Abstract
Background
Pulp necrosis is one of the main complications of dental trauma. When it happens on an immature tooth, pulp necrosis implies a lack of root maturation and apical closure. A therapy called apexification is required to induce the formation of a calcified apical barrier allowing a permanent and hermetic root filling. The aim of this prospective randomized clinical trial is to compare Mineral Trioxide Aggregate(MTA)with Calcium Hydroxide(CH)as materials used to induce root-end closure in necrotic permanent immature incisors.
Methods/Design
This study, promoted by AP-HP, was approved by the ethics committee(CPP Paris Ile de France IV). 34 children aged from 6 to 18 years and presenting a non-vital permanent incisor are selected. Prior to treatment, an appropriate written consent has to be obtained from both parents and from children. Patients are then randomly assigned to either the MTA(experimental)or CH(control)groups. Recalls are performed after 3, 6 and 12 months to determine the presence or absence of a calcified apical barrier through the use of clinical and radiographic exams. Additional criteria such as clinical symptoms, apical radiolucencies, periapical index(PAI)are also noted.
Trial registration
ClinicalTrials.gov no. NCT00472173 (First inclusion: May 10, 2007; Last inclusion: April 23, 2009; study completed: April 15, 2010)
Background
Dental injuries are very common in children between six and nine years old. A serious complication of these traumas is the pulp necrosis whose prevalence varies with the type of traumatism from 1-6% for crown fractures to nearly 100% for intrusions [1]. The pulp necrosis of permanent immature teeth implies the interruption of the root formation and apical closure. It is then necessary to implement a therapy, called apexification to induce a hard calcific barrier at the apical end of the root, to achieve the definitive root canal filling. For a long time, calcium hydroxide was the only material used in the apexification procedure: The treatment consists in repeated stimulations with calcium hydroxide, over a six to eighteen months' period, until the apical closure is achieved [2]. Many studies in the literature report inconveniences linked to that procedure:
- The treatment involves numerous visits over a prolonged period and the patients may therefore fail to attend their appointments. It may also lead to a loss of temporary dressings and a re-infection of teeth. It is moreover impossible to perform a permanent restoration [3].
- In retrospective studies, cervical root fractures were said to occur on teeth during or following treatment with calcium hydroxide due to thin dentinal walls in immature teeth as well as to weakened dental structure induced by calcium hydroxide [4-7].
To avoid these inconveniences, the use of new materials potentially inducing mineralisation such as Mineral Trioxyde Aggregate(MTA)was suggested [8]. A review of the existing literature was done to determine if MTA could be considered an alternative to calcium hydroxide in apexification of permanent immature teeth. The analysis of in vitro and animal studies reveals different properties of MTA which seem interesting as regards apexification, offering good sealing ability [9-15], antimicrobial properties [11,16,17], a setting ability uninhibited by blood or water [11,18], biocompatibility, low cytotoxicity [19-22], non-resorbable nature [11,23] and also an effect on the induction of odontoblasts and of a hard barrier [11,24-30]. The MTA(Proroot® MTA, Dentsply Maillefer France)is a medical device benefiting of EC marking. It is a powder mixed with sterile water to obtain a dental filling cement, used routinely for different clinical protocols in odontology. All clinical cases reported in the literature show, after a period of about six to twelve months, an absence of pathological clinical signs, the healing of a possible periapical lesion and the development of an apical closure [31-40]. However, the absence of a clinical comparative prospective study is to be deplored and we do not have the benefit of hindsight. That is why the implementation of a randomized clinical study in a public hospital is interesting to compare calcium hydroxide and MTA in the treatment of non vital immature teeth.
Methods/Study design
Objectives
- The main objective of this study is to test the ability of MTA, used as a root-end filling material, to induce a hard calcific barrier to seal hermetically non vital immature anterior permanent teeth with open apices over a 6 month's period.
MTA is a filling material allowing the creation of an artificial apical barrier-the MTA apical plug-but it is not good enough. The advantage of MTA is to induce a very close formation of mineralized tissue which is going to coat gradually the entire apex adhering to the MTA and the root walls. The interest of MTA is not only to create an immediate apical sealing(mechanical barrier)to induce a successful healing but also to promote the root-end growth or apexification, and finally the "bio-integration" of the non-vital immature permanent tooth.
- Secondary objectives:
• To assess the presence or absence of periapical radioluciency indicating the emergence or persistence of an apical periodontitis
• To compare the time required to obtain the clinical healing and the disappearance of clinical symptoms with the two materials: MTA and Calcium Hydroxide, when symptoms such as pain, swelling, sinus tract, abscess or abnormal mobility are present at the beginning of the study.
