SUMMARY
Endoscopic cryotherapy is a new technique for ablation of esophageal dysplasia and neoplasia. Preliminary studies have shown it to be safe and effective for this indication. The objective of this study is to characterize safety, tolerability, and efficacy of low-pressure liquid nitrogen endoscopic spray cryotherapy ablation in a large cohort across multiple study sites. Parallel prospective treatment studies at four tertiary care academic medical centers in the U.S. assessed spray cryotherapy in patients with Barrett’s esophagus with or without dysplasia, early stage esophageal cancer, and severe squamous dysplasia who underwent cryotherapy ablation of the esophagus. All patients were contacted between 1 and 10 days after treatment to assess for side effects and complications of treatment. The main outcome measurement was the incidence of serious adverse events and side effects from treatment. Complete response for high-grade dysplasia (HGD) (CR-HGD), all dysplasia (CR-D), intestinal metaplasia (CR-IM) and cancer (CR-C) were assessed in patients completing therapy during the study period. A total of 77 patients were treated for Barrett’s high-grade dysplasia (58.4%), intramucosal carcinoma (16.9%), invasive carcinoma (13%), Barrett’s esophagus without dysplasia (9.1%), and severe squamous dysplasia (2.6%). Twenty-two patients (28.6%) reported no side effects throughout treatment. In 323 procedures, the most common complaint was chest pain (17.6%) followed by dysphagia (13.3%), odynophagia (12.1%), and sore throat (9.6%). The mean duration of any symptoms was 3.6 days. No side effects were reported in 48% of the procedures (155/323). Symptoms did not correlate with age, gender, diagnosis, or to treatment early versus late in the patient’s or site’s experience. Logit analysis showed that symptoms were greater in those with a Barrett’s segment of 6 cm or longer. Gastric perforation occurred in one patient with Marfan’s syndrome. Esophageal stricture developed in three, all successfully treated with dilation. In 17 HGD patients, cryotherapy produced CR-HGD, CR-D, and CR-IM of 94%, 88%, and 53%, respectively. Complete regression of cancer and HGD was seen in all seven patients with intramucosal carcinoma or stage I esophageal cancer. Endoscopic spray cryotherapy ablation using low-pressure liquid nitrogen in the esophagus is safe, well-tolerated, and efficacious.
Keywords: Barrett esophagus, catheter ablation, cryosurgery, cryotherapy, esophageal neoplasm, safety
INTRODUCTION
Endoscopic spray cryotherapy ablation using low-pressure liquid nitrogen spray delivered through a standard endoscope is a novel technique for controlled ablation of malignant and pre-malignant esophageal disease. Preliminary results show this technique to be safe, well-tolerated, and effective in the ablation of Barrett’s esophagus and esophageal cancer.1,2 The aim of this study is to characterize the efficacy, safety, and tolerability of endoscopic spray cryotherapy ablation in a large cohort across multiple study sites.
MATERIALS AND METHODS
Setting
Between September 25, 2005 and November 27, 2007, patients treated with endoscopic spray cryotherapy ablation from the University of Maryland Medical Center (Baltimore, MD), Walter Reed Army Medical Center (Washington, DC), Columbia University Medical Center (New York, NY), and the Cleveland Clinic (Cleveland, OH) were enrolled in single-site prospective treatment trials.
Patients
Eligible patients included those with Barrett’s esophagus, Barrett’s esophagus with low-grade dysplasia (LGD), high-grade dysplasia (HGD) or intramucosal carcinoma (IMCA), esophageal cancer (T1 or T2 N0 M0, adenocarcinoma and squamous cell carcinoma), or severe squamous dysplasia enrolled in prospective, IRB-approved treatment protocols at each institution. For patients with Barrett’s esophagus with or without dysplasia and squamous dysplasia, the goal of therapy was endoscopic ablation of the abnormal mucosa. For esophageal cancer patients, the goal of the treatment was the elimination or decrease in size of the tumor. All patients with HGD and IMCA were deemed inoperable based on medical conditions or refused esophagectomy. Patients with invasive cancer were deemed inoperable based on medical conditions or refused esophagectomy and had refused, failed, or were ineligible for systemic therapy including chemotherapy or radiation therapy.
