Abstract
The meniscus plays important roles in proper knee function. It was recently reported that meniscus degeneration was associated with progression of osteoarthritis (OA). However, little is known about the effects of degradative enzymes on the meniscus matrix, primarily collagen type I and aggrecan, during OA. This study examined the effects of metalloproteinase (MMP) and aggrecanase on the destruction of aggrecan in the human meniscus. Eighteen trimmed meniscus portions were collected during partial menisectomy. Specimens were categorized into 3 groups according to the modified Copenhaver classification based on the degrees of damage to collagen bundles. Histological and immunohistochemical studies were conducted. Sections were stained with antibodies against MMP-3, aggrecanase 1 and 2, and their specific cleavage sites of aggrecan. Their localizations and staining ratios in the inner superficial and outer deep zones of the meniscus were determined separately. The population of chondrocyte-like cells increased with degeneration of the meniscus. MMP-3 and aggrecanase 1 and 2 are primarily expressed and activated in chondrocyte-like cells. MMP-3 expression and activation increased with degeneration and the population of chondrocyte-like cells. Changes in aggrecanase 1 expression with the degeneration were not clearly detected, whereas the expression of aggrecanase 2 was associated with progression of degeneration. MMP-3 and aggrecanases, particularly aggrecanase 2, expressed in chondrocyte-like cells could play important roles in aggrecan degradation in the human meniscus.
Key words: meniscus, degradation, aggrecan, aggrecanase, metalloproteinase
Introduction
The menisci perform several important biomechanical functions in the knee. They distribute stresses over wide areas of the articular cartilage, absorb shocks during dynamic loading, and assist in joint lubrication. Furthermore, the menisci are 2 crescentic fibrocartilaginous structures that serve to deepen the articular surface of the tibia for reception of the femoral condyles and provide mechanical stability in the knee.1 Clinically, it is well known that surgical removal of the damaged parts of the meniscus causes osteoarthritis (OA).2,3 A population-based study suggested that at least one-third of middle-aged or elderly persons had meniscal damage.4
Biochemically, approximately 65–75% of the meniscus is water. The extracellular matrix of the meniscus (60–70% of the dry weight) is primarily composed of collagen type I; however, types II, III, V, VI, and X have also been identified within the meniscus.5,6 Cartilage-like proteoglycan is an essential component found in the adult human meniscus.7 Histologically, collagen bundles are arranged in a circumferential pattern that is optimal for absorption of compressive loads. Radial fibers at the surface and in the midsubstance running parallel to the plateau probably increase structural rigidity and prevent longitudinal splitting.8 The proteoglycan content of the adult meniscus is approximately one-fifth that of dry articular cartilage. Aggrecan, which is a major proteoglycan in the articular cartilage, is a large proteoglycan that is also present in the meniscus.6 Collagen fibrils provide tensile strength to maintain tissue integrity, whereas aggrecan, interwoven with the collagen fibrils, contributes to compressive stiffness.
It is well known that both metalloproteinases (MMPs) and aggrecanase play roles in aggrecan cleavage in normal as well as OA cartilage.9 Cleavage of aggrecan could be an important and early event of cartilage matrix degradation during OA progression. Two major sites of enzymatic digestion have been identified within the interglobular domain between residues Asn341 and Phe342, and Glu373 and Ala374. The first site has been shown to be cleaved by a variety of matrix MMPs, whereas cleavage at the second site is catalyzed by aggrecanases.10 Aggrecanase 1 and 2 are members of a disintegrin and metalloproteinase with thrombospondin-like repeats (ADAM-TS) family and have been designated as ADAM-TS 4 and ADAM-TS 5, respectively.11
Based on a cohort study with a 3-year follow-up period, it was recently reported that the presence of meniscal damage itself, even without surgical removal, was a significant risk factor for development of radiographic OA. These results strongly suggested the need for understanding the mechanisms of meniscal damage during OA development.12 However, little is known about matrix degradation of the human meniscus.
In this study, we examined the localizations and expression of MMP-3 and aggrecanase 1 and 2 as well as their specific cleavage sites of aggrecan in the human meniscus. To the best of our knowledge, this is the first report on the roles of MMP-3 and aggrecanase in degeneration of the human meniscus.
