Abstract
We hypothesize that a visual prosthesis capable of evoking high-resolution visual perceptions can be produced using high-electrode-count arrays of penetrating microelectrodes implanted into the primary visual cortex of a blind human subject. To explore this hypothesis, and as a prelude to human psychophysical experiments, we have conducted a set of experiments in primary visual cortex (V1) of non-human primates using chronically implanted Utah Electrode Arrays (UEAs). The electrical and recording properties of implanted electrodes, the high-resolution visuotopic organization of V1, and the stimulation levels required to evoke behavioural responses were measured. The impedances of stimulated electrodes were found to drop significantly immediately following stimulation sessions, but these post-stimulation impedances returned to pre-stimulation values by the next experimental session. Two months of periodic microstimulation with currents of up to 96 μA did not impair the mapping of receptive fields from local field potentials or multi-unit activity, or impact behavioural visual thresholds of light stimuli that excited regions of V1 that were implanted with UEAs. These results demonstrate that microstimulation at the levels used did not cause functional impairment of the electrode array or the neural tissue. However, microstimulation with current levels ranging from 18 to 76 μA (46±19 μA, mean±std) was able to elicit behavioural responses on 8 out of 82 systematically stimulated electrodes. We suggest that the ability of microstimulation to evoke phosphenes and elicit a subsequent behavioural response may depend on several factors: the location of the electrode tips within the cortical layers of V1, distance of the electrode tips to neuronal somata, and the inability of nonhuman primates to recognize and respond to a generalized set of evoked percepts.
1. Introduction
The concept of a visual prosthesis to restore vision to individuals with profound blindness by electrically stimulating primary visual cortex (V1) was initially explored with experiments conducted in human patients [1, 2, 3]. These studies demonstrated that individual points of light, known as phosphenes, could be evoked by stimulating the surface of V1 via an array of macroelectrodes. Using this technique, they confirmed the global visuotopic organization of V1, and that subjects could assimilate spatial patterns of phosphenes with simultaneous stimulation via multiple macroelectrodes. However, large currents, in the milliampere range, were required to evoke individual phosphenes. Such large currents, when passed through groups of neighboring electrodes, caused non-linear interactions between the location and character of the evoked phosphenes and resulted in seizures in at least one patient [4]. Other researchers found that using penetrating intracortical microelectrodes to stimulate V1 in human patients evoked phosphenes at currents a few orders of magnitude lower than those required for surface stimulation using macroelectrodes [5, 6]. These studies demonstrated the ability of electrical stimulation of V1 to evoke subjective visual perceptions in human patients, but were constrained by the difficulty of performing research in human patients and the technological limitations of the time.
Additional insight into intracortical microstimulation of V1 has come from acute studies performed in nonhuman primates (NHPs). It was demonstrated that NHPs could be trained on a reaction time task using photic stimulation and continue to perform the task in response to intracortical microstimulation of visual cortex. This reaction time task was used to optimize microstimulation parameters for evoking behavioural responses [7]. Another extensive study explored the effectiveness of various V1 intracortical microstimulation parameters in evoking phosphenes [8]. However, these acute studies did not address the chronic performance and stability of V1 microstimulation; factors important in the eventual development of a human visual prosthesis. In a contemporaneous study [9], V1 of NHPs were chronically implanted with a large number of microelectrodes. NHPs made saccades to phosphenes evoked by microstimulation via these electrodes. Additionally, the spatial location of the phosphene was similar to the spatial location of the receptive field (RF) mapped from neurons recorded on that electrode. These studies showed that NHPs, in particular macaque monkeys, can serve as a good animal model for studying the psychophysics of microstimulation evoked phosphenes.
Several human studies have examined using a fixed-geometry penetrating intracortical microelectrode array, such as the Utah Electrode Array (UEA), for motor prosthetic applications [10, 11, 12, 13, 14, 15]. However, none of the human or NHP studies to date have characterized the chronic safety and efficacy of microstimulation via such any array for sensory prosthetic applications. In moving towards this goal, we obtained high-resolution visuotopic mapping of V1 by measuring the RFs of both the multi-unit activity (MUA) and local field potentials (LFPs) across UEAs over periods of several months. The in vivo impedances and the quality of the recorded neural signals and RFs were measured over time to examine the functionality of the UEAs and the implanted neural tissue. We found that microstimulation at levels required to evoke behavioural responses in the NHPs did not result in functional impairment of the UEAs or the neural tissue. However, stimuli delivered via only a small number of electrodes were able to evoke phosphenes to which the NHPs would respond. We discuss several potential challenges of using intracortical microstimulation as the basis of a visual prosthesis and the use of NHPs as a model for a human visual prosthesis.
