Abstract
In addition to osteosynthetic stabilizing techniques and autologous bone transplantations, so-called orthobiologics play an increasing role in the treatment of bone healing disorders. Besides the use of various growth factors, more and more new data suggest that cell-based therapies promote local bone regeneration. For ethical and biological reasons, clinical application of progenitor cells on the musculoskeletal system is limited to autologous, postpartum stem cells. Intraoperative one-step treatment with autologous progenitor cells, in particular, delivered promising results in preliminary clinical studies. This article provides an overview of the rationale for, and characteristics of the clinical application of cell-based therapy to treat osseous defects based on a review of existing literature and our own experience with more than 100 patients. Most clinical trials report successful bone regeneration after the application of mixed cell populations from bone marrow. The autologous application of human bone marrow cells which are not expanded ex vivo has medico-legal advantages. However, there is a lack of prospective randomized studies including controls for cell therapy for bone defects. Autologous bone marrow cell therapy seems to be a promising treatment option which may reduce the amount of bone grafting in future.
Key words: stem cell, cell therapy, bone defect, osteoblast.
Introduction
Treating bone healing disorders represents a huge challenge for orthopedic and trauma surgeons and frequently produces unsatisfactory results. Critical size bone defects, in particular, which appear after tumor surgery or trauma do not heal spontaneously and require special therapy. There are also diseases which, despite surgical intervention and the application of all conventional therapies to promote bone regeneration, are accompanied by insufficient bone healing. These include aneurysmal bone cysts, enchondroma and congenital pseudarthrosis. In the broadest sense, bone defects also include avascular osteonecrosis which is defined by the death of osteoblasts. In addition to successful bone healing through the use of growth factors, increasingly positive results of osseous regeneration through stem cells have been published in recent years.1 This article describes the current state of cell-based therapy for osseous regeneration.
Established treatments in bone healing disorders
Autologous bone transplantation
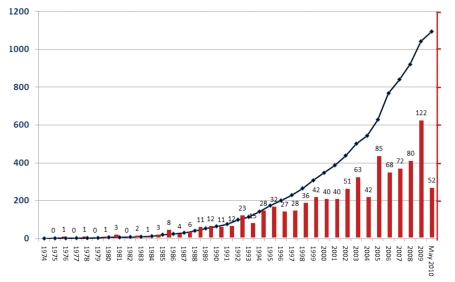
Autologous bone transplantation is the therapy of choice for treating bone healing disorders. Despite its high efficiency in regenerating bone tissue, autologous (cancellous) bone transplantation does have numerous disadvantages. These include a longer surgery time, damage by surgical exposure (e.g. subcutaneous nerves), persisting pain and swelling at the donor site, and impaired esthetics due to scar formation or osseous malformation. Furthermore, the potential for osseous regeneration of autologous bone grafts in elderly people is low compared to an increased donor site morbidity in this population.2 The growing interest among experts can also be seen in the increasing number of publications dealing with donor site morbidity(Figure 1).
Figure 1.
The increasing frequency of publications on “donor site morbidity” and “bone” listed by Medline reflects the growing interest and examination of bone harvesting-related damage.
Callus distraction
New bone formation in long bones is achieved using callus distraction, including the so-called segment transport and external fixation devices. There must be osseous interruption which is fracture-related or created by an osteotomy. Disadvantages include the fact that the process can continue for months, the risk of infections transmitted via the pin tracks of the fixation, and the lack of application possibilities to the pelvis, spine, thorax, skull or to the hand and foot skeletons.
Ultrasound and shock waves
Using extracorporeal shock wave therapy to regenerate bone is mostly restricted to treating atrophic pseudarthrosis. Critical size bone defects cannot be healed by this non-operative therapy.
Biological fundamentals and rationale of cell-based therapy of bone defects and bone healing disorders
The rationale for a cell-based therapy to induce bone tissue regeneration is based on the high osteogenic potency of undifferentiated or almost undifferentiated osteoblastic progenitor cells of various origins. This has been documented in a now vast number of pre-clinical studies.3 For ethical and biological reasons, stem cell therapy on the musculoskeletal system is limited to autologous transplantation of postpartum progenitor cells. Omnipotent (the potential to regenerate a complete, viable organism) or totipotent (potential to regenerate different types of tissue) embryonic stem cells, on the other hand, are used only in experimental studies.
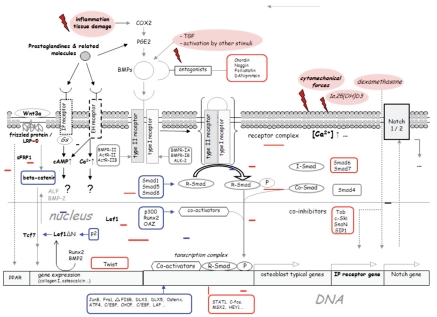
Quantitatively relevant amounts of mesenchymal, multipotent progenitor cells are found not only in human bone marrow, but particularly also in the periosteum and in adipose tissue.4–6 On the other hand, stem cells with osteoblastic potency, occur in lesser quantities in numerous other tissues, such as muscle,7 umbilical cord blood,8 placenta,9 skin,10 cartilage11 and synovium.12 Osteoblastic differentiation of mesenchymal stem cells passes through numerous intermediary stages, whereby it is less the original tissue of the MSC than the local environment with correspondingly different stimuli that influences the kinetics, gene expression and protein synthesis of the cells. The mechanisms of intracellular signal transduction are complex and most clinically oriented orthopedic surgeons can barely grasp the overall picture (Figure 2). Depending on the degree of differentiation of the osteoblastic precursor, different typical proteins and antigens are expressed in different amounts (Figure 2). As differentiation increases, the cellular proliferation rate falls.
Figure 2.
Summary of important intracellular pathways of signal transduction during osteoblastic differentiation. Cytomechanic stimuli, BMPs and inflammatory stimuli, in particular, encourage osteoblastic differentiation. The expression of some of the listed KO-factors, such as Lef1/Tcf7,13 decreases towards the end of osteogenic differentiation. On the other hand, other expression factors (e.g. Lef1▽N), increase in terminal osteoblastic differentiation.14 The differentiation paths of adipoblasts and osteoblasts from a common progenitor cell separate relatively late, whereby adipose tissue in addition to human bone marrow is suitable as the original tissue used in cell therapies for bone regeneration.15 Due to the small or even lack of expression of MHC-II, mesenchymal progenitor cells have a low immunogenetic potential.16 Moreover, in contrast to other cell types, they have an immunosuppressive effect on neighboring cells. ALK: activin receptor-like kinase; ALP: alkaline phosphatase; APC: activated protein C; BMP: bone morphogenic protein; cbfa: core binding factor; Cdk: cyclin-dependent kinases; CHOP: CCAAT enhancer-binding protein (C/EBP) homologous protein; CTGF: connective tissue growth factor; cAMP: cyclic adenosine monophosphate; COX: cyclo-oxygenase; ERK: extracellular signal-related kinase; LRP: LDL receptor-related protein; MAP: mitogen-activated protein kinase; MHC: major histocompatibility complex; OAZ: Olf-1/EBF-associated zinc finger; PG: prostaglandin(s); PPAR: peroxisome proliferator activated receptor; SF: short form; SnoN: Ski-related novel oncogene; STAT: transducer and activator of transcription; Tob: transducer of erbB2; VEGF: vascular derived growth factor; Wnt: wingless gene; sFRP: secreted frizzled related protein, Lef: lymphoid enhancer binding factor.