Materials and Methods
Eighteen menisci were collected from patients aged 23–75 years (Table 1) at the time of arthroscopic partial menisectomy in Hokuto Hospital. Institutional approval was obtained before the commencement of this study. All patients gave informed consent for samples to be harvested for this study.
Table 1. Patients' clinical characteristics.
| Variables | Meniscus degeneration (Copenhaver) | |||
|---|---|---|---|---|
| Stage 0 (N=6) | Stage 1 (N=5) | Stage 2 (N=7) | ||
| Sex | Male/Female | 3/3 | 3/2 | 3/4 |
| Age(year) | Mean (range) | 29.7 (23–35) | 39.8 (33–50) | 63.3 (47–75) |
| Duration after onset (day)mean (range) | 11.8 (3–20) | 16.0 (4–26) | 14.7 (7–28) | |
| X-P grade(Kellgren) | Grade 0 | 6 | 3 | 0 |
| 1 | 0 | 2 | 6 | |
| 2 | 0 | 0 | 1 | |
| Cartilage degeneration (Outerbridge) | Grade 0 | 3 | 0 | 0 |
| 1 | 3 | 3 | 0 | |
| 2 | 0 | 2 | 1 | |
| 3 | 0 | 0 | 4 | |
| 4 | 0 | 0 | 2 | |
At an initial visit, a detailed history was taken, and physical and X-ray photographic examinations (X-P) were performed. Radiological evaluation of the knee was performed according to the grading system described by Kellgren et al.13 In cases in which torn menisci were detected by magnetic resonance imaging, we performed arthroscopic examination and partial resection of the torn menisci, if needed. Patients with ligamentous lesions, such as anterior cruciate ligament (ACL) injury, were excluded from this study.
Evaluation of cartilage lesions was also performed. Articular cartilage lesions were graded according to the Outerbridge classification14: Grade 0, normal; Grade 1, articular softening or blistering; Grade 2, articular fissures or clefts measuring less than 1 cm in diameter; Grade 3, deep fissures extending to the subchondral bone measuring more than 1 cm; and Grade 4, exposed subchondral bone. The highest grade in all cartilage surfaces was recorded as the grade of the cartilage lesion for that patient. During removal of the torn menisci, the portion next to the lesion was removed for trimming; this portion was used for this study. After removal, samples were immediately fixed in 10% buffered formalin and embedded in paraffin.
Histological evaluation
Radial sections (4 µm thick) were cut from the embedded samples. Sequential sections were prepared for hematoxylin-eosin staining and immunohistochemistry. The degree of histological degeneration of the meniscus was defined according to the modified Copenhaver classification, based on degeneration of collagen bundles in the menisci.15 This classification is as follows: Stage 0, homogeneous eosinophilic staining collagen; Stage 1, mild cleft formation of collagen bundles; and Stage 2, severe cleft and cyst formation of collagen bundles. We divided the removed menisci (trimmed portions) into 3 groups based on this histological grading system.
Immunohistochemistry
Antibodies
The antibody against MMP-3 (mouse monoclonal) was purchased from Daiichi Fine Chemistry (Toyama, Japan). This antibody can recognize both the latent and active forms of MMP-3. Antibodies against aggrecanase 1 and 2 (rabbit polyclonal) were purchased from Sigma (St. Louis, MO, USA). They can recognize the latent and active forms of aggrecanase 1 and 2.
Rabbit polyclonal antibodies (Bio Synthesis, Lewisville, TX, USA) were prepared against neoepitopes generated by MMPs (DIPEN341) and aggrecanases (TEGE373), using amino acid sequences (NH2-GCGEDFVDIPEN and NH2-GCPLPRNITEGE, respectively) of human-specific cleavage sites. To confirm the specificity, a binding assay to the antigen peptide for each antibody was performed. Both antibodies had titers up to 1:25600, which is considered excellent. Furthermore, these antibodies could detect the specific cleavage sites extracted from interleukin (IL)-1-stimulated cartilage explants by Western blotting, as reported previously by Tortorella et al.11
Immunostaining
Sections were deparaffinized and immersed in 0.3% hydrogen peroxide methanol for 20 min. They were washed three times with phosphate-buffered saline (PBS) for 10 min each time, and the sections for DIPEN341 and TEGE373 antibodies were permeabilized with protease-free chondroitinase ABClyase [0.5 units per mL in 0.1 M Tris HCL (pH 8.0) containing 0.05 M sodium acetate; Seikagaku Corporation, Tokyo, Japan] for 30 min at 37°C. All sections were washed three times with PBS for 5 min each time and then permeabilized with 1% bovine serum albumin (BSA) for 10 min. These were then washed three times with PBS for 3 min each time. The sections were incubated for 90 min at 37°C with serum containing either DIPEN341 antibody (1:500 dilution of 1% BSA), TEGE373 antibody (1:500 dilution of 1% BSA), MMP-3 antibody (1:500 dilution of 1% BSA), or aggrecanase 1 and 2 antibodies (1:500 dilution of 1% BSA).