2. Methods
All surgical and experimental procedures were performed in accordance with the guidelines of the U.S. Department of Agriculture and were approved by the University of Utah’s Institutional Animal Care and Use Committee.
2.1. Instrumentation
Visual stimuli were generated using a real-time visual stimulator (ViSaGe, Cambridge Research Systems, Rochester, Kent, England) and displayed on a CRT monitor (G90fb, ViewSonic, Walnut, CA). The NHPs were head-fixed using a minimally invasive technique [16] and their eye positions were tracked with an infrared camera (1 kHz sampling rate, EyeLink 1000, SR Research, Mississauga, ON, Canada). Hand position was monitored and behavioural responses were registered with capacitive switches, which detected the presence of the NHP’s hand when it was within a few millimeters of the sensor.
2.2. Behavioural tasks
The NHP was placed in a primate chair inside of a dark chamber to dark-adapt. After 25 to 35 minutes, it was required to place each of its hands on a capacitance switch, one to its left and the other to its right. A small crosshair would then appear in the center of the CRT screen. Once the NHP directed its gaze within one degree of the center of the crosshair, the crosshair would change to a 0.1×0.1 visual degrees box indicating the start of the trial. The NHP had to maintain its gaze within one degree of the center of the box, or the trial was aborted.
For photic and electric stimulation tasks, stimuli were presented after fixation was maintained for a randomized duration (400–800ms). Photic stimuli were round, monochromatic Gaussian shapes with a diameter of one visual degree. Luminance levels were varied between 0 and 10,000 μcd/m2 with step sizes adapted in order to obtain several data points on the psychometric curve. The parameters of electrical microstimulation are described in Methods 2.6. Stimulus presentation was followed by a second randomized hold duration, and then an auditory cue was given indicating that the NHP should now make its response. The NHP would then respond by removing its right hand to indicate that it did not perceive, or its left hand to indicate that it did perceive, a stimulus. Photic threshold trials were rewarded with a bolus of juice 50% of the time regardless of the answer. Microstimulation trials were sparsely introduced into an ongoing photic task and were rewarded with juice 100% of the time regardless of the answer. Clearly visible or invisible catch photic trials were used to ensure that the animal was responding truthfully, and it was rewarded only if the correct response was made without any of the task constraints being violated. Using this forced-choice detection task, the NHP was required to indicate whether or not it perceived visual stimuli of varying luminance values, and phosphenes evoked by varying current amplitudes. Five or more responses to stimuli presented at a minimum of three intensity levels that spanned threshold (i.e. a minimum of 15 total responses) was considered a valid or consistent behavioural response. Psychometric data were fit using a Weibull cumulative distribution function, which was used to estimate response thresholds (intensity value at 50% probability of detection).
For the RF mapping task, the NHP had to maintain fixation while visual stimuli were presented. The animal was rewarded as long as it continued to maintain fixation and none of the task constraints were violated.
2.3. Subjects and surgery
Two adult male rhesus macaques (Macaca Mulatta) were used in these experiments and they are referred to as NHP1 and NHP2 in subsequent text. We followed human protocols during the surgeries and postoperative care. Two Cereport arrays were implanted (figure 1a) in V1 of each NHP using a pneumatic inserter [17]. Reference wires were placed in the subdural space. For NHP1, Array1 was implanted anterior to the calcarine fissure and lateral to the sagittal sulcus. Array2 was implanted anterior to the first array and lateral to the sagittal sulcus. This anatomical area was expected to represent the lower right visual quadrant of the visual field close to the vertical meridian at about eight degrees of eccentricity [18]. For NHP2, Array1 was implanted anterior to the calcarine fissure and lateral to the sagittal sulcus, and Array2 was implanted lateral to Array1. This anatomical area was expected to represent the visual field near the horizontal meridian from 2.5 to 9 degrees of eccentricity.