Hypothesis
In children with pulp necrosis of a permanent immature incisor, MTA is not better than calcium hydroxide in the rates of a calcified apical barrier, but MTA is more valuable to achieve a biologic periapical barrier before 6 months.
Research type
Randomized controlled trial(RCT), monocentric, open-labeled, superiority trial
Subjects
The studied sample is composed of 34 children and teenagers, aged from 6 to 18 years presenting a pulp necrosis of a permanent immature incisor. The root maturation is normally completed 3 or 4 years after the tooth eruption, that is to say around the age of 9 or 10 years as far as incisors are concerned. However some teenagers present teeth damaged by a trauma before their maturation was achieved. That is the reason why such patients are included in the study when those traumas were not or unsuccessfully dealt with. The number of patients needed for this study was calculated prior to investigation with the hypothesis of a higher successful apexification rate in the MTA group compared to the control CH group. The anticipated success rates at 6 months were 5% in the CH group and 50% in the MTA group; Given these hypotheses, it was necessary to include 15 patients in each group that is to say a total of 30 patients to have a 80% statistical power(with a α risk of 5%), to compare the proportion of apical closure at 6 months with a Chi-2 test(two sided). Taking into account an anticipated dropout of 5%, it was decided to include 34 patients. Sample size calculations were computed with nQuery Advisor® 6.01. For ethical reasons, the sample size was limited while remaining compatible with the ability to obtain a significant difference between the two materials. The case reports published in the literature show a periapical repair within the first 6 months whereas the apical bridging induced with calcium hydroxide is more often obtained after a period of 6 to 18 months. That is why we hypothesized that after a period of 6 months, at least half of the MTA cases would be a success for the main objective as opposed to a minimal percentage for the calcium hydroxide group.
Inclusion criteria
I1-Patient presenting a non-vital permanent immature incisor for which an apexification treatment is indicated
I2-Aged 6-18 years
I3-Written informed consent obtained from each parent and from the child
I4-Medical exam
Exclusion criteria
E1-General pathology
E1.1-History of uncontrolled diabetes
E1.2-Immunosuppression
E1.3-Severe asthma
E1.4-Chronic systemic disease if a treatment is required
E1.5-Eating disorder(anorexia, bulimia, malnutrition, ...)
E2-Oral pathology: Periodontal disease
E3-Corticosteroid treatment during the last 3 months
E4-Non-affiliation to a social security scheme
Suspension criteria
S1-Poor compliance
S2-New trauma or new complication
S3-Intercurrent illness requiring discontinuation of the trial
When patients are lost sight of, observations must be noted down in the CRF until the last effected visit. The investigators will do their best to contact the patient and find out why the patient ceased being in the trial.
Randomization
Participants are assigned in a treatment group by an independent statistician. Random numbers were randomly determined via a block randomization with a 1:1 ratio and blocks size of 4. The block size was concealed from all researchers. The randomization of the trial is centralized and conducted by an external authority: the Unity of Clinical Research(UCR). After the informed consent form is obtained, the investigator sends a randomization request fax to the UCR that sends back the random number according to the random sequence list. The latter fax is also sent to the hospital pharmacy that delivers the products necessary to the study.
Design of the study-Interventions
For each patient the protocol is composed of 7 sessions(Table 1). The follow-up takes place over a 12 months' period. Day 0 is considered as the day of the first treatment session.
Table 1.
Study protocol for each included patient
| Sessions | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Timing | D-15 to D0 | D0 | D + 15 | D+21 | 3 months | 6 months | 12 months |
| Written informed consent | X | ||||||
| Inclusion and exclusion criteria | X | ||||||
| Diagnosis of immature tooth pulp necrosis | X | ||||||
| Clinical exam: symptoms | X | X | X | X | X | X | X |
| Radiographic exam | X | X | X | X | X | X | X |
| Calcium hydroxide filling or renewal | X | X(CH group) | X(CH group-no barrier) | X(CH group-no barrier) | |||
| Apical filling with MTA | X(MTA group) | ||||||
| Complete filling with gutta-percha | X(MTA group) | X(CH group and apical barrier) | X(CH group and apical barrier) | ||||
1. Day 0-14/Day 0: Inclusion and selection visit
Diagnosis of pulp necrosis, clinical and radiographic exams
Written informed consent is obtained from both parents and from the child.