After washing with PBS, sections for rabbit polyclonal antibodies (DIPEN341, TEGE373, and aggrecanase 1 and 2) were incubated for 90 min at 37°C with serum containing anti-rabbit biotinylated secondary antibody (1:500 dilution of 1% BSA), whereas sections for MMP-3 antibody were incubated for 90 min at 37°C with serum containing anti-mouse biotinylated secondary antibody (1:500 dilution of 1% BSA). They were then washed three times with PBS for 5 min each time.
Finally, the sections were immersed in diaminobenzidine solution [150 mL 0.05 M Tris HCL (pH 7.6) containing 15 mL of 30% hydrogen peroxide and 30 mg of 3.3′-diaminobenzidine] for 30 min and washed three times with PBS for 5 min each time.
Statistical analysis
There are 2 histological features of the meniscus. One is that collagen bundles are arranged differently in each zone. The majority are arranged in a circumferential pattern in the deeper area, while radial fibers are found at the surface. The other is that the outer zone is vascularized, while the inner zone is non-vascularized. Therefore, we focused on 2 areas of the meniscus: the inner-superficial and outer-deeper zones.
Mean values of the staining ratio of MMP-3 and aggrecanase 1 and 2 in cells were determined from 2 randomly selected fields of microscopy (400×) in the inner-superficial and outer-deeper zones, respectively. Mean values of the staining ratio of cleavage sites (DIPEN341, TEGE373) in the territorial and pericellular regions of cells were also determined.
For statistical analysis, we used the non-parametric Kruskal-Wallis one-way analysis of variance on ranks to compare overall differences among the 3 collagen bundle degradation groups. If this analysis showed significant difference for a particular group, we compared this to the other groups using two-tailed Mann-Whitney U tests. A p<0.05 was considered significant.
Results
Clinical characteristics
Clinical characteristics of samples are summarized in Table 1. All 18 samples were from the medial meniscus. Study subjects were 9 men and 9 women. Their mean age was 45.6 years (range, 23–75 years). The mean duration after injury was 14.1 days (range, 3–28 days).
Patients with more degenerative menisci in stage 2 were significantly older than those with menisci in stage 0. Radiographic grading was also higher in patients with more degenerative menisci. More severe cartilage damage was detected in patients with more degenerative menisci in stage 2 compared to those with menisci in stage 0. Patients with the meniscus in stage 2 did not experience remarkable traumatic events at the onset of symptoms, whereas patients with the meniscus in stage 0 experienced traumatic events.
Histological findings
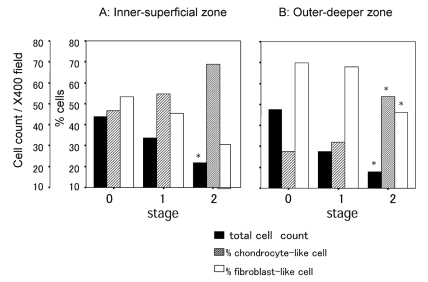
Two morphologically distinct populations of cells were identified. One was a fibroblast-like cell and the other was a chondrocyte-like cell. Cell density in both the inner-superficial (p=0.006; Figure 1A) and outer-deeper zones (p=0.007; Figure 1B) was remarkably decreased with these degenerative meniscal changes. Proportions of chondrocyte-like cells increased with these degenerative meniscal changes in the outer-deeper zone (p=0.002; Figure 1B); there was an increasing trend in the inner-superficial zone (p=0.055; Figure 1A). There was significant difference in the arrangement of collagen bundles between the superficial and deep zones, as described in the definitions of menisci degeneration. In the menisci in stage 0, there were clear structural distinctions between the superficial and deep zones (Figure 2); however, it was difficult to detect these structural differences between the superficial and deep zones in the menisci in stage 2 (Figure 3). We found that there was more cleft and cyst formation within the collagen bundles in the degenerative meniscus. There were some clusters consisting of chondrocyte-like cells in the degenerative meniscus (Figure 3).
