Abstract
Alcoholic cardiomyopathy is manifested as cardiac hypertrophy, disrupted contractile function and myofibrillary architecture. An ample amount of clinical and experimental evidence has depicted a pivotal role for alcohol metabolism especially the main alcohol metabolic product acetaldehyde, in the pathogenesis of this myopathic state. Findings from our group and others have revealed that the mitochondrial isoform of aldehyde dehydrogenase (ALDH2), which metabolizes acetaldehyde, governs the detoxification of acetaldehyde formed following alcohol consumption and the ultimate elimination of alcohol from the body. The ALDH2 enzymatic cascade may evolve as a unique detoxification mechanism for environmental alcohols and aldehydes to alleviate the undesired cardiac anomalies in ischemia-reperfusion and alcoholism. Polymorphic variants of the ALDH2 gene encode enzymes with altered pharmacokinetic properties and a significantly higher prevalence of cardiovascular diseases associated with alcoholism. The pathophysiological effects of ALDH2 polymorphism may be mediated by accumulation of acetaldehyde and other reactive aldehydes. Inheritance of the inactive ALDH2*2 gene product is associated with a decreased risk of alcoholism but an increased risk of alcoholic complications. This association is influenced by gene-environment interactions such as those associated with religion and national origin. The purpose of this review is to recapitulate the pathogenesis of alcoholic cardiomyopathy with a special focus on ALDH2 enzymatic metabolism. It will be important to dissect the links between ALDH2 polymorphism and prevalence of alcoholic cardiomyopathy, in order to determine the mechanisms underlying such associations. The therapeutic value of ALDH2 as both target and tool in the management of alcoholic tissue damage will be discussed.
Keywords: Alcohol, ALDH2, enzyme, metabolism, myocardial, transgenic mice
1. Introduction - Alcohol and cardiac complications
Alcoholism remains the most widespread and devastating form of substance abuse in the United States and the rest of world. While light to moderate alcohol consumption may be associated with a reduced risk of cardiovascular diseases possibly through reduced coronary artery-related events (Djousse & Gaziano, 2008; Guo & Ren, 2010; Kloner & Rezkalla, 2007; O'Keefe et al., 2007; Skotzko et al., 2007; Xie et al., 2011), long-term alcohol abuse or binge drinking results in detrimental consequences to the heart, leading to mitochondrial defects, cell death, contractile dysfunction, heart rate variability, arrhythmias and cardiac remodeling (Guo & Ren, 2010; Lang et al., 2005; Laonigro et al., 2009; O'Keefe et al., 2007; Preedy et al., 2001; Richardson et al., 1998; Romanowicz et al., 2011; Spies et al., 2001). Heavy, chronic alcohol consumption (> 90 g of ethanol per day for > 5 years) (Laonigro et al., 2009; Piano, 2002) usually results in cardiac remodeling and contractile dysfunction characterized by dilated cardiomyopathy, also known as alcoholic cardiomyopathy, which represents an important source of the morbidity and mortality associated with alcoholism (Awtry & Philippides 2010; Iacovoni et al., 2010; Laonigro et al., 2009; Liang et al., 1999; Spies et al., 2001; Vary et al., 2008).
It is estimated that one out of every three alcohol-dependent individuals (alcoholics) displays alcoholic cardiomyopathy of varying severity (Iacovoni et al., 2010; Laonigro et al., 2009; Ren & Wold, 2008; Spies et al., 2001). This distinct form of congestive heart failure is responsible for 21-36% of all cases of non-ischemic dilated cardiomyopathy in Western society. Without complete abstinence, the 4 year mortality for alcoholic cardiomyopathy is close to 50% (Laonigro et al., 2009). Alcoholic cardiomyopathy, or alcoholic heart muscle disease, is often characterized by cardiac hypertrophy, disruption in myofibrillary architecture, reduced myocardial contractility (and resultant reductions in ejection fraction and stroke volume), myocardial fibrosis (Wang et al., 2005) as well as enhanced risk of arrhythmias, stroke and hypertension (Djousse et al., 2004; Higashiyama et al., 2011; Jones, 2005; Romanowicz et al., 2011; Schoppet & Maisch, 2001). Apart from the history of alcoholism these features are consistent with other dilated cardiomyopathies (Skotzko et al., 2009; Spies et al., 2001).
Clinical studies have shown that the pathology of alcoholic cardiomyopathy can be reversed by abstinence from alcohol (Piano, 2002; Skotzko et al., 2009). Nonetheless, this reversibility is apparently lost once the disease has progressed beyond some, as yet poorly defined point of severity (Jacob et al., 1991; Seiva et al., 2009). The diagnosis of alcoholic cardiomyopathy is made on the basis of deteriorating cardiac function, increased heart size and a history of alcohol abuse (Iacovoni et al., 2010; Laonigro et al., 2009). The occurrence of cardiomyopathy in chronic alcoholism has been well documented (Piano, 2002; Ren & Wold, 2008; Skotzko et al., 2009; Spies et al., 2001) although the precise cause of the myopathy is still poorly understood.
Individuals with alcoholic cardiomyopathy do not usually suffer from vitamin or nutritional deficiencies, suggesting the development of alcoholic cardiomyopathy is a result of alcohol intake rather than malnutrition (Laonigro et al., 2009). Electron microscopy examination of alcoholic hearts reveals a loss or disruption of myofibrils and dilated sarcoplasmic reticulum (SR) (Jaatinen et al., 1994). Mitochondria, which are considered the main target organelles for ethanol and its metabolite (Guo & Ren, 2010), display enlargement and disorganized cristae (Zhang et al., 2010). These morphological and functional cardiac defects will eventually result in heart failure. In addition, ample amounts of clinical and experimental findings have confirmed alcoholic damage in the heart originating from non-myogenic alterations such as tachycardia, arrhythmias, hypertriglyceridemia, hypertension and altered sympathetic tone (Bessembinders et al., 2011; George & Figueredo, 2010; Hering et al., 2011; Ohira et al., 2009). Along the same line, alcohol misuse is also regarded as one of the excess burdens associated with certain sub-clinical vascular diseases (Hamer et al., 2010; Turcotte et al., 2002), which may affect cardiac function indirectly via undesirable hemodynamic regulation.
At present, a number of theories have been postulated for the onset and development of alcoholic cardiomyopathy including oxidative damage, deposition of triglycerides, altered fatty acid extraction, decreased myofilament Ca2+ sensitivity, impaired protein metabolism and mitochondrial anomalies (Awtry & Philippides, 2010; Djousse & Gaziano, 2008; Djousse et al., 2009; Iacovoni et al., 2010; Jing et al., 2011; Laonigro et al., 2009; Ren & Wold, 2008). Oxidative stress, apoptosis and mitochondrial damage have been observed in alcohol-induced myocardial dysfunction (Doser et al., 2009; Ge et al., 2011; Guo & Ren, 2010; McDonough, 2003). Altered intracellular Ca2+ homeostasis has been proposed to underscore the compromised mechanical function in alcoholic cardiomyopathy (Ren & Wold, 2008; Zhang et al., 2003).
Recent studies from our lab as well as others have revealed the participation of a number of intracellular Ca2+ cycling proteins, including sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA), Na+-Ca2+ exchanger and phospholamban in the impaired intracellular Ca2+ handling following alcohol consumption (Li et al., 2006; Oba et al., 2008; Zhang et al., 2003). The dynamic balance between protein synthesis and protein degradation is believed to play an essential role in the heart under normal and alcoholic conditions. Both actin and myosin content are decreased while the levels of β-myosin are elevated in the heart after prolonged alcohol consumption (Lang et al., 2005). More importantly, the loss of myofibrillar proteins occurs prior to the appearance of any detectable echocardiographic abnormalities in the heart (Lang et al., 2005; Vary & Summer, 2004), suggesting a critical role of protein synthesis in the pathogenesis of alcoholic cardiomyopathy.
Recent evidence from our laboratory also suggested that alcoholism may promote autophagy in an AMPK-dependent manner (Ge & Ren, 2011), although further evidence of alcoholism-related changes in protein quality control machineries, such as ubiquitination and proteolysis, is still lacking in alcoholic hearts. Ethanol and its enzymatic metabolism also play a pivotal role through direct toxicity or indirect action of cell stress signaling activation. For example, the major ethanol metabolite acetaldehyde may contribute to cardiac dysfunction, hypertrophy and heart failure by either its direct toxicity or promoting elevated levels of catecholamines and reactive oxygen species (ROS) (Zhang et al., 2004, 2010). Other scenarios have also been speculated for alcoholic cardiomyopathy such as aldehyde-protein adduct formation (Niemela, 2001), acetaldehyde-derived DNA adduct formation (Yu et al., 2010), accumulation of fatty acid ethyl esters (Patel et al., 1997), or modifications of lipoprotein and apolipoprotein particles (Hannuksela et al., 2002).
Despite the ample clinical and experimental evidence endorsing most of these theories for the pathogenesis of alcoholic cardiomyopathy, none of these mechanisms may be considered as the ultimate culprit responsible for the development of alcoholic cardiomyopathy. It appears that the susceptibility to the detrimental effect of alcohol intake is a result of the complex interplay between genes and environmental factors (the latter including alcohol itself and other nutrients) (Crabb et al., 2004). As illustrated in Fig. 1, alcohol is metabolized mainly through a two-step enzymatic process involving alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH), which break down ethanol into acetaldehyde and then acetate (Crabb et al., 2004; Manzo-Avalos & Saavedra-Molina, 2010). These two enzymes involved in alcohol metabolism are polymorphic and affect the elimination rate of ethanol or its metabolite acetaldehyde. As a result, genetic polymorphisms of ADH and ALDH2 alter the susceptibility to ethanol intake and the risk of alcoholism and alcoholic complications (Pautassi et al., 2010). A highly active ADH or low (or mutant) ALDH protects against alcoholism but predisposes the organism to more severe alcoholic damage, an effect related to a pre-steady state burst in arterial acetaldehyde (Pautassi et al., 2010; Ren, 2007). The main ALDH isoform – the mitochondrial isozyme of ALDH or ALDH2 – and its cardiac effects along with its genetic variation are the subject of the present review.
Fig. 1.
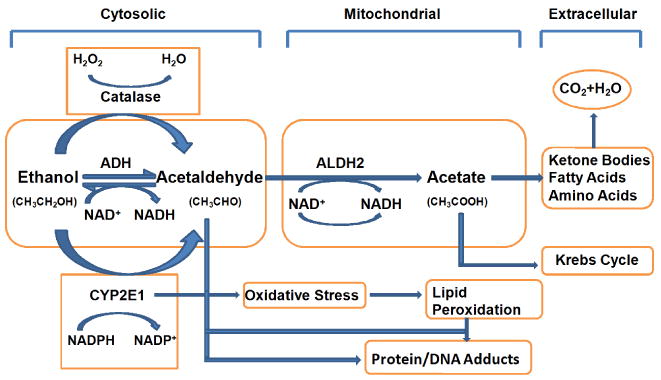
Ethanol metabolism pathway. Ethanol is metabolized into acetaldehyde through the cytosolic enzyme alcohol dehydrogenase (ADH), the microsomal enzyme cytochrome P450 2E1 (CYP2E1) and the peroxisomal enzyme catalase. The ADH enzyme reaction is the main ethanol metabolic pathway involving an intermediate carrier of electrons, namely nicotinamide adenine dinucleotide (NAD+), which is reduced by two electrons to form NADH. Acetaldehyde is metabolized mainly by aldehyde dehydrogenase 2 (ALDH2) in the mitochondria to acetate and NADH before being cleared into the systemic circulation.