Cryotherapy
All patients were treated with twice a day proton pump inhibitor therapy beginning at least 1 week prior to first treatment, and maintained on this dose until treatment was complete. Patients were prepared for EGD in the standard fashion using an overnight fast with only sips of clear liquids and required medications allowed up to 2 h before the procedure. Moderate sedation with intravenous meperidine or fentanyl and midazolam or monitored anesthesia care with propofol was administered. EGD using a standard upper endoscope was performed in the standard fashion with standard monitoring. During the procedure, a 16 French modified orogastric tube (cryodecompression tube) was passed over a guide wire through the mouth into the stomach. This tube contains decompression ports spanning the distal 12 inches of the tube to allow decompression of both the stomach and the esophagus. This was connected to continuous suction to allow decompression during the cryotherapy procedure.
The cryotherapy catheter was passed through the working channel of the endoscope and extended beyond the distal tip of the scope. Liquid nitrogen was sprayed through the catheter, forming a white frost and freezing the adjacent mucosa (Fig. 1). At the start of the study, each mucosal site was frozen for 20 seconds times three cycles, with at least 45 seconds between freezes to allow tissue thawing. Beginning January 2007, tissue was frozen for 10 seconds times four cycles. This decision was made after a patient with Marfan’s syndrome developed gastric perforation during treatment (discussed later). By decreasing the total tissue freeze time from 60 to 40 seconds, the total volume of nitrogen sprayed was reduced to minimize gastric distension and potentially reduce the risk of further perforations. In all cases, the freeze-thaw process was repeated throughout the abnormal esophageal segment, typically until all abnormal tissue had been treated. At the end of the procedure, the endoscope and orogastric decompression tube were removed. Patients were recovered in the usual fashion and discharged the same day. Procedures were repeated every 4 to 6 weeks until the target lesion was ablated or reduced in size (tumors). Prescriptions were given for narcotic analgesics and anti-emetics to be used as needed after each procedure.
Fig. 1.

Endoscopic appearance during endoscopic cryotherapy in the esophagus. The decompression tube is seen in the lumen of the esophagus.
Post-treatment assessment
Patients were interviewed in person or contacted by telephone after every treatment to assess for side effects or complications. Patients were specifically asked for the presence of dysphagia, odynophagia, chest pain, abdominal pain, sore throat, irregular heart beat, or other symptoms. Symptoms were self-reported as mild, moderate, or severe. The duration of the symptoms was assessed only at some sites, when patients were contacted typically 7 days after their procedure. Symptoms persisting beyond that date were assessed at the next patient visit, typically a cryotherapy treatment session. Symptom assessment at other sites was obtained by phone call 1–3 days after the procedure.
Efficacy assessment
Patients who completed cryotherapy and had at least one follow-up endoscopy with biopsy were assessed for histologic regression of their underlying lesion. The rates of complete response (regression) of cancer (CR-C) – if appropriate, HGD (CR-HGD), any dysplasia (CR-D), and all intestinal metaplasia (CR-IM) were recorded for this group.
Statistical analysis
Data were analyzed using SAS version 9.1.3 (SAS Institute, Cary, NC, USA). Descriptive variables are reported using mean and standard deviation (SD) or median and range. Cochran-Mantel-Haenszel statistics for independence were calculated for all symptoms. This showed that symptom occurrence was random in the study. The Spearman rank-order correlation coefficient was calculated to assess for symptom differences between groups. Factors assessed included patient age, diagnosis, gender, likelihood of symptoms at the beginning versus end of treatment, or timing of treatment within the study period. A generalized logit model was used to determine the relationship between symptom severity and length of esophageal segment treated. Tests of significance with multiple comparisons were calculated using the Bonferroni method.
RESULTS
Seventy-seven patients were enrolled at the participating institutions (Table 1). The mean age (SD) was 69 ± 12.2 years, with a range of 36 to 93 years, and 74% were male. The mean esophageal segment length (SD) treated was 4 ± 3.6 cm, with the longest segment measuring 15 cm. The median number of treatments was 4 with a range of 1 to 10 treatments administered per patient. Procedures were performed in 45 patients with HGD (58.4%), 13 with IMCA (16.9%), 10 with invasive cancer (13%), 7 with Barrett’s esophagus without dysplasia (9.1%), and 2 with severe squamous dysplasia (2.6%).
Table 1.