Figure 1.
Changes in cell density and population of chondrocyte- and fibroblast-like cells. Cell count, cell/one microscopic field of 400×; % cells, percentage of the number of chondrocyte- or fibroblast-like cell to the total number of cells.*p<0.05, Kruskal-Wallis test.
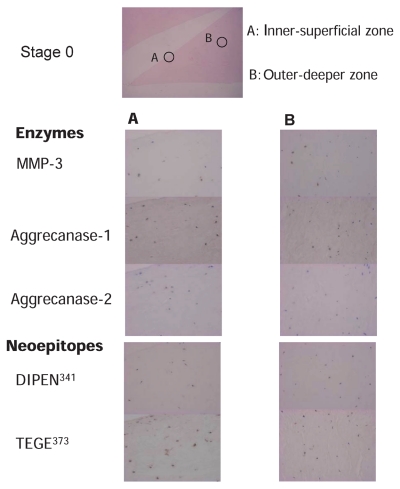
Figure 2.
Immunostaining of the degradative enzymes and their specific cleavage sites of aggrecan in the meniscus in stage 0. Column A. Inner-superficial zone of the meniscus (400×) Column B. Outer-deeper zone of the meniscus (400×). Immunostaining of MMP-3, aggrecanase 1, aggrecanase 2, DIPEN341 (neoepitope of aggrecan generated by MMPs), and TEGE373 (neoepitope of aggrecan generated by aggrecanases) are shown.
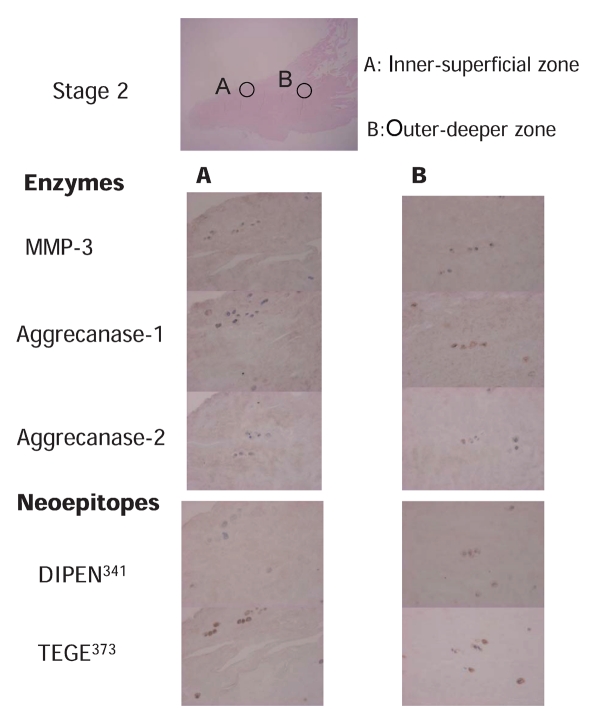
Figure 3.
Immunostaining of the degradative enzymes and their specific cleavage sites of aggrecan in the meniscus in stage 2. Column A. Inner-superficial zone of meniscus (400×). Column B. Outer-deeper zone of meniscus (400×). Immunostaining of MMP-3, aggrecanase 1, aggrecanase 2, DIPEN341 (neoepitope of aggrecan generated by MMPs), and TEGE373 (neoepitope of aggrecan generated by aggrecanases) are shown.