2. ALDH2, metabolism of ethanol and acetaldehyde
2.1. Enzymology of alcohol metabolism
The effect of alcohol (ethanol) in various tissues depends on its blood concentration over time. After oral absorption, alcohol is readily absorbed by the gastrointestinal tract by passive diffusion through the stomach wall (∼ 20%) or the small-intestinal wall (∼ 80%). Elimination of alcohol is achieved mostly through metabolism (95-98%) with a small unchanged fraction being excreted through exhalation, sweating or urination (Manzo-Avalos & Saavedra-Molina, 2010). Ethanol distributed in the body fluid space is metabolized mainly through the hepatic oxidation catalyzed by the ADH, ALDH, cytochrome P450 2E1 (CYP2E1) and catalase enzymes, as depicted in Fig. 1 (Manzo-Avalos & Saavedra-Molina, 2010; Wu & Cederbaum, 2009; Wu et al., 2010). In particular, alcohol is metabolized into acetaldehyde (CH3CHO) by ADH and CYP2E1 in cytoplasm and microsomes, respectively. Although CYP2E1 is pretty important in ethanol metabolism and toxicity, and is responsible for a number of ethanol-related drug interactions, it will not be emphasized in our present review. Due to the high capacity and the relatively high affinity (Km = 0.05–0.1 g/L) of ADH in hepatocytes, the enzyme gets saturated after only a few drinks, which decreases the rate at which ethanol is effectively metabolized. Once formed in the liver, acetaldehyde is oxidized by the mitochondrial isoform of ALDH (ALDH2) in an irreversible reaction to acetate. ALDH2 has a very low Km value, which makes the elimination of toxic acetaldehyde soon after its formation highly efficient. The activated form of acetate, acetyl CoA, can be further metabolized into ketone bodies, fatty acids, amino acids and steroids in addition to oxidation in the Krebs cycle, leading to the formation of CO2 and water as the end-products of ethanol oxidation (George & Figueredo, 2010; Manzo-Avalos & Saavedra-Molina, 2010).
Rates of ethanol metabolism by ADH and ALDH2 enzymes are, therefore, deemed critical in determining its toxicity because the intermediate product of this pathway acetaldehyde is highly toxic (ten times higher than ethanol). The maximal activities of ADH and ALDH are similar in the liver, making a comparable contribution for both enzymes in the overall control of the rate of alcohol oxidation (George & Figueredo, 2010; Manzo-Avalos & Saavedra-Molina, 2010). Both enzymes use the cofactor nicotinamide adenine dinucleotide (NAD+), which is reduced to NADH. As a result, the ratio NADH/NAD+ is significantly elevated following ethanol oxidation, resulting in an altered cellular redox state and adverse effects associated with alcohol consumption (Manzo-Avalos & Saavedra-Molina, 2010; McDonough, 2003).
In human subjects with ADH and ALDH2 variants, the rate of ethanol oxidation may be substantially influenced by enzyme kinetic properties such as the Km, Vmax and sensitivity to product inhibition of the variants (Crabb et al., 2004). Thus it is possible that components of alcohol metabolism and the concentrations of metabolic intermediates such as acetaldehyde may change despite an unaltered alcohol elimination rate under certain conditions.
2.2. ALDH2, alcohol metabolism and genetic polymorphisms
ALDH2 is one of 19 members of the ALDH gene family in humans that play a crucial role in the oxidation and detoxification of reactive aldehydes including the ethanol metabolite acetaldehyde in various organs and cells (Budas et al., 2009). Besides metabolism of acetaldehyde, ALDH2 also serves as a metabolic enzyme in the detoxification of other reactive aldehydes such as 4-hydroxy-2-nonenal (4-HNE) and conversion of nitroglycerin (from glyceryl trinitrate to 1,2-glyceryl dinitrate) (Budas et al., 2010; Chen et al., 2010; Ren, 2007; Zhang et al., 2004). Two major isoforms of ALDH, cytosolic and mitochondrial, are present and can be distinguished by electrophoretic mobilities, kinetic properties, and subcellular localizations.
The gene for mitochondrial ALDH, or ALDH2, in humans is found on chromosome 12. This gene encodes a mitochondrial isoform with a low Km for acetaldehyde, and is localized in the mitochondrial matrix. All Caucasians studied are deemed homozygous for ALDH2 while approximately 50% of Asians are heterozygous and possess only one normal copy of the ALDH2 gene and one mutant copy encoding an inactive mitochondrial isozyme. Epidemiological studies have revealed a remarkably reduced risk of alcoholism due to alcohol intolerance, albeit with an increased risk of alcoholic complications, in Asians as compared with Caucasians; this is mainly associated with the greatly reduced activity of the mutant ALDH2 isozyme (Nishida et al., 2004; Peng & Yin, 2009). Similarly, incidence rates for alcoholic complication are greater in African-Americans and Native-Americans than in Caucasians, largely due to genetic polymorphisms in ADH and ALDH2, nutrition, and other factors (Chou et al., 1999; Russo et al., 2004). Although genetic variation in ALDH2 has been reported to affect alcohol metabolism in Europeans, it does not appear that such genetic variation in ALDH2 leads to the alteration in alcohol sensitivity, consumption, or risk of dependence in Europeans (Dickson et al., 2006). ALDH2 genetic polymorphisms have been shown to contribute to the effects of alcohol intake on liver (Takeshita et al., 2000) and bone formation (Shimizu et al., 2011). A meta-analysis of seven East Asian populations showed the association between ALDH2*2 allele and low HDL-C level (Hao et al., 2010). The ALDH2*2 allele encodes a protein with an amino acid change from glutamate to lysine (derived from the ALDH2*1 allele) and devoid of enzymatic activity. Allelic variation of ALDH genes, especially deficiency in ALDH2 due to such a point mutation in the active ALDH2*1 gene, alters blood acetaldehyde levels and decreases vulnerability for the development of alcoholism (Chen et al., 2009; Peng et al., 1999, 2002, 2007). Up to 50% of Asians carry mutant alleles of ALDH (ALDH2*2/1 and ALDH2*2/2) that resulted from a single point mutation of the active ALDH2*1 gene, producing a ∼ 10 fold increase in blood acetaldehyde levels in the ALDH2-deficient individuals following alcohol intake compared with the ALDH2-intact populations (Nishimura et al., 2002; Peng & Yin, 2009; Yin & Peng, 2007).
Table 1 summarizes some of the most commonly seen biological and pathophysiological effects resulting from ALDH2 genetic variation. Interestingly, due to the acetaldehyde-associated feeling of discomfort, the gene of ALDH2*2/2 may protect against the development of alcohol dependence and alcohol-related disease by discouraging alcohol consumption (Peng & Yin, 2009). In addition to the cardiac depressant response elicited by acetaldehyde as mentioned earlier, contribution of acetaldehyde to alcoholic cardiomyopathy was substantiated by the fact that the ALDH inhibitor, cyanamide, potentiates alcohol intake-induced rise of plasma cardiac troponin-T levels, a key index for myocardial cell death. It is believed that homozygosity for the ALDH2*2 allele should help to inhibit the development of alcoholism. After a small dose of alcohol, cardiac and extracranial/intracranial arterial hemodynamic parameters as well as self-rated subjective sensations were strikingly responsive in homozygous ALDH2*2 individuals as evidenced by pronounced cardiovascular hemodynamic effects as well as subjective perception of general discomfort for as long as 2 hr after alcohol ingestion.
Table 1.
Examples of ALDH2 polymorphisms and associated pathophysiological responses.
| ALDH2 genotype | Glu487Lys | ALDH activity | Effect | Reference |
|---|---|---|---|---|
| ALDH2*2/2 | Lys/Lys | Inactive | Less alcohol consumption, highest alcohol sensitivity, less periodontitis progression | (Nishida et al., 2010) |
| Facial flushing, nausea, drowsiness, headache, positive patch testing after drinking | (Harada et al., 1981; Ishibashi et al., 2010) | |||
| A risk conferring factor for alcohol dependence | (Vaswani et al., 2009) | |||
| Decreased suicide behavior | (Hishimoto et al., 2010) | |||
| Increased risk of myocardial infarction | (Jo et al., 2007) | |||
| Increased morbidity of osteoporosis | (Yamaguchi et al., 2006) | |||
| Increased risk of esophageal cancer | (Yang et al., 2010) | |||
| ALDH2*1/2 | Glu/Lys | Inactive | Suicide behavior in male | (Hishimoto et al., 2010) |
| Increased risk of squamous cell carcinoma of upper aerodigestive tract, head and neck cancer in moderate and heavy drinkers | (Yokoyama et al., 2010) | |||
| Increased risk of myocardial infarction | (Jo et al., 2007) | |||
| Increased risk of esophageal cancer among never/rare, moderate and heavy drinkers, as well as among ex-drinkers. | (Yang et al., 2010) | |||
| ALDH2*1/1 | Glu/Glu | Active | Higher alcohol consumption, lowest alcohol sensitivity | (Nishida et al., 2004) |
| Decreased facial flushing, nausea, drowsiness, headache, positive patch testing after drinking | (Harada et al., 1981; Ishibashi et al., 2010) | |||
| Increased suicide behavior | (Hishimoto et al., 2010) | |||
| Increased colorectal cancer risk | (Gao et al., 2008) | |||
| Decreased risk of myocardial infarction | (Jo et al., 2007) |
3. Acetaldehyde and the heart
As mentioned above, acetaldehyde is formed when ethanol is oxidized primarily through cytosolic ADH (Fig. 1). It is a chemically reactive organic compound with a low molecular weight (44.05 Da) and boiling point (21°C). While liver is considered the primary site of oxidation, other organs (including the heart) participate in ethanol metabolism. Other than classic ethanol metabolism, acetaldehyde may be produced endogenously through the degradation of biological molecules such as that occurring during lipid peroxidation, in a manner similar to other reactive aldehydes including 4-HNE and malondialdehyde (Uchida, 2000; Wang et al., 2008). Acetaldehyde is about ten times more toxic than ethanol based on its LD50 value. An ample amount of recent evidence from our lab and others has consolidated a pivotal role for acetaldehyde in the pathogenesis of alcoholic cardiomyopathy (Aberle et al., 2003; Aberle & Ren, 2003; Brown et al., 1999, 2001; Cai, 2008; Guo & Ren, 2010). Acetaldehyde may elicit a direct toxic effect on the heart or react with amino, hydroxyl, and sulfhydryl groups to interfere with or modify the structure and function of macromolecules such as proteins and enzymes.
Elevated circulating and cardiac acetaldehyde levels are seen in individuals consuming excessive amounts of alcohol (Espinet & Argiles, 1984; Hintz et al., 2003; Jankala et al., 2000; Nishimura et al., 2002; Watanabe et al., 1985). Moreover, blood acetaldehyde levels are found to be much higher in alcohol-dependent individuals after alcohol administration (Nuutinen et al., 1983; Oba et al., 2005). Impaired ALDH capacity such as in ALDH2 polymorphism may predominantly contribute to the elevated blood acetaldehyde levels (Nuutinen et al., 1983). Blood acetaldehyde levels were found to be ∼ 5 μM in normal subjects and 30 to 125 μM in Asians with defective ALDH2 following heavy alcohol intake (Chen et al., 1999; Nishimura et al., 2002; Watanabe et al., 1985).
