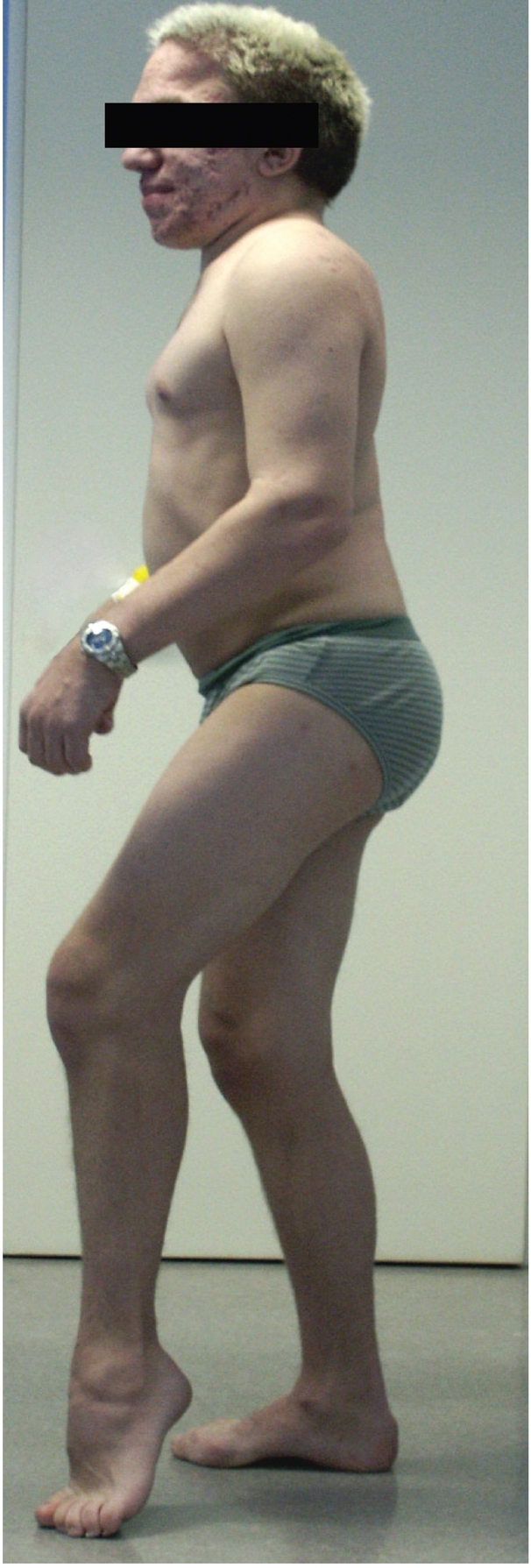
Typical skeletal manifestations of mucopolysaccharidosis type II in an 18-year old patient. The patient has flexion contraction of the elbow, knee and hip, as well as shortening of the Achilles tendon and claw hands.
Table 3A. Prevalence of skeletal involvement in patients with MPS II (n=124).
| Sign or symptom | N. patientsa | Median age (10th–90th percentile) at onset (years) |
|---|---|---|
| Kyphosis/scoliosis | 42 (33.8%) | 6.4 (0.6–15.9) |
| Claw hand | 66 (53.2%) | 4.2 (1.8–9.4) |
| Any joint stiffness | 93 (75.0%) | 4.0 (1.5–10.3) |
| Spine | 26 (21.0%) | 7.7 (1.6–15.4) |
| Shoulder | 68 (54.8%) | 4.6 (1.8–12.1) |
| Elbow | 74 (59.7%) | 5.1 (1.9–11.6) |
| Hand | 80 (64.5%) | 4.4 (1.9–10.2) |
| Hip | 35 (28.2%) | 5.6 (2.4–11.5) |
| Knee | 53 (42.7%) | 5.1 (2.3–11.6) |
| Ankle | 42 (33.9%) | 6.1 (2.6–11.6) |
Prevalences are calculated from birth to last visit for untreated patients and from birth to treatment start for treated patients.
Table 3B. Prevalence of orthopedic surgeries in patients with MPS II (n=124).
| Location and type of surgery | N. |
|---|---|
| Spine | |
| Spinal decompression | 1 (<1%) |
| Spine fusion | 1 (<1%) |
| Hip | |
| Femoral osteotomy | 3 (2.4%) |
| Pelvic osteotomy | 0 |
| Hip replacement | 0 |
| Knee | |
| Arthroscopy | 0 |
| Hand | |
| Carpal tunnel decompression | 24 (19.4%) |
| Trigger finger operation | 2 (1.6%) |
Prevalences are calculated from birth to last visit for untreated patients and from birth to treatment start for treated patients, and based on the number of patients with available surgery data (n=124).
For a small number of the total group of patients, detailed data on spinal and hip imaging were also available (Table 4).
Table 4. Prevalence of spinal and hip abnormalities identified based on available imaging data (n=39 for spine; n=30 for hip).
| Location | Abnormality | N. patients (%) | na |
|---|---|---|---|
| Any spinal abnormalityb | 87.2 | 39 | |
| Cervical | Vertebral deformity | 68.8 | 32 |
| Instability | 3.1 | 32 | |
| Spinal cord compression | 9.4 | 32 | |
| Thoracic | Vertebral deformity | 79.3 | 29 |
| Spinal cord compression | 3.4 | 29 | |
| Lumbar | Vertebral deformity | 93.8 | 32 |
| Spinal cord compression | 3.1 | 32 | |
| Any hip abnormalitiesc | 86.7 | 30 | |
| Hip | Femoral head dysplasia | 26.7 | 30 |
| Acetabulum dysplasia | 53.3 | 30 | |
| Coxa valga | 16.7 | 30 | |
| Coxa vera | 13.3 | 30 | |
| Femoral head necrosis | 13.3 | 30 | |
| Degenerative changes | 16.7 | 30 | |
| Other abnormality | 23.3 | 30 |
Number of patients for whom data were available on that variable.