Other characteristics which make autologous mesenchymal progenitor cells an attractive candidate for the treatment of bone defects are:
simple availability and an uncomplicated harvesting technique by aspiration without the disadvantage of significant harvesting morbidity;
the standardized and well-established isolation technique using density gradient centrifugation or flow cytometry technology (e.g. fluorescence activated cell sorter, FACS);
a consensual definition of the term “mesenchymal stem cells” (MSC) that has now been worked out, with well-defined biological properties17–19 (Table 1);
the simple cultivation technique for in vitro expansion and determination of the proliferation rate through colony forming units (CFU);
that osteoblastic differentiation can be well controlled in in vitro cultivation with stimuli such as dexamethasone, ascorbic acid and β-glycerol phosphate (DAG), and the decades of experience with this stimulation method;
the availability of defined cellular expression markers by which osteoblastic differentiation can be reliably documented (e.g. osteocalcin, osteopontin, osteoprotegerin, Cbf1/Runx2, collagen type I, alkaline phosphatase, osterix, bone sialo protein, signs of biomineralization, RANKL);20
the immunosuppressive and immunomodulatory effects of MSC which lead to a limitation of local inflammatory reactions at the transplantation site;21
the good adherence to surfaces, which favors the use of scaffolds and advocates local concentration of in vivo introduced cells at the transplantation site;22
the decades of experience in bone marrow transplantations in hemato-oncology, which is supported by the low transplantation risk especially in autologous transplantations.
Table 1. Consensual definition of the term “mesenchymal stem cell”. A large number of synonyms exist, however, in scientific literature, e.g. precursors of non-hematopoietic tissue, colony forming units-fibroblasts, marrow stromal cells, bone marrow stroma/stem cells.
| Properties of mesenchymal stem cells | |
|---|---|
| Expression of mesenchymal markers | CD49a, CD73, CD90, CD105, CD146, Stro-1, |
| Expression of matrix receptors | CD44, CD29, CD71 |
| Absence of hematopoietic markers | CD45, CD34, CD14, CD11b, HLA-DR |
| Biological properties | Spindle-shaped morphology Good adherence to plastic Evidence of CFU-F Self-renewal potential Mesenchymal multipotency (osteoblast, chondroblast, adipoblast, myoblast, fibroblast and their precursors) High proliferation rate, particularly in the presence of defined growth factors, e.g. FGF-2 |
Clinical application of cell therapies in bone healing disorders
In contrast to the extensive in vitro and animal experiment data, there are only a few studies that show clinical results for cell therapy treatments to regenerate bone.
There are two clinical application forms of cell therapies to regenerate bone. Besides the biological differences, various health law-related consequences also emerge for the manufacturer and the orthopedic surgeon in attendance.
- Cell therapies without expansion in culture:
- with the isolation of defined primary cells;
- without the isolation of defined primary cells.
- Cell therapies with ex vivo expansion:
- with typical cell differentiation;
- without typical cell differentiation.
Cell therapies without expansion in culture
What is generally meant here is cell therapies that are harvested or produced during an operation. The tissue used for this does not leave the operation theatre or operation area and is, therefore, under the direct supervision and responsibility of the operator in attendance. Bone marrow aspiration concentrate (BMAC) is a typical example of this form of application. At the beginning of the operation, a defined volume of bone marrow is harvested by Jamshidi-vacuum aspiration of the ventral or dorsal iliac crest and suspended in an anti-coagulating heparin and ACDA solution in a transfusion bag. Mononuclear cells are then isolated from the harvested bone marrow aspirate in a density gradient centrifuge in the closed system that we have been using since 2005.
Possible quality controls of the cell therapy (BMAC) are to compare the number of cells in the BMAC with that in the initial aspirated bone marrow, and determine the CFU-F and ALP activity during in vitro cultivation.23–25 Despite these quality parameters, the individual potency of in vivo applied cell therapies cannot be reliably predicted. Some publications indicate, however, that compared to the transplantation of a defined type of cell, applying mixed populations of mesenchymal and hematopoietic progenitor cells at different stages of differentiation is more effective for osteogenic regeneration.26
In a prospective clinical study and in various experimental treatments, our research group has so far successfully treated over 100 patients with local bone healing disorders using a BMAC biomaterial composite. Fifty percent of the bone defects were grafted with autologous cancellous bone and the remaining 50% with a BMAC biomaterial composite (hydroxylapatite, Orthoss®, Geistlich, Wolhusen, Switzerland vs. collagen sponge, Gelaspon®, Chauvin Ankerpharm, Berlin, Germany). So far, our study has found that the use of BMAC reduces the harvest of autogenous bone by 50% with no slowing down or absence of bone healing being observed.27, 28 No complications with the application were observed in any of the patients. The low complication risk of this procedure29 and the osteogenic potency in the parallel application of different biomaterials has also been reported by other research groups.30, 31
A high variance in the number of harvested cells was observed in the human bone marrow aspirate.32, 33 To maximize the yield of bone marrow cells, the following procedure is recommended.
Create a sufficiently high vacuum: this is necessary to create sufficient local force to retrieve the (in contrast to hematopoietic cells) strongly adherent mesenchymal cells from the tissue mass. If the vacuum is too low, the amount of peripheral blood in the aspirate will be higher. As well as the plunger pressure created by the operator, the geometry of the syringe also plays an important role in creating sufficient negative pressure(Figure 3).
Draw several small portions in small aspiration volumes: the number of mesenchymal progenitors (and the vacuum) per aspiration volume decreases during an aspiration procedure.34 It is recommended, therefore, that you draw a maximum 5 mL of bone marrow and then create a new vacuum.
The number of mesenchymal progenitor cells falls with repeated aspiration procedures at the same spot. A maximum 3 aspirations at the same spot before positioning the needle at another spot is, therefore, recommended.
More cells are retrieved with parallel insertions of the aspiration needle than with diverging insertions in one area(Figure 4).
Figure 3.
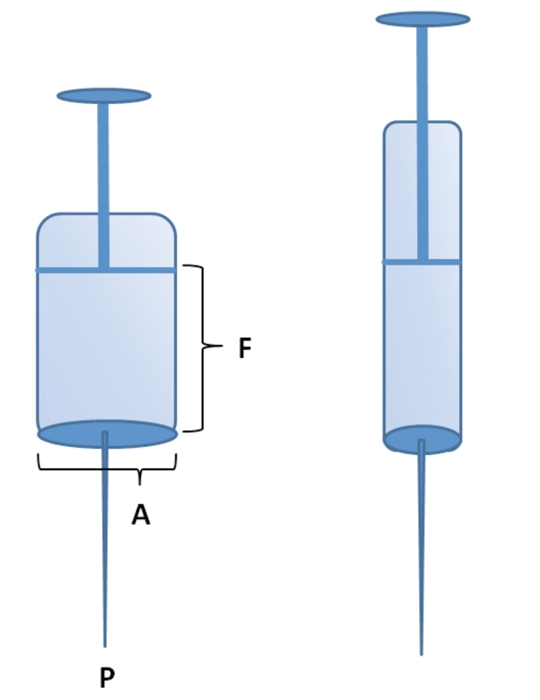
In iliac crest-vacuum aspiration, the geometry of an aspiration syringe influences the proportion of MSCs in the aspirate. The pressure required to retrieve the mesenchymal cells is exerted at the tip of the needle and is defined by the formula: pressure (P) = force (F)/area (A), whereby the force used to create a vacuum is created by withdrawing the plunger of the syringe. This force remains relatively constant. Narrow, long syringes are, therefore, advantageous when harvesting MSCs using bone marrow aspiration.
Figure 4.