Elevation in blood acetaldehyde levels may not be directly and proportionally correlated with the rise in blood alcohol levels due to the apparent difference in ethanol metabolism in various populations. It is perceived that blood levels of acetaldehyde rather than ethanol play a more significant role in the pathogenesis of alcoholic cardiomyopathy (Zhang et al., 2004). Acetaldehyde may trigger cardiac hypertrophy or dilated cardiomyopathy associated with a significant increase in the hypertrophic marker skeletal actin and ANF (Li & Ren, 2008; Liang et al., 1999). Direct effects of acute (5 to 10 min) acetaldehyde exposure on cardiovascular function have been extensively studied (Aberle & Ren, 2003; Aistrup et al., 2006; Brown et al., 1999, 2001; Brown & Savage 1996; Ren et al., 1997; Savage et al., 1995). Acetaldehyde produces vasoconstriction and positive inotropic and chronotropic responses at concentrations of 3 mM or below. Higher concentrations of acetaldehyde (>3 mM) elicit cardiac depression, vasodilatation and hypotension (Brown & Carpentier, 1989, 1990). The acetaldehyde-induced negative inotropic response in the heart seems to be associated with decreased SR Ca2+ release (Ren et al., 1997; Savage et al., 1995) or inhibition of voltage-dependent Ca2+ channels (Morales et al., 1997). Our earlier studies revealed that acetaldehyde depresses cardiomyocyte contractile amplitude, maximal velocity of contraction/relaxation, and prolonged duration of relaxation and intracellular Ca2+ clearance (Aberle et al., 2004; Aberle & Ren, 2003; Ren et al., 1997). In addition to its apparent cardiac toxicity, acetaldehyde may also interfere with gene expression and protein synthesis in the heart (Siddiq et al., 1993). Acetaldehyde may regulate the expression of apoptosis-related genes en route to development of alcoholic cardiomyopathy (Fernandez-Sola et al., 2006; Jankala et al., 2002). However, due to the impracticality of administering acetaldehyde to humans, it is difficult to assess the pathophysiological and epigenetic effects of acetaldehyde exposure in human subjects to determine its role in alcoholic cardiomyopathy. On the other hand, using metabolic inhibitors to alter acetaldehyde levels has been proven to be rather nonspecific, ineffective, toxic and difficult to manage (Preedy et al., 2007; Ren, 2007; Ren & Wold, 2008).
To better assess the role of acetaldehyde in alcohol-induced tissue damage, cardiac-specific ADH overexpression transgenic mice were generated in our labs (Duan et al., 2002; Hintz et al., 2003; Liang et al., 1999). The mice with overexpression of ADH driven by the α-myosin heavy chain promoter displayed exacerbated alcoholic cardiomyopathy following alcohol consumption (Duan et al., 2002; Hintz et al., 2003; Liang et al., 1999). Moreover, ADH exacerbated alcohol exposure-induced mitochondrial dysfunction manifested as decreased mitochondrial membrane potential (MMP) and accumulation of mitochondrial O2-. Myocardium from ethanol-treated mice displayed enhanced apoptosis shown as elevated expression of Bax and Caspase-3 and decreased expression of Bcl-2, the effects of which with the exception of Caspase-3 were augmented by ADH. ADH accentuated ethanol-induced increase in the mitochondrial death domain components pro-caspase-9 and cytochrome c in the cytoplasm (Guo & Ren, 2010), suggesting that acetaldehyde toxicity possibly through mitochondrial damage is permissive to the development of alcoholic cardiomyopathy. Our study further revealed that the ADH transgene-accentuated cardiac contractile depression in response to ethanol exposure was more pronounced in females than males despite similar cardiac acetaldehyde levels between the two genders following alcohol challenge (Duan et al., 2003). These findings attribute a role of acetaldehyde in the gender-related difference of alcoholic cardiomyopathy. In addition, ADH transgene itself does not affect morphological, mechanical and intracellular Ca2+ properties, suggesting that the transgene is not innately harmful. Moreover, the NADH/NAD+ ratio was similar in ADH and FVB wild-type mice chronically consuming alcohol (Liang et al., 1999), thus not favoring a key role of depletion of NAD+ as an adequate factor for enhanced cardiac damage in ADH transgenic mice after chronic alcohol intake.
Although the availability of ADH transgenic mice has greatly supported the acetaldehyde theory in the development of alcoholic cardiomyopathy, caution needs to be taken in data interpretation. Acetaldehyde often initiates cell and tissue injury at a level of 50 to 100 μM or higher. However, the concentrations of acetaldehyde usually achieved in the body are in the low micromolar range following moderate ethanol intoxication (Tominaga, 2009; Tsukamoto et al., 1989). Certain tissues such as the brain exhibit an even lower level of acetaldehyde. Therefore, the jury is still out as to whether acetaldehyde is the main mediator of cytotoxic effects induced by ethanol. Other hypotheses postulated for alcoholic cardiomyopathy include oxidative damage, lipid peroxidation, altered membrane integrity as well as acetaldehyde-induced hemodynamic effects in the vasculature (Ren et al., 2002; Ren & Wold, 2008; Zhang et al., 2004). These pathological processes may work in concert with acetaldehyde to produce a synergistic effect on the function of protein and membrane phospholipids following alcohol intake (Cederbaum et al., 2009; Wu & Cederbaum, 2009; Wu et al., 2010). Given the absence of convincing human case study data on heart function following chronic alcohol intake, it is still premature to conclude that acetaldehyde is the ultimate cause of alcoholic cardiomyopathy.
4. ALDH2 and cardiac function in alcoholism
Public health guidelines usually recommend avoidance of excessive alcohol consumption in order to lessen the alcohol-associated health burden (Russo et al., 2004). In particular, alcoholism is an avoidable risk factor for cancer (Druesne-Pecollo et al., 2009). Functional variants in genes involved in alcohol metabolism lead to dramatic differences in exposure to carcinogenic acetaldehyde, suggesting a likely interaction of genetic susceptibility and alcoholism in cancer. Polymorphisms in alcohol metabolism alter folate metabolism and thus cancer risk. Not surprisingly, inactive or mutant forms of ALDH2 are considered an independent risk factor for aerodigestive tract cancers including carcinomas of the pancreas, liver and esophagus due to the carcinogenicity of acetaldehyde (Druesne-Pecollo et al., 2009; Yang et al., 2010). Fig. 2 summarizes the spectrum of pathophysiological and behavioral effects of ALDH2 in multiple organ systems.
Fig. 2.
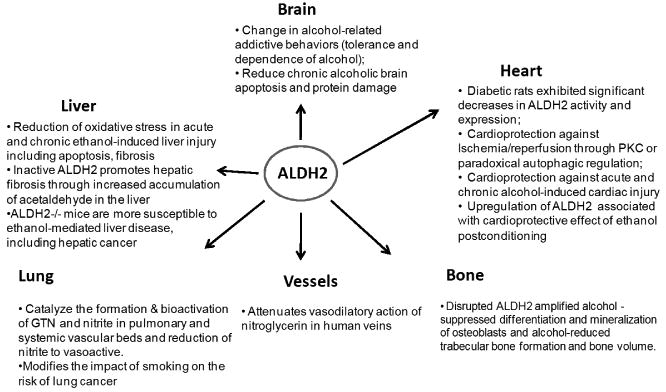
Involvement of ALDH2 enzyme in the regulation of multiple organ functions. GTN = glyceryl trinitrate; PKC = protein kinase C.
Substantial clinical and experimental evidence recently revealed a novel beneficial role of ALDH2 in the pathological process of cardiovascular diseases including ischemia-reperfusion injury, arrhythmia and alcoholism (Budas et al., 2009; Chen et al., 2008, 2010; Doser et al., 2009; Koda et al., 2010; Ma et al., 2009, 2010; Ren et al., 2009). To better understand the role of ALDH2 in the onset and development of alcoholic cardiomyopathy, we made transgenic mice overexpressing low Km ALDH2 using the chicken β-actin promoter to achieve non-specific overexpression of the enzyme (Doser et al., 2009). The cardiac-specific α-myosin heavy chain promoter was not chosen for ALDH2 overexpression because diffusion of acetaldehyde from peri-cardiac regions would easily offset the facilitated acetaldehyde removal from cardiac tissue. Reports from our lab have provided convincing evidence that ALDH2 is capable of attenuating both acute and chronic alcohol exposure-induced myocardial morphological and functional injury (Doser et al., 2009; Li & Ren, 2008; Ma et al., 2009). In the absence of alcohol intake, the ALDH2 transgene did not exhibit any effect on myocardial and cardiomyocyte function or any of the other biochemical markers tested. Chronic alcohol intake triggered cardiac geometric and contractile dysfunction including decreased left ventricular wall thickness and septal thickness, enlarged left ventricular systolic and diastolic diameters, reduced fractional shortening, cardiomyocyte peak shortening, maximal velocities of contraction and relaxation as well as prolonged relaxation duration associated with dysregulated intracellular Ca2+ release and SR Ca2+ uptake (Doser et al., 2009). Interestingly, myocardium and cardiomyocytes from ALDH2 transgenic mice displayed mitigated alcohol-induced mechanical anomalies. Oxidative stress, as indicated by lipid peroxidation and protein carbonyl formation, was significantly elevated in hearts and other tissues in wild-type FVB mice following chronic ethanol consumption, the effects of which were attenuated by the ALDH2 transgene. These findings are somewhat consistent with the previous findings of myopathic alteration following alcohol intake featured by compromised myocardial contractility (Iacovoni et al., 2010; Ma et al., 2009; Skotzko et al., 2009; Spies et al., 2001; Zhang et al., 2004). Several hypotheses have been put forward with regards to chronic alcohol intake-induced cardiac anomalies including lipid peroxidation (Hintz et al., 2003), oxidative damage (Ren & Wold, 2008), mitochondrial dysfunction (Ma et al., 2009), and altered membrane properties (Cederbaum et al., 2001). Although earlier findings from our lab demonstrated that overexpression of ALDH2 alleviates alcohol and acetaldehyde-induced cell injury caused by alcohol and acetaldehyde both in vivo and in vitro (Li et al., 2004, 2006; Ma et al., 2009), little is known about the precise mechanism of the protection due to ALDH2 transgene. Interestingly, seminal findings from Mochly-Rosen's laboratory have unveiled an emerging role of ALDH2 against myocardial ischemic injury courtesy of its dual dehydrogenase and reductase activities (Budas et al., 2009; Chen et al., 2008, 2010). The concept of ALDH2 as a new and promising therapeutic target in cardiovascular diseases received further consolidation from our recent work that revealed that overexpression of the ALDH2 transgene may alleviate ischemia/reperfusion injury, post-ischemic-reperfusion injury, and ischemic cardiac dysfunction. Consistent with this, the ischemia-reperfusion injury may be exacerbated by ALDH2 knockout (Ma et al., 2011). Nonetheless, the mechanism(s) of action behind protection by ALDH2 against ischemia-reperfusion injury may be diverse, involving bioactivation of nitroglycerin, preventing the degranulating effects of toxic aldehydes and lessening the production of free radicals, 4-HNE and ultimately mitochondrial dysfunction (Budas et al., 2009; Chen et al., 2010). ALDH2 is known for its role in the metabolism of the ethanol metabolite acetaldehyde. Besides acetaldehyde, ALDH2 serves as a critical metabolic enzyme in the detoxification of other reactive aldehydes such as 4-HNE and conversion of nitroglycerin (from glyceryl trinitrate [GTN]) to 1,2-glyceryl dinitrate (1,2-GDN) (Budas et al., 2009; Chen et al., 2010).
Using a transgenic model with inactive ALDH2, ethanol and acetaldehyde concentrations in blood, brain, and liver were scrutinized between ALDH2-/- and ALDH2+/+ wild-type mice following alcohol gavages. Much higher blood acetaldehyde but not alcohol levels were found in ALDH2-/- mice compared with ALDH2+/+ mice 1 hr after alcohol challenge, consistent with the observation of elevated blood acetaldehyde levels (by ∼10 fold) in patients with defective ALDH2 as compared to those in normal individuals (Nishimura et al., 2002). ALDH2 enzyme metabolized 94% of acetaldehyde produced from ethanol (Isse et al., 2005). These data indicate that ALDH2 is a major enzyme for acetaldehyde metabolism. To evaluate the role of facilitated acetaldehyde metabolism on tissue and cell injury caused by alcohol or acetaldehyde, we overexpressed ALDH2 driven by the non-specific chicken β-actin promoter in human umbilical vein endothelial cells (HUVEC) and fetal human cardiac myocytes. Our results demonstrated that ALDH2 overexpression significantly attenuates ethanol and acetaldehyde-induced oxidative stress and apoptosis (Li et al., 2004, 2006), suggesting that facilitation of acetaldehyde breakdown lessens its cellular toxicity. These results support the notion that acetaldehyde may directly elicit cell injury because facilitation of its metabolism by ALDH2 alleviates cellular toxicity. These data from our group suggest that facilitated acetaldehyde breakdown with overexpression of ALDH2 may protect against alcohol-induced detrimental effects in the heart, liver and brain (Doser et al., 2009; Guo et al., 2009; Li et al., 2009; Ren et al., 2009), indicating the therapeutic potential of ALDH2 enzyme in alcoholic tissue damage. To the contrary, knockdown of ALDH2 accentuated the severity of alcoholic cardiomyopathy (Ma et al., 2010).