Prevalences are based on the number of patients with available data for each section of the spine (cervical n=32; thoracic n=29; lumbar n=32). Data on the spine were based on X-ray (n=34), magnetic resonance imaging (n=11), and computed tomography scan (n=1) (more than one imaging method was used in some patients).
Data on the hip were based on X-rays (n=30).
A spinal abnormality was present in 34 of the 39 patients (87.2%) for whom imaging data were available. The most common abnormalities were cervical, thoracic and lumber vertebral deformities (affecting 68.8–93.8%). Cervical spinal cord compression was reported in 3 patients. Cervical instability, thoracic spinal cord compression and lumbar spinal cord compression were each reported in one patient. A hip abnormality was present in 26 of the 30 patients (86.7%) for whom hip images were included in the HOS (all X-rays). The most common abnormality was acetabulum dysplasia (affecting 53.3%), followed by femoral head dysplasia (affecting 26.7%) (Figure 2).
Figure 2.
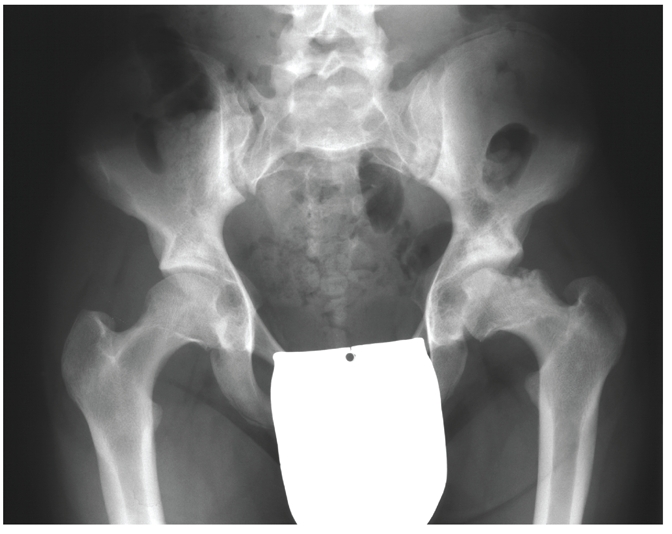
X-ray of an 18-year old patient with mucopolysaccharidosis type II showing femoral head necrosis and the typical features of dysostosis multiplex.
JROM and the 6-minute walk test
JROM measurements are shown in Table 5. There was evidence of restriction in the range of motion for all of the joints assessed. For the majority of joints that were studied (the elbow, wrist, hip, knee and ankle), the movement that was most severely affected was extension. Other movements of these joints were generally near-normal or less severely affected. For the shoulder, the most severely affected joint motions were abduction and flexion.32,33
Table 5. Joint range of motion measurements in patients with MPS II (n=124).
| Joint and movement | na | Median (10th–90th percentile) JROM (°) |
Normal JROM (°)b |
|---|---|---|---|
| Predominantly affected joint movements | |||
| Shoulder flexion | 108 | 119 (90–153) | 150–180 |
| Shoulder abduction | 117 | 98 (75–130) | 180 |
| Hip flexion, knee bent | 105 | 110 (85–130) | 100–120 |
| Hip extension | 96 | 0 (–25 to 20) | 20–30 |
| Hip internal rotation | 78 | 25 (10–40) | 40–45 |
| Other joint movements | |||
| Wrist flexion | 109 | 55 (30–75) | 60–80 |
| Wrist extension | 116 | 30 (8–64) | 60–70 |
| Shoulder internal rotation | 83 | 57 (36–85) | 70–90 |
| Shoulder external rotation | 85 | 61 (30–85) | 90 |
| Shoulder extension | 81 | 54 (35–73) | 50–60 |
| Elbow flexion | 107 | 135 (110–150) | 140–150 |
| Elbow extension | 106 | −27 (−51 to 10) | 0–10 |
| Hip abduction | 91 | 33 (20–46) | 40 |
| Hip adduction | 44 | 20 (13–30) | 20 |
| Hip external rotation | 79 | 32 (23–61) | 45–50 |
| Knee flexion | 105 | 130 (105–145) | 150 |
| Knee extension | 101 | −10 (−25 to 5) | 0–10 |
| Ankle dorsal extension | 85 | 4 (−15 to 20) | 20 |
| Ankle plantar flexion | 45 | 41 (20–50) | 40–50 |
Joint restriction increased with age. Simple linear regression analysis showed a negative relationship between age and JROM for each of the major joints studied. This relationship was significant for shoulder abduction, hip flexion with the knee bent, hip extension and hip internal rotation (Table 6). The relationship between hip flexion and age is illustrated in Figure 3.
Table 6. Simple linear regression parameters between joint range of motion and age for each of the joints that are known to be predominantly affected in patients with MPS II.
| Joint | All values | Pa | |
|---|---|---|---|
| N | Parameter estimate (SE) | ||
| Shoulder flexion | 108 | −0.36 (0.27) | 0.181 |
| Shoulder abduction | 117 | −0.77 (0.23) | <0.001 |
| Hip flexion, knee bent | 105 | −0.83b (0.22) | <0.001 |
| Hip extension | 96 | −0.46c (0.21) | 0.030 |
| Hip internal rotation | 78 | −0.59 (0.14) | <0.001 |
SE, standard error.
