Decreased cellular oxygen levels, known as hypoxia, normally occur during mammalian embryogenesis, and this physiological condition promotes formation of new blood vessels to provide sufficient O2 to these rapidly growing tissues. Hypoxia also occurs during pathophysiological processes, such as tumorigenesis.1 Rapidly growing tumors become hypoxic because their nascent blood vessels are functionally abnormal, resulting in a reduced blood supply. Tumor hypoxia is known to induce treatment resistance and is correlated with poor prognosis. In this regard, it is important to understand how cells respond and adapt to these low O2 conditions.
The cellular response to hypoxia is mediated by Hypoxia Inducible Factors (HIFs), which are transcription factors belonging to the bHLH/PAS protein family. HIFs promote adaptation to hypoxia through regulation of more than 150 genes involved mainly in angiogenesis, metabolism, proliferation and cell migration.2 These transcription factors act as heterodimers, composed of two subunits: HIF-α and HIF-β, also known as aryl hydrocarbon nuclear translocator (ARNT). While ARNT is constitutively and ubiquitously expressed, HIF-α subunits are regulated by intracellular O2 concentrations. Under normoxic conditions, HIF-α is prolyl-hydroxylated by O2-dependent prolyl-hydroxylases (PHDs). This prolyl-hydroxylation promotes interaction of HIF-α with the von Hippel Lindau ubiquitin-ligase complex, initiating rapid ubiquitination and subsequent HIF-α protein destruction via the 26S proteasome.
There are two major HIF-α subunits that mediate cellular adaptation to hypoxia, HIF-1α 3 and HIF-2α also known as Endothelial PAS domain protein (EPAS).4 Although HIF-1α is broadly expressed, the expression of HIF-2α is restricted predominantly to vascular endothelial cells (ECs) during embryonic development, and to various cell types in kidneys, liver, lungs and pancreas, suggesting HIF-2α has distinct functions from HIF- 1α . Moreover, HIF-2α is expressed in tumor vascular cells, parenchymal cells, and infiltrating macrophages, and is known to regulate several angiogenic factors in ECs, indicating that this subunit might play an important role in tumorigenesis through promoting angiogenesis.5 Furthermore, recent studies have shown that although HIF-1α and HIF-2α are structurally alike and regulate many overlapping target genes, they can perform different and even counteracting roles. For example, in murine embryos, HIF-2α, but not HIF-1α regulates the stem cell factor Oct-4,6 and whereas HIF-1α inhibits c-Myc activity, HIF-2α potentiates it.7 Thus, it is becoming increasingly clear that HIF-1α and HIF-2α have distinct roles in both embryonic development and tumor biology, and a thorough understanding of the different functions of HIF-1α and HIF-2α is essential for creating targeted cancer therapies.
In order to investigate the role of HIF-2α in angiogenesis, specifically its function in endothelial cells and vascular biology, our lab developed a genetic model designed to test the physiological consequences of deleting HIF-2α in murine ECs.8 One study has already shown the importance of HIF-1α in ECs. Tang and colleagues created a conditional deletion of HIF-1α in ECs using mice with HIF-1α “ floxed” alleles and expressing Cre recombinase through the Tie2 promoter, allowing specific deletion in the endothelium.9 In our study, we used the VE-cadherin Cre transgene to delete HIF-2α specifically in the endothelium. In contrast to global HIF-2α mutant animals, we did not observe embryonic defects with endothelial-specific deletion of HIF-2α, nor did we observe any defects in blood cell counts or in the vasculature of several organs. These results strongly suggest that HIF-1α compensates for HIF-2α loss in ECs. Of note, ARNT specific deletion in ECs results in vascular defects and high embryonic lethality.10 However, assessment of vessel integrity by injection of Evans blue dye revealed increased vessel permeability, particularly in lungs, skin and adipose tissues of mutant mice, indicating that HIF-1α does not completely compensate for HIF-2α deletion in ECs. Electron microscopic analysis of mutant blood vessels confirmed the leaky phenotype and shows that ECs exhibit decreased attachment to the basement membrane, leading to discontinuous areas between ECs and basal lamina. Moreover, pulmonary artery pressure measurements and echocardiographic studies reveal that endothelial-specific deletion of HIF-2α leads to pulmonary hypertension and cardiac hypertrophy, probably due to the lung vascular defects we observed. These results demonstrate that HIF-2α expression in ECs is clearly important for vascular integrity in multiple organ systems.