Results from our group showed that transgenic overexpression of ALDH2 effectively antagonizes myocardial hypertrophy and contractile defects elicited by alcohol intake through a mechanism that is associated, at least in part, with phosphorylation of ASK-1, GSK-3β, GATA4, and CREB (Doser et al., 2009). Moreover, alcohol treatment dampened phosphorylation of Akt and AMPK associated with up-regulated PP2A and PP2C, which was abrogated by ALDH2. ALDH2 significantly attenuated the decrease in Akt- and AMPK-stimulated phosphorylation of Foxo3 at Thr32 and Ser413, respectively, caused by ethanol. These results suggested that ALDH2 is cardioprotective against acute ethanol toxicity possibly through inhibition of protein phosphatases, leading to enhanced Akt and AMPK activation. Subsequently, inhibition of Foxo3 occurs followed by apoptosis and mitochondrial dysfunction (Ma et al., 2009). In contrast, ALDH2 deficiency led to a worsened cardiomyocyte function caused by ethanol, which may be due to upregulated expression of protein phosphatase, depressed Akt activation, and subsequently impaired mitochondrial function (Ma et al., 2010). ALDH2 also reversed the myocardial endoplasmic reticulum (ER) stress caused by ethanol. ALDH2 overexpression antagonizes the cardiac insulin insensitivity and contractile defect caused by chronic alcohol intake, possibly via improvement of insulin signaling at the levels of the insulin receptor, IRS, Akt, Foxo3a and JNK (Li et al., 2009).
5. Aldehyde accumulation, protein adduct formation and ALDH2 in alcoholic cardiac disease
Generation of protein-aldehyde adducts or acetaldehyde-derived DNA adducts as a result of excessive alcohol intake has been documented (Badger et al., 2003; Niemela 2001; Yu et al., 2010). Acetaldehyde can bind to reactive lysine residues, some aromatic amino acids, cysteine, or free α-amino groups (Niemela, 2001). The preferential targets of aldehyde or acetaldehyde adduct formation include erythrocyte membrane proteins, albumin, lipoproteins, hemoglobin, collagens, tubulin and cytochrome enzymes (Niemela, 1999, 2001; Yu et al., 2010). Although most aldehyde adducts are located in the liver (Jeong et al., 2000; Worrall et al., 2001), some may be found in the muscle, brain and blood cells. As a consequence of adduct formation, the physicochemical properties of proteins, nucleic acids and lipids may be compromised (Niemela, 1999). Chronic alcohol consumption contributes to formation of various DNA adducts. The acetaldehyde-DNA binding was demonstrated to overtly promote carcinogenesis in alcohol-dependent individuals (Niemela, 2001). Although formation of DNA adducts is deemed to be one of the early steps in carcinogenesis, whether these alcohol-related DNA adducts are true factors or initiators of cancer is still elusive. It was reported that acetaldehyde protein adducts and lipid peroxidation products may increase collagen mRNA levels and thus the levels of connective tissue proteins (Aroor & Shukla, 2004; Lieber, 1991). Moreover, the presence of specific protein adducts in alcohol-dependent individuals has prompted the effort to identify new biological markers targeted for alcohol-induced diseases. Nonetheless, adduct assays often display insufficient sensitivities to be adopted in clinical practice at this time. Last but not least, the acetaldehyde-biogenic amine condensation products have been implicated to play an essential role in ethanol and acetaldehyde reinforcement (Melis et al., 2009; Talhout et al., 2007), although little is known about the effect of these acetaldehyde-biogenic amine condensation products on heart geometry or function. One of these condensation products, salsolinol, was shown to regulate cardiac contractile function and thus may mediate the myocardial responses elicited by acetaldehyde (Sokolova et al., 1990). Further study is warranted to elucidate the precise role of the acetaldehyde-biogenic amine condensation products in the alcohol and acetaldehyde-elicited myocardial structural and functional responses, and the effect of ALDH2 on such biogenic amine condensation products.
6. Autophagy and ALDH2 in alcoholic heart disease
Autophagy plays a pivotal role in the heart to maintain physiological cardiac function by engulfing damaged proteins or macromolecular structures (Gottlieb & Carreira, 2010; Gottlieb & Mentzer, 2010; Gurusamy & Das, 2009a, 2009b; Zhang & Ren, 2010). Despite the beneficial effects, an unfavorable role of autophagy has been documented in a number of human diseases such as cancer and cardiovascular and neurodegenerative diseases (Levine & Kroemer, 2008). Thus, autophagy has been considered as a double-edged sword for both disease pathogenesis and prevention (Gurusamy & Das, 2009b). Elevated autophagy promotes survival in response to mild stress, such as brief ischemia and a low grade of oxidative stress, by removing damaged organelles and by recycling of macromolecules to maintain cellular homeostasis (Levine & Kroemer, 2008; Ma et al., 2011; Zhang & Ren, 2010). On the other hand, prolonged ischemic injury may elicit excessive upregulation of autophagy, resulting in cell death due to excessive self-digestion of essential organelles and proteins (Ma et al., 2011; Sciarretta et al., 2010; Zhang & Ren, 2010). Manipulation of autophagy has been deemed as a potential therapeutic target for heart diseases (Levine & Kroemer, 2008). However, the role of autophagy in alcoholic cardiomyopathy remains unclear.
Recent study from our laboratory revealed that ALDH2 promotes cardiomyocyte survival during ischemia, whereas inhibition of autophagy improves cell survival during reperfusion (or reoxygenation), indicating a paradoxical role of autophagy in ischemia and ischemia/reperfusion phases. This is consistent with the observation that inhibition and induction of autophagy mitigate, respectively, the ALDH2-offered protection against ischemia and ischemia/reperfusion (Ma et al., 2011). Our data further demonstrated that ischemia-induced AMPK activation is increased and decreased, by ALDH overexpression and knockout, respectively. Likewise, reperfusion-induced Akt phosphorylation is augmented and attenuated by ALDH2 overexpression and knockout, respectively. These data favor the notion that ALDH2 turns on AMPK to inhibit mTOR signaling and facilitate autophagy during ischemia. However, AMPK is replaced by Akt during reperfusion. Akt activation turns on mTOR to suppress autophagy. Thus, mTOR serves as a converging point for ALDH2-mediated activation of Akt and AMPK signaling molecules in ischemia and ischemia-reperfusion (Ma et al., 2011). This dual regulatory paradox appears to underscore the homeostatic machinery for ALDH2-elicited cardioprotection against ischemia-reperfusion injury (Zhang & Ren, 2010).
Our data also revealed a role of ALDH2 as a metabolic enzyme in the detoxification of 4-HNE. 4-HNE along with malondialdehyde, are highly reactive aldehydes known to directly compromise cardiomyocyte contractile dysfunction (Aberle et al., 2004; Folden et al., 2003). Our data revealed that ALDH2 attenuates 4-HNE-induced cardiomyocyte contractile dysfunction, while cardiac 4-HNE accumulation is accentuated in the ALDH2 knockout mice in response to ischemia-reperfusion (Ma et al., 2011; Zhang & Ren, 2010). These findings further suggest a role for aldehyde detoxification in the protection by ALDH2 against ischemia-reperfusion injury. This protection may be mediated by lifting the inhibition of 4-HNE on LKB1/PTEN-mediated regulation of AMPK and Akt, respectively.
Somewhat similar to its beneficial properties in ischemia-reperfusion injury (Ma et al., 2011), ALDH2 overexpression was found to protect against cardiac geometric and contractile anomalies caused by alcohol likely through inhibition of autophagy (Ge & Ren, 2011). Very recent findings from our group revealed that the change in cardiac mechanical and autophagic responses caused by alcohol intake were associated with dampened activation of Akt/AMPK and their downstream signal mTOR. ALDH2 transgene appears to offer its protection in the heart by reversing alcohol-induced changes in AMPK, Akt and mTOR, en route to mitigating alcohol-induced autophagy induction and contractile dysfunction. Akt and AMPK are essential regulators of autophagy, survival, energy metabolism and contractile function in the heart (Arad et al., 2007; Clerk et al., 2003; Li & Ren, 2006). Our previous report indicated that cardiomyocyte contractile dysfunction caused by acute alcohol exposure is associated with a reduced Akt activity but an enhanced AMPK activation (Guo et al., 2010; Li & Ren, 2006). ALDH2 overexpression reconciled the dampened phosphorylation of Akt and AMPK along with facilitated autophagy in response to alcohol intake. Given that activation of AMPK promotes while activation of Akt suppresses autophagy (Ma et al., 2011), our data favor a pivotal role of Akt- rather than AMPK-dependent regulation of autophagy in chronic alcoholism and ALDH2 transgene-elicited myocardial responses. Our results revealed that inhibition in phosphorylation of Akt, mTOR and STAT3 caused by alcohol intake was restored by ALDH2 overexpression, favoring the notion that overexpression of ALDH2 rescues geometric and contractile anomalies caused by alcohol intake by inhibiting alcohol-induced autophagy through an Akt-mTOR-STAT3 dependent mechanism (Ge & Ren, 2011). However, given that acute alcohol challenge compromises cardiac contractile function in conjunction with facilitating the activation of AMPK, which should trigger autophagy induction (Guo et al., 2010), caution needs to be taken with regards to the role of autophagy in the regulation of cardiac function during various stages of alcoholism.
More recent findings from our group suggested that ALDH2 may execute its protective effect against alcoholic heart injury and autophagy by restoring Notch signaling (Ge & Ren, 2011). Inhibition of Notch1 with the γ-secretase inhibitor N-[N-(3,5-difluorophenacetyl)-1-alany1]-S-phenyglycine t-butyl ester (DAPT) exaggerated acute ethanol exposure-induced cardiomyocyte contractile dysfunction, apoptosis and autophagy. In our hands, ALDH2 ablated alcohol-induced suppression of phosphorylation of mTOR and STAT3. mTOR complex 1 (mTORC1) was shown to positively regulate Notch signaling through STAT3 in the regulation of cell differentiation (Ma et al., 2010). In addition, development of tumors as a result of hyperactive mTOR signaling is often associated with aberrant high STAT3/Notch activity, while inhibition of Notch signaling extends survival (Ma et al., 2010). Although the role of Notch has not been elucidated in the regulation of autophagy and alcoholic complications, the fact that the Notch pathway acts as a positive regulator of the PI3K/Akt/mTOR pathway favors its likely important role in the regulation of autophagy and consequently cardiac function (Chan et al., 2007).
7. Summary and clinical perspectives
ALDH2 is capable of mitigating cardiac remodeling and myocardial dysfunction following chronic alcohol ingestion (Doser et al., 2009), possibly through facilitated acetaldehyde detoxification. Blood acetaldehyde levels are ∼10-fold higher in humans with defective ALDH2 (e.g., Asians and African Americans) than normal individuals following alcohol ingestion. Allelic variation of ALDH genes, especially ALDH2, due to a point mutation in the active ALDH2*1 gene, significantly alters vulnerability to alcoholism and alcoholic complications. Using genetically modified ALDH2 models, several studies have suggested a cardioprotective role of ALDH2 to counteract cardiac remodeling and myocardial dysfunction following alcohol intake. Therefore, ALDH2 may possess important therapeutic potential against alcoholic and other forms of myocardial damage. Because convincing human case studies on interaction between ALDH2 gene polymorphisms and heart function following chronic alcohol intake are lacking, caution must be taken when evaluating the role of ALDH2 and acetaldehyde detoxification in the pathogenesis and management of alcoholic cardiomyopathy.