P value based on t-test for the hypothesis: parameter estimate = 0.
Parameter estimate = −0.87 (SE, 0.15) when excluding outliers.
Parameter estimate = −0.33 (SE, 0.18) when excluding the outlier.
Figure 3.
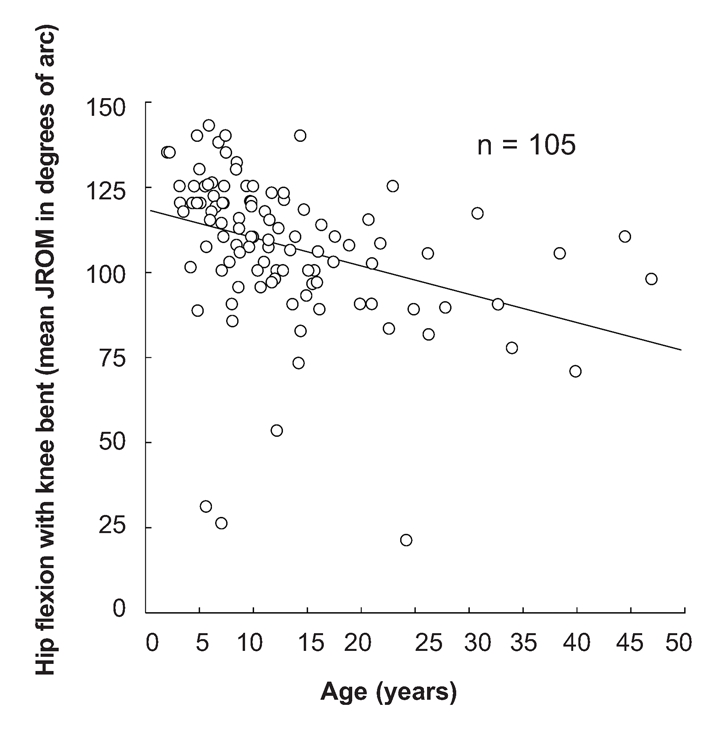
The relationship between hip flexion with knee bent and age.
The median distance walked in the 6-minute walk test was 362 meters (range 37–569 meters; n=84). Distance walked was not correlated with age at assessment (r=0.17, P=0.122).
Relationship between orthopedic manifestations of MPS II and other disease-related manifestations
Skeletal manifestations were usually accompanied by other serious disease-related manifestations. In general, there was a clear association between the presence of individual orthopedic manifestations and the presence of CNS and pulmonary involvement, but not so clearly with the presence of cardiovascular involvement (Table 7). Patients with orthopedic manifestations were generally more likely to have CNS and pulmonary manifestations than those without orthopedic manifestations. However, patients with orthopedic manifestations were only very slightly more likely to have cardiovascular manifestations than patients who did not have orthopedic manifestations.
Table 7. Relationship between selected orthopedic manifestations and CNS, pulmonary and cardiovascular manifestations (Fisher's exact test).
| Orthopedic manifestation | CNS manifestations | Pulmonary manifestation | Cardiovascular manifestation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | P value | Yes | No | P value | Yes | No | P value | ||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||||
| Joint stiffness | Yes | 52 (55.9) | 41 (44.1) | < 0.001 | 74 (79.6) | 19 (20.4) | < 0.001 | 84 (90.3) | 9 (9.7) | 0.117 |
| No | 6 (19.4) | 25 (80.6) | 12 (40.0) | 18 (60.0) | 24 (77.4) | 7 (22.6) | ||||
| Scoliosis | Yes | 12 (60.0) | 8 (40.0) | 0.227 | 19 (95.0) | 1 (5.0) | 0.007 | 20 (100) | 0 (0) | 0.072 |
| No | 46 (44.2) | 58 (55.8) | 67 (65.0) | 36 (35.0) | 88 (84.6) | 16 (15.4) | ||||
| Kyphosis | Yes | 21 (72.4) | 8 (27.6) | 0.003 | 25 (86.2) | 4 (13.8) | 0.037 | 29 (100) | 0 (0) | 0.022 |
| No | 37 (38.9) | 58 (61.1) | 61 (64.9) | 33 (35.1) | 79 (83.2) | 16 (16.8) | ||||
| Claw hand | Yes | 41 (62.1) | 25 (37.9) | < 0.001 | 54 (81.8) | 12 (18.2) | 0.003 | 61 (92.4) | 5 (7.6) | 0.067 |
| No | 17 (29.3) | 41 (70.7) | 32 (56.1) | 25 (43.9) | 47 (81.0) | 11 (19.0) | ||||
Joint stiffness
Patients with joint stiffness were more likely to have CNS and pulmonary manifestations than those without joint stiffness: 55.9% of patients with and 19.4% of those without joint stiffness had CNS signs/symptoms, and 79.6% of patients with and 40.0% of patients without joint stiffness had pulmonary manifestations. Overall, 89.7% of patients with CNS manifestations and 86.0% of patients with pulmonary manifestations also had joint stiffness. Cardiovascular manifestations were common both in patients with and without joint stiffness (90.3% and 77.4%, respectively).
Having early joint stiffness and/or spine deformity (onset before five years of age) was associated with the presence of CNS involvement in patients who were eight years or older at latest HOS visit (P=0.001). In total, 67.3% of patients with early-onset joint stiffness/spine deformity had developed CNS involvement. Among patients who did not have early-onset joint stiffness/spine deformity, 34.0% developed CNS manifestations (data reported on Table 7).