Clinical characteristics of 77 patients undergoing endoscopic cryotherapy ablation
| Mean age (years) (SD) | 69 ± 12.2 |
| Gender (No. [%]) | |
| Female | 10 (36) |
| Male | 57 (74) |
| Mean length of esophagus treated (cm) (SD) | 4 ± 3.6 |
| Median number of treatments (range) | 4 (1–10) |
| Diagnosis (%) | |
| Barrett’s esophagus | 7 (9.1) |
| Barrett’s esophagus with HGD | 45 (58.4) |
| Barrett’s esophagus with IMCA | 13 (16.9) |
| Esophageal cancer | 10 (13.0) |
| Severe esophageal squamous dysplasia | 2 (2.6) |
HGD, high-grade dysplasia; IMCA, intramucosal carcinoma.
Overall, cryotherapy was well tolerated. A total of 323 procedures were performed in the study period. In 155 procedures (48%), no side effects or complications were reported. Side effects were generally mild in severity and are reported in Table 2. Mild chest pain or discomfort was the most common complaint, reported in 13.9% of procedures, with more severe chest pain described in 3.7%. Dysphagia (13.3%), odynophagia (12.1%), and sore throat (9.6%) were reported with diminishing frequency. Symptoms were no more likely to occur early in a center’s experience when the 20 second times three dosimetry was used (P = 0.99). Patients also were not more likely to report side effects earlier in their course of treatment (P = 0.99).
Table 2.
Number and percent of side effects for all cryotherapy procedures (total procedures = 323)
| None | Mild | Moderate | Severe | |
|---|---|---|---|---|
| Chest pain (n [%]) | 266 (82.4) | 45 (13.9) | 10 (3.1) | 2 (0.6) |
| Dysphagia (n [%]) | 280 (86.7) | 24 (7.4) | 19 (5.9) | 0 (0) |
| Odynophagia (n [%]) | 284 (87.9) | 27 (8.4) | 11 (3.4) | 1 (0.3) |
| Sore throat (n [%]) | 292 (90.4) | 29 (9.0) | 1 (0.3) | 1 (0.3) |
| Nausea (n [%]) | 307 (95.0) | 16 (5.0) | 0 (0) | 0 (0) |
| Abdominal pain (n [%]) | 309 (95.7) | 10 (3.1) | 1 (0.3) | 3 (0.9) |
| Irregular heartbeat (n [%]) | 315 (97.5) | 8 (2.5) | 0 (0) | 0 (0) |
| Fever (n [%]) | 320 (99.1) | 3 (0.9) | 0 (0) | 0 (0) |
Symptom duration information was available for 100 procedures in which patients were contacted typically 7 days after the procedure (Table 3). Mean (SD) symptom duration for all symptoms was 3.6 ± 2.2 days. Chest pain, the most commonly reported symptom, was reported after 34 procedures and lasted a mean (SD) of 3.7 ± 2.0 days. The duration of dysphagia and odynophagia were longer, lasting 4.9 ± 2.4 and 4.5 ± 2.5 days after 34 and 18 procedures respectively.
Table 3.
Duration of symptoms after endoscopic cryotherapy
| No. of procedures | Mean duration (days) (SD) | |
|---|---|---|
| All symptoms | 100 | 3.6 ± 2.2 |
| Chest pain | 34 | 3.7 ± 2.0 |
| Dysphagia | 18 | 4.9 ± 2.4 |
| Odynophagia | 12 | 4.5 ± 2.5 |
| Abdominal pain | 11 | 2.7 ± 1.8 |
| Sore throat | 9 | 1.9 ± 1.0 |
| Nausea/Vomiting | 4 | 2.2 ± 1.2 |
| Fever | 3 | 1.7 ± 1.1 |
Twenty-two patients (28.6%) reported no side effects or complications during their entire treatment. For those with symptoms, no statistically significant differences in symptom frequency or severity was seen in symptoms by diagnosis (P = 0.63), gender (P = 0.91), or age (less than 65 years compared to 65 years and over, P = 0.49). The correlation of symptoms to first treatment compared to all other treatments for each patient, and to treatment of patients early versus later in the endoscopists’ experience, were both negative. Symptoms did correlate with length of treated segment. Patients were more likely to have a symptom when the treated esophagus length was greater than 6 cm (P = 0.0105).
We assessed efficacy in 72 patients undergoing 311 cryotherapy procedures. After the initial procedure (23% of the total), only one patient did not demonstrate reduction in the size of the esophageal lesion. Cryotherapy resulted in a complete response in 24 patients with HGD or greater during the study period (Table 4). In the 17 patients with HGD, results for CR-HGD, CR-D, and CR-IM were 94%, 88%, and 53%, respectively. In the four patients with intramucosal carcinoma, results for CR-C, CR-HGD, CR-D, and CR-IM were 100%, 100%, 100%, and 75%, respectively. Three patients with stage I esophageal adenocarcinoma completed therapy, with CR-C, CR-HGD, CR-D, and CR-IM of 100%, 100%, 67%, and 67%, respectively.