Acknowledgments
The research in the Ren laboratory is supported by NIH R15 AA13575, NIH/NIAAA R01 AA013412 and NIH P20RR016474. Editorial assistance from Ms. Kacy L. Richmond from the University of Wyoming is greatly appreciated. We sincerely express our apology to those authors whose important work cannot be cited here due to space limitation.
Abbreviations
- 1,2-GDN
1,2-glyceryl dinitrate
- 4-HNE
4-hydroxy-2-nonenal
- ADH
Alcohol dehydrogenase
- ALDH
Aldehyde dehydrogenase
- CYP2E1
Cytochrome P450 2E1
- DAPT
N-[N-(3,5-difluorophenacetyl)-1-alany1]-S-phenyglycine t-butyl ester
- ER
Endoplasmic reticulum
- GTN
Glyceryl trinitrate
- HUVEC
Human umbilical vein endothelial cells
- MMP
Mitochondrial membrane potential
- NAD+
Nicotinamide adenine dinucleotide
- ROS
Reactive oxygen species
- SERCA
Sarco(endo)plasmic reticulum Ca2+-ATPase
- SR
Sarcoplasmic reticulum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yingmei Zhang, Department of Cardiology, Xijing Hospital, Fourth Military Medical University, Xi'an, China 710032.
Jun Ren, Center for Cardiovascular Research and Alternative Medicine, University of Wyoming, Laramie, WY 82071, USA.
References
- Aberle IN, Ren J. Experimental Assessment of the Role of Acetaldehyde in Alcoholic Cardiomyopathy. Biol Proced Online. 2003;5:1–12. doi: 10.1251/bpo41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberle NS, Burd L, 2nd, Zhao BH, Ren J. Acetaldehyde-induced cardiac contractile dysfunction may be alleviated by vitamin B1 but not by vitamins B6 or B12. Alcohol Alcohol. 2004;39:450–4. doi: 10.1093/alcalc/agh085. [DOI] [PubMed] [Google Scholar]
- Aberle NS, 2nd, Picklo MJ, Sr, Amarnath V, Ren J. Inhibition of cardiac myocyte contraction by 4-hydroxy-trans-2-nonenal. Cardiovasc Toxicol. 2004;4:21–8. doi: 10.1385/ct:4:1:21. [DOI] [PubMed] [Google Scholar]
- Aberle NS, 2nd, Privratsky JR, Burd L, Ren J. Combined acetaldehyde and nicotine exposure depresses cardiac contraction in ventricular myocytes: prevention by folic acid. Neurotoxicol Teratol. 2003;25:731–6. doi: 10.1016/j.ntt.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Aberle NS, 2nd, Ren J. Short-term acetaldehyde exposure depresses ventricular myocyte contraction: role of cytochrome P450 oxidase, xanthine oxidase, and lipid peroxidation. Alcohol Clin Exp Res. 2003;27:577–83. doi: 10.1097/01.ALC.0000060522.40447.8E. [DOI] [PubMed] [Google Scholar]
- Aistrup GL, Kelly JE, Piano MR, Wasserstrom JA. Biphasic changes in cardiac excitation-contraction coupling early in chronic alcohol exposure. Am J Physiol Heart Circ Physiol. 2006;291:H1047–57. doi: 10.1152/ajpheart.00214.2006. [DOI] [PubMed] [Google Scholar]
- Arad M, Seidman CE, Seidman JG. AMP-activated protein kinase in the heart: role during health and disease. Circ Res. 2007;100:474–88. doi: 10.1161/01.RES.0000258446.23525.37. [DOI] [PubMed] [Google Scholar]
- Aroor AR, Shukla SD. MAP kinase signaling in diverse effects of ethanol. Life Sci. 2004;74:2339–64. doi: 10.1016/j.lfs.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Awtry EH, Philippides GJ. Alcoholic and cocaine-associated cardiomyopathies. Prog Cardiovasc Dis. 2010;52:289–99. doi: 10.1016/j.pcad.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Badger TM, Ronis MJ, Seitz HK, Albano E, Ingelman-Sundberg M, Lieber CS. Alcohol metabolism: role in toxicity and carcinogenesis. Alcohol Clin Exp Res. 2003;27:336–47. doi: 10.1097/01.ALC.0000052583.87673.37. [DOI] [PubMed] [Google Scholar]
- Bessembinders K, Wielders J, van de Wiel A. Severe Hypertriglyceridemia Influenced by Alcohol (SHIBA) Alcohol Alcohol. 2011 Jan; doi: 10.1093/alcalc/agq088. [DOI] [PubMed] [Google Scholar]
- Brown RA, Anthony MJ, Petrovski P, Ren J. The influence of gender, diabetes, and acetaldehyde on the intrinsic contractile properties of isolated rat myocardium. Cardiovasc Toxicol. 2001;1:35–42. doi: 10.1385/ct:1:1:35. [DOI] [PubMed] [Google Scholar]
- Brown RA, Carpentier RG. Effects of acetaldehyde on the automaticity of the guinea pig sinus node. Alcohol. 1989;6:103–7. doi: 10.1016/0741-8329(89)90033-5. [DOI] [PubMed] [Google Scholar]
- Brown RA, Carpentier RG. Effects of acetaldehyde on membrane potentials of sinus node pacemaker fibers. Alcohol. 1990;7:33–6. doi: 10.1016/0741-8329(90)90057-j. [DOI] [PubMed] [Google Scholar]
- Brown RA, Jefferson L, Sudan N, Lloyd TC, Ren J. Acetaldehyde depresses myocardial contraction and cardiac myocyte shortening in spontaneously hypertensive rats: role of intracellular Ca2+ Cell Mol Biol (Noisy-le-grand) 1999;45:453–65. [PubMed] [Google Scholar]
- Brown RA, Savage AO. Effects of acute acetaldehyde, chronic ethanol, and pargyline treatment on agonist responses of the rat aorta. Toxicol Appl Pharmacol. 1996;136:170–8. doi: 10.1006/taap.1996.0021. [DOI] [PubMed] [Google Scholar]
- Budas GR, Churchill EN, Disatnik MH, Sun L, Mochly-Rosen D. Mitochondrial import of PKCepsilon is mediated by HSP90: a role in cardioprotection from ischaemia and reperfusion injury. Cardiovasc Res. 2010;88:83–92. doi: 10.1093/cvr/cvq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budas GR, Disatnik MH, Mochly-Rosen D. Aldehyde dehydrogenase 2 in cardiac protection: a new therapeutic target? Trends Cardiovasc Med. 2009;19:158–64. doi: 10.1016/j.tcm.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L. Alcoholic cardiomyopathy: acetaldehyde, insulin insensitization and ER stress. J Mol Cell Cardiol. 2008;44:979–82. doi: 10.1016/j.yjmcc.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol. 2009;83:519–48. doi: 10.1007/s00204-009-0432-0. [DOI] [PubMed] [Google Scholar]
- Cederbaum AI, Wu D, Mari M, Bai J. CYP2E1-dependent toxicity and oxidative stress in HepG2 cells. Free Radic Biol Med. 2001;31:1539–43. doi: 10.1016/s0891-5849(01)00743-2. [DOI] [PubMed] [Google Scholar]
- Chan SM, Weng AP, Tibshirani R, Aster JC, Utz PJ. Notch signals positively regulate activity of the mTOR pathway in T-cell acute lymphoblastic leukemia. Blood. 2007;110:278–86. doi: 10.1182/blood-2006-08-039883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–5. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Sun L, Mochly-Rosen D. Mitochondrial aldehyde dehydrogenase and cardiac diseases. Cardiovasc Res. 2010;88:51–7. doi: 10.1093/cvr/cvq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Lu RB, Peng GS, Wang MF, Wang HK, Ko HC, Chang YC, Lu JJ, Li TK, Yin SJ. Alcohol metabolism and cardiovascular response in an alcoholic patient homozygous for the ALDH2*2 variant gene allele. Alcohol Clin Exp Res. 1999;23:1853–60. [PubMed] [Google Scholar]
- Chen YC, Peng GS, Wang MF, Tsao TP, Yin SJ. Polymorphism of ethanol-metabolism genes and alcoholism: correlation of allelic variations with the pharmacokinetic and pharmacodynamic consequences. Chem Biol Interact. 2009;178:2–7. doi: 10.1016/j.cbi.2008.10.029. [DOI] [PubMed] [Google Scholar]
- Chou WY, Stewart MJ, Carr LG, Zheng D, Stewart TR, Williams A, Pinaire J, Crabb DW. An A/G polymorphism in the promoter of mitochondrial aldehyde dehydrogenase (ALDH2): effects of the sequence variant on transcription factor binding and promoter strength. Alcohol Clin Exp Res. 1999;23:963–8. [PubMed] [Google Scholar]
- Clerk A, Cole SM, Cullingford TE, Harrison JG, Jormakka M, Valks DM. Regulation of cardiac myocyte cell death. Pharmacol Ther. 2003;97:223–61. doi: 10.1016/s0163-7258(02)00339-x. [DOI] [PubMed] [Google Scholar]
- Crabb DW, Matsumoto M, Chang D, You M. Overview of the role of alcohol dehydrogenase and aldehyde dehydrogenase and their variants in the genesis of alcohol-related pathology. Proc Nutr Soc. 2004;63:49–63. doi: 10.1079/pns2003327. [DOI] [PubMed] [Google Scholar]
- Dickson PA, James MR, Heath AC, Montgomery GW, Martin NG, Whitfield JB, Birley AJ. Effects of variation at the ALDH2 locus on alcohol metabolism, sensitivity, consumption, and dependence in Europeans. Alcohol Clin Exp Res. 2006;30:1093–100. doi: 10.1111/j.1530-0277.2006.00128.x. [DOI] [PubMed] [Google Scholar]
- Djousse L, Gaziano JM. Alcohol consumption and heart failure: a systematic review. Curr Atheroscler Rep. 2008;10:117–120. doi: 10.1007/s11883-008-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djousse L, Gaziano JM. Alcohol consumption and heart failure: a systematic review. Curr Atheroscler Rep. 2008;10:117–20. doi: 10.1007/s11883-008-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djousse L, Lee IM, Buring JE, Gaziano JM. Alcohol consumption and risk of cardiovascular disease and death in women: potential mediating mechanisms. Circulation. 2009;120:237–44. doi: 10.1161/CIRCULATIONAHA.108.832360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djousse L, Levy D, Benjamin EJ, Blease SJ, Russ A, Larson MG, Massaro JM, D'Agostino RB, Wolf PA, Ellison RC. Long-term alcohol consumption and the risk of atrial fibrillation in the Framingham Study. Am J Cardiol. 2004;93:710–3. doi: 10.1016/j.amjcard.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Doser TA, Turdi S, Thomas DP, Epstein PN, Li SY, Ren J. Transgenic overexpression of aldehyde dehydrogenase-2 rescues chronic alcohol intake-induced myocardial hypertrophy and contractile dysfunction. Circulation. 2009;119:1941–9. doi: 10.1161/CIRCULATIONAHA.108.823799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Druesne-Pecollo N, Tehard B, Mallet Y, Gerber M, Norat T, Hercberg S, Latino-Martel P. Alcohol and genetic polymorphisms: effect on risk of alcohol-related cancer. Lancet Oncol. 2009;10:173–80. doi: 10.1016/S1470-2045(09)70019-1. [DOI] [PubMed] [Google Scholar]
- Duan J, Esberg LB, Ye G, Borgerding AJ, Ren BH, Aberle NS, Epstein PN, Ren J. Influence of gender on ethanol-induced ventricular myocyte contractile depression in transgenic mice with cardiac overexpression of alcohol dehydrogenase. Comp Biochem Physiol A Mol Integr Physiol. 2003;134:607–14. doi: 10.1016/s1095-6433(02)00347-1. [DOI] [PubMed] [Google Scholar]