Kyphosis
The presence of kyphosis was associated with CNS manifestations in 72.4% of patients; however, CNS manifestations were recorded for only 38.9% of patients who did not have kyphosis. The majority of patients with kyphosis (86.2%) had pulmonary manifestations, whereas 64.9% of those without kyphosis had pulmonary involvement. It is noteworthy that all patients with kyphosis had cardiovascular involvement, compared with 83.2% of patients without kyphosis (data reported Table 7).
Discussion
Musculoskeletal manifestations are known to be common in patients with MPS II, and are associated with complex changes in bone, cartilage and soft tissues. This was the first study to investigate the prevalence and severity of musculoskeletal manifestations and orthopedic surgical procedures in such a large group of patients with this rare disorder. Detailed investigations were based on data obtained during routine clinical practice by physicians treating patients with MPS II and collected in the HOS database. Despite the obvious limitations of survey-based data, the results obtained from this analysis provide insight into clinical experience with the orthopedic/skeletal manifestations of MPS II.
Skeletal manifestations were found to be prevalent in patients with MPS II, affecting about 80% of the group of 124 patients in the HOS for whom data were available. Skeletal deformities were normally seen first in the hand and wrist, with 53.2% of patients having clawed hands at a median age of 4.2 years. Claw hands are typically associated with shortening of the ligaments and broadened bones, often leading to carpal tunnel syndrome and a significant reduction in fine motor control. Restriction of movement of the wrist was documented in terms of flexion and extension, probably leading to a reduction in hand strength. The hand was also the area in which joint stiffness was most commonly reported (64.5%). Joint stiffness was also common in the elbow (59.7%), shoulder (54.8%), knee (42.7%) and ankle (33.9%). Joint stiffness appeared in the hand at a median age of 4.4 years and in the shoulder at a median age of 4.6 years. Joint stiffness generally became apparent in the elbow and knee when children were slightly older (median onset, 5.1 years). The next two joints in which stiffness was reported to have developed were the hip (median onset 5.6, years) and ankle (median onset, 6.1 years). Spinal deformities (kyphosis or scoliosis) and spine stiffness generally developed later in the disease, with a median age at onset of 6.4 and 7.7 years, respectively. It is noteworthy that there was an association between kyphosis and CNS involvement, and scoliosis and CNS involvement. All patients with kyphosis or scoliosis also had cardiovascular manifestations. Although kyphosis and scoliosis together affected only 33.8% of patients studied, kyphosis in particular may be indicative of a severe disease course. Early onset of joint stiffness and/or spinal deformities (kyphosis or scoliosis) (before five years of age) was associated with the presence of CNS involvement in patients who were eight years or older at latest HOS visit; however, it was not possible to determine whether the development of skeletal manifestations preceded the onset of CNS involvement.
Contractures of nearly all of the main joints (elbow, wrist, shoulder, hip and knee) were consistent features of MPS II with onset in early childhood. Of note, the movement that was most severely restricted was extension. Joint contractures often prevent patients with MPS II from standing erect and may limit mobility, as shown by the reported abnormal gait in 25.0% of the patients in the present study, as well as the limited distance walked in the 6-minute walk test. It is clinically logical to speculate that the severely limited extension of the hip (by up to 55°), in combination with the extension deficits in the knee (by up to 45°) and foot (by up to 25°), contributed to the characteristic abnormal gait of patients with MPS II. Regarding the severe limitation in shoulder mobility found in the current analysis, particularly in terms of flexion (by up to 90°) and abduction (by up to 105°), we hypothesize that this was the result of damage to the soft tissues surrounding the shoulder joint owing to GAG storage. In combination with deficits in the flexion contraction of the elbow by up to 51°, this would result in a significant impairment in the motion of the arm. Overall, our analysis showed that assessment of flexion and abduction of the shoulder, and extension of the elbow, wrist, knee and ankle, appear to be useful for documenting the disease burden in terms of joint restriction.
Radiographic examination of patients with MPS disorders has generally revealed the typical features of dysostosis multiplex, with abnormal thickness of all bones, irregular epiphysial ossification of many joints and anterior breaking of vertebral bodies of the entire spine.22,34 In the current study, imaging data showed evidence of some characteristics of dysostosis multiplex. Hip deformity in terms of acetabulum dysplasia was apparent in more than half of those patients for whom hip images were available, and was associated with degenerative changes in the femoral heads in some, but not all, cases. Acetabulum dysplasia is probably the result of the disturbances in the architecture of the growth plate and the decreased ability of bone cells to synthesize new bone on calcified cartilage.23,25,28 Hip dysplasia is predictive of osteoarthritis and poor mobility35,36 and, in otherwise healthy patients, surgery is indicated to preserve long-term mobility. In the current investigation, 3 patients with MPS II were reported to have undergone femoral osteotomy. Limited experience with other MPS disorders suggests that patients may also benefit from innominate osteotomy.37
Descriptions of spine deformities in MPS II are relatively rare in the literature;8,13 however, it is clear that the spine is affected in the majority of patients with MPS II. Radiographic data in this study suggested a high prevalence of spinal abnormalities (87.2%), with the most common malformation being vertebral deformity; however, it is important to bear in mind that these data may be biased as little imaging data were available in the HOS. Spinal cord compression caused by dural thickening and/or by instability of the atlanto-axial joint has been reported in patients with MPS disorders.18,38,39 In the current study, unusual and extensive changes in the anatomy of the spine led to spinal cord compression or cervical instability in up to 12.5% of the patients for whom imaging data were available in the HOS. One patient showed cervical, thoracic and lumbar spinal cord compression, 2 patients showed cervical spinal cord compression and one patient showed cervical instability.
