Abstract
Forced-air-warming (FAW) is an effective and widely used means for maintaining surgical normothermia, but FAW also has the potential to generate and mobilize airborne contamination in the operating room.
We measured the emission of viable and non-viable forms of airborne contamination from an arbitrary selection of FAW blowers (n=25) in the operating room. A laser particle counter measured particulate concentrations of the air near the intake filter and in the distal hose airstream. Filtration efficiency was calculated as the reduction in particulate concentration in the distal hose airstream relative to that of the intake. Microbial colonization of the FAW blower's internal hose surfaces was assessed by culturing the microorganisms recovered through swabbing (n=17) and rinsing (n=9) techniques.
Particle counting revealed that 24% of FAW blowers were emitting significant levels of internally generated airborne contamination in the 0.5 to 5.0 µm size range, evidenced by a steep decrease in FAW blower filtration efficiency for particles 0.5 to 5.0 µm in size. The particle size-range-specific reduction in efficiency could not be explained by the filtration properties of the intake filter. Instead, the reduction was found to be caused by size-range-specific particle generation within the FAW blowers. Microorganisms were detected on the internal air path surfaces of 94% of FAW blowers.
The design of FAW blowers was found to be questionable for preventing the build-up of internal contamination and the emission of airborne contamination into the operating room. Although we did not evaluate the link between FAW and surgical site infection rates, a significant percentage of FAW blowers with positive microbial cultures were emitting internally generated airborne contamination within the size range of free floating bacteria and fungi (<4 µm) that could, conceivably, settle onto the surgical site.
Key words: forced-air warming.
Introduction
Forced-air warming (FAW) has been widely adopted in clinical practice to prevent inadvertent surgical hypothermia. This is based upon the well established benefits of surgical normothermia which include reduced operative blood loss,1 improved wound healing,2 reduced duration of hospital stay,3 increased survival,4 and reduced wound infection.3 Although FAW is one of several methods available for maintaining surgical normothermia, it has the potential to mobilize and generate airborne contamination in the operating room from FAW airflow which other methods of warming do not.
Airflow-free alternatives to FAW, such as resistive-heating technologies, have been shown to be comparably effective to or better than FAW for maintaining surgical normothermia.5–15 Given these clinically validated warming alternatives, research is needed to assess the relationship between FAW and airborne contamination, as a surrogate risk of infection, in the operating room. This is particularly relevant as there has not been a study of surgical site infection (SSI) rates after FAW compared with a normothermic control population, nor is there likely to be, bearing in mind the numbers of patients that might be needed to show a statistically significant difference if one exists.
Airborne contamination consists of all particulate matter suspended in the operating room air. Common forms include microbialladen dust, lint, skin squames, and respiratory droplets.16–18 These contaminants are mobilized by air currents and have been shown to settle out of the air onto the surgical site, contributing to the risk of a surgical site infection (SSI) through at least two possible mechanisms: pathogenic contaminants can be the direct cause of SSI; non-pathogenic contaminants can enable SSI through the forming of a nidus for pathogen growth and attachment.19 Most FAW devices contain a “0.2 µm rated” intake filter20 to prevent the devices from becoming internally contaminated and to lessen the mobilization of airborne contamination in the operating room. However, several studies have reported colonization21–23 on the internal surfaces of the warm-air blower devices and one study was able to repeatedly culture microbes from the blower's airstream;21 this study recommended the placement of a distal hose end filter to lessen FAW microbial emissions.
In contrast, other studies assessing settle plate colonization levels did not detect significant differences following the use of FAW24–26 in the operating room; the conclusion made was that FAW posed no incremental airborne contamination risk. To the authors' knowledge, studies have not examined the relationship between FAW and the spread of non-viable forms of airborne contamination.
Therefore, in this study we investigated the emission of both viable and non-viable forms of airborne contamination from FAW blowers in several hospitals, with assessment of microbial colonization on the internal hose surfaces.