2. Day 0: Calcium hydroxide canal conditioning regardless of the treatment group
This treatment is performed under local anesthesia with Articaïne with adrenalin 1/200000(Septanest, Septodont, France), after placement of a rubber dam. After preparing an access cavity and establishing the working length by taking a radiograph with a file inserted into the root canal within 1 mm of the radiographic apex, the canal is cleaned by irrigation with 3% sodium hypochlorite(Parcan, Septodont, France)and the use of manual files. The cleaning and shaping are realized with files with a very light parietal action to avoid the canal widening and the weakening of the root walls. Above all, it consists in removing the pulp remnants. The most important thing is to observe the vacuity and cleanliness of the canal using operative microscopy. Considering the root immaturity, it is actually a cleaning and desinfection without shaping. Then the canal is dried with paper point and can be filled with calcium hydroxide. Calcium hydroxide paste is prepared by mixing the calcium hydroxide powder(obtained from the hospital pharmacy)with an anaesthetic solution(Scandicaïne, Septodont, Saint Maur des Fossés, France). A plug of calcium hydroxide is deposed in the canal and condensed to the apical end of the root with a plugger. Other applications of calcium hydroxide are realized, until complete canal filling. The excess moisture is dried between each input with sterile paper points. The intracanal dressing quality is checked with a radiograph. The access cavity is temporarily sealed with a resin modified glass ionomer cement(Fuji II LC, GC, Bonneuil sur Marne, France). This calcium hydroxide canal conditioning is performed for all patients to allow the complete disinfection already obtained thanks to irrigation with sodium hypochlorite and the use of manual files, to control acute symptoms and to allow further treatments.
3. Day0+15: MTA apical filling for the testing group/calcium hydroxide renewal for the control group
This session starts with a local anesthesia, the placement of a rubber dam and the removal of all the calcium hydroxide. For this procedure, the canal has to be cleaned by irrigation and the use of ultrasonic files to remove all the calcium hydroxide. The use of EDTA allows a more complete elimination but due to these teeth immaturity the EDTA is not recommended. Then, the treatment differs among the treatment group:
For the MTA group, a MTA plug is placed into the canal with a root canal messing gun and condensed to the apical end of the root with a plugger to create a 4 mm apical plug of MTA. The pipe of the MTA gun is adjusted for the piston to deliver a 3 mm plug. Coarse paper points are used upside down to perfect the MTA adaptation to the apical walls. A radiographic control allows the practitioner to verify the good placement of MTA. A paper point moistened with sterile water is placed in the canal to produce an ambient humidity for the MTA to achieve its solidification. The access cavity is filled with conventional glass ionomer cement(Fuji IX, GC, Bonneuil sur Marne, France).
- For the CH group, the complete filling with calcium hydroxide is obtained as the first treatment session.
4. Day 0+21: Control for the CH group/complete filling with gutta-percha for the MTA group. The hardness of the MTA is checked with an endodontic file and the root canal filling with gutta-percha can be realized in contact with MTA. This filling is realized with the Schilder's technique: warm gutta-percha compacted with the Touch'n Heat(SybronEndo, Henry Schein, Alfortville, France). The access cavity is then sealed with a composite resin.
5. 3 months: clinical and radiographic control for all patients, and calcium hydroxide renewal for CH group patients.
6. 6 months: clinical and radiographic control for all patients and for CH group patients, calcium hydroxide renewal or complete root filling with gutta-percha if an apical barrier is present.
7. 12 months: clinical and radiographic control
For the teeth of CH group not filled with gutta-percha, the following treatment is realized outside study.
All the treatment sessions are realized in the same dental office, under local anesthesia, after placement of a rubber dam and with optical aids, by the same two operators, one assistant and the other alternately.
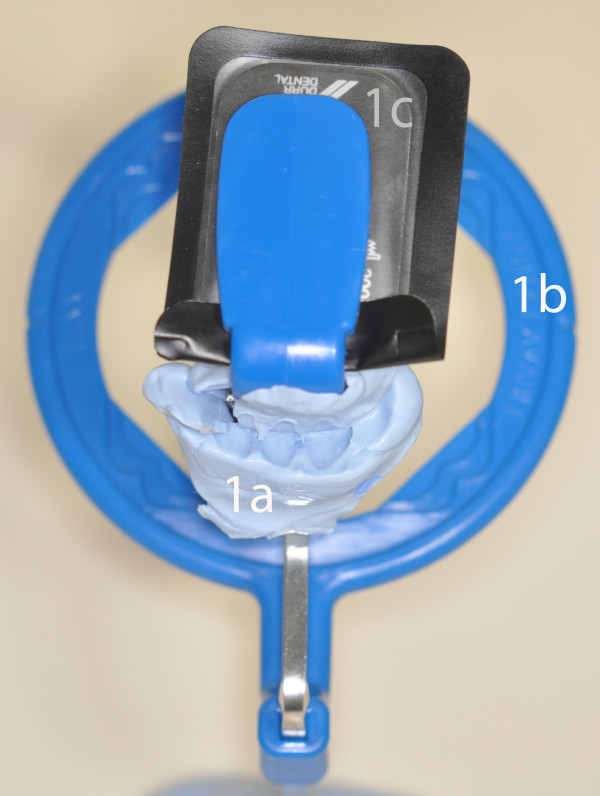
The X-Rays of each patient are standardized so that they can be compared. When each patient has his first X-Ray taken, the patient is asked to bite into a piece of silicone which enables the operator to put the film holder in the same position at each session(Figure 1a, b, c). Thus the radiographs can be reproducible.(Figures 2a, b, c)
Figure 1.