- Duan J, McFadden GE, Borgerding AJ, Norby FL, Ren BH, Ye G, Epstein PN, Ren J. Overexpression of alcohol dehydrogenase exacerbates ethanol-induced contractile defect in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2002;282:H1216–22. doi: 10.1152/ajpheart.00780.2001. [DOI] [PubMed] [Google Scholar]
- Espinet C, Argiles JM. Ethanol and acetaldehyde concentrations in the rat foeto-maternal system after an acute ethanol administration given to the mother. Arch Int Physiol Biochim. 1984;92:339–44. doi: 10.3109/13813458409080609. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sola J, Fatjo F, Sacanella E, Estruch R, Bosch X, Urbano-Marquez A, Nicolas JM. Evidence of apoptosis in alcoholic cardiomyopathy. Hum Pathol. 2006;37:1100–10. doi: 10.1016/j.humpath.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Folden DV, Gupta A, Sharma AC, Li SY, Saari JT, Ren J. Malondialdehyde inhibits cardiac contractile function in ventricular myocytes via a p38 mitogen-activated protein kinase-dependent mechanism. Br J Pharmacol. 2003;139:1310–6. doi: 10.1038/sj.bjp.0705384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao CM, Takezaki T, Wu JZ, Zhang XM, Cao HX, Ding JH, Liu YT, Li SP, Cao J, Matsuo K, Hamajima N, Tajima K. Polymorphisms of alcohol dehydrogenase 2 and aldehyde dehydrogenase 2 and colorectal cancer risk in Chinese males. World J Gastroenterol. 2008;14:5078–83. doi: 10.3748/wjg.14.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge W, Li Q, Turdi S, Wang XM, Ren J. Deficiency of insulin-like growth factor 1 reduces vulnerability to chronic alcohol intake-induced cardiomyocyte mechanical dysfunction: Role of AMPK. J Cell Mol Med. 2011 doi: 10.1111/j.1582-4934.2010.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge W, Ren J. mTOR-STAT3-Notch signaling contributes to ALDH2-induced protection against cardiac contractile dysfunction and autophagy under alcoholism. J Cell Mol Med. 2011 doi: 10.1111/j.1582-4934.2011.01347.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George A, Figueredo VM. Alcohol and arrhythmias: a comprehensive review. J Cardiovasc Med (Hagerstown) 2010;11:221–8. doi: 10.2459/JCM.0b013e328334b42d. [DOI] [PubMed] [Google Scholar]
- Gottlieb RA, Carreira RS. Autophagy in health and disease. 5. Mitophagy as a way of life. Am J Physiol Cell Physiol. 2010;299:C203–10. doi: 10.1152/ajpcell.00097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb RA, Mentzer RM. Autophagy during cardiac stress: joys and frustrations of autophagy. Annu Rev Physiol. 2010;72:45–59. doi: 10.1146/annurev-physiol-021909-135757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R, Ren J. Alcohol and acetaldehyde in public health: from marvel to menace. Int J Environ Res Public Health. 2010;7:1285–301. doi: 10.3390/ijerph7041285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R, Ren J. Alcohol dehydrogenase accentuates ethanol-induced myocardial dysfunction and mitochondrial damage in mice: role of mitochondrial death pathway. PLoS One. 2010;5:e8757. doi: 10.1371/journal.pone.0008757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R, Scott GI, Ren J. Involvement of AMPK in alcohol dehydrogenase accentuated myocardial dysfunction following acute ethanol challenge in mice. PLoS One. 2010;5:e11268. doi: 10.1371/journal.pone.0011268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R, Zhong L, Ren J. Overexpression of aldehyde dehydrogenase-2 attenuates chronic alcohol exposure-induced apoptosis, change in Akt and Pim signalling in liver. Clin Exp Pharmacol Physiol. 2009;36:463–8. doi: 10.1111/j.1440-1681.2009.05152.x. [DOI] [PubMed] [Google Scholar]
- Gurusamy N, Das DK. Autophagy, redox signaling, and ventricular remodeling. Antioxid Redox Signal. 2009a;11:1975–88. doi: 10.1089/ars.2009.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurusamy N, Das DK. Is autophagy a double-edged sword for the heart? Acta Physiol Hung. 2009b;96:267–76. doi: 10.1556/APhysiol.96.2009.3.2. [DOI] [PubMed] [Google Scholar]
- Hamer M, Malan L, Schutte AE, Huisman HW, van Rooyen JM, Schutte R, Fourie CM, Malan NT, Seedat YK. Conventional and behavioral risk factors explain differences in sub-clinical vascular disease between black and Caucasian South Africans: The SABPA study. Atherosclerosis. 2010 Dec; doi: 10.1016/j.atherosclerosis.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Hannuksela ML, Liisanantti MK, Savolainen MJ. Effect of alcohol on lipids and lipoproteins in relation to atherosclerosis. Crit Rev Clin Lab Sci. 2002;39:225–83. doi: 10.1080/10408360290795529. [DOI] [PubMed] [Google Scholar]
- Hao PP, Xue L, Wang XL, Chen YG, Wang JL, Ji WQ, Xu F, Wei SJ, Zhang Y. Association between aldehyde dehydrogenase 2 genetic polymorphism and serum lipids or lipoproteins: a meta-analysis of seven East Asian populations. Atherosclerosis. 2010;212:213–6. doi: 10.1016/j.atherosclerosis.2010.05.024. [DOI] [PubMed] [Google Scholar]
- Harada S, Agarwal DP, Goedde HW. Aldehyde dehydrogenase deficiency as cause of facial flushing reaction to alcohol in Japanese. Lancet. 1981;2:982. doi: 10.1016/s0140-6736(81)91172-7. [DOI] [PubMed] [Google Scholar]
- Hering D, Kucharska W, Kara T, Somers VK, Narkiewicz K. Potentiated sympathetic and hemodynamic responses to alcohol in hypertensive vs. normotensive individuals. J Hypertens. 2011 Jan; doi: 10.1097/HJH.0b013e328342b2a9. [DOI] [PubMed] [Google Scholar]
- Higashiyama A, Wakabayashi I, Ono Y, Watanabe M, Kokubo Y, Okayama A, Miyamoto Y, Okamura T. Association With Serum Gamma-Glutamyltransferase Levels and Alcohol Consumption on Stroke and Coronary Artery Disease: The Suita Study. Stroke. 2011 doi: 10.1161/STROKEAHA.110.608307. [DOI] [PubMed] [Google Scholar]
- Hintz KK, Relling DP, Saari JT, Borgerding AJ, Duan J, Ren BH, Kato K, Epstein PN, Ren J. Cardiac overexpression of alcohol dehydrogenase exacerbates cardiac contractile dysfunction, lipid peroxidation, and protein damage after chronic ethanol ingestion. Alcohol Clin Exp Res. 2003;27:1090–8. doi: 10.1097/01.ALC.0000075823.73536.DD. [DOI] [PubMed] [Google Scholar]
- Hishimoto A, Fukutake M, Mouri K, Nagasaki Y, Asano M, Ueno Y, Nishiguchi N, Shirakawa O. Alcohol and aldehyde dehydrogenase polymorphisms and risk for suicide: a preliminary observation in the Japanese male population. Genes Brain Behav. 2010;9:498–502. doi: 10.1111/j.1601-183X.2010.00577.x. [DOI] [PubMed] [Google Scholar]
- Iacovoni A, De Maria R, Gavazzi A. Alcoholic cardiomyopathy. J Cardiovasc Med (Hagerstown) 2010;11:884–92. doi: 10.2459/JCM.0b013e32833833a3. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Taguchi A, Yamamoto Y, Harada S. Evaluation of the use of self-reported facial flushing and ethanol patch test for ALDH2 genotypes. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2010;45:464–76. [PubMed] [Google Scholar]
- Isse T, Matsuno K, Oyama T, Kitagawa K, Kawamoto T. Aldehyde dehydrogenase 2 gene targeting mouse lacking enzyme activity shows high acetaldehyde level in blood, brain, and liver after ethanol gavages. Alcohol Clin Exp Res. 2005;29:1959–64. doi: 10.1097/01.alc.0000187161.07820.21. [DOI] [PubMed] [Google Scholar]
- Jaatinen PI, Saukko P, Sarviharju M, Kiianmaa K, Hervonen A. Effects of lifelong ethanol consumption on the ultrastructure and lipopigmentation of rat heart. Alcohol Alcohol. 1994;29:269–82. [PubMed] [Google Scholar]
- Jacob AJ, McLaren KM, Boon NA. Effects of abstinence on alcoholic heart muscle disease. Am J Cardiol. 1991;68:805–7. doi: 10.1016/0002-9149(91)90662-5. [DOI] [PubMed] [Google Scholar]
- Jankala H, Eriksson CJ, Eklund KK, Harkonen M, Maki T. Combined calcium carbimide and ethanol treatment induces high blood acetaldehyde levels, myocardial apoptosis and altered expression of apoptosis-regulating genes in rat. Alcohol Alcohol. 2002;37:222–8. doi: 10.1093/alcalc/37.3.222. [DOI] [PubMed] [Google Scholar]
- Jankala H, Eriksson CJP, Petersen NE, Harkonen M, Maki T. Role of acetaldehyde in the induction of heart left ventricular atrial natriuretic peptide gene expression in rats. Alcohol Alcohol. 2000;35:331–5. doi: 10.1093/alcalc/35.4.331. [DOI] [PubMed] [Google Scholar]
- Jeong KS, Soh Y, Jeng J, Felder MR, Hardwick JP, Song BJ. Cytochrome P450 2E1 (CYP2E1)-dependent production of a 37-kDa acetaldehyde-protein adduct in the rat liver. Arch Biochem Biophys. 2000;384:81–7. doi: 10.1006/abbi.2000.2119. [DOI] [PubMed] [Google Scholar]
- Jing L, Zhou LJ, Li WM, Zhang FM, Yuan L, Li S, Song J, Sang Y. Carnitine regulates myocardial metabolism by Peroxisome Proliferator-Activated Receptor-alpha (PPARalpha) in alcoholic cardiomyopathy. Med Sci Monit. 2011;17:BR1–9. doi: 10.12659/MSM.881311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo SA, Kim EK, Park MH, Han C, Park HY, Jang Y, Song BJ, Jo I. A Glu487Lys polymorphism in the gene for mitochondrial aldehyde dehydrogenase 2 is associated with myocardial infarction in elderly Korean men. Clin Chim Acta. 2007;382:43–7. doi: 10.1016/j.cca.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Jones WK. A murine model of alcoholic cardiomyopathy: a role for zinc and metallothionein in fibrosis. Am J Pathol. 2005;167:301–4. doi: 10.1016/S0002-9440(10)62975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloner RA, Rezkalla SH. To drink or not to drink? That is the question. Circulation. 2007;116:1306–17. doi: 10.1161/CIRCULATIONAHA.106.678375. [DOI] [PubMed] [Google Scholar]