Materials and Methods
Sampling procedures
FAW blowers, from hospitals in the vicinity of Minneapolis and St. Paul, MN, USA, were sampled after-hours in the operating room to quantify the levels of contamination caused by airborne emissions and bacterial colonization on internal hose surfaces.
Contamination caused by airborne emissions from the FAW blowers was recorded using a Handilaz™ laser particle counter (Particle Measuring Systems, Boulder, CO, USA) with a 0.1 ft3 sample volume. Particle counts were taken at the intake and distal hose end airstreams: for the intake sample, the probe was placed at 1–2 inches from the intake filter; for the distal sample, the probe was placed 1–2 inches inside the distal hose end. Two or more samples were taken at each location.
Bacterial colonization of the internal hose surfaces was sampled through the following swabbing and rinsing techniques: pre-moistened swabs were rubbed against portions of the internal air-path surfaces of the injection molded proximal (unit-end) and distal (output-end) hose fittings; 100 mL of sterile water was poured into the unit hose and mechanically agitated by gently rolling and elevating the hose until the internal surfaces had been rinsed twice.
Assessments
Microbiological culturing and analysis was performed by PACE Analytical, Oakdale, MN, USA.
The filtration efficiency of FAW blowers was calculated as the mean within-device reduction in particle counts for the distal as compared to intake airstream. Filtration efficiencies were segmented for the particle size ranges of 0.3 to 0.5 µm, 0.5 to 5.0 µm, and greater than 5.0 µm. FAW blowers which displayed an abnormal filtration efficiency pattern, where the efficiency at 0.3 to 0.5 µm exceeded the efficiency at 0.5 to 5.0 µm, were classified as “abnormally operating”; FAW blowers which displayed a normal filtration efficiency pattern, where the efficiency at 0.5 to 5.0 µm exceeded the efficiency at 0.3 to 0.5 µm, were classified as “normally operating”. Scatter plots of average FAW blower intake compared with distal air-stream particle counts, grouped by FAW blower classification, were used to calculate expected filtration efficiencies and identify outlying units (see Statistical analysis). Bar charts of statistically significant abnormally operating units were created by plotting intake, distal, and expected distal particle counts by unit, with expected distal particle counts calculated as the product of expected filtration efficiency and observed intake particle count.
Colony forming units (CFU) per swab were assessed by the following process: swabs were transported from the site in 10 mL of Butterfield's buffer on ice; the diluent and swab were vortexed in transport container for 30 seconds; the diluent was filtered through a 0.45 µm nitrocellulose membrane filter; the filter was plated on tryptic soy agar, a non-selective medium; incubation was performed for 48 hours at 36.5±2ºC; and plates were inspected for growth and micro-organisms counted as CFU per swab.
CFU per rinse were assessed by the following process: the rinse solution was transported from the site in sterile whirl pack bags on ice; the rinse solution was filtered through a 0.45 µm nitrocellulose membrane filter; the filter was plated on tryptic soy agar, a non-selective medium; incubation was performed for 48 hours at 36.5±2ºC; and plates were inspected for growth micro-organisms counted as CFU per 100 mL rinse.
Statistical analysis
For the normally operating population, a simple no-intercept ANCOVA model was fitted to within-unit means of the following: a response variable for distal hose end particles 0.5 to 5.0 µm/ft3; and a predictor variable for intake particles 0.5 to 5.0 µm/ft3. Expected FAW blower filtration efficiency for 0.5 to 5.0 µm particle size range is defined as the predicted percent reduction in distal hose end particles/ft3, relative to intake particles/ft3, based upon the ANCOVA least squares parameter estimates. For each FAW blower in the abnormally operating population, tests of hypothesis were conducted to determine the probability that the observed distal hose end particle deviation from expected was due to random variation using the following procedure: the 0.5 to 5.0 µm particles/ft3 deviation from expected was calculated using the normally operating population ANCOVA model parameter estimates; a t-value was calculated by dividing this deviation by the square root of the ANCOVA model's mean square error; and a one-tailed probability was assessed from a t-distribution with degrees of freedom equal to that of the ANCOVA model. Reported P-values were not adjusted for family confidence intervals.