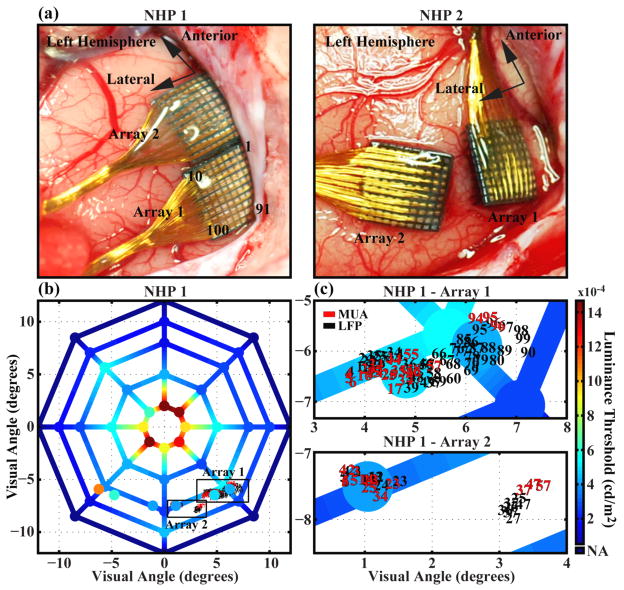
Figure 1.
Comparison of the anatomic and visuotopic locations of the electrode arrays and the spatial distribution of luminance thresholds across visual space for a behaving non-human primate. (a) Placement of arrays in cortex for NHP 1 and NHP 2. Arrays were placed in the left occipital cortex near the calcarine fissure and lateral to the sagittal sulcus for both animals. Electrode numbering scheme (with respect to lead wire location) is shown and is the same for all arrays. (b) Luminance threshold values obtained from NHP 1 for points across visual space. Values for spatial locations at the vertices of the plot were obtained before implantation of the arrays. Values located in the lower left and lower right quadrants of the plot that are not located at the vertices were obtained after the completion of all microstimulation experiments more than 290 days post-implantation. The center of the plot represents the fixation point. MUA-RF and LFP-RF center locations on each array are superimposed with the number indicating the electrode upon which they were recorded. (c) Enlarged views of the boxed regions in (b) showing RF center locations. Data was obtained from an experimental session five months post-implantation.
After a week of data collection, NHP2 damaged its connectors. Limited data was collected from this animal and is included in figure 6a. NHP1 was implanted with two arrays (Array1 and Array2), but the quality of the recordings from Array2 was poor and, with the exception of data in figures 4c and 6c, it was not included in this work. Neither one of the NHPs exhibited any observable signs of neurological deficiencies throughout the course of the experiments.
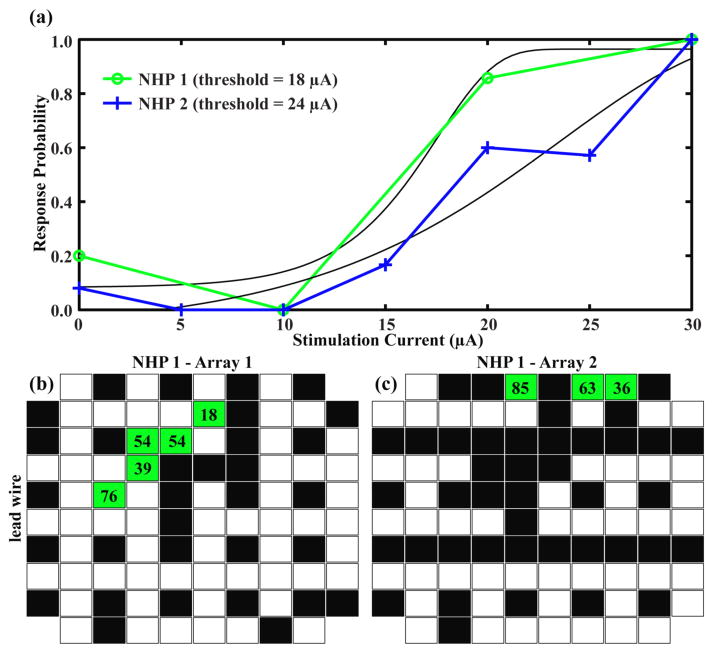
Figure 6.