Device used to realize reproducible radiographs at each session. The patient is asked to bite into a piece of silicone, which enables us to put the film holder and so the radiograph in the same position at each session. 1a: piece of silicone. 1b: film holder. 1c: Radiograph.
Figure 2.
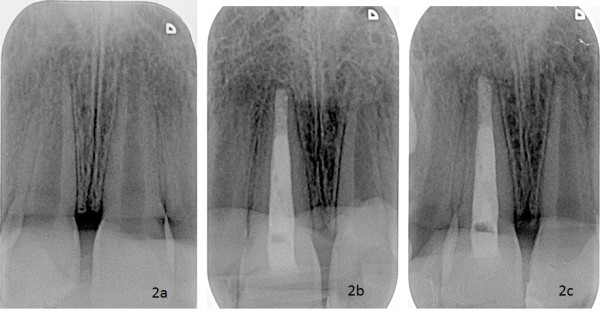
The reproducibility of the radiographs taken at each session allowing us to compare them. 2a: Radiograph taken during the inclusion session. 2b: Radiograph taken at the 6th month session. 2c: radiograph taken at the 12th month session.
Evaluation criteria
The assessment criteria are both clinical and radiographic(Table 2).
Table 2.
Data reported in the CRF
| Visits Data | V1 | V2 | V3 | V4 | V5 | V6 | V7 |
|---|---|---|---|---|---|---|---|
| Sex | X | ||||||
| Age | X | ||||||
| Clinical criteria | |||||||
| Pain | X | X | X | X | X | X | X |
| Mobility(I, II, III, IV) | X | X | X | X | X | X | X |
| Swelling | X | X | X | X | X | X | X |
| Sinus tract | X | X | X | X | X | X | X |
| Abcess | X | X | X | X | X | X | X |
| Percussion tenderness | X | X | X | X | X | X | X |
| Radiographic criteria | |||||||
| Degree of immaturity: Nolla stages: 7, 8, 9 | X | ||||||
| Endodontic filling | X | ||||||
| Root resorption | X | ||||||
| Periodontal ligament enlargement | X | X | X | X | X | X | X |
| Presence of an apical barrier | X | X | X | X | |||
| Radioluscent image | X | X | X | X | X | X | X |
| PAI | X | X | X | X | X | X | X |
| Form of the apical barrier | X | X | X | ||||
Assessment with the patient at each session
First, the evaluation is realized at each session by the investigators-operators, who assess the presence or absence of clinical symptoms such as pain, abnormal mobility, swelling, sinus tract, abcess and percussion tenderness. Radiographic exams allow the evaluation of the main success criterion, the presence or absence of a calcified apical barrier as well as the presence of a radioluscent image and the form and thickness of the apical barrier. These investigators can compare all the patient's radiographs.
All these data are immediately entered in the CRF.
Anonymous evaluation at the end of the study
Two independent investigators will study all the anonymized X-Rays. This assessment will be done without the investigators having any information about the treated patients, their treatment or the nature of the × rays(preoperative, postoperative, after 3, 6 or 12 months). If the conclusions of the two investigators diverge, a third one will settle the matter. These anonymous data will be sent to the UCR that will then remove the anonymity before sending the service statistics for analysis.
Expected benefits
The dental root canal can be hermetically filled immediately after the MTA hardens thus reducing the number of sessions and the length of the treatment.
Predictable risks
Many in Vitro and In Vivo studies on MTA biocompatibility have been realized [21,25,26,41]. Ribeiro et al.(2006)demonstrated in an in vivo study, the absence of harmful effects of MTA [42]. Souza et al.(2006)carried out a study about the toxicity of 6 dental biomaterials and proved MTA was the least harmful of all [43]. This protocol does not involve any risks of serious side-effects entailed by the treatment.