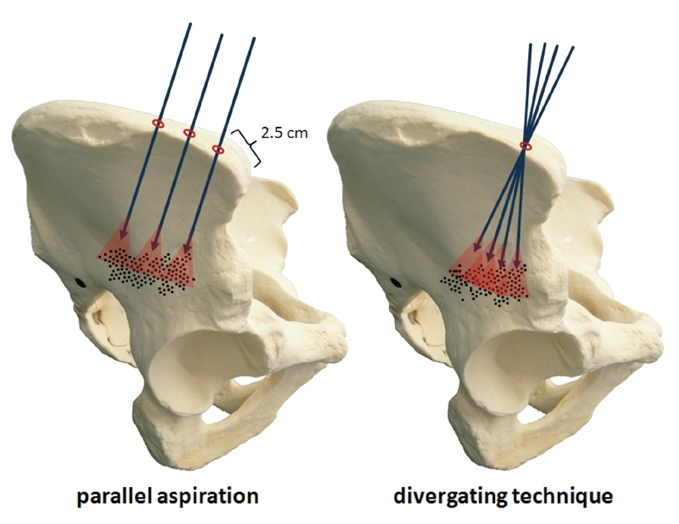
The areas of the iliac crest reached by the tip of the aspiration needle if inserted at diverging angles at the same point overlap, so that areas that have been perforated and aspirated once already are subjected to the procedure several times. This leads to a drop in the amount of MSCs per volume of bone marrow aspirate. If the positions of the inserted needle are parallel, then new MSC harvesting areas will always be accessed.
Also ensure sufficient anti-coagulation of the bone marrow aspirate during the harvesting procedure. Heparin and ACDA solutions are used for this. The aspiration needles and syringes should be flushed with the solution before use. Density gradient centrifugation is particularly suitable for isolating mononuclear cells for bone regeneration therapy.23
Cell therapy treatments with temporary ex vivo expansion
In orthopedics and traumatology, autologous cell therapies have been used regularly on the musculoskeletal system after ex vivo cultivation, at least since the clinical introduction of autologous chondrocyte transplantation (ACI). Unlike cartilage regeneration, for which ACI was used in more than 12,000 patients between 1987 and 2005,35, 36 there are no reliable data on osseous regeneration after temporary in vitro cultivation. In the treatment of necrosis of the femoral head, for instance, whereas numerous one-step transplantations are documented, only three case studies with a maximal observation period of three months can be found. Here, a mixed cell population from bone marrow cells (so-called tissue repair cells, TRCs), was expanded over 12 days under GMP conditions and then transplanted autologously together with a scaffold made of tricalcium phosphate (TCP) within the framework of core decompression.37
The particular drawbacks of temporary cultivation of MSCs lie not only in the considerable logistical effort to ensure the quality of the cell therapy treatment but especially in the biological characteristics of this cell population. As soon as MSCs are isolated from their tissue mass and transferred to a culture dish, differentiation proceeds in accordance with the culture conditions.38–40 The yet inconclusive biological effects when fetal bovine serum is used in the culture, as well as telomere shortening, and thus cell aging with ex vivo cultivation also have to be considered. Furthermore, analysis of 170 neoplasia-associated DNA promoters was able to show that despite the relatively high genetic stability of MSCs from human bone marrow or adipose tissue, damage in the genome could occur at later stages.41 The question as to whether these genotoxic effects of prolonged in vitro cultivation are also clinically manifested after re-transplantation remains unanswered, however. The potential effects of changes in the chromatin structure due to epigenetic factors at the beginning of osteoblastic differentiation also remain largely unknown.42
Cell therapy in local bone defects, bone healing disorders and osteonecrosis
Other research groups have also reported positive clinical results after using human bone marrow cells. Giannini et al. showed that in patients with osteochondral defects in the talus, functional improvements were achieved through autologous bone marrow cell transplantation by arthroscopic surgery.43 As early as 1991, Conolly et al.44 reported equivalent healing rates for autologous bone marrow grafting to treat post-traumatic pseudarthrosis of the tibia. Other authors also support the high osseous regeneration potency of the percutaneous implantation of autologous bone marrow concentrate to treat pseudarthrosis32, 33 and discuss supplementary osteoblastic stimulation using platelet rich plasma (PRP).45
Some authors, on the other hand, have reason to believe that bone regeneration through cell therapy also depends very much on the transplantation site and the local blood supply. According to a study by Kitho et al., in which ex vivo -cultivated MSCs were used together with PRP in 51 lengthening osteotomies, cell therapy accelerated bone healing in the femur compared to in the tibia.46 Overall, however, the cell therapy showed no advantages over the untreated control group and, moreover, no relation between the bone healing rate and the number of transplanted cells or the PRP concentration was found. Hernigou et al., however, reported that grafting over 50,000 osteoblastic progenitor cells particularly encouraged healing in atrophic tibial pseudarthrosis.33
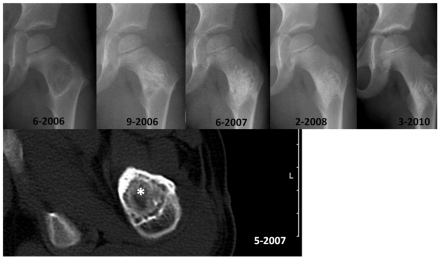
Minimally invasive cell therapy of solitary or aneuryamal bone cysts via percutaneous implantation of autologous bone marrow is favored by a number of authors owing to the healing rate of over 80% 47, 48(Figure 5). On the other hand, a randomized clinical study showed that in the treatment of simple bone cysts, autologous bone marrow injections were inferior to local steroid injections.49
Figure 5.
Healing course after autologous cell therapy with bone marrow aspiration concentrate (“BMAC”) augmented with HA granules in a 4-year old male patient with a large aneurysmal bone cyst of the proximal femur. Ten months after surgery, relevant new bone formation starting within the transplant is observed in computed tomography scan (star, *). The clinical and radiological 3.5 year follow up after treatment showed no recurrence and an asymptomatic patient. Based on the reduced amount of autologous bone available for grafting, pediatric patients in particular might benefit from the minimally invasive cell therapy.
Initial results are also available on cell therapy treatments to promote osseous fusion at the spine. After an observation period of 34 months, fusion rates of over 90% were found for tricalcium phosphate (TCP)-bone marrow composite transplantations.50 For an HA-collagen-I composite incubated with autologous bone marrow, the posterolateral lumbal fusion rate was found to be the same as for autologous bone transplantation, but fusion rates were not the same for intracorporeal fusions.51
Cell therapies have been used successfully to treat avascular osteonecrosis (AVN) for many years by Hernigou et al.52, 53 and, in the meantime, also by other research teams in experimental treatments and clinical studies.54–56 Patients with sickle cell anemia-related AVN, in particular, benefit from a local injection of mononuclear bone marrow cells. Patients with steroid-related AVN have a worse prognosis when treated with MSCs to regenerate bone.57
The number of implanted cells and their proliferation potency, as measured by the CFU-F, are positive predictors for successful bone marrow concentrate therapy in the treatment of osteonecrosis.52 It is unclear whether the reduced number of MSCs in the proximal femur observed in patients with AVN is an independent risk factor in the development of an AVN, or is resulting from AVN.57 Other authors, however, report comparatively high numbers of osteoblasts in the major trochanter region with necrosis of the femoral head.58
It has been shown that for ARCO stages I and II local cell therapy with autologous bone marrow in combination with core decompression diminished the risk of a medium-term progression of necrosis of the femoral head.59
A multi-center study on patients with peripheral artery disease (PAD) amply documented that mixed cell populations from human bone marrow not only have an osteoblastic but also an angiogenetic effect.60
Because of the lack of control groups, however, cell therapy is mentioned in the current S1-recommended treatments of atraumatic necrosis of the femoral head in adults under “Operation methods without good documentation support”.61 Besides direct transplantation of the cell suspension into the AVN area within the framework of a core decompression, a cancellous bone graft can also be combined with the autologous cell therapy.62 For this, a Krohn hollow mill is used to extract a cylinder of cancellous bone and the AVN area subjected to curettage through the resulting cavity. After removing the macroscopic avascular tissue for histopathological diagnosis, the cylinder of cancellous bone is incubated with the cell therapy and then re-implanted in the osseous defect. Medium- and long-term results of this cell therapy treatment are pending. Table 2 is a summary of the results of clinical applications of cell therapies to regenerate bone.