Patients with MPS II should be screened for clinical and radiological signs of spinal cord compression. Signs of spinal cord compression may include abnormal gait, muscle weakness, dysesthesia and bladder dysfunction. Instability of the atlanto-axial joint can be identified by flexion-extension radiographs. Spinal cord compression, secondary to dural thickening, must be confirmed by magnetic resonance imaging.18 In this cohort, only one patient was documented as having undergone spinal decompression and one had undergone surgery for spine fusion at baseline. In the literature, experience of spinal cord decompression in patients with MPS II is also limited.38 However, because compression of the spinal cord is often associated with irreversible neurological dysfunction, decompression surgery should be considered in patients with MPS II as soon as possible after signs of compression are confirmed.
Carpal tunnel syndrome is the most common entrapment neuropathy in adults, but it is rarely seen in children.12 However, carpal tunnel syndrome was commonly seen in young patients with MPS II in the current study (affecting 27.4% of patients with a median onset at 7.0 years). This is consistent with a review of published literature that found MPS disorders to be the major cause of carpal tunnel syndrome during childhood.12 Carpal tunnel syndrome may result in contracture of distal interphalangeal joints, as well as dysesthesia, loss of feeling in the first three fingers, and paresis of the thenar muscles.40 Decompression surgery is recommended in patients for whom either loss of hand function or large decreases in nerve conduction are demonstrated, and is reported to result in rapid and sustained improvement of function.41 In this cohort, 19.4% of the patients underwent the procedure, although based on the proportion of patients in whom carpal tunnel syndrome was reported (27.4%), more patients would probably have benefited from this surgery. In summary, patients with MPS II may benefit from regular assessment to detect the development of carpal tunnel syndrome.
When considering any surgical procedure in a patient with MPS II it is important to keep other organ system manifestations in mind. Results of the current analysis showed that the majority of patients with MPS II, both with and without skeletal manifestations, have cardiovascular involvement. Orthopedic/skeletal manifestations were, however, frequently accompanied by both CNS and pulmonary involvement. Among patients with joint stiffness, for example, 90% also had cardiovascular involvement, 80% also had pulmonary involvement, and 57% also had CNS manifestations. This shows that patients with skeletal manifestations generally have clear multisystemic involvement. It is, therefore, important that concurrent disease manifestations are identified and taken into account before undertaking any surgical procedures. In addition, anatomical changes including short neck, atlanto-axial instability, lung impairment and cardiovascular problems may complicate general anesthesia in patients with MPS II.14,42,43 Intubation and extubation, in particular, are usually difficult in patients with MPS II.42 Despite these considerations, orthopedic surgery clearly has a role in the management of MPS II.
Patients may also benefit from physical therapy although there are no published studies as of yet providing evidence of benefit of physical therapy in MPS II. Physical therapy is designed to preserve and improve physical function and offers a non-surgical approach to the management of joint involvement in MPS II. Techniques that may be beneficial include physiotherapy, and alternative therapies like hippotherapy, dolphin therapy and dance movement therapy, as well as occupational therapy. Before initiating physical therapy, the first step should be to rule out the possibility that the musculoskeletal manifestations are of neurological origin. Subsequently, it is possible to design a program directed at addressing the specific problem areas for the individual. This program may involve mobilization, strength and endurance training, enhancement of fine motor skills for the hands, and gait training for lower limb joints. It is especially important to document progress by performing baseline assessments and conducting periodic evaluations. In addition to physical therapy, the mobility of patients may be improved by the use of orthopedic aids and devices. Although physical therapy and the use of orthopedic devices are intended to improve musculoskeletal and sometimes cardiopulmonary function, by improving patient mobility they can also address some of the psychological aspects of the condition by increasing independence and reducing anxiety. In recent years, improvements in patient identification, care and management mean that patients with MPS II are living longer.44 With the introduction of ERT it is clear that we can expect additional benefits in terms of improvements in visceral manifestations and increased mobility, as evident from increased distances walked on the 6-minute walk test.9 Although there is some indication that joint contractures may be improved by ERT, both ERT and bone marrow transplantation are likely to have only a limited impact on bone and joint disease based on the results of studies in animal models.45,46 This may be due partly to some of the irreversible changes in bone structure known to be induced by GAG accumulation.26 Thus, physical therapy and corrective surgery should be considered, although the long-term outcome for patients with MPS II remains unclear because ERT is unlikely to influence the cognitive aspects of the condition. Owing to the heterogeneous nature of MPS II, it is clear that no single management strategy can be used for all patients. Instead, individual management programs should be developed based on the disease progression in each patient, with input from the multidisciplinary specialists caring for the patient. Pediatricians, neurologists, neurosurgeons, cardiologists, anesthesiologists and pulmonologists may all play a role in orthopedic decisions.
The authors would like to thank all the HOS investigators listed below who submitted data from their patients to the HOS database.