Undesirable events
Any adverse event observed during the study or the follow-up has to be reported in the CRF. Some information including occurrence time, duration, severity and treatment of this undesirable event should be noted as well. The relationship between adverse event and the apexification treatment has to be assessed(biological and mechanical failure).
Monitoring and inspection
Monitor should regularly visit the dental office to control the process of study and the CRF, check storage of investigational dental products and record of data.
Legal and ethical considerations
This study has been promoted by AP-HP(Paris Public Hospital)and approved by the ethics committee(CPP Paris Ile de France IV)and other French institutions:
- CNIL: French data protection watchdog
- AFSSAPS: the French Medecine and healthcare products regulatory agency, equivalent of the Food and Drug Administration in the United States.
Informed consent form(ICF)has been reviewed and approved by the ethics committee before study. Information about the protocol should be given to children and their parents both orally and in writing in an easily understandable language. The ICF must be dated and signed by the child and the parents(or representatives), and 3 copies must be made to be kept by the participants, the investigators and the UCR(15 years).
All information about the participants must be kept confidential.
Statistical analysis
All the analyses followed an intention-to-treat principle and will be conducted to compare the efficacy of MTA with CH to induce root-end closure in necrotic permanent immature incisors at 6 months. Secondary efficacy endpoints are apical radiolucencies and PeriApical Index(PAI = 1, 2: healthy or PAI = 3, 4, 5: pathological)
Primary endpoints, ie, proportions of patients with an apical barrier close at 6 months will be compared between treatment groups with the Fisher's exact test, such as with the secondary endpoints.
To handle missing data on the primary endpoint, several procedures will be used where the missing values will be either dropped-out or replaced according to 3 simple imputation methods as follows:
- Observed data: patients with missing values are dropped out.
- Worst-case analysis: missing values are considered as a failure(apical barrier not closed at 6 months)in MTA group and as a success in CH group.
- Best-case analysis: missing values are considered as a success(apical barrier closed at 6 months)in MTA group and as a failure in CH group.
- Last Observation Carried Forward(LOCF):previous diagnostic is used to replace missing values.
The assessment of concordance between the two independent investigators about the closure of apical barrier at baseline, months 3, months 6 and months 12 will be given with the Kappa coefficient and the 95% confidence interval.
Analyses will be performed by two statisticians of Biostatistics and Epidemiology unit of Cochin Hospital(Paris)using SAS 9.1(SAS Institute Inc, Cary, North Carolina); tests will be 2-sided, and 0.05 will be used as the threshold for statistical significance.
List of abbreviations
AFSSAPS: Agence Française de Sécurité Sanitaire des aliments et produits de santé; AP-HP: Assistance Publique-Hôpitaux de Paris; CH: Calcium Hydroxide; CNIL: Commission Nationale Informatique et Liberté; CRF: Case Report Form; EDTA: Ethylenediaminetetraacetic acid; ICF: Informed Consent Form; MTA: Mineral Trioxide Aggregate; PAI: Periapical Index; UCR: Unity of Clinical Research
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
ABN, RS, JJL: participated in the design of the study. RS: is in charge of the trial management. FA is in charge of monitoring. BB manage the dental materials. ABN, JJL: do treatment and data collection. ABN, EB, JJL: are analyzing X-rays data. LQ, SG: are responsible of the statistical analysis. ABN particularly developed this publication. JJL: chair.
All authors read and approved the final manuscript.
Author details
Aurélie Beslot-Neveu: DDS, Hôpital Bretonneau, Assistance Publique-Hôpitaux de Paris(AP-HP), Department of Pediatric Dentistry, Paris Descartes University, Unité de Recherche Biomatériaux Innovants et Interfaces EA 4462.
Eric Bonte: DDS, Associate Professor, Paris Descartes University, AP-HP, Hôpital Bretonneau, Department of conservative dentistry and endodontics, Paris
Bruno Baune: Hospital Pharmacist, AP-HP Hôpital Bretonneau, Paris
Raphaël Serreau: MD, PhD, pharmacologist, Project Manager, Unity of Clinical Research CIC-URC Necker Cochin Paris
Fawzia Aissat: Monitor, Unity of Clinical Research CIC-URC Necker Cochin Paris
Laurent Quinquis: Statistician, Unité de Biostatistique et d'Epidémiologie Hôpital Cochin, Paris
Sophie Grabar: MD, PhD, Paris Descartes University, AP-HP, Hôpital Cochin, Unité de Biostatistique et d'Epidémiologie, Paris
Jean-Jacques Lasfargues: DDS, Pr PhD, PD, Paris Descartes University, AP-HP, Hopital Bretonneau, Department of conservative dentistry and endodontics, Paris
Contributor Information
Aurélie Beslot-Neveu, Email: aurelie.beslot@yahoo.fr.