Table 2. A summary of published studies including more than 10 patients after cell therapy in bone defects or bone healing disorders. The medline review showed that cell therapy by bone marrow is not a new technique but has been applied for almost 20 years. According to the scaffold, causative disease, body region and other patient-related factors, most studies demonstrate promising results for bone regeneration by bone marrow cells.
| Author | Year | Journal | Bone defect | N patients | Results |
|---|---|---|---|---|---|
| Connolly et al.44 | 1991 | CORR | Pseudarthrosis | 20 | Application of autologous bone marrow (BM) in tibial pseudarthrosis or “non-union”. Post-operative treatment with plaster cast. Additional intramedullary nailing in 10 cases. The authors report that autologous BM application produced the same results as for autologous bone transplantation. |
| Lokiec et al.63 | 1996 | JBJS-Br | Simple | 10 bone cysts | Percutaneous injection of autogenous bone marrow: all the patients became pain-free after two weeks and resumed full activities within six weeks. The cysts were radiologically consolidated and showed remarkable remodeling within four months. Bone healing was achieved 12–48 months after treatment (no complications). |
| Köse et al.64 | 1999 | Bull Hosp J T Dis | Simple | 12 bone cysts | Autologous bone marrow injection in bone cysts: complete healing occurred in 2 patients, whereas 3 cysts showed residual defects. In 6 patient, cysts recurred. Authors concluded that factors such as the size, multi-loculation, and completeness of the filling of the cyst with bone marrow grafting might influence the post-operative outcome. |
| Hernigou et al.52 | 2002 | CORR | AVN (Hip) | 116 (189 hips) | Evaluation of the clinical outcome 5–10 years after core decompression in combination with injection of autologous BM concentrate in the treatment AVN of the femoral head. Very good results in pre-collapse stages (ARCO I-II): 9 out of 145 hips were replaced endoprosthetically. In post-collapse stages, 25 out of 44 hips replaced endoprosthetically. Better results with higher CFU-F and cell numbers. |
| Rougraff et al.65 | 2002 | JBJS-Am | Unicameral | 23 bone cyst | Percutaneous injection of allogeneic demineralized bone matrix augmented with autogenous bone marrow is an effective treatment for unicameral bone cysts. |
| Chang et al.66 | 2002 | JBJS-Br | Unicameral bone cyst | 79 | 14 patients treated with BM ( 27 injections) vs. 65 patients with steroid application (99 injections). Repeated injections were required in 57% of patients after BM had been used and in 49% after steroid. No complications. No advantage could be shown for the use of autogenous injection of BM compared with injection of steroid in the management of unicameral bone cysts. |
| Price et al.67 | 2003 | Spine | Spinal fusion | 77 | Retrospective study with 3 different bone grafting techniques: autologous iliac crest bone graft (ICBG) vs. freeze-dried corticocancellous allograft vs. composite graft of autologous bone marrow (BM) and demineralized bone matrix. Segmental instrumentation with dual-rod fixation was used in 77 patients. No BM aspiration-associated morbidity. Fusion rates were comparable for ICBG and BM group. |
| Docquier et al.68 | 2003 | J Pediatr Orthop | Simple bone cyst | 17 | Percutaneous aspiration and injection of BM. FU: 33.9 months. Slow regression of the cyst and progressive healing: 13 cases (76%). No response: 2 cases (12%), recurrence: 2 cases (12%). |
| Gangji et al59 | 2004 | JBJS-Am | AVN (hip) | 13 (18 hips) | Necrosis of the femoral head in ARCO stages I-II. Core decompression (vs. core decompression + BM aspirate (10 patients). Within 24 months, significant reduction in pain, functional improvement and lower AVN progression rate after cell therapy. No transplantation-related complications. |
| Hernigou et al.33 | 2005 | JBJS-Am | Pseudarthrosis /non-unions (atrophic, tibia) | 60 | Injection of 20 cm3 BM concentrate: 612±34 progenitor cells/cm3 in the aspirate compared to 2579±1121 progenitor cells/cm3 after density gradient centrifugation: healing in 53 cases. Positive correlation between callus regeneration and the number of CFUs. |
| Kanellopoulos69 | 2005 | J Pediatric Orthop | Active unicameral bone cyst | 19 | BM injection in bone cysts. All patients were asymptomatic at the latest follow up. Two patients required a second intervention to achieve complete cyst healing. Radiographic outcome was improved in all patients according to the Neer classification at the latest FU. There were no significant complications related to the procedure, nor did any fracture occur after initiation of the above regimen. |
| Neen et al.51 | 2006 | Spine | Spinal fusions | 50 | Therapy using HA-collagen I composite incubated with autologous BM aspirate (incubation time: 20 min) vs. autologous bone transplantation The same posterolateral lumbar fusion rates for both groups, similar functional results for both groups. Autologous bone transplantations raised the fusion rate in “interbody fusions”, but donor-site morbidity in 14% of the cases. |
| Yan et al.70 | 2006 | Chin J Traumatol | AVN (hip) | 28 (44 hips) | Percutaneous multiple hole decompression combined with autologous BMCs. The earlier the stage, the better the result. A randomized prospective study needed in the future to compare with routine core decompression. |
| Dallari et al.45 | 2007 | JBJS-Am | Proximal | 33 tibia osteotomies | Prospective, randomized study with 2 therapy groups: lyophilized bone chips + PRP (A, 11 patients) vs. lyophilized bone chips + PRP + bone marrow (B, 12patients). Control group: lyophilized bone chips only. CT-controlled biopsies six weeks post-OP showed increased callus formation in A and B compared to the control group. Improved bone healing in A and B within one year. |
| Deng et al.71 | 2007 | Chin J Regen Reconstr Surg | Bone cyst | 13 | Transplantation of the autologous bone marrow combined with the allograft bone. Complete healing within 3.5–8 months (Ø 5.2 months). No recurrence, no pathological fracture occurred. Complete recovery of function. |
| Cho et al.72 | 2007 | JBJS-Br | Bone cysts | 28 (58) | 30 patients treated by steroid injection vs. 28 individuals by bone marrow grafting. Overall success rates: 86.7% vs. 92.0%, respectively (P>0.05). Initial success rate: 23.3% in the steroid group vs. 52.0% in the BM group. Mean number of procedures: 2.19 (1 to 5) vs. 1.57 (1 to 3) (P<0.05). Average healing interval: 12.5 months (4–32) P =14.3 months (7–36) (P>0.05). Rate of recurrence after initial procedure: 41.7% vs. 13.3% (P<0.05). Although the overall rates of success of both methods were similar, the steroid group showed higher recurrences after a single procedure and required more injections to achieve healing. |