| Austria | |
| Graz: | Barbara Plecko |
| Salzburg: | Olaf Bodamer |
| Belgium | |
| Brussels: | Linda DeMeirleir |
| Brazil | |
| Porto Alegre: | Roberto Giugliani |
| Rio de Janeiro: | Raquel Tavares Boy da |
| Silva | |
| Fortaleza: | Erlane Ribeiro |
| São Paulo: | Ana Maria Martins |
| Bulgaria | |
| Sofia: | Radka Tincheva |
| Canada | |
| Toronto: | Joe Clarke |
| Vancouver: | Lorne Clarke |
| Croatia | |
| Zagreb: | Ingeborg Barišić |
| Ivo Barić | |
| Czech Republic | |
| Prague: | Jiri Zeman |
| Denmark | |
| Copenhagen: | Allan Meldgaard Lund |
| France | |
| Lyon: | Nathalie Guffon |
| Paris: | Vassili Valayannopoulos |
| Bénédicte Héron | |
| Germany | |
| Mainz: | Michael Beck |
| Gudrun Schulze | |
| Frenking | |
| Hamburg: | Nicole Muschol |
| Salzburg: | Barbara Volkmar |
| Magdeburg: | Silke Klose |
| Berlin: | Julia Hennermann |
| Greece | |
| Thessaloniki: | Dimitrios Zafeiriou |
| Hungary | |
| Budapest: | Zsuzsanna Almássy |
| Italy | |
| Ancona: | Orazio Gabrielli |
| Rome: | Claudio Feliciani |
| Padova: | Maurizio Scarpa |
| Genova: | Maja Di Rocco |
| Bologna: | Alessandro Cicognani |
| Monza: | Rossella Parini |
| Bari: | Francesco Papadia |
| Portugal | |
| Porto: | Elisa Leao Teles |
| Esmeralda Martins | |
| Lisbon: | Ana Gaspar |
| Russia | |
| Moscow: | Peter Novikov |
| Spain | |
| Barcelona: | Mireia del Toro |
| Merce Pined | |
| Badalona: | Guillem Pintos |
| Badajoz: | Enrique Galán |
| Bilbao: | Luis Aldámiz |
| Las Palmas: | Milagros Marti |
| Linares: | Pilar Munguira |
| Madrid: | Luis González |
| Murcia: | Rosario Domingo |
| Palma de Mallorca: | Begoña de Azua |
| Salamanca: | Aránzazu Hernández |
| Seville: | Dolores Lluch |
| Ourense: | Gemma Novoa |
| Valencia: | Jaime Dalmau |
| Valladolid: | José Manuel Muro |
| Zaragoza: | Juan Pérez |
| Antonio Baldellou | |
| Carlos Alcalde | |
| Sweden | |
| Stockholm: | Gunilla Malm |
| Lund: | Dominiki Papadopoulou |
| Halmstad: | Nils Nilsson |
| Gothemburg: | Niklas Darin |
| Taiwan | |
| Taipei: | Shuan-Pei Lin |
| The Netherlands | |
| Rotterdam: | Ans van der Ploeg |
| United Kingdom | |
| Manchester: | Ed Wraith |
| Simon Jones | |
| Stephen Waldek | |
| Cambridge: | Uma Ramaswami |
| Birmingham: | Chris Hendriksz |
| London: | Ashok Vellodi |
| Amersham: | UK MPS Society |
| United States | |
| Houston, TX: | Christine Eng |
| Iowa City, IA: | Sara Copeland |
| Minneapolis, MN: | Nancy Mendelsohn |
| Charlottesville, VA: | William Wilson |
| Norfolk, VA: | Virginia Proud |
| Baltimore, MD: | Ada Hamosh |
| Kansas City, MO: | Laurie Smith |
| Salt Lake City, UT: | David Viskochil |
| Omaha, NE: | William Rizzo |
| Lebanon, NH: | John Moeschler |
| New York, NY: | Greg Pastores |
| Cincinnati, OH: | Nancy Leslie |
| Columbus, OH: | Kim McBride |
| Seattle, WA: | Ronald Scott |
| Chicago, IL: | Barbara Burton |
| Chapel Hill, NC: | Joseph Muenzer |
| Atlanta, GA: | Paul Fernhoff |
| Hartford, CT: | Robert Greenstein |
| Oakland, CA: | Paul Harmatz |
| Denver, CO: | Janet Thomas |
| Miami, FL: | Parul Jayaker |
| Phoenix, AZ: | Kirk Aleck |
| Minneapolis, MN: | Chet Whitley |
| Portland, OR: | Robert Steiner |
| Sioux Falls, SD: | Laura Keppen |
| Washington, DC: | Cynthia Tifft |
| Jackson, MS: | Omar Abdul-Rahman |
| Boston, MA: | Kathleen Sims |
| St. Louis, MO: | Dorothy Grange |
| Greenville, SC: | Curtis Rogers |
Acknowledgments:
HOS is supported by Shire Human Genetic Therapies (HGT) Inc., which is responsible for maintaining the central database and for performing statistical analyses at the request of the HOS advisory board. The authors would like to thank Ms Isabelle Morin for her excellent statistical support. Shire HGT had no role in the interpretation of data. Editorial assistance to the authors was provided by Dr Harriet Crofts (Oxford PharmaGenesis™ Ltd) and was paid for by Shire HGT Inc.