Eric Bonte, Email: eric.bonte@brt.aphp.fr.
Bruno Baune, Email: bruno.baune@brt.ap-hop-paris.fr.
Raphaël Serreau, Email: raphael.serreau@cch.aphp.fr.
Fawzia Aissat, Email: fawzia.aissat@cch.aphp.fr.
Laurent Quinquis, Email: laurent.quinquis@cch.aphp.fr.
Sophie Grabar, Email: sophie.grabar@parisdescartes.fr.
Jean-Jacques Lasfargues, Email: jean-jacques.lasfargues@brt.aphp.fr.
Acknowledgements
Pr JM Treluyer, head of the Clinical Research Unit Paris Centre is gratefully acknowledged. We also acknowledge Pr. D Buch, director of the odontologic center of Hopital Bretonneau, for the provision of technical plateform. We especially wish to acknowledge Dr F Villette and Dr F Courson for their help in recruiting patients.
Funding
This trial is funding by Assistance Publique-Hôpitaux de Paris(AP-HP).
References
- Andreasen FM. Pulpal healing following acute dental trauma: clinical and radiographic review. Pract Proced Aesthet Dent. 2001;13(4):315–22. [PubMed] [Google Scholar]
- Sheehy EC, Roberts GJ. Use of calcium hydroxide for apical barrier formation and healing in non-vital immature permanent teeth: a review. Br Dent J. 1997;183(7):241–6. doi: 10.1038/sj.bdj.4809477. [DOI] [PubMed] [Google Scholar]
- Rafter M. Apexification: a review. Dent Traumatol. 2005;21(1):1–8. doi: 10.1111/j.1600-9657.2004.00284.x. [DOI] [PubMed] [Google Scholar]
- Cvek M. Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta-percha. A retrospective clinical study. Endod Dent Traumatol. 1992;8:45–55. doi: 10.1111/j.1600-9657.1992.tb00228.x. [DOI] [PubMed] [Google Scholar]
- Mackie IC, Worthington HV, Hill FJ. A follow-up study of incisor teeth which has been treated by apical closure and root-filling. Br Dent J. 1993;175:99–101. doi: 10.1038/sj.bdj.4808243. [DOI] [PubMed] [Google Scholar]
- Andreasen JO, Farik B, Munksgaard EC. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent. Traumatol. 2002;18:134–137. doi: 10.1034/j.1600-9657.2002.00097.x. [DOI] [PubMed] [Google Scholar]
- White JD, Lacefield WR, Chavers LS, Eleazer PD. The effect of three commonly used endodontic materials on the strength and hardness of root dentin. J Endod. 2002;28(12):828–830. doi: 10.1097/00004770-200212000-00008. [DOI] [PubMed] [Google Scholar]
- Schwartz RS, Mauger M, Clement DJ, Walker WA. Mineral trioxide aggregate: a new material for endodontics. J Am Dent Assoc. 1999;130(7):967–75. doi: 10.14219/jada.archive.1999.0337. [DOI] [PubMed] [Google Scholar]
- Torabinejad M, Watson TF, Pitt Ford TR. Sealing ability of Mineral Trioxide Aggregate when used as a root-end filling material. J Endod. 1993;19(12):591–595. doi: 10.1016/S0099-2399(06)80271-2. [DOI] [PubMed] [Google Scholar]
- Torabinejad M, Rastegar AF, Kettering JD, Pitt Ford TR. Bacterial leakage of Mineral Trioxide Aggregate as a root-end filling material. J Endod. 1995;21(3):109–112. doi: 10.1016/S0099-2399(06)80433-4. [DOI] [PubMed] [Google Scholar]
- Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of an new root-end filling material. J Endod. 1995;21(7):349–352. doi: 10.1016/S0099-2399(06)80967-2. [DOI] [PubMed] [Google Scholar]
- Bates CF, Carnes DL, Del Rio CE. Longitudinal sealing ability of mineral trioxide aggregate when used as a root-end filling material. J Endod. 1996;22(11):575–578. doi: 10.1016/S0099-2399(96)80023-9. [DOI] [PubMed] [Google Scholar]
- Fisher EJ, Arens DE, Miller CH. Bacterial leakage of mineral trioxide aggregate as compared with zinc free amalgame, IRM and Super-EBA as a root-end filling material. J Endod. 1998;24(3):176–178. doi: 10.1016/S0099-2399(98)80178-7. [DOI] [PubMed] [Google Scholar]