| Wright et al.49 | 2008 | JBJS-Am | Bone cysts | 77 | Randomized, prospective study. Two therapy groups: injection of autologous BM (A) vs. injection of methylprednisolone (B). Healing rate within two years: 23% (A) vs. 42% (B). No significant difference in the functional outcome. |
| Park et al.47 | 2008 | Foot Ankle | Bone cysts | 20 (23 cysts) | Therapy of unicameral bone cysts of the calcaneus. Two therapy groups: open surgery application of avital allogenic donor bone + autologous BM (A) vs. injection of demineralized bone powder + autologous BM (B). Healing rate within 49.4 months: A: 9 out of 13 cysts vs. B: 5 out of 10 cysts. No infections. |
| Gan et al.50 | 2008 | Biomaterials | Spinal fusions | 41 | Application of TCP incubated with BM concentrate (duration circa 2 h). Concentration factor (CFUs-ALP: 4.3). Drop in MSCs with increasing age, but no dependency on gender. After 34.5 months, spinal fusion in 95.1% of the cases. |
| Zamzam et al.48 | 2008 | Int Orthop | Solitary bone cysts | 28 | A minimum one-off percutaneous injection of autologous BM. No complications. Within 34.7±6.87 months, bone healing in 82% of the cases. |
| Jäger et al.73 | 2009 | CSCRT | Bone defects | 10 | Significant bone regeneration through bone marrow concentrate (BMAC) in combination with autologous cancellous bone. |
| Hendrich et al.29 | 2009 | Orthop Rev | Bone defects, AVN | 101 | Proof of the low complication risk of autologous BMAC in 101 applications. |
| Giannini et al.43 | 2009 | CORR | Osteochondral lesions (talus) | 48 | Functional improvements after arthroscopy-assisted application of autologous BM aspirate in osteochondral defects in the talus. |
| Sir et al.74 | 2009 | Vnitr Lek | Fracture-related bone defects, pseudarthrosis | 11 | Local and one-step injection of MSCs from human BM. Results pending. |
| Kitoh et al.46 | 2009 | J Pediatr Orthop | Tibial vs.femoral lengthening osteotomies | 28 (51 osteotomies) | Retrospective study. Application of ex vivo cultivated MSCs together with PRP Control group: 60 patients without MSC/PRP. No stimulation of bone healing by MSC/PRP. Worse results for the tibia. |
| Hernigou et al.56 | 2009 | Indian J Orthop | AVN (hip) | 342 (534 hips) | Autologous cell therapy in ARCO stages I–II in combination with a core decompression. After 8–18 years, 94 endoprosthetic hip replacements. Predictor for a therapy success was a high number of progenitor cells. |
| Wang et al.75 | 2009 | Arch Orthop Trauma Surg | AVN (hip) | 45 (59 hips) | BMAC injection in AVN of the femoral head (ARCO stage I–III).Clinically successful in 79.7%. Hip replacement within FU in 11.9% of the hips. Radiologically, 14 of the 59 hips exhibited femoral head collapse or narrowing of the joint space. Overall failure rate: 23.7%. The concentration factor of mononuclear cells from BM vs. BMAC was about 3. |
| Miller et al.76 | 2010 | Int Orthop | Non-union or segmental defect | 13 | Bone marrow cells harvested by a reamer-irrigator-aspirator (RIA) were treated by dexamethason and transplanted into segmental bone defects. Promising results were achieved using this technique; and given the complexity of these cases, the observed success is of great value and warrants controlled study into both standardization of the procedure and concentration of the grafting material. |
| Yamasaki et al.77 | 2010 | JBJS-Br | AVN (hip) | 22 (30 hips) | Transplantation of bone-marrow-derived mononuclear cells (BMMNCs) combined with hydroxypapatite (HA) vs. HA only in AVN of the femoral head. Reduction of the osteonecrotic lesion was observed subsequent to hypertrophy of the bone in the transition zone in the BM group. In 3 patients of the BMMNC group, progression to extensive collapse occurred. Control group showed bone hypertrophy, but severe collapse of the femoral head occurred in 6 of 8 hips. |
Cell therapy in skeletal diseases
In addition to the local application of MSCs, data are also available for MSCs in the successful treatment of skeletal diseases which are accompanied with deficiencies in the bone structure.78, 79
In 1999, after numerous in vitro experiments and animal experimental studies, Horwitz et al., for the first time, treated 3 children with osteogenesis imperfecta (OI) with allogenic transplantations of mesenchymal bone marrow cells.80 The cells were introduced intravenously after ablative pre-treatment of the patient (chemotherapy and immunosuppression). Post-operative bone biopsies after 216 days and bone density measurements showed a significant quantitative and qualitative improvement in the bone structure. In another publication from the year 2001, the same research group81 reported their findings on 5 OI patients who had been treated with cell therapy and on 2 other OI patients without cell therapy treatment (OI type III). After an investigation period of six months, children who had received cell-based therapy showed an accelerated growth rate.
Osteopetrosis is another skeletal disease involving insufficient osteoclast activity that is currently being treated with autologous bone marrow transplantation. Driessen et al.82 found that the probability of 5-year disease free survival was 73% after cell-based therapy. Treatment before the age of three years improved the chances of success of cell therapy in osteopetrosis.83 However, due to the severe side-effects and possible complications (e.g. graft rejection, hypercalcemic crises, pulmonary hypertension, delayed hematopoiesis, veno-occlusive disease), allogeneic cell therapy treatment of patients with osteopetrosis is limited to severe manifestation of disease.84 Three case reports also report the successful treatment of an 8-month old infant with infantile hypophosphatasia who underwent transplantation of T-cell depleted bone marrow from the sister. The positive effects of the cell therapy ceased, however, after six months. Twenty-one months after the first transplantation, a second transplantation of ex vivo expanded bone marrow cells took place resulting in an increase in bone mass. At the age of six, the patient in question still showed signs of stunted growth but displayed normal intelligence.85 Another approach in cell therapy treatment of infantile hypophosphatasia consists of intra-peritoneal, subcutaneous or intraosseous bone transplantation from a related donor parallel to the intravenous bone marrow injection. The postulate here is that migration of the donor MSCs in the recipient organism will positively influence bone healing and the rejection reactions.86, 87 It is unclear whether cell-based therapy will also gain acceptance in other skeletal diseases, such as osteoporosis.