Table 4.
Efficacy of cryotherapy in 23 patients completing therapy
| Barrett HGD | Barrett intramucosal carcinoma | Barrett carcinoma | |
|---|---|---|---|
| n | 17 | 4 | 3 |
| Complete response – cancer | – | 100% | 100% |
| Complete response – HGD | 94% | 100% | 100% |
| Complete response – dysplasia | 88% | 100% | 100% |
| Complete response – intestinal metaplasia | 53% | 75% | 67% |
| Mean segment length (range, cm) | 4.1 (1,12) | 4 (2,8) | 2.7 (1,6) |
| Mean number of treatments (range, cm) | 4.7 (1,9) | 4.3 (3,6) | 3.7 (3,4) |
| Mean follow-up (range, months) | 9.9 (2,20) | 13.8 (10,18) | 9.3 (3,13) |
Serious adverse events related to treatment were seen in two patients. Gastric perforation occurred in a patient with Marfan’s syndrome. This patient completed a cryotherapy treatment session uneventfully but returned to the hospital later that night with abdominal pain and free intra-abdominal air. Laparotomy showed a perforation in the posterior wall of the stomach, not at the cryotherapy treatment site. One lip ulcer developed because of cold injury from contact with the endoscope. This ulcer resolved in four days without specific treatment. Minor adverse events included three patients (4%) with stricture that responded to dilation therapy. All three had Barrett’s HGD from 6 to 12 cm in length. The stricture was seen after five cryotherapy sessions and resolved after a single balloon dilation in two cases. The remaining patient had a 6-cm segment of Barrett’s esophagus with HGD and ulcerated stricture noted prior to ablation therapy likely due to peptic esophagitis. This stricture developed after one cryotherapy session and required three dilations during the course of cryotherapy ablation.
DISCUSSION
Safety and tolerability are important factors impacting physician choice and patient acceptance of ablation procedures for esophageal neoplasia. Adverse events of other ablation modalities, including photodynamic therapy (PDT), argon plasma coagulation (APC), and multipolar electrocoagulation (MPEC) are well studied. In a randomized multi-center study,3,4 94% of patients treated with PDT for Barrett’s HGD developed adverse events or side effects. Most serious were photosensitivity (69%) and stricture formation (35%). Side effects of this therapy included vomiting (32%), chest pain (20%), fever (20%), dysphagia (19%), nausea (11%), and hiccups (10%). Serious adverse events and side effects are also associated with APC.5–12 Perforation (0–3.6%), stricture (0–15.4%), and major bleeding (0–3.9%) are the most serious events recorded. Chest pain was reported in all studies (1.8%–54.5%), with one study reporting dysphagia and odynophagia in over half the patients.8 In MPEC, strictures were reported in 0–6.5% of patients. The reported rate of significant bleeding was 3.2%, with side effects including chest pain (0–37.5%), throat pain (0–56.3%), and dysphagia (0–37.5%) noted.5,11,13 Published data on radiofrequency ablation (RFA) is limited, with one paper reporting on results using dosimetry (10 J/cm2) less than currently used in ablation of HGD (12 J/cm2).14 In this study, no serious adverse events were reported. Twenty-four minor adverse events were seen in 106 circumferential ablation procedures (23%) in 16 patients, and included chest/throat pain (9), nausea (8), fever (2), superficial linear mucosal injury (1), and minor bleeding (1). A registry study of RFA in patients with HGD and dosimetry of 12 J/cm2 reported no serious adverse events and stricture rate of 0.4%.15 Tolerability and minor adverse events were not reported. A recent abstract comparing the side effects of PDT, RFA, and cryotherapy showed that dysphagia, odynophagia, and chest pain were the most common complaints for all modalities; however, stricture and weight loss were only seen after PDT and RFA.16 An alternative means of assessing the safety profile of a device is the Manufacturer and User Facility Device Experience (MAUDE) database, an FDA-maintained database of device adverse events reported by manufacturers and others (http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/search.CFM). In this database (updated on October 31, 2008, accessed on November 14, 2008), 24 adverse events are reported for RFA including stricture (16), perforation requiring surgery (1), esophageal tear requiring surgery (1), gastrointestinal bleeding (1), superficial mucosal tear (3), pleural effusion (1), and unknown (1).