- Koda K, Salazar-Rodriguez M, Corti F, Chan NY, Estephan R, Silver RB, Mochly-Rosen D, Levi R. Aldehyde dehydrogenase activation prevents reperfusion arrhythmias by inhibiting local renin release from cardiac mast cells. Circulation. 2010;122:771–81. doi: 10.1161/CIRCULATIONAHA.110.952481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CH, Frost RA, Summer AD, Vary TC. Molecular mechanisms responsible for alcohol-induced myopathy in skeletal muscle and heart. Int J Biochem Cell Biol. 2005;37:2180–95. doi: 10.1016/j.biocel.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Laonigro I, Correale M, Di Biase M, Altomare E. Alcohol abuse and heart failure. Eur J Heart Fail. 2009;11:453–62. doi: 10.1093/eurjhf/hfp037. [DOI] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Ren J. Cardiac overexpression of metallothionein rescues chronic alcohol intake-induced cardiomyocyte dysfunction: role of Akt, mammalian target of rapamycin and ribosomal p70s6 kinase. Alcohol Alcohol. 2006;41:585–92. doi: 10.1093/alcalc/agl080. [DOI] [PubMed] [Google Scholar]
- Li SY, Gilbert SA, Li Q, Ren J. Aldehyde dehydrogenase-2 (ALDH2) ameliorates chronic alcohol ingestion-induced myocardial insulin resistance and endoplasmic reticulum stress. J Mol Cell Cardiol. 2009;47:247–55. doi: 10.1016/j.yjmcc.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SY, Gomelsky M, Duan J, Zhang Z, Gomelsky L, Zhang X, Epstein PN, Ren J. Overexpression of aldehyde dehydrogenase-2 (ALDH2) transgene prevents acetaldehyde-induced cell injury in human umbilical vein endothelial cells: role of ERK and p38 mitogen-activated protein kinase. J Biol Chem. 2004;279:11244–52. doi: 10.1074/jbc.M308011200. [DOI] [PubMed] [Google Scholar]
- Li SY, Li Q, Shen JJ, Dong F, Sigmon VK, Liu Y, Ren J. Attenuation of acetaldehyde-induced cell injury by overexpression of aldehyde dehydrogenase-2 (ALDH2) transgene in human cardiac myocytes: role of MAP kinase signaling. J Mol Cell Cardiol. 2006;40:283–94. doi: 10.1016/j.yjmcc.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Li SY, Ren J. Cardiac overexpression of alcohol dehydrogenase exacerbates chronic ethanol ingestion-induced myocardial dysfunction and hypertrophy: role of insulin signaling and ER stress. J Mol Cell Cardiol. 2008;44:992–1001. doi: 10.1016/j.yjmcc.2008.02.276. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Liang Q, Carlson EC, Borgerding AJ, Epstein PN. A transgenic model of acetaldehyde overproduction accelerates alcohol cardiomyopathy. J Pharmacol Exp Ther. 1999;291:766–72. [PubMed] [Google Scholar]
- Lieber CS. Alcohol and fibrogenesis. Alcohol Alcohol Suppl. 1991;1:339–44. [PubMed] [Google Scholar]
- Ma H, Guo R, Yu L, Zhang Y, Ren J. Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury: role of autophagy paradox and toxic aldehyde. Eur Heart J. 2011;32:1025–38. doi: 10.1093/eurheartj/ehq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Li J, Gao F, Ren J. Aldehyde dehydrogenase 2 ameliorates acute cardiac toxicity of ethanol: role of protein phosphatase and forkhead transcription factor. J Am Coll Cardiol. 2009;54:2187–96. doi: 10.1016/j.jacc.2009.04.100. [DOI] [PubMed] [Google Scholar]
- Ma H, Yu L, Byra EA, Hu N, Kitagawa K, Nakayama KI, Kawamoto T, Ren J. Aldehyde dehydrogenase 2 knockout accentuates ethanol-induced cardiac depression: role of protein phosphatases. J Mol Cell Cardiol. 2010;49:322–9. doi: 10.1016/j.yjmcc.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Meng Y, Kwiatkowski DJ, Chen X, Peng H, Sun Q, Zha X, Wang F, Wang Y, Jing Y, Zhang S, Chen R, Wang L, Wu E, Cai G, Malinowska-Kolodziej I, Liao Q, Liu Y, Zhao Y, Sun Q, Xu K, Dai J, Han J, Wu L, Zhao RC, Shen H, Zhang H. Mammalian target of rapamycin regulates murine and human cell differentiation through STAT3/p63/Jagged/Notch cascade. J Clin Invest. 2010;120:103–14. doi: 10.1172/JCI37964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzo-Avalos S, Saavedra-Molina A. Cellular and mitochondrial effects of alcohol consumption. Int J Environ Res Public Health. 2010;7:4281–304. doi: 10.3390/ijerph7124281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough KH. Antioxidant nutrients and alcohol. Toxicology. 2003;189:89–97. doi: 10.1016/s0300-483x(03)00155-0. [DOI] [PubMed] [Google Scholar]
- Melis M, Diana M, Enrico P, Marinelli M, Brodie MS. Ethanol and acetaldehyde action on central dopamine systems: mechanisms, modulation, and relationship to stress. Alcohol. 2009;43:531–9. doi: 10.1016/j.alcohol.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales JA, Ram JL, Song J, Brown RA. Acetaldehyde inhibits current through voltage-dependent calcium channels. Toxicol Appl Pharmacol. 1997;143:70–4. doi: 10.1006/taap.1996.8072. [DOI] [PubMed] [Google Scholar]
- Niemela O. Aldehyde-protein adducts in the liver as a result of ethanol-induced oxidative stress. Front Biosci. 1999;4:D506–13. doi: 10.2741/niemela. [DOI] [PubMed] [Google Scholar]
- Niemela O. Distribution of ethanol-induced protein adducts in vivo: relationship to tissue injury. Free Radic Biol Med. 2001;31:1533–8. doi: 10.1016/s0891-5849(01)00744-4. [DOI] [PubMed] [Google Scholar]
- Nishida N, Tanaka M, Hayashi N, Nagata H, Takeshita T, Nakayama K, Morimoto K, Shizukuishi S. Association of ALDH(2) genotypes and alcohol consumption with periodontitis. J Dent Res. 2004;83:161–5. doi: 10.1177/154405910408300215. [DOI] [PubMed] [Google Scholar]
- Nishida N, Tanaka M, Sekine S, Takeshita T, Nakayama K, Morimoto K, Shizukuishi S. Association of ALDH2 genotypes with periodontitis progression. J Dent Res. 2010;89:138–42. doi: 10.1177/0022034509356045. [DOI] [PubMed] [Google Scholar]
- Nishimura FT, Fukunaga T, Kajiura H, Umeno K, Takakura H, Ono T, Nishijo H. Effects of aldehyde dehydrogenase-2 genotype on cardiovascular and endocrine responses to alcohol in young Japanese subjects. Auton Neurosci. 2002;102:60–70. doi: 10.1016/s1566-0702(02)00206-0. [DOI] [PubMed] [Google Scholar]
- Nuutinen H, Lindros KO, Salaspuro M. Determinants of blood acetaldehyde level during ethanol oxidation in chronic alcoholics. Alcohol Clin Exp Res. 1983;7:163–8. doi: 10.1111/j.1530-0277.1983.tb05432.x. [DOI] [PubMed] [Google Scholar]
- O'Keefe JH, Bybee KA, Lavie CJ. Alcohol and cardiovascular health: the razor-sharp double-edged sword. J Am Coll Cardiol. 2007;50:1009–14. doi: 10.1016/j.jacc.2007.04.089. [DOI] [PubMed] [Google Scholar]
- Oba T, Maeno Y, Ishida K. Differential contribution of clinical amounts of acetaldehyde to skeletal and cardiac muscle dysfunction in alcoholic myopathy. Curr Pharm Des. 2005;11:791–80. doi: 10.2174/1381612053381891. [DOI] [PubMed] [Google Scholar]
- Oba T, Maeno Y, Nagao M, Sakuma N, Murayama T. Cellular redox state protects acetaldehyde-induced alteration in cardiomyocyte function by modifying Ca2+ release from sarcoplasmic reticulum. Am J Physiol Heart Circ Physiol. 2008;294:H121–33. doi: 10.1152/ajpheart.00520.2007. [DOI] [PubMed] [Google Scholar]
- Ohira T, Tanigawa T, Tabata M, Imano H, Kitamura A, Kiyama M, Sato S, Okamura T, Cui R, Koike KA, Shimamoto T, Iso H. Effects of habitual alcohol intake on ambulatory blood pressure, heart rate, and its variability among Japanese men. Hypertension. 2009;53:13–9. doi: 10.1161/HYPERTENSIONAHA.108.114835. [DOI] [PubMed] [Google Scholar]
- Patel VB, Why HJ, Richardson PJ, Preedy VR. The effects of alcohol on the heart. Adverse Drug React Toxicol Rev. 1997;16:15–43. [PubMed] [Google Scholar]
- Pautassi RM, Camarini R, Quadros IM, Miczek KA, Israel Y. Genetic and environmental influences on ethanol consumption: perspectives from preclinical research. Alcohol Clin Exp Res. 2010;34:976–87. doi: 10.1111/j.1530-0277.2010.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng GS, Chen YC, Tsao TP, Wang MF, Yin SJ. Pharmacokinetic and pharmacodynamic basis for partial protection against alcoholism in Asians, heterozygous for the variant ALDH2*2 gene allele. Pharmacogenet Genomics. 2007;17:845–55. doi: 10.1097/FPC.0b013e3282609e67. [DOI] [PubMed] [Google Scholar]
- Peng GS, Wang MF, Chen CY, Luu SU, Chou HC, Li TK, Yin SJ. Involvement of acetaldehyde for full protection against alcoholism by homozygosity of the variant allele of mitochondrial aldehyde dehydrogenase gene in Asians. Pharmacogenetics. 1999;9:463–76. [PubMed] [Google Scholar]
- Peng GS, Yin JH, Wang MF, Lee JT, Hsu YD, Yin SJ. Alcohol sensitivity in Taiwanese men with different alcohol and aldehyde dehydrogenase genotypes. J Formos Med Assoc. 2002;101:769–74. [PubMed] [Google Scholar]
- Peng GS, Yin SJ. Effect of the allelic variants of aldehyde dehydrogenase ALDH2*2 and alcohol dehydrogenase ADH1B*2 on blood acetaldehyde concentrations. Hum Genomics. 2009;3:121–7. doi: 10.1186/1479-7364-3-2-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piano MR. Alcoholic cardiomyopathy: incidence, clinical characteristics, and pathophysiology. Chest. 2002;121:1638–50. doi: 10.1378/chest.121.5.1638. [DOI] [PubMed] [Google Scholar]
- Preedy VR, Adachi J, Peters TJ, Worrall S, Parkkila S, Niemela O, Asamo M, Ueno Y, Takeda K, Yamauchi M, Sakamoto K, Takagi M, Nakajima H, Toda G. Recent advances in the pathology of alcoholic myopathy. Alcohol Clin Exp Res. 2001;25:54S–59S. doi: 10.1097/00000374-200105051-00010. [DOI] [PubMed] [Google Scholar]
- Preedy VR, Crabb DW, Farres J, Emery PW. Alcoholic myopathy and acetaldehyde. Novartis Found Symp. 2007;285:158–77. doi: 10.1002/9780470511848.ch12. discussion 177-82, 198-9. [DOI] [PubMed] [Google Scholar]
- Ren J. Acetaldehyde and alcoholic cardiomyopathy: lessons from the ADH and ALDH2 transgenic models. Novartis Found Symp. 2007;285:69–76. doi: 10.1002/9780470511848.ch5. discussion 76-9, 198-9. [DOI] [PubMed] [Google Scholar]
- Ren J, Babcock SA, Li Q, Huff AF, Li SY, Doser TA. Aldehyde dehydrogenase-2 transgene ameliorates chronic alcohol ingestion-induced apoptosis in cerebral cortex. Toxicol Lett. 2009;187:149–56. doi: 10.1016/j.toxlet.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Davidoff AJ, Brown RA. Acetaldehyde depresses shortening and intracellular Ca2+ transients in adult rat ventricular myocytes. Cell Mol Biol (Noisy-le-grand) 1997;43:825–34. [PubMed] [Google Scholar]