Results
FAW blowers were sampled in the operating rooms from 5 locations, representing a full spectrum of hospital sizes (Table 1); particle counts were performed on 25 blowers, swabs were collected from 17 blowers, and hoses were rinsed on 9 blowers. The three forms of sampling were not undertaken on mutually exclusive blowers.
Table 1. Hospital demographics and number of FAW blowers sampled via particle counting, swabbing, and rinsing.
| Hospitals sampled (number of operating rooms) | |
| Hospital A | 1 to 5 |
| Hospital B | 6 to 12 |
| Hospital C | 13 or more |
| Hospital D | 1 to 5 |
| Hospital E | 1 to 5 |
| Forced-air warming blowers sampled, (n) | |
| Particle counting | 25 |
| Swabbing | 17 |
| Rinsing | 9 |
Airborne contamination (particle counting)
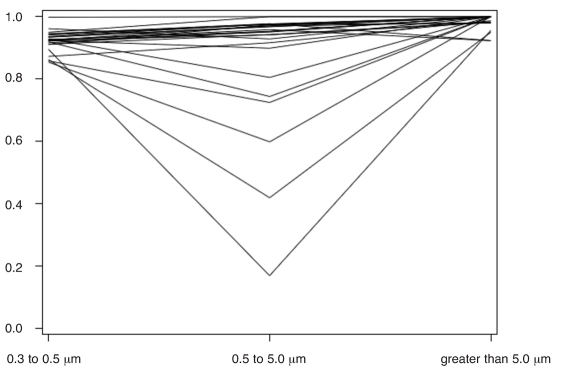
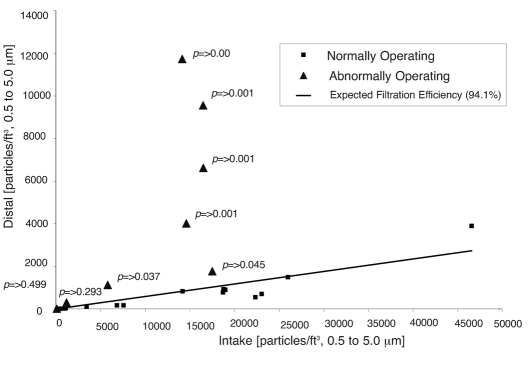
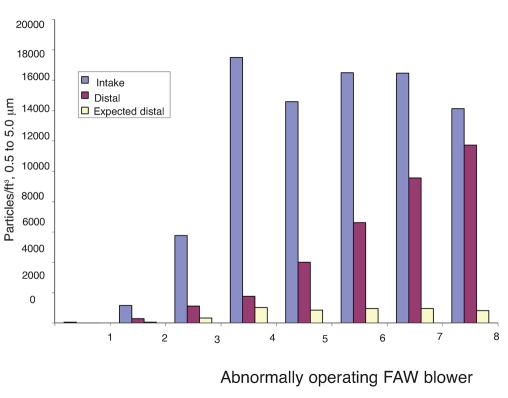
A line plot of FAW blower filtration efficiency by particle size range revealed that 8 of 25 blowers were operating abnormally, meaning they had lower filtration efficiencies in the particle size range of 0.5 to 5.0 µm than in the size range of 0.3 to 0.5 µm (Figure 1). The magnitude of airborne contamination generated by abnormally operating units is displayed as a plot of average distal versus intake particle counts per cubic foot by unit in the size range of 0.5 to 5.0 µm (Figure 2). As indicated, particle emissions from normally operating blowers are clustered around the trend line of expected filtration efficiency (94.1%); particle emissions from abnormally operating blowers are generally greater than expected, with 6 of 8 blowers showing significant deviations from the trend line. The magnitude of this deviation is further highlighted in a bar chart of intake, distal, and expected distal particle counts per cubic foot for abnormally operating blowers in the size range of 0.5 to 5.0 µm (Figure 3). As shown, distal particle emissions are greater than expected for blowers 3 through 8, resulting in lowered filtration efficiencies ranging from 17% to 81% for these blowers.