Localization of degradative enzymes MMP-3 and aggrecanase 1 and 2, and their specific cleavage sites
Enzymes were stained mainly in the chondrocyte-like cells; they were less evident in the fibroblast-like cells. Specific cleavage sites of these enzymes were also primarily detected in the surrounding regions (pericellular and territorial region) of chondrocyte-like cells but rarely in the surrounding regions of fibroblast-like cells. A representative case with the meniscus in stage 0 is shown in Figure 2. This case was a trimmed portion of a middle transverse tear of the right medial meniscus removed from a 26-year old man with X-P grade (Kellgren) 0 and Cartilage grade (Outerbridge) 0. The superficial area in the inner-superficial zone contained more chondrocyte-like cells than the deeper area. The cleavage site stained with DIPEN341(staining ratio 27%) was not always colocalized with that stained with MMP-3 (staining ratio 65.5%), whereas TEGE373, the cleavage site of aggrecanases (staining ratio 50.5%), was mostly detected in the cells stained with aggrecanase 1 (staining ratio 41%) and aggrecanase 2 (staining ratio 43.5%). In the menisci in stage 0, the population of fibroblast-like cells in the outer-deeper zone was greater than that in the superficial area. The cleavage site stained with DIPEN341 (staining ratio 13.5%) was not often colocalized with that stained with MMP-3 (staining ratio 35.5%), whereas TEGE373, the cleavage site of aggrecanases (staining ratio 35%), was mostly detected in the cells stained with aggrecanase 1 (staining ratio 27%) and aggrecanase 2 (staining ratio 33%). A representative case with the meniscus in stage 2 is shown in Figure 3. This case was a trimmed portion of a posterior complex tear of the right medial meniscus removed from a 66-year old woman with X-P grade (Kellgren) II and Cartilage grade (Outerbridge) III. There were some clusters in the inner-superficial zone, and the number of cells in this meniscus was smaller than that in the meniscus in stage 0. Most of these cells were chondrocyte-like cells. The cleavage site stained with DIPEN341 (staining ratio 32%) was not completely colocalized with that stained with MMP-3 (staining ratio 58.5%), whereas the cleavage site of aggrecanases (staining ratio 42%) was mostly detected in the cells stained with aggrecanase 1 (staining ratio 33%) and aggrecanase 2 (staining ratio 59%). There were relatively more cells stained with aggrecanase 2 than TEGE373. The number and morphology of cells in the outer-deeper zone of this meniscus was similar to that in the inner-superficial area. The ratio of chondrocyte-like cells in stage 2 was greater than that in stage 0. The cleavage site stained with DIPEN341 (staining ratio 34.5%) was not completely colocalized with that stained with MMP-3 (staining ratio 58.5%), whereas TEGE373, the cleavage site of aggrecanase (staining ratio 30%), was often detected in cells stained with aggrecanase 1 (staining ratio 33%) and aggre-canase 2 (staining ratio 76%). Cells stained with aggrecanase 2 were more frequent than those stained with TEGE373.
Staining ratio of the degradative enzymes MMP-3 and aggrecanase 1 and 2, and their specific cleavage sites
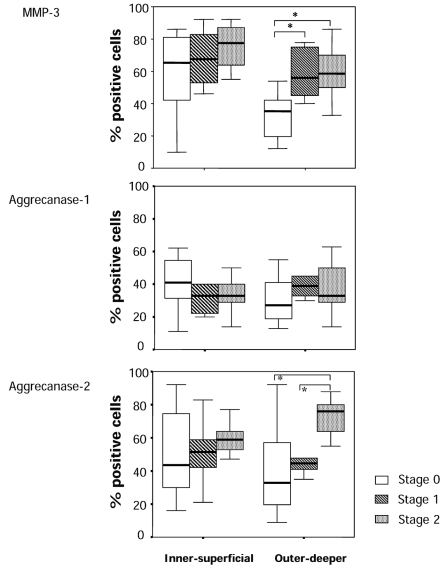
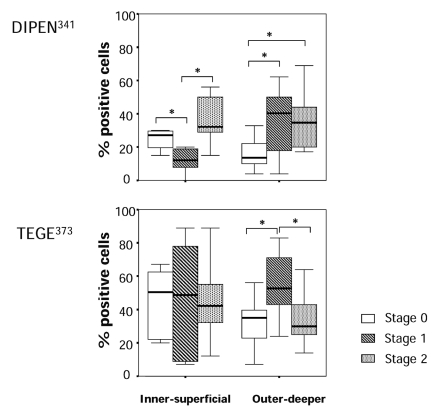
The staining ratio of degradative enzymes in the cells and their specific cleavage sites around the cells (territorial and pericellular region) are summarized in Figures 4 and 5. The cell staining ratio of aggrecanase 2 in the menisci in stage 0 had greater variance compared to that in stage 2 in both the inner-superficial and outer-deeper zones, whereas the staining ratio of MMP-3 had greater variance in stage 0 compared to that in stage 2. Aggrecanase 1 showed no significant difference in variance of the staining ratio in stage 0 compared to that in stage 2. In the inner-superficial zone, there were no significant differences in the staining ratio of MMP-3 and aggrecanase 1 and 2, although there was an increasing trend in the staining ratio of MMP-3 and aggrecanase 2 but not aggrecanase 1. In the outer-deeper zone, we found a significant increase in the staining ratio of MMP-3 in the menisci in stages 1 and 2 compared to that in the menisci in stage 0. We also found a higher staining ratio of aggrecanase 2 in the menisci in stage 2 than that in the menisci in stage 0 and 1. Because the staining ratio of aggrecanase 2 of stage 0 in this region had large variance, we could not find significant differences between stage 0 and stages 1. We detected a significant difference in the staining ratio of DIPEN341 between stage 0 and stage 1 and also between stages 1 and 2 in the inner-superficial zone. There was significant increase in the staining ratio of DIPEN341 in the menisci in stages 1 and 2 compared to that in the menisci in stage 0 in the outer deeper zone. We found large variances of the staining ratio of TEGE373 in each stage, and there were no significant differences in the staining ratio of TEGE373 among the stage groups of the menisci in the inner-superficial zone. In the outer-deeper zone, the menisci in stage 1 had a higher staining ratio of TEGE373 than those in stages 0 and 2.