Cryotherapy differs from other thermal ablative modalities in that the mucosal surface of the esophagus is intact immediately after the procedure rather than destroyed.17,18 In animal studies, the immediate effect of cryotherapy is hemorrhage seen in the submucosa with minimal inflammation. Mucosal hyperemia is typically the only endoscopic finding seen at the conclusion of an endoscopic cryotherapy session (Fig. 2). Blistering and sloughing occurs in the following days to weeks with subsequent healing. A study using carbon dioxide based cryotherapy showed that treatment time of 15 seconds resulted in minimal esophageal necrosis or injury limited to the mucosa alone, while a 30-second treatment time produced treatment effect into the submucosa.19
Fig. 2.
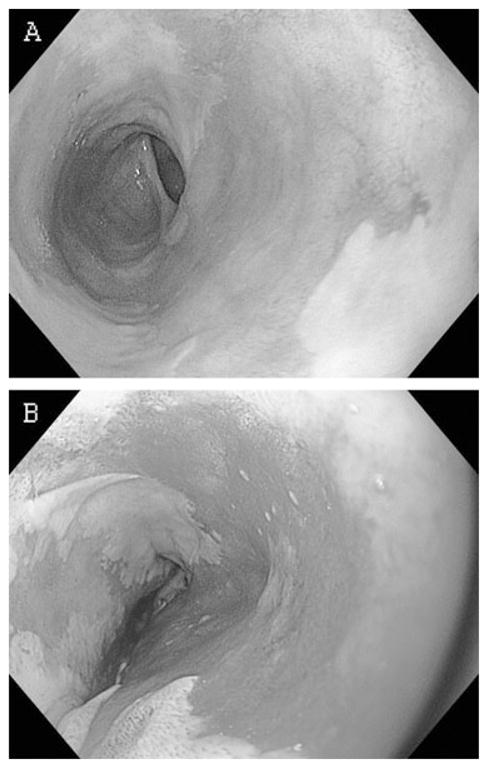
Endoscopic appearance of the esophagus (a) before and (b) immediately after endoscopic cryotherapy ablation. The mucosa appears hyperemic but remains intact.
This study is the largest to date evaluating the safety and efficacy of endoscopic low-pressure spray cryotherapy for treatment of diseases of the esophagus. Overall, cryotherapy appears to be safe and efficacious. In the 17 patients completing therapy within the study interval, the response to therapy for HGD is comparable to other modalities, with complete elimination of HGD in 94% and complete elimination of intestinal metaplasia in 53%. Impressive response was also seen in three patients with stage I cancer and four with intramucosal carcinoma who were not candidates for traditional therapies, with all seven patients showing complete regression of cancer and six showing complete regression of dysplasia.
Gastric perforation occurred in one patient with Marfan’s syndrome. Liquid nitrogen expands almost 25 times in volume as it evaporates, and proper venting of the esophagus and stomach are required during endoscopic cryotherapy. This is accomplished by a 16 French orogastric tube inserted prior to treatment and placed on continuous suction during liquid nitrogen spray. Continuous monitoring for gastric distension is performed by nursing personnel during the procedure, further minimizing the risk of over distension. It is theorized that abnormal gastric distensibility because of abnormal collagen formation contributed to the perforation in this patient. Cryotherapy is now contraindicated in patients with Marfan’s syndrome and in those with limited ability to distend the stomach such as partial or total gastrectomy. In addition, a new version of the cryotherapy decompression tube appears to minimize esophageal and gastric distension during the procedure. The lip ulcer because of contact freezing occurred early in the treatment trial and has not recurred in this or any other patient.
Cryotherapy appears to be well tolerated, with no side effects reported in almost half of all procedures performed. As in other modalities, chest pain was the most common complaint after treatment; however it was usually mild and short-lived. Chest pain, dysphagia, and odynophagia are likely due to tissue injury from the therapy, while sore throat is a consequence of endoscope and decompression tube placement. The change in dosimetry from 20 second freeze times three to 10 second freeze times four did not appear to change symptom frequency or severity. Stricture formation, a known complication of ablation therapy, occurred in 3/77 patients (4%). This stricture rate is lower than PDT, higher than reported for RFA in non-dysplastic Barrett’s esophagus, and comparable with the rate reported in a recent study of RFA in Barrett’s dysplasia.20 The resolution of the stricture after one dilation in 2/3 cases suggests the formation of minimal fibrotic tissue, which likely developed in the mucosa/submucosa as a consequence of repeated cryotherapy sessions.