The hypoxic response of ECs is a crucial mediator of tumor angiogenesis, and loss of HIF-1α in ECs impairs tumor angiogenesis and is associated with an increase of necrosis and a decrease in tumor vessel density.9 In order to assess the effect of HIF-2α deficiency in ECs on tumor angiogenesis, we performed subcutaneous injection of tumor cells. Tumors grown in HIF-2α mutant mice exhibit reduced tumor growth and metastasis compared to the control mice, illustrating the importance of HIF-2α in tumor progression. This inhibition of tumor growth was correlated with an increase in tumor hypoxia and cell death. The decreased tumor growth and metastasis is likely due to defective tumor angiogenesis, as HIF-2α deficiency in ECs leads to abnormal tumor vasculature with a decrease in vessel density and in the number of functional tumor vessels consistent with reduction in perfusion and generation of hypoxic areas within tumors. Interestingly, our study can be correlated with a recent report showing that PHD2 haplodeficiency, leading to HIF-2α upregulation particularly in ECs, did not affect tumor vessel density but “ normalized” the endothelial lining and increased oxygenation.11 These experiments highlight the importance of the EC hypoxic response in tumor progression and maintenance and demonstrate the critical role that HIF-2α plays in this process.
In order to investigate the cell-autonomous basis of the phenotypes we observed in vivo, we isolated HIF-2α-deficient ECs. As has been demonstrated for HIF-1α, HIF-2α also regulates several aspects of endothelial cell behavior, including migration, capillary structure formation and induction of angiogenic gene expression under hypoxic conditions. HIF-2α-deficient ECs expressed reduced levels of transcripts encoding fibronectin, integrins, angiopoietin2 and, interestingly, Delta-like ligand 4 (Dll4).8 The Notch ligand Dll4 has a crucial role in both normal and tumor-related development of blood vessels and is known to regulate EC functions.12 Hypoxic conditions lead to induction of Dll4 by activation of both HIF-2α and HIF-1α 13 Interestingly, it has been shown that Dll4/Notch pathway down-regulation increases sprouting, tube formation, migration and invasion,14 but also decreases adhesion of ECs15 comparable to what we observe for the HIF-2α-deficient ECs. Moreover, tumor growth and angiogenesis is affected by Dll4 inhibition.16 Indeed, several studies have shown that inhibition of Dll4 inhibits tumor growth by promoting a non-productive angiogenesis associated with limited perfusion and increased tumor hypoxia. Therefore, defects resulting from HIF-2α deletion in ECs are driven by modulation of the Dll4/Notch pathway, identifying unique cell-autonomous functions for HIF-2α through the Dll4/Notch pathway in murine vascular ECs.
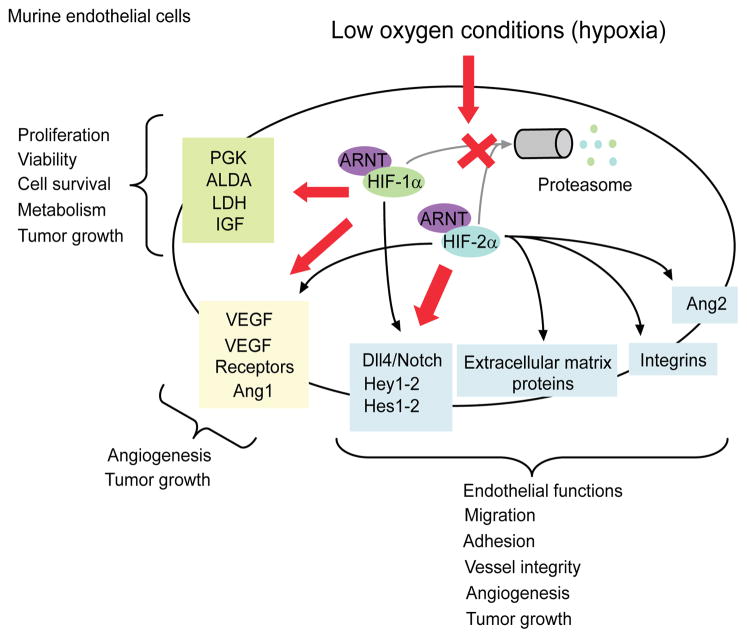
Finally, despite their distinct functions and compensatory effects, it is still unclear which HIF-α subunit predominantly drives the transcriptional outcome in response to a specific stimulus, and whether this is also determined by specific cellular contexts. However, both HIF-1α and HIF-2α appear to be important in ECs, vascular functions and angiogenic processes. In the endothelium under low O2 conditions, HIF-1α controls proliferation, metabolism and survival, and HIF-2α triggers cell migration, adhesion and vessel integrity (Figure 1). Targeting the HIF pathway has become an attractive approach for development of new anticancer agents.17 Therefore, it will be important to determine whether inhibition of HIF-1α and HIF-2α alone or in combination produces the best therapeutic outcome in terms of inhibiting tumor growth and angiogenesis in vivo.