Outlook for the future
Due to the accelerated aging of osteoblastic progenitor cells after in vitro cultivation, the limited resources, the diminished osteoblastic potency with increasing age and the improved standardizations, immortalized human MSCs are currently undergoing pre-clinical investigations for their suitability for cell therapy.88, 89 One way of avoiding aging of MSCs is to transfer the cDNA of telomerase reverse transcriptase (hTERT). With this enzyme, the telomeres that have been shortened during the course of replication are returned to their original length. Some authors were able to demonstrate a high osteoblastic potency in vitro and in animal experiments with this process.88 Nevertheless, in view of the current legislation, it is uncertain whether these new therapy procedures can also be tested clinically. In addition to cell-based therapies, there are other innovative “orthobiologics” with bone regeneration as the goal. These include anchor proteins that stimulate osteoblastic adherence, e.g. RGD sequences, fibronectin or peptide 15. α-granules of thrombocytes, in particular, contain large amounts of growth factors with osteoblastic and cell proliferation potency, such as transforming growth factor-β (TGF), vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF).90 Whether it makes sense and is necessary, within the context of the clinical application of cell therapies, to increase the osseous potency of osteoblastic progenitor cells using additional growth factors must first be investigated in controlled clinical studies given that the hitherto existing data are contradictory and not sufficiently reliable.46,91,92
References
- 1.Satija NK, Singh VK, Verma YK, et al. Mesenchymal Stem Cell-based Therapy: A New Paradigm in Regenerative Medicine. J Cell Mol Med. 2009;13:4385–402. doi: 10.1111/j.1582-4934.2009.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coipeau P, Rosset P, Langonne A, et al. Impaired differentiation potential of human trabecular bone mesenchymal stromal cells from elderly patients. Cytotherapy. 2009;11:584–94. doi: 10.1080/14653240903079385. [DOI] [PubMed] [Google Scholar]
- 3.El Tamer MK, Reis RL. Progenitor and stem cells for bone and cartilage regeneration. J Tissue Eng Regen Med. 2009;3:327–37. doi: 10.1002/term.173. [DOI] [PubMed] [Google Scholar]
- 4.Lin G, Garcia M, Ning H, et al. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008;17:1053–63. doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackay DL, Tesar PJ, Liang LN, Haynesworth SE. Characterizing medullary and human mesenchymal stem cell-derived adipocytes. J Cell Physiol. 2006;207:722–8. doi: 10.1002/jcp.20617. [DOI] [PubMed] [Google Scholar]
- 6.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 7.Jackson WM, Aragon AB, Djouad F, et al. Mesenchymal progenitor cells derived from traumatized human muscle. J Tissue Eng Regen Med. 2009;3:129–38. doi: 10.1002/term.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park J, Setter V, Wixler V, Schneider H. Umbilical cord blood stem cells: induction of differentiation into mesenchymal lineages by cell-cell contacts with various mesenchymal cells. Tissue Eng Part A. 2009;15:397–406. doi: 10.1089/ten.tea.2007.0379. [DOI] [PubMed] [Google Scholar]
- 9.Miao Z, Jin J, Chen L, et al. Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biol Int. 2006;30:681–7. doi: 10.1016/j.cellbi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Lavoie JF, Biernaskie JA, Chen Y, et al. Skin-derived precursors differentiate into skeletogenic cell types and contribute to bone repair. Stem Cells Dev. 2009;18:893–906. doi: 10.1089/scd.2008.0260. [DOI] [PubMed] [Google Scholar]
- 11.Segawa Y, Muneta T, Makino H, et al. Mesenchymal stem cells derived from synovium, meniscus, anterior cruciate ligament, and articular chondrocytes share similar gene expression profiles. J Orthop Res. 2009;27:435–41. doi: 10.1002/jor.20786. [DOI] [PubMed] [Google Scholar]
- 12.Zhang S, Muneta T, Morito T, et al. Autologous synovial fluid enhances migration of mesenchymal stem cells from synovium of osteoarthritis patients in tissue culture system. J Orthop Res. 2008;26:1413–8. doi: 10.1002/jor.20659. [DOI] [PubMed] [Google Scholar]
- 13.Kahler RA, Galindo M, Lian J, et al. Lymphocyte enhancer-binding factor 1 (Lef1) inhibits terminal differentiation of osteoblasts. J Cell Biochem. 2006;97:969–83. doi: 10.1002/jcb.20702. [DOI] [PubMed] [Google Scholar]
- 14.Hoeppner LH, Secreto F, Jensen ED, et al. Runx2 and bone morphogenic protein 2 regulate the expression of an alternative Lef1 transcript during osteoblast maturation. J Cell Physiol. 2009;221:480–9. doi: 10.1002/jcp.21879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takada I, Kouzmenko AP, Kato S. Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol. 2009;5:442–7. doi: 10.1038/nrrheum.2009.137. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Tang T, Shi Q, et al. The immunologic properties of undifferentiated and osteogenic differentiated mouse mesenchymal stem cells and its potential application in bone regeneration. Immunobiology. 2009;214:179–86. doi: 10.1016/j.imbio.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 17.Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301–16. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 19.Horwitz EM, Le Blanc K, Dominici M, et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–5. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 20.Granchi D, Ochoa G, Leonardi E, et al. Gene expression patterns related to osteogenic differentiation of bone marrow-derived mesenchymal stem cells during ex vivo expansion. Tissue Eng Part C Methods. 2010;16:511–24. doi: 10.1089/ten.TEC.2009.0405. [DOI] [PubMed] [Google Scholar]
- 21.Kode JA, Mukherjee S, Joglekar MV, Hardikar AA. Mesenchymal stem cells: immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy. 2009;11:377–91. doi: 10.1080/14653240903080367. [DOI] [PubMed] [Google Scholar]
- 22.Au A, Boehm CA, Mayes AM, et al. Formation of osteogenic colonies on well-defined adhesion peptides by freshly isolated human marrow cells. Biomaterials. 2007;28:1847–61. doi: 10.1016/j.biomaterials.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hermann PC, Huber SL, Herrler T, et al. Concentration of bone marrow total nucleated cells by a point-of-care device provides a high yield and preserves their functional activity. Cell Transplant. 2008;16:1059–69. [PubMed] [Google Scholar]
- 24.Lange C, Cakiroglu F, Spiess AN, et al. Accelerated and safe expansion of human mesenchymal stromal cells in animal serum-free medium for transplantation and regenerative medicine. J Cell Physiol. 2007;213:18–26. doi: 10.1002/jcp.21081. [DOI] [PubMed] [Google Scholar]
- 25.Sauerbier S, Stricker A, Kuschnierz J, et al. In vivo comparison of hard tissue regeneration with human mesenchymal stem cells processed with either the FICOLL method or the BMAC method. Tissue Eng Part C Methods. 2010;16:215–23. doi: 10.1089/ten.TEC.2009.0269. [DOI] [PubMed] [Google Scholar]
- 26.Yin D, Wang Z, Gao Q, et al. Determination of the fate and contribution of ex vivo expanded human bone marrow stem and progenitor cells for bone formation by 2.3ColGFP. Mol Ther. 2009;17:1967–78. doi: 10.1038/mt.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jäger M, Herten M, Jelinek EM, et al. Bone marrow concentrate - Optimal for bone repair! Langenbeck's Archives of Surgery. 2009a:394–940. [Google Scholar]
- 28.Jäger M. Gelenkerhaltende Operationen bei atraumatischer Femurkopfnekrose. Osteologie. 2010;19:24–8. [Google Scholar]