Figure 1.
Forced-air warming blower filtration efficiency by particle size range.
Figure 2.
Average distal versus intake particles/ft3 in the 0.5 to 5.0 µm size range by FAW blower
Figure 3.
Average intake and distal particles/ft3 plotted alongside expected distal particles/ft3 for the abnormally operating blower population by blower.
Bacterial contamination (swabbing and rinsing)
Swabs taken from the internal hose surface detected bacterial colonization rates of 71% and 88% for the proximal and distal locations respectively (Table 2), with 2 of 17 distal samples showing colonization levels above the limits of detection used in the study, and only one of 17 units showing no colonization at either location. Rinsing detected bacterial colonization of 89% on the internal hose surfaces of the 9 units sampled.
Table 2. CFU detected per site for swabbing and rinsing sampling techniques.
| Swabbing | Rinse | |||||
|---|---|---|---|---|---|---|
| Proximal hose end | Distal hose end | Internal hose surface | ||||
| (CFU/Site) | (CFU/Site) | (CFU/100mL) | ||||
| Hospital A | ||||||
| Bair hugger 505, unit 1 | 3 | 6 | 8 | |||
| Bair hugger 505, unit 2 | 2 | 3 | 6 | |||
| Bair hugger 505, unit 3 | 2 | 1 | 7 | |||
| Bair hugger 505, unit 4 | 0 | 0 | ||||
| Bair hugger 505, unit 5 | 0 | 2 | ||||
| Hospital B | ||||||
| Bair hugger 505, unit 6 | 2 | 11 | 4 | |||
| Bair hugger 505, unit 7 | 6 | 102 | 6 | |||
| Bair hugger 505, unit 8 | 0 | 9 | 5 | |||
| Hospital C | ||||||
| Bair hugger 505, unit 9 | 1 | >300* | 0 | |||
| Bair hugger 505, unit 10 | 1 | >300* | 1 | |||
| Bair hugger 505, unit 11 | 24 | 0 | 1 | |||
| Hospital D | ||||||
| Bair hugger 505, unit 12 | 1 | 3 | ||||
| Bair hugger 505, unit 13 | 0 | 7 | ||||
| Bair hugger 505, unit 14 | 1 | 2 | ||||
| Bair hugger 505, unit 15 | 1 | 7 | ||||
| Bair hugger 505, unit 16 | 0 | 32 | ||||
| Bair hugger 505, unit 17 | 2 | 7 | ||||
| Percentage of samples | 71 | 88 | 89 | |||
| colonized, (%) | ||||||
Bacterial colonies were too numerous to count on plates.
Discussion
This study quantified levels of airborne particle emission from FAW blowers which were in use in a hospital operating room environment. Thirty-two percent of the blowers investigated appeared to exhibit abnormal filtration efficiency patterns that were suggestive of airborne contaminant generation inside the blowers. The filtration efficiency trends shown in Figure 1 illustrate the differences between “normally” and “abnormally” operating blowers. Normal depth-filters exhibit increasing filtration efficiency as particle sizes increase from 0.3 µm,27 and this trend is clearly shown in the blowers classified as “normal”. This is understandable because depth-filters are most easily penetrated by 0.3 µm sized particles and less easily penetrated by larger particles. Blowers classified as “abnormal” showed decreasing filtration efficiency in the 0.5 to 5.0 µm particle range. In other words, more particles were emitted from the blowers in the 0.5 to 5.0 µm size range than expected. If air leaks in or around the filter were responsible for the 0.5 to 5.0 µm reduction in efficiency, one would expect to see a proportional reduction in filtration efficiency for each particle size range. It appears, therefore, that 32% of the blowers tested were emitting internally generated airborne contamination with a mean particle size of 0.5 to 5.0 µm. Furthermore, 6 of the 8 abnormal blowers were emitting significant levels of airborne contamination (Figures 2 and 3).