Limitations of this study include lack of a standardized scale to characterize post-treatment symptoms and the use of different time points to contact patients at different study sites. The cross-sectional design of this study did not allow complete evaluation of efficacy since some patients had not completed therapy within the study interval. Efficacy data was available for only a subset of patients who completed treatment and had at least one follow-up endoscopy with biopsy during the study period.
In summary, low-pressure liquid nitrogen endoscopic spray cryotherapy ablation is a new ablative modality with significant promise for the treatment of dysplasia and neoplasia in the esophagus. The treatment is well tolerated and effective, and serious complications are rare. Studies are ongoing to further determine effectiveness, safety and tolerability.
Acknowledgments
The authors wish to acknowledge Andrea Waldt, RN, BSN; Kevin Bukowski, RN; and Lorraine Frey, RN. for their invaluable assistance.
The views expressed in this article are those of the author(s) and do not reflect the official policy of the Department of Army, Department of Defense, or U.S. Government.
Grant support
CSA Medical, Inc. provided financial support for data collection and statistical analysis. However, CSA Medical, Inc. had no role in the analysis and interpretation of the data, the preparation, review, or approval of the manuscript, or the decision to publish the findings. CSA Medical, Inc. also provided research support at the University of Maryland and Cleveland Clinic Foundation to conduct the treatment studies. The Maryland Industrial Partnerships Program provided research funding at the University of Maryland. Dr. Abrams was supported in part by a K07 award from the National Cancer Institute (CA132892).
Footnotes
Meeting presentations
Partial data from this study was presented at DDW 2008, San Diego, CA. (Gastrointest Endosc 2008; 67(5); AB76)
Author contributions:
| Greenwald | Horwhat | Abrams | Lightdale | Dumot | |
|---|---|---|---|---|---|
| Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data | X | X | X | X | X |
| Drafting the article or revising it critically for important intellectual content | X | X | X | X | X |
| Final approval of the version to be published | X | X | X | X | X |
Disclosures
Dr. Greenwald: Consultant for CSA Medical, Inc. Received research support from CSA Medical, Inc.
Dr. Horwhat, Nothing to disclose.
Dr. Abrams, Nothing to disclose.
Dr. Lightdale, Consultant for CSA Medical, Inc.
Dr. Dumot, Consultant for CSA Medical, Inc. Received research support from CSA Medical Inc.
References
- 1.Johnston MH, Eastone JA, Horwhat JD, Cartledge J, Mathews J, Foggy JR. Cryoablation of Barrett’s Esophagus: a pilot study. Gastrointest Endosc. 2005;62:842–8. doi: 10.1016/j.gie.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Cash BD, Johnston LR, Johnston MH. Cryospray ablation (CSA) in the palliative treatment of squamous cell carcinoma of the esophagus. World J Surg Oncol. 2007;5:34. doi: 10.1186/1477-7819-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Overholt BF, Lightdale CJ, Wang KK, et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett’s esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc. (Published erratum appears in Gastrointest Endosc. 2006 Feb; 63(2): 359.) Gastrointest Endosc. 2005;62:488–98. doi: 10.1016/j.gie.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 4.Overholt BF, Wang KK, Burdick JS, et al. Five-year efficacy and safety of photodynamic therapy with Photofrin in Barrett’s high-grade dysplasia. Gastrointest Endosc. 2007;66:460–8. doi: 10.1016/j.gie.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 5.Dulai GS, Jensen DM, Cortina G, Fontana L, Ippoliti A. Randomized trial of argon plasma coagulation vs. multipolar electrocoagulation for ablation of Barrett’s esophagus. Gastrointest Endosc. 2005;61:232–40. doi: 10.1016/s0016-5107(04)02576-3. [DOI] [PubMed] [Google Scholar]