- Hachmeister DR, Schindler WG, Walker WA, Thomas DD. The sealing ability and retention characteristics of mineral trioxide aggregate in a model of apexification. J Endod. 2002;28(5):386–90. doi: 10.1097/00004770-200205000-00010. [DOI] [PubMed] [Google Scholar]
- Al-Khatani A, Shostad S, Schifferle R, Bhambhani S. In-vitro evaluation of microleakage of an orthograde apical plug of mineral trioxide aggregate in permanent teeth with simulated immature apices. J Endod. 2005;31(2):117–119. doi: 10.1097/01.don.0000136204.14140.81. [DOI] [PubMed] [Google Scholar]
- Estrela C, Bamman LL, Estrela CR, Silva RS, Pecora JD. Antimicrobial and chemical study of mineral trioxide aggregate, Portland cement, calcium hydroxyde paste, Sealapex and Dycal. Braz Dent J. 2000;11:3–9. [PubMed] [Google Scholar]
- Torabinejad M, Hong CU, Pitt Ford TR, Kettering JD. Antibacterial effects of some root-end filling materials. J Endod. 1995;21(8):403–406. doi: 10.1016/S0099-2399(06)80824-1. [DOI] [PubMed] [Google Scholar]
- Torabinejad M, Higa RK, McKendry DJ, Pitt Ford TR. Dye leakage of four root-end filling materials: effects of blood contamination. J Endod. 1994;20(4):159–163. doi: 10.1016/S0099-2399(06)80326-2. [DOI] [PubMed] [Google Scholar]
- Keiser K, Johnson CC, Tipton DA. Cytotoxicity of mineral trioxide aggregate using human periodontal ligament fibroblasts. J Endod. 2000;26(5):288–290. doi: 10.1097/00004770-200005000-00010. [DOI] [PubMed] [Google Scholar]
- Kettering J, Torabinejad M. Investigation of mutagenicity of mineral trioxide aggregate and other commonly used root-end filling materials. J Endod. 1995;21(11):537–539. doi: 10.1016/S0099-2399(06)80980-5. [DOI] [PubMed] [Google Scholar]
- Mitchell PJC, Pitt Ford TR, Torabinejad M, Mc Donald F. Osteoblast biocompatibility of mineral trioxide aggregate. Biomaterials. 1999;20:: 167–173. doi: 10.1016/s0142-9612(98)00157-4. [DOI] [PubMed] [Google Scholar]
- Saidon J, He J, Zhu Q, Safavi K. Cell and tissue reactions to mineral trioxide aggregate and Portland cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:483–489. doi: 10.1067/moe.2003.20. [DOI] [PubMed] [Google Scholar]
- Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999;25(3):197–205. doi: 10.1016/S0099-2399(99)80142-3. [DOI] [PubMed] [Google Scholar]
- Apaydin ES, Shabahang S, Torabinejad M. Hard tissue healing after application of fresh or set MTA as root-end-filling material. J Endod. 2004;30(1):21–24. doi: 10.1097/00004770-200401000-00004. [DOI] [PubMed] [Google Scholar]
- Holland R, De Souza V, Nery MJ, Otoboni Filho JA, Bernabe PFE, Dezane E. Reaction of rat connective tissue to implanted dentin tubes filled with mineral trioxide aggregate or calcium hydroxide. J Endod. 1999;25(3):161–166. doi: 10.1016/S0099-2399(99)80134-4. [DOI] [PubMed] [Google Scholar]
- Torabinejad M, Hong CU, Pitt Ford TR, Kariyawasam SP. Tissue reaction to implanted Super EBA and MTA in the mandible of Guinea Pigs: Preliminary Report. J Endod. 1995;21(11):569–571. doi: 10.1016/S0099-2399(06)80987-8. [DOI] [PubMed] [Google Scholar]
- Torabinejad M, Pitt Ford TR, Mc Kendry DJ, Abedi HR, Miller DA, Kariyawaasam SP. Histologic assessment of mineral trioxide aggregate as a root-end filling in monkeys. J Endod. 1997;23(4):225–228. doi: 10.1016/S0099-2399(97)80051-9. [DOI] [PubMed] [Google Scholar]
- Torabinejad M, Pitt Ford TR, Abedi HR, Kariyawasam SP, Tang HM. Tissue reaction to implanted root-end filling materials in the tibia and mandible of Guinea Pigs. J Endod. 1998;24(7):468–471. doi: 10.1016/S0099-2399(98)80048-4. [DOI] [PubMed] [Google Scholar]