The presence of microbes on air path surfaces in 94% of the blowers suggests that a viable component could be present in the emitted contaminants (Table 2). Common operating room airborne microbes in the 0.5 to 5.0 µm size range include unclumped bacteria (<4 µm) and fungi (<4 µm).28 Non-viable sources may have included particles generated from moving components, which can become buoyant airborne carriers of microbes. Additionally, CFUs detected by rinsing were lower than CFUs detected by swabbing, even though the rinsing technique sampled a larger surface area than the swabbing technique. The most likely explanation for this is that the bacteria were encapsulated in a biofilm barrier that required mechanical debridement to dislodge them during sampling.19 The implication is that the measured bacterial colonization at each site could be artificially low.
The clinical significance of these findings relates to the link between airborne contamination and SSI, which has been well established.29 It has been estimated that 98% of the bacterial contamination found in a surgical site is deposited from the air.30,31 Research has also shown that implantation of foreign materials, such as vascular or orthopedic prostheses, greatly reduces the inoculum of bacterium needed to initiate an infection; for some materials the inoculum required to cause a SSI is reduced 10,000-fold.31 Therefore, SSI may result from the implant being contaminated by a few organisms,32 or in some orthopedic cases even a single Staphylococcus aureus bacterium may be sufficient.33 Although the present study did not evaluate the link between FAW and SSI rates, the findings in this study and those of others21–23 suggest that bacteria colonize the internal air path surfaces of the majority of FAW blowers. The findings also suggest that a significant percentage of FAW blowers are emitting particulates, which were shown to originate inside the blowers. Given that the air effluent from FAW blowers passes through a warming blanket that vents the effluent in close proximity to the surgical site, particulate emission from FAW blowers could, conceivably, be deposited onto the surgical site, which would be of particular importance for the most contamination-sensitive procedures.
This study has also shown that the design of forced-air warming equipment is questionable for preventing the emission of airborne contamination. European Union Medical Device Directives require that reusable medical equipment should allow decontamination;34 US Food and Drug Administration and Health Canada make a similar statement,35,36 but the statement is currently a recommendation, and not a requirement. Operating instructions from FAW manufacturers do not provide a method for decontaminating the inside of the hose or the blower. Additionally, particle counting showed that the intake filter was not HEPA rated. The observed efficiency of the intake filter was 93.5% for particles over 0.3 µm in the current study, which is below the rating of a “true HEPA” filter, which by definition eliminates more than 99.97% of particles over 0.3 µm in size. With 93.5% efficient intake filtration, 6.5% of particulates over 0.3 µm are passing through the intake filter into the blower. The passage of these particulates may lead to contamination of the blower's interior surfaces or emission of the particulates into the warming blanket. As such, other authors have suggested the implementation of a distal hose end-filter.21
Based upon the results of this study, FAW manufacturs should consider re-designing FAW blowers to ensure compliance with mandates for internal decontamination and provide certifiable “true HEPA” filtration. Clinicians should be aware that FAW blowers emit more than just hot air and that alternative technologies to prevent inadvertent perioperative hypothermia exist.