Figure 4.
Ratio (%) of positive staining cells of the degradative enzymes in the meniscus of each stage. Upper, immunostaining of MMP-3; Middle, immunostaining of aggrecanase 1; Lower, immunostaining of aggrecanase 2. p were determined by the Kruskal-Wallis test, followed by the Mann-Whitney U test. *p<0.05.
Figure 5.
Ratio (%) of positive staining regions around the cells of specific cleavage sites generated by the enzymes in the meniscus of each stage. Upper, immunostaining of DIPEN341 (neoepitope of aggrecan generated by MMPs); Lower, immunostaining of TEGE373 (neoepitope of aggrecan generated by aggrecanases). p were determined by the Kruskal-Wallis test, followed by the Mann-Whitney U test. *p<0.05.
Discussion
In this study, we examined how MMP-3 and aggrecanase 1 and 2 play roles in aggrecan degradation in the human meniscus during progression of degeneration. Our results indicated that the expression of MMP-3 and aggrecanase 1 and 2 and their specific cleavage sites of aggrecan were expressed in chondrocyte-like cells and that this population of cells increased with meniscus degeneration. This suggests that these enzymes play important roles in the cleavage of aggrecan during progression of meniscus degeneration, similar to the progression of cartilage degradation.
We studied the expression of degradative enzymes and their specific cleavage sites together. There are several natural inhibitors directed against these degradative enzymes in tissues, such as tissue inhibitors of matrix metalloproteinases. The mechanisms for activation of these enzymes are quite complex.9 Therefore, to study matrix destruction, it is important to study the expression of specific cleavage sites of matrix molecules, including aggrecan and collagen, generated by each degradative enzyme as well as the expression of the enzymes themselves.
As the antibodies of MMP-3 and aggrecanase 1 and 2 used in this study could recognize both the latent and activated forms, there was a difference in the staining ratio between the enzymes and their specific cleavage sites around the cells. We showed that expression and activation of MMP-3 were more pronounced in the outer deeper zones of the menisci during OA progression. Of note, in the outer-deeper zone, the staining ratio of aggrecanase 2 increased with meniscus degeneration, whereas the cleavage site (AG) significantly decreased in the meniscus in stage 2. When cleavage of aggrecan by MMP-3 at DIPEN341 increases, the cleavage site for aggrecanase containing TEGE373 should be removed. MMP-3 activation could be pronounced in the meniscus in stage 2.
Glasson et al.16 reported that ADAM-TS 5 is the primary aggrecanase responsible for aggrecan degradation in a murine model of OA. Stanton et al.17 reported that ADAM-TS 5 is the major aggrecanase in mouse cartilage, both in vitro and in a mouse model of inflammatory arthritis. Using an ACL transaction model, the levels of gene expression for aggrecanase 1 or 2 remained remarkably stable in both the cartilage and meniscus during OA development.18
Even in human cartilage, it is still unclear how ADAM-TS 4 and/or ADAM-TS 5 play roles in the degradation of aggrecan during OA progression. One study reported that aggrecanase 2 was most strongly expressed in normal and arthritic cartilage, whereas aggrecanase 1 was expressed only at a very low level in normal cartilage and was only slightly upregulated in OA cartilage. Furthermore, it has also been reported that aggrecanase 1 was induced in vitro after stimulation by IL-1β, although it is not clear whether IL-1-induced cartilage degradation is a suitable model for OA.19 Another study showed that both cartilage aggrecanase 1 and 2 are present in osteoarthritic cartilage of the knee and that their inhibition could block degradation in an explant culture.20 To explain these discrepancies, there is a possibility that expression of aggrecanases could change during OA progression.