References
- 1.Poorthuis BJ, Wevers RA, Kleijer WJ, et al. The frequency of lysosomal storage diseases in The Netherlands. Hum Genet. 1999;105:151–6. doi: 10.1007/s004399900075. [DOI] [PubMed] [Google Scholar]
- 2.Baehner F, Schmiedeskamp C, Krummenauer F, et al. Cumulative incidence rates of the mucopolysaccharidoses in Germany. J Inherit Metab Dis. 2005;28:1011–7. doi: 10.1007/s10545-005-0112-z. [DOI] [PubMed] [Google Scholar]
- 3.Neufeld EF, Muenzer J. The Mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. McGraw-Hill; New York: 2001. pp. 3421–52. [Google Scholar]
- 4.Schwartz IV, Ribeiro MG, Mota JG, et al. A clinical study of 77 patients with mucopolysaccharidosis type II. Acta Paediatr. 2007;96:63–70. doi: 10.1111/j.1651-2227.2007.00212.x. [DOI] [PubMed] [Google Scholar]
- 5.Wraith JE, Scarpa M, Beck M, et al. Mucopolysaccharidosis type II (Hunter syndrome): a clinical review and recommendations for treatment in the era of enzyme replacement therapy. Eur J Pediatr. 2008;167:267–77. doi: 10.1007/s00431-007-0635-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vellodi A, Young E, Cooper A, et al. Long-term follow-up following bone marrow transplantation for Hunter disease. J Inherit Metab Dis. 1999;22:638–48. doi: 10.1023/a:1005525931994. [DOI] [PubMed] [Google Scholar]
- 7.Coppa GV, Gabrielli O, Zampini L, et al. Bone marrow transplantation in Hunter syndrome (mucopolysaccharidosis type II): two-year follow-up of the first Italian patient and review of the literature. Pediatr Med Chir. 1995;17:227–35. [PubMed] [Google Scholar]
- 8.Guffon N, Bertrand Y, Forest I, et al. Bone marrow transplantation in children with Hunter syndrome: outcome after 7 to 17 years. J Pediatr. 2009;154:733–7. doi: 10.1016/j.jpeds.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 9.Muenzer J, Wraith JE, Beck M, et al. A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome) Genet Med. 2006;8:465–73. doi: 10.1097/01.gim.0000232477.37660.fb. [DOI] [PubMed] [Google Scholar]
- 10.Muenzer J, Gucsavas-Calikoglu M, McCandless SE, et al. A phase I/II clinical trial of enzyme replacement therapy in mucopolysaccharidosis II (Hunter syndrome) Mol Genet Metab. 2007;90:329–37. doi: 10.1016/j.ymgme.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Kato T, Kato Z, Kuratsubo I, et al. Evaluation of ADL in patients with Hunter disease using FIM score. Brain Dev. 2007;29:298–305. doi: 10.1016/j.braindev.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Van Meir N, De Smet L. Carpal tunnel syndrome in children. Acta Orthop Belg. 2003;69:387–95. [PubMed] [Google Scholar]
- 13.Benson PF, Button LR, Fensom AH, et al. Lumbar kyphosis in Hunter's disease (MPS II) Clin Genet. 1979;16:317–22. doi: 10.1111/j.1399-0004.1979.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 14.Man TT, Tsai PS, Rau RH, et al. Children with mucopolysaccharidoses - three cases report. Acta Anaesthesiol Sin. 1999;37:93–6. [PubMed] [Google Scholar]
- 15.Bona I, Vial C, Brunet P, et al. Carpal tunnel syndrome in Mucopolysaccharidoses. A report of four cases in child. Electromyogr Clin Neurophysiol. 1994;34:471–5. [PubMed] [Google Scholar]
- 16.Al Sawaf S, Mayatepek E, Hoffmann B. Neurological findings in Hunter disease: pathology and possible therapeutic effects reviewed. J Inherit Metab Dis. 2008;31:473–80. doi: 10.1007/s10545-008-0878-x. [DOI] [PubMed] [Google Scholar]
- 17.Parsons VJ, Hughes DG, Wraith JE. Magnetic resonance imaging of the brain, neck and cervical spine in mild Hunter's syndrome (mucopolysaccharidoses type II) Clin Radiol. 1996;51:719–23. doi: 10.1016/s0009-9260(96)80246-7. [DOI] [PubMed] [Google Scholar]
- 18.Vinchon M, Cotten A, Clarisse J, et al. Cervical myelopathy secondary to Hunter syndrome in an adult. AJNR Am J Neuroradiol. 1995;16:1402–3. [PMC free article] [PubMed] [Google Scholar]
- 19.Vieira T, Schwartz I, Munoz V, et al. Mucopolysaccharidoses in Brazil: what happens from birth to biochemical diagnosis? Am J Med Genet A. 2008;146A:1741–7. doi: 10.1002/ajmg.a.32320. [DOI] [PubMed] [Google Scholar]
- 20.Wakai S, Minami R, Kameda K, et al. Skeletal muscle involvement in mucopolysaccharidosis type IIA: severe type of Hunter syndrome. Pediatr Neurol. 1988;4:178–80. doi: 10.1016/0887-8994(88)90009-4. [DOI] [PubMed] [Google Scholar]
- 21.Schmitt HP. Changes in the voluntary muscles and the peripheral nerves in an autopsy case of MPS type II (Hunter) Neuro-pediatrics. 1981;12:83–91. doi: 10.1055/s-2008-1059642. [DOI] [PubMed] [Google Scholar]
- 22.Martin R, Beck M, Eng C, et al. Recognition and diagnosis of mucopolysaccharidosis II (Hunter syndrome) Pediatrics. 2008;121:e377–86. doi: 10.1542/peds.2007-1350. [DOI] [PubMed] [Google Scholar]
- 23.Simonaro CM, Haskins ME, Schuchman EH. Articular chondrocytes from animals with a dermatan sulfate storage disease undergo a high rate of apoptosis and release nitric oxide and inflammatory cytokines: a possible mechanism underlying degenerative joint disease in the mucopolysaccharidoses. Lab Invest. 2001;81:1319–28. doi: 10.1038/labinvest.3780345. [DOI] [PubMed] [Google Scholar]