References
- 1.Schmied H, Kurz A, Sessler DI, et al. Mild hypothermia increases blood loss and transfusion requirements during total hip arthroplasty. Lancet. 1996;347:289–92. doi: 10.1016/s0140-6736(96)90466-3. [DOI] [PubMed] [Google Scholar]
- 2.Leaper DJ, Melling AC. Antibiotic prophylaxis in clean surgery: clean non-implant wounds. J Chemother. 2001;13:96–101. doi: 10.1179/joc.2001.13.Supplement-2.96. [DOI] [PubMed] [Google Scholar]
- 3.Kurz A, Sessler DI, Lenhardt R. Peri-operative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334:1209–15. doi: 10.1056/NEJM199605093341901. [DOI] [PubMed] [Google Scholar]
- 4.Bush HL, Hydo LJ, Fischer E, et al. Hypothermia during elective abdominal aortic aneurysm repair: the high price of avoidable morbidity. J Vasc Surg. 1995;21:392–402. doi: 10.1016/s0741-5214(95)70281-4. [DOI] [PubMed] [Google Scholar]
- 5.Scheck T, Kober A, Bertalanffy P, et al. Active warming of critically ill trauma patients during intrahospital transfer: a prospective, randomized trial. Wiener Klinische Wochenschrift. 2004;116:94–7. doi: 10.1007/BF03040703. [DOI] [PubMed] [Google Scholar]
- 6.Ng V, Lai A, Ho V. Comparison of forced-air warming and electric heating pad for maintenance of body temperature during total knee replacement. Anaesthesia. 2006;61:1100–4. doi: 10.1111/j.1365-2044.2006.04816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janke EL, Pilkington SN, Smith DC. Evaluation of two warming systems after cardiopulmonary bypass. Br J Anaesthesia. 1996;77:268–70. doi: 10.1093/bja/77.2.268. [DOI] [PubMed] [Google Scholar]
- 8.Kimberger O, Held C, Stadelmann K, et al. Resistive polymer versus forced-air warming: comparable heat transfer and core rewarming rates in volunteers. Anesth Analg. 2008;107:1621–6. doi: 10.1213/ane.0b013e3181845502. [DOI] [PubMed] [Google Scholar]
- 9.Negishi C, Hasegawa K, Mukai S, et al. Resistive-heating and forced-air warming are comparably effective. Anesth Analg. 2003;96:1683–7. doi: 10.1213/01.ANE.0000062770.73862.B7. [DOI] [PubMed] [Google Scholar]
- 10.Pathi V, Berg GA, Morrison J, et al. The benefits of active rewarming after cardiac operations: a randomized prospective trial. J Thor Cardiovasc Surg. 1996;111:637–41. doi: 10.1016/s0022-5223(96)70316-1. [DOI] [PubMed] [Google Scholar]
- 11.Matsuzaki Y, Matsukawa T, Ohki K, et al. Warming by resistive heating maintains perioperative normothermia as well as forced air heating. BnJ Anaesth. 2003;90:689–91. doi: 10.1093/bja/aeg106. [DOI] [PubMed] [Google Scholar]
- 12.Melling AC, Ali B, Scott EM, Leaper DJ. The effects of preoperative warming on the incidence of wound infection after clean surgery. Lancet. 2001;358:876–80. doi: 10.1016/S0140-6736(01)06071-8. [DOI] [PubMed] [Google Scholar]
- 13.Wong PF, Kumar S, Bohra A, et al. Randomised clinical trial of perioperative warming in major elective abdominal surgery. Br J Surg. 2007;94:421–6. doi: 10.1002/bjs.5631. [DOI] [PubMed] [Google Scholar]
- 14.Wong PF, Kumar S, Leaper DJ. Systemic warming as an adjunct to resuscitation in peritonitis: a pilot, randomised controlled trial. Surg Infect (Larchmt) 2007;8:387–96. doi: 10.1089/sur.2006.016. [DOI] [PubMed] [Google Scholar]
- 15.Satheesan KS, Whetter D, Melling AC, et al. The role of systemic warming of surgical patients during the initial hospital phase. Surg Infect. 2007;8:323–4. [Google Scholar]
- 16.Davies R, Noble WC. Dispersal of bacteria on desquamated skin. Lancet. 1962;2:1295–7. doi: 10.1016/s0140-6736(62)90849-8. [DOI] [PubMed] [Google Scholar]