Information regarding this important issue is much more limited in human meniscus studies. Interestingly, in this study, we showed that there was no significant difference in the expression of aggrecanase 1, in spite of the changes in meniscus degradative grades. In the inner-superficial zone, the staining ratio of TEGE373 was higher than that of aggrecanase 1 and similar to that of aggrecanase 2, suggesting that cleavage of aggrecan by aggrecanase could be better reflected by aggrecanase 2 expression during matrix degradation of the human meniscus, although we could not detect any significant increase with the stage of degradation. These results suggested that aggrecanase 1 could be constitutively expressed in the meniscus and that aggrecanase 2 might be inducible during the progression of matrix degradation.
During the process of OA, matrix degradation progresses with the imbalance between destruction and repair. In this study, we only focused on degradative enzymes and their products in the menisci. The features of the degenerative meniscus, including increased proportion of chondrocyte-like cells and cluster formation, have been reported by a previous study.21 As for the features of meniscus repair, Takeuchi et al.22 reported that the cells observed in meniscal repair tissues in rabbits initially were fibroblast-like cells, the proportion of chondrocyte-like cells increased with the advance of repair, and the repair tissue in the meniscus might have been hyaline-like cartilage, which contains 5 times the amount of proteoglycans than the meniscus. Therefore, there is a possibility that morphological changes during OA progression could be related to repair processes. In fact, aggrecan cleaved by MMPs and aggrecanase were mainly present around these chondrocyte-like cells. Chondrocyte-like cells should be related to the distribution and amount of aggrecan in the meniscus during OA progression. Further study on matrix synthesis is needed to understand the balance between repair and destruction of the meniscus matrix during the progression of OA.
There are some limitations in this study. Although MMP-3 and aggrecanase 1 and 2 are probably important enzymes for aggrecan degradation,9 there is a possibility that other degradative enzymes, including MMPs and aggrecanases, play significant roles. Further studies will be required to understand the roles of other enzymes during the progression of meniscus degeneration. The sample number was limited because of the difficulty in sampling the meniscus in humans, especially the normal meniscus. In this study, the menisci in stage 0 had a nearly normal structure histologically. However, parts of the menisci were torn due to trauma. The median time after injury was 11.8 days at the sampling. This should still be in a subacute phase. In fact, the menisci in stage 0 had large variances in MMP-3 and aggrecanase 2 expression, especially in the inner-superficial zone, and aggrecanase 2 expression in the outer-deeper zone. This could be reflective of the traumatic events, including bleeding, followed by acute inflammation. Further studies are needed to determine the effects of inflammation, including cytokines, during the progression of OA.
In conclusion, MMP-3 and aggrecanase 1 and 2 are expressed and activated in the cells of the degenerative meniscus. Based on the changes in expression and activation with degeneration of the meniscus, MMP-3 and aggrecanase 2 could play important roles in progression of meniscus degeneration. Increased population of chondrocyte-like cells in the human degenerative meniscus affects aggrecan metabolism.
Acknowledgments:
we would like to thank Mr. Yukimasa Miwa for his technical assistance. This work was funded by Aichi D. R. G. Foundation, Aichi, Japan to TK.