- Ren J, Wang GJ, Petrovski P, Ren BH, Brown RA. Influence of hypertension on acetaldehyde-induced vasorelaxation in rat thoracic aorta. Pharmacol Res. 2002;45:195–9. doi: 10.1006/phrs.2002.0947. [DOI] [PubMed] [Google Scholar]
- Ren J, Wold LE. Mechanisms of alcoholic heart disease. Ther Adv Cardiovasc Dis. 2008;2:497–506. doi: 10.1177/1753944708095137. [DOI] [PubMed] [Google Scholar]
- Richardson PJ, Patel VB, Preedy VR. Alcohol and the myocardium. Novartis Found Symp. 1998;216:35–45. doi: 10.1002/9780470515549.ch4. discussion 45-50. [DOI] [PubMed] [Google Scholar]
- Romanowicz M, Schmidt JE, Bostwick JM, Mrazek DA, Karpyak VM. Changes in Heart Rate Variability Associated With Acute Alcohol Consumption: Current Knowledge and Implications for Practice and Research. Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2011.01442.x. [DOI] [PubMed] [Google Scholar]
- Russo D, Purohit V, Foudin L, Salin M. Workshop on Alcohol Use and Health Disparities 2002: a call to arms. Alcohol. 2004;32:37–43. doi: 10.1016/j.alcohol.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Savage AO, Dunbar JC, Brown RA. Effects of acetaldehyde on the isolated papillary muscle of diabetic rats. J Cardiovasc Pharmacol. 1995;26:251–8. [PubMed] [Google Scholar]
- Schoppet M, Maisch B. Alcohol and the heart. Herz. 2001;26:345–52. doi: 10.1007/pl00002037. [DOI] [PubMed] [Google Scholar]
- Sciarretta S, Hariharan N, Monden Y, Zablocki D, Sadoshima J. Is Autophagy in Response to Ischemia and Reperfusion Protective or Detrimental for the Heart? Pediatr Cardiol. 2010 doi: 10.1007/s00246-010-9855-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiva FR, Amauchi JF, Rocha KK, Ebaid GX, Souza G, Fernandes AA, Cataneo AC, Novelli EL. Alcoholism and alcohol abstinence: N-acetylcysteine to improve energy expenditure, myocardial oxidative stress, and energy metabolism in alcoholic heart disease. Alcohol. 2009;43:649–56. doi: 10.1016/j.alcohol.2009.09.028. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Sakai A, Menuki K, Mori T, Isse T, Oyama T, Kawamoto T, Nakamura T. Reduced bone formation in alcohol-induced osteopenia is associated with elevated p21 expression in bone marrow cells in aldehyde dehydrogenase 2-disrupted mice. Bone. 2011 Jan; doi: 10.1016/j.bone.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Siddiq T, Richardson PJ, Mitchell WD, Teare J, Preedy VR. Ethanol-induced inhibition of ventricular protein synthesis in vivo and the possible role of acetaldehyde. Cell Biochem Funct. 1993;11:45–54. doi: 10.1002/cbf.290110106. [DOI] [PubMed] [Google Scholar]
- Skotzko CE, Vrinceanu A, Krueger L, Freudenberger R. Alcohol use and congestive heart failure: incidence, importance, and approaches to improved history taking. Heart Fail Rev. 2007 doi: 10.1007/s10741-007-9048-8. [DOI] [PubMed] [Google Scholar]
- Skotzko CE, Vrinceanu A, Krueger L, Freudenberger R. Alcohol use and congestive heart failure: incidence, importance, and approaches to improved history taking. Heart Fail Rev. 2009;14:51–5. doi: 10.1007/s10741-007-9048-8. [DOI] [PubMed] [Google Scholar]
- Sokolova NA, Chudakov LI, Ashmarin IP, Vinogradova TM, Volodin ND, Vlasov GP, Nikonova IN. The positive chronotropic effects of salsolinol on the isolated rat heart. Fiziol Zh SSSR Im I M Sechenova. 1990;76:1043–7. [PubMed] [Google Scholar]
- Spies CD, Sander M, Stangl K, Fernandez-Sola J, Preedy VR, Rubin E, Andreasson S, Hanna EZ, Kox WJ. Effects of alcohol on the heart. Curr Opin Crit Care. 2001;7:337–43. doi: 10.1097/00075198-200110000-00004. [DOI] [PubMed] [Google Scholar]
- Takeshita T, Yang X, Morimoto K. The ALDH2 genotype, alcohol intake, and liver-function biomarkers among Japanese male workers. Hum Genet. 2000;106:589–93. doi: 10.1007/s004390000314. [DOI] [PubMed] [Google Scholar]
- Talhout R, Opperhuizen A, van Amsterdam JG. Role of acetaldehyde in tobacco smoke addiction. Eur Neuropsychopharmacol. 2007;17:627–36. doi: 10.1016/j.euroneuro.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Tominaga Y. Use of acetaldehyde and methanol as markers of alcohol abuse and their measurement. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2009;44:26–37. [PubMed] [Google Scholar]
- Tsukamoto S, Muto T, Nagoya T, Shimamura M, Saito M, Tainaka H. Determinations of ethanol, acetaldehyde and acetate in blood and urine during alcohol oxidation in man. Alcohol Alcohol. 1989;24:101–8. doi: 10.1093/oxfordjournals.alcalc.a044872. [DOI] [PubMed] [Google Scholar]
- Turcotte LA, Aberle NS, Norby FL, Wang GJ, Ren J. Influence of prenatal ethanol exposure on vascular contractile response in rat thoracic aorta. Alcohol. 2002;26:75–81. doi: 10.1016/s0741-8329(01)00198-7. [DOI] [PubMed] [Google Scholar]
- Uchida K. Role of reactive aldehyde in cardiovascular diseases. Free Radic Biol Med. 2000;28:1685–96. doi: 10.1016/s0891-5849(00)00226-4. [DOI] [PubMed] [Google Scholar]
- Vary TC, Deiter G, Lantry R. Chronic alcohol feeding impairs mTOR(Ser 2448) phosphorylation in rat hearts. Alcohol Clin Exp Res. 2008;32:43–51. doi: 10.1111/j.1530-0277.2007.00544.x. [DOI] [PubMed] [Google Scholar]
- Vary TC, Summer AD. Deleterious effects of alcohol intoxication on heart: arrhymias to cardiomyopathies. In: Watson RR, Preedy V, editors. Nutrition and Alcohol: Linking Nutrient Interactions and Dietary Intake. CRC Press; Boca Raton: 2004. pp. 117–141. [Google Scholar]
- Vaswani M, Prasad P, Kapur S. Association of ADH1B and ALDH2 gene polymorphisms with alcohol dependence: a pilot study from India. Hum Genomics. 2009;3:213–20. doi: 10.1186/1479-7364-3-3-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Konig R, Ansari GA, Khan MF. Lipid peroxidation-derived aldehyde-protein adducts contribute to trichloroethene-mediated autoimmunity via activation of CD4+ T cells. Free Radic Biol Med. 2008;44:1475–82. doi: 10.1016/j.freeradbiomed.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhou Z, Saari JT, Kang YJ. Alcohol-induced myocardial fibrosis in metallothionein-null mice: prevention by zinc supplementation. Am J Pathol. 2005;167:337–44. doi: 10.1016/S0002-9440(10)62979-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A, Kobayashi M, Hobara N, Nakatsukasa H, Nagashima H, Fujimoto A. A report of unusually high blood ethanol and acetaldehyde levels in two surviving patients. Alcohol Clin Exp Res. 1985;9:14–6. doi: 10.1111/j.1530-0277.1985.tb05040.x. [DOI] [PubMed] [Google Scholar]
- Worrall S, Niemela O, Parkkila S, Peters TJ, Preedy VR. Protein adducts in type I and type II fibre predominant muscles of the ethanol-fed rat: preferential localisation in the sarcolemmal and subsarcolemmal region. Eur J Clin Invest. 2001;31:723–30. doi: 10.1046/j.1365-2362.2001.00848.x. [DOI] [PubMed] [Google Scholar]
- Wu D, Cederbaum AI. Oxidative stress and alcoholic liver disease. Semin Liver Dis. 2009;29:141–54. doi: 10.1055/s-0029-1214370. [DOI] [PubMed] [Google Scholar]
- Wu D, Wang X, Zhou R, Cederbaum A. CYP2E1 enhances ethanol-induced lipid accumulation but impairs autophagy in HepG2 E47 cells. Biochem Biophys Res Commun. 2010;402:116–22. doi: 10.1016/j.bbrc.2010.09.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Ma YT, Yang YN, Fu ZY, Ma X, Huang D, Li XM, Chen BD, Liu F, Huang Y, Liu C, Zhang XL, Zheng YY, Baituola G, Wang BZ, Du L, Gao X. Alcohol consumption and carotid atherosclerosis in China: the Cardiovascular Risk Survey. Eur J Cardiovasc Prev Rehabil. 2011 doi: 10.1177/1741826711404501. [DOI] [PubMed] [Google Scholar]
- Yamaguchi J, Hasegawa Y, Kawasaki M, Masui T, Kanoh T, Ishiguro N, Hamajima N. ALDH2 polymorphisms and bone mineral density in an elderly Japanese population. Osteoporos Int. 2006;17:908–13. doi: 10.1007/s00198-006-0077-2. [DOI] [PubMed] [Google Scholar]
- Yang SJ, Yokoyama A, Yokoyama T, Huang YC, Wu SY, Shao Y, Niu J, Wang J, Liu Y, Zhou XQ, Yang CX. Relationship between genetic polymorphisms of ALDH2 and ADH1B and esophageal cancer risk: a meta-analysis. World J Gastroenterol. 2010;16:4210–20. doi: 10.3748/wjg.v16.i33.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin SJ, Peng GS. Acetaldehyde, polymorphisms and the cardiovascular system. Novartis Found Symp. 2007;285:52–63. doi: 10.1002/9780470511848.ch4. discusion 63-8, 198-9. [DOI] [PubMed] [Google Scholar]
- Yokoyama A, Omori T, Yokoyama T. Alcohol and aldehyde dehydrogenase polymorphisms and a new strategy for prevention and screening for cancer in the upper aerodigestive tract in East Asians. Keio J Med. 2010;59:115–30. doi: 10.2302/kjm.59.115. [DOI] [PubMed] [Google Scholar]
- Yu HS, Oyama T, Isse T, Kitagawa K, Pham TT, Tanaka M, Kawamoto T. Formation of acetaldehyde-derived DNA adducts due to alcohol exposure. Chem Biol Interact. 2010;188:367–75. doi: 10.1016/j.cbi.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Zhang B, Turdi S, Li Q, Lopez FL, Eason AR, Anversa P, Ren J. Cardiac overexpression of insulin-like growth factor 1 attenuates chronic alcohol intake-induced myocardial contractile dysfunction but not hypertrophy: Roles of Akt, mTOR, GSK3beta, and PTEN. Free Radic Biol Med. 2010;49:1238–53. doi: 10.1016/j.freeradbiomed.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Klein AL, Alberle NS, Norby FL, Ren BH, Duan J, Ren J. Cardiac-specific overexpression of catalase rescues ventricular myocytes from ethanol-induced cardiac contractile defect. J Mol Cell Cardiol. 2003;35:645–52. doi: 10.1016/s0022-2828(03)00080-4. [DOI] [PubMed] [Google Scholar]
- Zhang X, Li SY, Brown RA, Ren J. Ethanol and acetaldehyde in alcoholic cardiomyopathy: from bad to ugly en route to oxidative stress. Alcohol. 2004;32:175–86. doi: 10.1016/j.alcohol.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ren J. Autophagy in ALDH2-elicited cardioprotection against ischemic heart disease: slayer or savior? Autophagy. 2010;6:1212–3. doi: 10.4161/auto.6.8.13652. [DOI] [PubMed] [Google Scholar]