- 6.Manner H, May A, Miehlke S, et al. Ablation of nonneoplastic Barrett’s mucosa using argon plasma coagulation with concomitant esomeprazole therapy (APBANEX): a prospective multicenter evaluation. Am J Gastroenterol. 2006;101:1762–9. doi: 10.1111/j.1572-0241.2006.00709.x. [DOI] [PubMed] [Google Scholar]
- 7.Morris CD, Byrne JP, Armstrong GR, Attwood SE. Prevention of the neoplastic progression of Barrett’s oesophagus by endoscopic argon beam plasma ablation. Br J Surg. 2001;88:1357–62. doi: 10.1046/j.0007-1323.2001.01926.x. [DOI] [PubMed] [Google Scholar]
- 8.Pereira-Lima JC, Busnello JV, Saul C, et al. High power setting argon plasma coagulation for the eradication of Barrett’s esophagus. Am J Gastroenterol. 2000;95:1661–8. doi: 10.1111/j.1572-0241.2000.02197.x. [DOI] [PubMed] [Google Scholar]
- 9.Ragunath K, Krasner N, Raman VS, Haqqani MT, Phillips CJ, Cheung I. Endoscopic ablation of dysplastic Barrett’s oesophagus comparing argon plasma coagulation and photodynamic therapy: a randomized prospective trial assessing efficacy and cost-effectiveness. Scand J Gastroenterol. 2005;40:750–8. doi: 10.1080/00365520510015737. [DOI] [PubMed] [Google Scholar]
- 10.Schulz H, Miehlke S, Antos D, et al. Ablation of Barrett’s epithelium by endoscopic argon plasma coagulation in combination with high-dose omeprazole. Gastrointest Endosc. 2000;51:659–63. [PubMed] [Google Scholar]
- 11.Sharma P, Wani S, Weston AP, et al. A randomised controlled trial of ablation of Barrett’s oesophagus with multipolar electrocoagulation versus argon plasma coagulation in combination with acid suppression: long term results. Gut. 2006;55:1233–9. doi: 10.1136/gut.2005.086777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Laethem JL, Cremer M, Peny MO, Delhaye M, Devière J. Eradication of Barrett’s mucosa with argon plasma coagulation and acid suppression: immediate and mid term results. Gut. 1998;43:747–51. doi: 10.1136/gut.43.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sampliner RE, Faigel D, Fennerty MB, et al. Effective and safe endoscopic reversal of nondysplastic Barrett’s esophagus with thermal electrocoagulation combined with high-dose acid inhibition: a multicenter study. Gastrointest Endosc. 2001;53:554–8. doi: 10.1067/mge.2001.114418. [DOI] [PubMed] [Google Scholar]
- 14.Fleischer DE, Overholt BF, Sharma VK, et al. Endoscopic ablation of Barrett’s Esophagus: a multicenter study with 2.5-year follow-up. Gastrointest Endosc. 2008;68:867–76. doi: 10.1016/j.gie.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Ganz RA, Overholt BF, Sharma VK, et al. Circumferential ablation of Barrett’s esophagus that contains high-grade dysplasia: a U.S. multicenter registry. Gastrointest Endosc. 2008;68:35–40. doi: 10.1016/j.gie.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Gross SA, Gill KRS, Hemminger LL, Greenwald BD, Wolfsen HC. Burn, freeze or photo-ablate? Comparative Symptom Profile in Patients with Barrett’s High Grade Dysplasia Undergoing Endoscopic Ablation [abstract] Gastrointest Endosc. 2008;67:AB180–1. [Google Scholar]
- 17.Johnston MH, Schoenfeld P, Mysore J, Kita JA. Endoscopic cryotherapy: a new technique for tissue ablation in the esophagus [abstract] Am J Gastroenterol. 1997;92:A44. [Google Scholar]
- 18.Eastone JA, Horwhat JD, Haluszka O, Mathews J, Johnston MH. Cryoablation of Swine Esophageal Mucosa: a direct comparison to Argon Plasma Coagulation (APC) and Multipolar Electrocoagulation (MPEC) [abstract] Gastrointest Endosc. 2001;53:A3448. [Google Scholar]
- 19.Raju GS, Ahmed I, Xiao SY, Brining D, Bhutani MS, Pasricha PJ. Graded esophageal mucosal ablation with cryotherapy, and the protective effects of submucosal saline. Endoscopy. 2005;37:523–6. doi: 10.1055/s-2005-861312. [DOI] [PubMed] [Google Scholar]
- 20.Shaheen NJ, Sharma P, Overholt BF, et al. A randomized, multicenter, sham-controlled trial of radiofrequency ablation (RFA) for subjects with Barrett’s esophagus (BE) containing dysplasia: interim results of the AIM dysplasia trial. Gastroenterol. 2008;134:A-37. [Google Scholar]