References
- 1.Fithian DC, Kelly MA, Mow VC. Material properties and structure-function relationships in the menisci. Clin Orthop Relat Res. 1990:19–31. [PubMed] [Google Scholar]
- 2.Cicuttini FM, Forbes A, Yuanyuan W, et al. Rate of knee cartilage loss after partial meniscectomy. J Rheumatol. 2002;29:1954–6. [PubMed] [Google Scholar]
- 3.Englund M, Lohmander LS. Risk factors for symptomatic knee osteoarthritis fifteen to twenty-two years after meniscectomy. Arthritis Rheum. 2004;50:2811–9. doi: 10.1002/art.20489. [DOI] [PubMed] [Google Scholar]
- 4.Englund M, Guermazi A, Gale D, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med. 2008;359:1108–15. doi: 10.1056/NEJMoa0800777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bluteau G, Labourdette L, Ronziere M, et al. Type X collagen in rabbit and human meniscus. Osteoarthritis Cartilage. 1999;7:498–501. doi: 10.1053/joca.1999.0245. [DOI] [PubMed] [Google Scholar]
- 6.Radin E. Structure and Function of Joints. In: Koopman , editor. Arthritis and Allied Conditions. 14th ed. Philadelphia: Williams & Wilkins; 2000. pp. 157–73. [Google Scholar]
- 7.Roughley PJ, McNicol D, Santer V, Buckwalter J. The presence of a cartilage-like proteoglycan in the adult human meniscus. Biochem J. 1981;197:77–83. doi: 10.1042/bj1970077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan I, Arnoczky SP. Biology of Meniscal Healing and Replacement. In: Callaghan J, Rosenberg AG, Rubash HE, Simonian P, Wickiewicz TL, editors. The Adult Knee. Philadelphia: Williams & Wilkins; 2003. pp. 187–201. [Google Scholar]
- 9.Poole AR. Cartilage in Health and Disease. In: Koopman, editor. Arthritis and Allied Conditions. 14th ed. Philadelphia: Williams & Wilkins;; 2000. pp. 226–84. [Google Scholar]
- 10.Pratta MA, Tortorella MD, Arner EC. Age-related changes in aggrecan glycosylation affect cleavage by aggrecanase. J Biol Chem. 2000;275:39096–102. doi: 10.1074/jbc.M006201200. [DOI] [PubMed] [Google Scholar]
- 11.Tortorella MD, Malfait AM, Deccico C, Arner E. The role of ADAM-TS4 (aggrecanase-1) and ADAM-TS5 (aggrecanase-2) in a model of cartilage degradation. Osteoarthritis Cartilage. 2001;9:539–52. doi: 10.1053/joca.2001.0427. [DOI] [PubMed] [Google Scholar]
- 12.Englund M, Guermazi A, Roemer FW, et al. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum. 2009;60:831–9. doi: 10.1002/art.24383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43-B:752–7. doi: 10.1302/0301-620X.43B4.752. [DOI] [PubMed] [Google Scholar]
- 15.Copenhaver W, Kelly DR, Wood RL. The Connective tissues: Cartilage and Bone. In: Wilfred M, Douglas EK, Richard LW, editors. Bailey's textbook of histology. 17th ed. Philadelphia: Williams & Wilkins; 1978. pp. 170–8. [Google Scholar]
- 16.Glasson SS, Askew R, Sheppard B, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–8. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 17.Stanton H, Rogerson FM, East CJ, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648–52. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- 18.Bluteau G, Conrozier T, Mathieu P, et al. Matrix metalloproteinase-1, -3, -13 and aggrecanase-1 and -2 are differentially expressed in experimental osteoarthritis. Biochim Biophys Acta. 2001;1526:147–58. doi: 10.1016/s0304-4165(01)00122-2. [DOI] [PubMed] [Google Scholar]
- 19.Bau B, Gebhard PM, Haag J, et al. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum. 2002;46:2648–57. doi: 10.1002/art.10531. [DOI] [PubMed] [Google Scholar]
- 20.Malfait AM, Liu RQ, Ijiri K, Komiya S, Tortorella MD. Inhibition of ADAM-TS4 and ADAM-TS5 prevents aggrecan degradation in osteoarthritic cartilage. J Biol Chem. 2002;277:22201–8. doi: 10.1074/jbc.M200431200. [DOI] [PubMed] [Google Scholar]
- 21.Mesiha M, Zurakowski D, Soriano J, et al. Pathologic characteristics of the torn human meniscus. Am J Sports Med. 2007;35:103–12. doi: 10.1177/0363546506293700. [DOI] [PubMed] [Google Scholar]
- 22.Takeuchi N, Suzuki Y, Sagehashi Y, et al. Histologic examination of meniscal repair in rabbits. Clin Orthop Relat Res. 1997:253–61. doi: 10.1097/00003086-199705000-00034. [DOI] [PubMed] [Google Scholar]