- 29.Hendrich C, Engelmaier F, Waertel G, et al. Safety of autologous bone marrow aspiration concentrate transplantation: initial experiences in 101 patients. Orthop Rev. 2009:1–e32. doi: 10.4081/or.2009.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurkalli BG, Gurevitch O, Sosnik A, et al. Repair of bone defect using bone marrow cells and demineralized bone matrix supplemented with polymeric materials. Curr Stem Cell Res Ther. 2010;5:49–56. doi: 10.2174/157488810790442831. [DOI] [PubMed] [Google Scholar]
- 31.Yoshimi R, Yamada Y, Ito K, et al. Self-assembling peptide nanofiber scaffolds, platelet-rich plasma, and mesenchymal stem cells for injectable bone regeneration with tissue engineering. J Craniofac Surg. 2009;20:1523–30. doi: 10.1097/SCS.0b013e3181b09b7e. [DOI] [PubMed] [Google Scholar]
- 32.Hernigou P, Mathieu G, Poignard A, et al. Percutaneous autologous bone-marrow grafting for nonunions.Surgical technique. J Bone Joint Surg Am. 2006;88(Suppl 1 Pt 2):322–7. doi: 10.2106/JBJS.F.00203. [DOI] [PubMed] [Google Scholar]
- 33.Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87:1430–7. doi: 10.2106/JBJS.D.02215. [DOI] [PubMed] [Google Scholar]
- 34.Muschler GF, Nitto H, Boehm CA, Easley KA. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res. 2001;19:117–25. doi: 10.1016/S0736-0266(00)00010-3. [DOI] [PubMed] [Google Scholar]
- 35.Marlovits S, Striessnig G, Kutscha-Lissberg F, et al. Early postoperative adherence of matrix-induced autologous chondrocyte implantation for the treatment of full-thickness cartilage defects of the femoral condyle. Knee Surg Sports Traumatol Arthrosc. 2005;13:451–7. doi: 10.1007/s00167-004-0535-3. [DOI] [PubMed] [Google Scholar]
- 36.Marlovits S, Zeller P, Singer P, et al. Cartilage repair: generations of autologous chondrocyte transplantation. Eur J Radiol. 2006;57:24–31. doi: 10.1016/j.ejrad.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 37.Nöth U, Reichert J, Reppenhagen S, et al. [Cell based therapy for the treatment of femoral head necrosis] Orthopade. 2007;36:466–71. doi: 10.1007/s00132-007-1087-2. [DOI] [PubMed] [Google Scholar]
- 38.Jäger M, Wild A, Lensing-Höhn S, Krauspe R. Influence of different culture solutions on osteoblastic differentiation in cord blood and bone marrow derived progenitor cells. Biomed Tech (Berl) 2003;48:241–4. doi: 10.1515/bmte.2003.48.9.241. [DOI] [PubMed] [Google Scholar]
- 39.Turnovcova K, Ruzickova K, Vanecek V, et al. Properties and growth of human bone marrow mesenchymal stromal cells cultivated in different media. Cytotherapy. 2009:1–12. doi: 10.3109/14653240903188947. [DOI] [PubMed] [Google Scholar]
- 40.Zilkens C, Lögters T, Bittersohl B, et al. Spinning around or stagnation- what do osteoblasts and chondroblast really like? Eur J Med Res. 2009;14:1–9. doi: 10.1186/2047-783X-15-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dahl JA, Duggal S, Coulston N, et al. Genetic and epigenetic instability of human bone marrow mesenchymal stem cells expanded in autologous serum or fetal bovine serum. Int J Dev Biol. 2008;52:1033–42. doi: 10.1387/ijdb.082663jd. [DOI] [PubMed] [Google Scholar]
- 42.Siddiqi S, Mills J, Matushansky I. Epigenetic remodeling of chromatin architecture: exploring tumor differentiation therapies in mesenchymal stem cells and sarcomas. Curr Stem Cell Res Ther. 2010;5:63–73. doi: 10.2174/157488810790442859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giannini S, Buda R, Vannini F, et al. Onestep bone marrow-derived cell transplantation in talar osteochondral lesions. Clin Orthop Relat Res. 2009;467:3307–20. doi: 10.1007/s11999-009-0885-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Connolly JF, Guse R, Tiedeman J, Dehne R. Autologous marrow injection as a substitute for operative grafting of tibial nonunions. Clin Orthop Relat Res. 1991:259–70. [PubMed] [Google Scholar]
- 45.Dallari D, Savarino L, Stagni C, et al. Enhanced tibial osteotomy healing with use of bone grafts supplemented with platelet gel or platelet gel and bone marrow stromal cells. J Bone Joint Surg Am. 2007;89:2413–20. doi: 10.2106/JBJS.F.01026. [DOI] [PubMed] [Google Scholar]
- 46.Kitoh H, Kawasumi M, Kaneko H, Ishiguro N. Differential effects of culture-expanded bone marrow cells on the regeneration of bone between the femoral and the tibial lengthenings. J Pediatr Orthop. 2009;29:643–9. doi: 10.1097/BPO.0b013e3181b2afb2. [DOI] [PubMed] [Google Scholar]
- 47.Park IH, Micic ID, Jeon IH. A study of 23 unicameral bone cysts of the calcaneus: open chip allogeneic bone graft versus percutaneous injection of bone powder with autogenous bone marrow. Foot Ankle Int. 2008;29:164–70. doi: 10.3113/FAI.2008.0164. [DOI] [PubMed] [Google Scholar]
- 48.Zamzam MM, Abak AA, Bakarman KA, et al. Efficacy of aspiration and autogenous bone marrow injection in the treatment of simple bone cysts. Int Orthop. 2009;33:1353–8. doi: 10.1007/s00264-008-0619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wright JG, Yandow S, Donaldson S, et al. A randomized clinical trial comparing intralesional bone marrow and steroid injections for simple bone cysts. J Bone Joint Surg Am. 2008;90:722–30. doi: 10.2106/JBJS.G.00620. [DOI] [PubMed] [Google Scholar]
- 50.Gan Y, Dai K, Zhang P, et al. The clinical use of enriched bone marrow stem cells combined with porous beta-tricalcium phosphate in posterior spinal fusion. Biomaterials. 2008;29:3973–82. doi: 10.1016/j.biomaterials.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 51.Neen D, Noyes D, Shaw M, et al. Healos and bone marrow aspirate used for lumbar spine fusion: a case controlled study comparing healos with autograft. Spine (Phila Pa 1976) 2006;31:E636–40. doi: 10.1097/01.brs.0000232028.97590.12. [DOI] [PubMed] [Google Scholar]
- 52.Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res. 2002:14–23. doi: 10.1097/00003086-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 53.Hernigou P, Daltro G, Filippini P, et al. Percutaneous implantation of autologous bone marrow osteoprogenitor cells as treatment of bone avascular necrosis related to sickle cell disease. Open Orthop J. 2008;2:62–5. doi: 10.2174/1874325000802010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gangji V, Hauzeur JP. Cellular-based therapy for osteonecrosis. Orthop Clin North Am. 2009;40:213–21. doi: 10.1016/j.ocl.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 55.Gangji V, Toungouz M, Hauzeur JP. Stem cell therapy for osteonecrosis of the femoral head. Expert Opin Biol Ther. 2005;5:437–42. doi: 10.1517/14712598.5.4.437. [DOI] [PubMed] [Google Scholar]
- 56.Hernigou P, Poignard A, Zilber S, Rouard H. Cell therapy of hip osteonecrosis with autologous bone marrow grafting. Indian J Orthop. 2009;43:40–5. doi: 10.4103/0019-5413.45322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hernigou P, Beaujean F, Lambotte JC. Decrease in the mesenchymal stem-cell pool in the proximal femur in corticosteroid-induced osteonecrosis. J Bone Joint Surg Br. 1999;81:349–55. doi: 10.1302/0301-620x.81b2.8818. [DOI] [PubMed] [Google Scholar]
- 58.Tingart M, Beckmann J, Opolka A, et al. Analysis of bone matrix composition and trabecular microarchitecture of the femoral metaphysis in patients with osteonecrosis of the femoral head. J Orthop Res. 2009;27:1175–81. doi: 10.1002/jor.20873. [DOI] [PubMed] [Google Scholar]
- 59.Gangji V, Hauzeur JP, Matos C, et al. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. A pilot study. J Bone Joint Surg Am. 2004;86-A:1153–60. doi: 10.2106/00004623-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 60.Amann B, Luedemann C, Ratei R, Schmidt-Lucke JA. Autologous bone marrow cell transplantation increases leg perfusion and reduces amputations in patients with advanced critical limb ischemia due to peripheral artery disease. Cell Transplant. 2009;18:371–80. doi: 10.3727/096368909788534942. [DOI] [PubMed] [Google Scholar]