- 17.Noble WC, Habbema JD, van Furth R, et al. Quantitative studies on the dispersal of skin bacteria into the air. J Med Microbiol. 1976;9:53–61. doi: 10.1099/00222615-9-1-53. [DOI] [PubMed] [Google Scholar]
- 18.Noble WC. Dispersal of skin microorganisms. Br J Dermatol. 1975;93:477–85. doi: 10.1111/j.1365-2133.1975.tb06527.x. [DOI] [PubMed] [Google Scholar]
- 19.Mangram AJ, Horan TC, Pearson ML, et al. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97–132. [PubMed] [Google Scholar]
- 20.Bair Hugger Therapy Product Specifications. Available at: http://www. arizant.com/
- 21.Avidan MS, Jones N, Ing R, et al. Convection blowers-not just hot air. Anaesthesia. 1997;52:1073–6. doi: 10.1111/j.1365-2044.1997.250-az0384.x. [DOI] [PubMed] [Google Scholar]
- 22.Bernards AT, Harinck HIJ, Dijkshoorn L, et al. Persistent Acinetobacter baumannii? Look inside your medical equipment. Infect Control Hosp Epidemiol. 2004;25:1002–4. doi: 10.1086/502335. [DOI] [PubMed] [Google Scholar]
- 23.Baker N, King D, Smith EG. Infection control hazards of intraoperative forced air warming. J Hosp Infect. 2002;51:153–4. doi: 10.1053/jhin.2002.1226. [DOI] [PubMed] [Google Scholar]
- 24.Tumia N, Ashcroft GP. Convection blowers-a possible source of contamination in laminar airflow operating theatres? J Hosp Infect. 2002;52:171–4. doi: 10.1053/jhin.2002.1297. [DOI] [PubMed] [Google Scholar]
- 25.Zink RS, Iaizzo PA. Convective warming therapy does not increase the risk of wound contamination in the operating room. Anesth Analg. 1993;76:50–3. doi: 10.1213/00000539-199301000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Huang JKC, Shah EF, Vinodkumar N, et al. The Bair Hugger patient warming system in prolonged vascular surgery: an infection risk? Critical Care (London, England) 2003;7:R13–16. doi: 10.1186/cc1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Avery RH. Nafa Guide to Air Filtration. National Air Filtration Association; 1993. [Google Scholar]
- 28.Jensen PA, Schafer MP. Sampling and characterization of bioaerosols. NIOSH Manual of Analytical Methods. 1998:82–112. [Google Scholar]
- 29.Hoffman PN, Williams J, Stacey A, et al. Microbiological commissioning and monitoring of operating theatre suites. J Hosp Infect. 2002;52:1–28. doi: 10.1053/jhin.2002.1237. [DOI] [PubMed] [Google Scholar]
- 30.Lidwell OM. Air, antibiotics and sepsis in replacement joints. J Hosp Infect. 1988;11:18–40. doi: 10.1016/0195-6701(88)90020-5. [DOI] [PubMed] [Google Scholar]
- 31.Petty W, Spanier S, Shuster JJ, Silverthorne C. The influence of skeletal implants on in-cidence of infection. Experiments in a canine model. J Bone Joint Surg. 1985;67:1236–44. [PubMed] [Google Scholar]
- 32.Whyte W. The role of clothing and drapes in the operating room. J Hosp Infect. 1988;11:2–17. doi: 10.1016/0195-6701(88)90019-9. [DOI] [PubMed] [Google Scholar]
- 33.Lidwell OM, Lowbury EJ, Whyte W, et al. Bacteria isolated from deep joint sepsis after operation for total hip or knee replacement and the sources of the infections with Staphylococcus aureus. J Hosp Infect. 1983;4:19–29. doi: 10.1016/0195-6701(83)90061-0. [DOI] [PubMed] [Google Scholar]
- 34.Council Directive 93/42/EEC (Medical Devices Directive) [ published on 14 June, 1993];Official Journal of the European Community No L169. 1993 Jul 12;:1–43.
- 35.Food and Drug Administration (FDA) [April 1996];Labeling Reusable Medical Devices for Reprocessing in Healthcare Facilities. Available at: http://www.fda.gov/
- 36.Health Canada. Draft Guidance Document Information to be Provided by Manufacturers for the Reprocessing and Sterilization of Reusable Medical Devices, 2.3.2 (2006)