Figure 1. HIF-1α versus HIF-2α in endothelial cells and vascular functions.
HIF-1α and HIF-2α act in a cell-specific manner on both common and specific targets. Differences in HIF-1α and HIF-2α targets may contribute to the differential effects of these factors on cell proliferation, survival, migration, metabolism, tumor growth or angiogenesis. In endothelial cells, whereas HIF-2α was shown to trigger cell migration, adhesion and vessel integrity under hypoxia, HIF-1α mainly controls proliferation, metabolism and survival. Future experiments will determine the individual contributions of HIF-1α and HIF-2α in these processes. ALDA, Aldehyde Dehydrogenase; Ang1-2, Angiopoietin1-2; IGF, Insulin Growth Factor; LDH, Lactate Dehydrogenase; PGK, Phosphoglycerate Kinase; VEGF, Vascular Endothelial Growth Factor.
Acknowledgments
We thank the Simon lab for helpful comments, and particularly Dr. Bryan Krock and Erin Podewils for editing. This work was funded by the Howard Hughes Medical Institute (M. Celeste Simon) and NIH (grant HL66130) (Brian Keith and M. Celeste Simon).
References
- 1.Bertout JA, et al. Nat Rev Cancer. 2008;8:967–975. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hickey MM, et al. Curr Top Dev Biol. 2006;76:217–57. doi: 10.1016/S0070-2153(06)76007-0. [DOI] [PubMed] [Google Scholar]
- 3.Wang GL, et al. J Biol Chem. 1995;270:1230–7. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 4.Tian H, et al. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 5.Peng J, et al. Proc Natl Acad Sci USA. 2000;97:8386–8391. doi: 10.1073/pnas.140087397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Covello KL, et al. Genes Dev. 2006;20:557–70. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordan JD, et al. Cancer Cell. 2007;11:335–47. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skuli N, et al. Blood. 2009;114:469–477. doi: 10.1182/blood-2008-12-193581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang N, et al. Cancer Cell. 2004;6:485–495. doi: 10.1016/j.ccr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 10.Yim SH, et al. Hepatology. 2006;44:550–60. doi: 10.1002/hep.21284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazzone M, et al. Cell. 2009;136:839–51. doi: 10.1016/j.cell.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel NS, et al. Cancer Res. 2005;65:8690–8697. doi: 10.1158/0008-5472.CAN-05-1208. [DOI] [PubMed] [Google Scholar]
- 13.Diez H, et al. Exp Cell Res. 2007;313:1–9. doi: 10.1016/j.yexcr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Scehnet JS, et al. Blood. 2007;109:4753–4760. doi: 10.1182/blood-2006-12-063933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodkinson PS, et al. J Biol Chem. 2007;282:28991–29001. doi: 10.1074/jbc.M703601200. [DOI] [PubMed] [Google Scholar]
- 16.Noguera-Troise I, et al. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 17.Melillo G. Cancer Metastasis Rev. 2007;26:341–52. doi: 10.1007/s10555-007-9059-x. [DOI] [PubMed] [Google Scholar]