- 61.Roth A, Jäger M, Maus U, et al. Atraumatische Femurkopfnekrose des Erwachsenen. Evidenz als Voraussetzung für die Erstellung aktueller Leitlinien. Osteologie. 2010;19:60–64. [Google Scholar]
- 62.Jäger M, Herten M, Fochtmann U, et al. Bridging the gap: Bone marrow aspiration concentrate reduces autologous bone grafting in osseous defects. J Orthop Res. 2010 doi: 10.1002/jor.21230. (in press) [DOI] [PubMed] [Google Scholar]
- 63.Lokiec F, Ezra E, Khermosh O, Wientroub S. Simple bone cysts treated by percutaneous autologous marrow grafting. A preliminary report. J Bone Joint Surg Br. 1996;78:934–7. doi: 10.1302/0301-620x78b6.6840. [DOI] [PubMed] [Google Scholar]
- 64.Köse N, Gokturk E, Turgut A, et al. Percutaneous autologous bone marrow grafting for simple bone cysts. Bull Hosp Jt Dis. 1999;58:105–10. [PubMed] [Google Scholar]
- 65.Rougraff BT, Kling TJ. Treatment of active unicameral bone cysts with percutaneous injection of demineralized bone matrix and autogenous bone marrow. J Bone Joint Surg Am. 2004;84-A:921–9. doi: 10.2106/00004623-200206000-00005. [DOI] [PubMed] [Google Scholar]
- 66.Chang CH, Stanton RP, Glutting J. Unicameral bone cysts treated by injection of bone marrow or methylprednisolone. J Bone Joint Surg Br. 2002;84:407–12. doi: 10.1302/0301-620x.84b3.12115. [DOI] [PubMed] [Google Scholar]
- 67.Price CT, Connolly JF, Carantzas AC, Ilyas I. Comparison of bone grafts for posterior spinal fusion in adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2003;28:793–8. [PubMed] [Google Scholar]
- 68.Docquier PL, Delloye C. Treatment of simple bone cysts with aspiration and a single bone marrow injection. J Pediatr Orthop. 2003;23:766–73. doi: 10.1097/00004694-200311000-00015. [DOI] [PubMed] [Google Scholar]
- 69.Kanellopoulos AD, Yiannakopoulos CK, Soucacos PN. Percutaneous reaming of simple bone cysts in children followed by injection of demineralized bone matrix and autologous bone marrow. J Pediatr Orthop. 2005;25:671–5. doi: 10.1097/01.bpo.0000164874.36770.42. [DOI] [PubMed] [Google Scholar]
- 70.Yan ZQ, Chen YS, Li WJ, et al. Treatment of osteonecrosis of the femoral head by percutaneous decompression and autologous bone marrow mononuclear cell infusion. Chin J Traumatol. 2006;9:3–7. [PubMed] [Google Scholar]
- 71.Deng G, Ling Q, Li T. [Treatment of bone cyst by transplantation of autologous bone marrow combined with allograft bone] Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2007;21:801–3. [PubMed] [Google Scholar]
- 72.Cho HS, Oh JH, Kim HS, et al. Unicameral bone cysts: a comparison of injection of steroid and grafting with autologous bone marrow. J Bone Joint Surg Br. 2007;89:222–6. doi: 10.1302/0301-620X.89B2.18116. [DOI] [PubMed] [Google Scholar]
- 73.Jäger M, Jelinek EM, Wess KM, et al. Bone marrow concentrate: a novel strategy for bone defect treatment. Curr Stem Cell Res Ther. 2009;4:34–43. doi: 10.2174/157488809787169039. [DOI] [PubMed] [Google Scholar]
- 74.Sir M, Prochazka V, Gumulec J, et al. [Our first experiences with autologous transplantation of bone marrow stem cells to treat pseudarthrosis, delayed fracture healing and long bone defects fracture] Vnitr Lek. 2009;55:187–9. [PubMed] [Google Scholar]
- 75.Wang BL, Sun W, Shi ZC, et al. Treatment of nontraumatic osteonecrosis of the femoral head with the implantation of core decompression and concentrated autologous bone marrow containing mononu-clear cells. Arch Orthop Trauma Surg. 2010;130:859–65. doi: 10.1007/s00402-009-0939-0. [DOI] [PubMed] [Google Scholar]
- 76.Miller MA, Ivkovic A, Porter R, et al. Autologous bone grafting on steroids: preliminary clinical results. A novel treatment for nonunions and segmental bone defects. Int Orthop. 2010 doi: 10.1007/s00264-010-1013-9. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamasaki T, Yasunaga Y, Ishikawa M, et al. Bone-marrow-derived mononuclear cells with a porous hydroxyapatite scaffold for the treatment of osteonecrosis of the femoral head: a preliminary study. J Bone Joint Surg Br. 2010;92:337–41. doi: 10.1302/0301-620X.92B3.22483. [DOI] [PubMed] [Google Scholar]
- 78.Jethva R, Otsuru S, Dominici M, Horwitz EM. Cell therapy for disorders of bone. Cytotherapy. 2009;11:3–17. doi: 10.1080/14653240902753477. [DOI] [PubMed] [Google Scholar]
- 79.Undale AH, Westendorf JJ, Yaszemski MJ, Khosla S. Mesenchymal stem cells for bone repair and metabolic bone diseases. Mayo Clin Proc. 2009;84:893–902. doi: 10.4065/84.10.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Horwitz EM, Prockop DJ, Fitzpatrick LA, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–13. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 81.Horwitz EM, Prockop DJ, Gordon PL, et al. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood. 2001;97:1227–31. doi: 10.1182/blood.v97.5.1227. [DOI] [PubMed] [Google Scholar]
- 82.Driessen GJ, Gerritsen EJ, Fischer A, et al. Long-term outcome of haematopoietic stem cell transplantation in autosomal recessive osteopetrosis: an EBMT report. Bone Marrow Transplant. 2003;32:657–63. doi: 10.1038/sj.bmt.1704194. [DOI] [PubMed] [Google Scholar]
- 83.Stark Z, Savarirayan R. Osteopetrosis. Orphanet J Rare Dis. 2009:4–5. doi: 10.1186/1750-1172-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Steward CG, Pellier I, Mahajan A, et al. Severe pulmonary hypertension: a frequent complication of stem cell transplantation for malignant infantile osteopetrosis. Br J Haematol. 2004;124:63–71. doi: 10.1046/j.1365-2141.2003.04739.x. [DOI] [PubMed] [Google Scholar]
- 85.Whyte MP, Kurzberg J, McAlister WM, et al. Marrow cell transplantation for infantile hypophosphatasia. J Bone Miner Res. 2003:624–36. doi: 10.1359/jbmr.2003.18.4.624. [DOI] [PubMed] [Google Scholar]
- 86.Cahill RA, Jones OY, Klemperer M, et al. Replacement of recipient stromal/mesenchymal cells after bone marrow transplantation using bone fragments and cultured osteoblast-like cells. Biol Blood Marrow Transplant. 2004;10:709–17. doi: 10.1016/j.bbmt.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 87.Cahill RA, Wenkert D, Perlman SA, et al. Infantile hypophosphatasia: transplantation therapy trial using bone fragments and cultured osteoblasts. J Clin Endocrinol Metab. 2007;92:2923–30. doi: 10.1210/jc.2006-2131. [DOI] [PubMed] [Google Scholar]
- 88.Nakahara H, Misawa H, Hayashi T, et al. Bone repair by transplantation of hTERT-immortalized human mesenchymal stem cells in mice. Transplantation. 2009;88:346–53. doi: 10.1097/TP.0b013e3181ae5ba2. [DOI] [PubMed] [Google Scholar]
- 89.Yang J, Cao C, Wang W, et al. Proliferation and osteogenesis of immortalized bone marrow-derived mesenchymal stem cells in porous polylactic glycolic acid scaffolds under perfusion culture. J Biomed Mater Res A. 2010;92:817–29. doi: 10.1002/jbm.a.32378. [DOI] [PubMed] [Google Scholar]
- 90.Alsousou J, Thompson M, Hulley P, et al. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone Joint Surg Br. 2009;91:987–96. doi: 10.1302/0301-620X.91B8.22546. [DOI] [PubMed] [Google Scholar]
- 91.Pradeep AR, Pai S, Garg G, et al. A randomized clinical trial of autologous platelet-rich plasma in the treatment of mandibular degree II furcation defects. J Clin Periodontol. 2009;36:581–8. doi: 10.1111/j.1600-051X.2009.01428.x. [DOI] [PubMed] [Google Scholar]
- 92.Pradeep AR, Shetty SK, Garg G, Pai S. Clinical effectiveness of autologous platelet-rich plasma and Peptide-enhanced bone graft in the treatment of intrabony defects. J Periodontol. 2009b;80:62–71. doi: 10.1902/jop.2009.080214. [DOI] [PubMed] [Google Scholar]