- 24.Simonaro CM, D'Angelo M, Haskins ME, et al. Joint and bone disease in mucopolysaccharidoses VI and VII: identification of new therapeutic targets and biomarkers using animal models. Pediatr Res. 2005;57:701–7. doi: 10.1203/01.PDR.0000156510.96253.5A. [DOI] [PubMed] [Google Scholar]
- 25.Simonaro CM, D'Angelo M, He X, et al. Mechanism of glycosaminoglycan-mediated bone and joint disease: implications for the mucopolysaccharidoses and other connective tissue diseases. Am J Pathol. 2008;172:112–22. doi: 10.2353/ajpath.2008.070564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalteis T, Schubert T, Caro WC, et al. Arthroscopic and histologic findings in Morquio's syndrome. Arthroscopy. 2005;21:233–7. doi: 10.1016/j.arthro.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 27.Monroy MA, Ross FP, Teitelbaum SL, et al. Abnormal osteoclast morphology and bone remodeling in a murine model of a lysosomal storage disease. Bone. 2002;30:352–9. doi: 10.1016/s8756-3282(01)00679-2. [DOI] [PubMed] [Google Scholar]
- 28.Nuttall JD, Brumfield LK, Fazzalari NL, et al. Histomorphometric analysis of the tibial growth plate in a feline model of mucopolysaccharidosis type VI. Calcif Tissue Int. 1999;65:47–52. doi: 10.1007/s002239900656. [DOI] [PubMed] [Google Scholar]
- 29.Haddad FS, Jones DH, Vellodi A, et al. Carpal tunnel syndrome in the mucopolysaccharidoses and mucolipidoses. J Bone Joint Surg Br. 1997;79:576–82. doi: 10.1302/0301-620x.79b4.7547. [DOI] [PubMed] [Google Scholar]
- 30.Wraith JE, Beck M, Giugliani R, et al. Initial report from the Hunter Outcome Survey. Genet Med. 2008;10:508–16. doi: 10.1097/gim.0b013e31817701e6. [DOI] [PubMed] [Google Scholar]
- 31.Norkin CC, White DJ. 3rd ed. F.A. Davis Company; 2003. Measurement of joint range of motion: a guide to goniometry. [Google Scholar]
- 32.Third Edition, Revised ed. Chicago, IL: AMA Press; 1990. American Medical Association guides to evaluation of permanent impairment. [Google Scholar]
- 33.American Academy of Orthopedic Surgeons. Joint motion: method of measuring and recording. Chicago, IL: American Academy of Orthopedic Surgeons; 1965. [Google Scholar]
- 34.Hunter C. A rare disease in two brothers. Proc R Soc Med. 1917;10:104–16. doi: 10.1177/003591571701001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imhof H, Nöbauer-Huhmann I, Trattnig S. [Coxarthrosis - an update] Radiologe. 2009;49:400–9. doi: 10.1007/s00117-009-1832-0. [DOI] [PubMed] [Google Scholar]
- 36.Oniankitan O, Kakpovi K, Fianyo E, et al. [Risk factors of hip osteoarthritis in Lome, Togo] Med Trop (Mars) 2009;69:59–60. [PubMed] [Google Scholar]
- 37.Masterson EL, Murphey PG, O'Meara A, et al. Hip dysplasia in Hurler's syndrome: orthopaedic management after bone marrow transplantation. J Pediatr Orthop. 1996;16:731–3. doi: 10.1097/00004694-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 38.O'Brien DP, Cowie RA, Wraith JE. Cervical decompression in mild mucopolysaccharidosis type II (Hunter syndrome) Childs Nerv Syst. 1997;13:87–90. doi: 10.1007/s003810050049. [DOI] [PubMed] [Google Scholar]
- 39.Müller-Forell W, Schulze Frenking G, Amraoui Y, et al. Mucopolysaccharidoses (MPS): clinical and neuroradiological aspects of the different types. Clin Neuroradiol. 2007;17:141–58. [Google Scholar]
- 40.Norman-Taylor F, Fixsen JA, Sharrard WJ. Hunter's syndrome as a cause of childhood carpal tunnel syndrome: a report of three cases. J Pediatr Orthop B. 1995;4:106–9. doi: 10.1097/01202412-199504010-00018. [DOI] [PubMed] [Google Scholar]
- 41.Muenzer J, Beck M, Eng CM, et al. Multidisciplinary management of Hunter syndrome. Pediatrics. 2009;124:e1228–39. doi: 10.1542/peds.2008-0999. [DOI] [PubMed] [Google Scholar]
- 42.Kamin W. Diagnosis and management of respiratory involvement in Hunter syndrome. Acta Paediatr. 2008;97:57–60. doi: 10.1111/j.1651-2227.2008.00650.x. [DOI] [PubMed] [Google Scholar]
- 43.Sjogren P, Pedersen T, Steinmetz H. Mucopolysaccharidoses and anaesthetic risks. Acta Anaesthesiol Scand. 1987;31:214–8. doi: 10.1111/j.1399-6576.1987.tb02553.x. [DOI] [PubMed] [Google Scholar]
- 44.Jones SA, Almassy Z, Beck M, et al. Mortality and cause of death in mucopolysaccharidosis type II - a historical review based on data from the Hunter Outcome Survey (HOS) J Inherit Metab Dis. 2009;32:534–43. doi: 10.1007/s10545-009-1119-7. [DOI] [PubMed] [Google Scholar]
- 45.Crawley AC, Niedzielski KH, Isaac EL, et al. Enzyme replacement therapy from birth in a feline model of mucopolysacharidosis type VI. J Clin Invest. 1997;99:651–62. doi: 10.1172/JCI119208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Norrdin RW, Simske SJ, Gaarde S, et al. Bone changes in mucopolysaccharidosis VI in cats and the effects of bone marrow transplantation: mechanical testing of long bones. Bone. 1995;17:485–9. doi: 10.1016/8756-3282(95)00333-4. [DOI] [PubMed] [Google Scholar]