- Shabahang S, Torabinejad M, Boyne PP, Abedi H, McMillan P. A comparative study of root-end induction using osteogenic protein 1, calcium hydroxide and mineral trioxide aggregate in dogs. J Endod. 1999;25(1):1–5. doi: 10.1016/S0099-2399(99)80388-4. [DOI] [PubMed] [Google Scholar]
- Economides N, Pantelidou O, Kokkas A, Tziafas D. Short-term periradicular tissue response to mineral trioxide aggregate(MTA)as root-end filling material. Int Endod J. 2003;36:44–48. doi: 10.1046/j.0143-2885.2003.00611.x. [DOI] [PubMed] [Google Scholar]
- Nayar S, Bishop K, Alani A. A report on the clinical and radiographic outcomes of 38 cases of apexification with mineral trioxide aggregate. Eur J Prosthodont Restor Dent. 2009;17(4):150–6. Erratum in: Eur J Prosthodont Restor Dent. 2010, 18(1):42. [PubMed] [Google Scholar]
- Raldi DP, Mello I, Habitante SM, Lage-Marques JL, Coil J. Treatment options for teeth with open apices and apical periodontitis. J Can Dent Assoc. 2009;75(8):591–6. [PubMed] [Google Scholar]
- Bogen G, Kuttler S. Mineral trioxide aggregate obturation: a review and case series. J Endod. 2009;35(6):777–90. doi: 10.1016/j.joen.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Erdem AP, Sepet E. Mineral trioxide aggregate for obturation of maxillary central incisors with necrotic pulp and open apices. Dent Traumatol. 2008;24(5):e38–41. doi: 10.1111/j.1600-9657.2008.00636.x. [DOI] [PubMed] [Google Scholar]
- Ghaziani P, Aghasizadeh N, Sheikh-Nezami M. Endodontic treatment with MTA apical plugs: a case report. J Oral Sci. 2007;49(9):325–9. doi: 10.2334/josnusd.49.325. [DOI] [PubMed] [Google Scholar]
- Sarris S, Tahmassebi JF, Duggal MS, Cross IA. A clinical evaluation of mineral trioxide aggregate for root-end closure of non-vital immature permanent incisors in children-a pilot study. Dent Traumatol. 2008;24(1):79–85. doi: 10.1111/j.1600-9657.2006.00485.x. [DOI] [PubMed] [Google Scholar]
- D'Arcangelo C, D'Amario M. Use of MTA fr orthograde obturation of nonvital teeth with open paices: report of two cases. Oral Surg Oral Med Oral Pathol Oral Endod. 2007;104(4):e98–101. doi: 10.1016/j.tripleo.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Maroto M, Barberia E, Planells P, Vera V. Treatment of a non-vital immature incisor with mineral trioxide aggregate(MTA) Dent Traumatol. 2003;19(3):165–9. doi: 10.1034/j.1600-9657.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- Giuliani V, Baccetti T, Pace R, Pagavino G. The use of MTA in teeth with necrotic pulps and open apices. Dent Traumatol. 2002;18(4):217–21. doi: 10.1034/j.1600-9657.2002.02107.x. [DOI] [PubMed] [Google Scholar]
- Levenstein H. Obturation of teeth with wide open apices using mineral trioxide aggregate: a case report. SADJ. 2002;57(7):270–3. [PubMed] [Google Scholar]
- Kok ET, McDonald F, Pitt Ford TR, Torabinejad M. Cellular response to mineral trioxide aggregate. J Endod. 1998;24(8):543–547. doi: 10.1016/S0099-2399(98)80074-5. [DOI] [PubMed] [Google Scholar]
- Ribeiro DA, Matsumoto MA, Duarte MA, Marques ME, Salvadori DM. Ex-vivo biocompatibility tests of regular and white forms of mineral trioxide aggregate. Int Endod J. 2006;39(1):26–30. doi: 10.1111/j.1365-2591.2005.01043.x. [DOI] [PubMed] [Google Scholar]
- Souza NJ, Justo GZ, Oliveira CR, Haun M, Bincoletto C. Cytotoxicity of materials used in perforation repair tested using the V79 fibroblast cell line and the granulocyte-macrophage progenitor cells. Int Endod J. 2006;39(1):40–7. doi: 10.1111/j.1365-2591.2005.01045.x. [DOI] [PubMed] [Google Scholar]