Effective microstimulation occurred on electrodes in the same region of the array where the largest MUA and the negative-going visually-evoked LFP responses were recorded. (a) Behavioral responses to microstimulation of a single electrode in each of two NHPs. Each data point represents the average of ~5 yes/no responses to that level of stimulation. Stimulation parameters for both animals consisted of a train of 40 cathodic-first pulses of width 200 μs and frequency 200 Hz. The green psychometric curve was generated from data collected from NHP 1 by stimulation via electrode 15 on Array 1. The blue psychometric curve was generated from data collected from NHP 2 by stimulation via electrode 56 on Array 1. Black curves are the non-linear fits of the Weibull function to the data. Thresholds are taken at the 50% response probability. (b) Distribution of stimulated electrodes and the resulting behavioral responses across Array 1 for NHP 1. Black boxes represent electrodes that were stimulated up to 96 μA and did not elicit a behavioral response. Green boxes show electrodes that generated psychometric curves in response to stimulation. The numbers in each box represent the lowest current threshold acquired for that electrode. White boxes were never stimulated. (c) Same plot as (b) for NHP 1 Array 2.
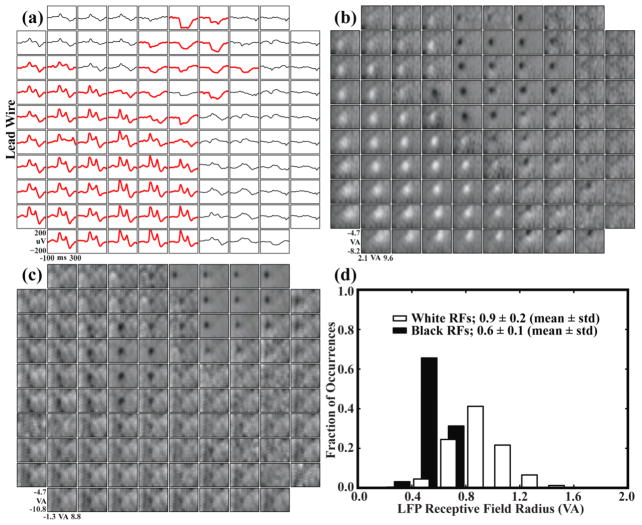
Figure 4.
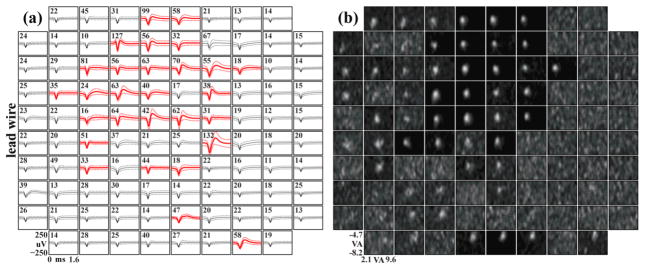
Visually-evoked LFP recordings and LFP-RFs demonstrated an amplitude phase reversal across the array. The region of the array with negative-going LFP responses correlated with the region where the largest MUA was observed. (a) Largest visually-evoked LFP for each electrode compiled from ten recording sessions spanning three months. Each waveform represents the average of ~50 LFP responses to a visual stimulus. Responses with a peak-to-peak amplitude greater than 75 μV are shown in bold red. Stimulus onset occurred at time zero. A shift from a positive to a negative-going response can be seen when moving diagonally across the array. (b) Each box represents a region of visual space vertically from −4.7 to −8.2 visual degrees and horizontally from 2.1 to 9.6 visual degrees with the fixation point near the upper left corner. Pixel intensities represent the area under the evoked LFP response for a stimulus at that location in visual space. White RFs were generated from positive-going LFP responses and black RFs from negative-going responses. (c) LFP-RFs across Array 2. An amplitude phase reversal was not observed for this array. (d) LFP-RF sizes for Array 1 compiled from ten recording sessions over three months and separated into black (negative-going LFP) versus white (positive-going LFP) responses. RFs were fit to a Gaussian curve, and the radius represents the standard deviation of the fit. Black RFs were significantly smaller than white RFs.
2.4. Microelectrode arrays
Fixed-geometry arrays of penetrating microelectrodes were used for neural recording and microstimulation (Cereport Array (UEA), Blackrock Microsystems, Salt Lake City, UT). These arrays have been successfully implanted in the motor cortex [19, 20, 21, 22], the auditory cortex [23, 24], the auditory nerve [25, 26, 27], the sciatic nerve [28], and the visual cortex [29, 30] of various animal models. Moreover, these electrodes have been acutely implanted in the middle temporal gyrus of epilepsy patients undergoing temporal lobectomy surgery [31, 32, 33, 34, 35, 36] and chronically implanted in the motor cortex of paralyzed human patients [10, 11, 12, 13, 14, 15].
Each array consisted of one hundred 1 mm long microelectrodes arranged in a 10×10 grid spaced 400 μm apart. The length of the electrode was chosen so that the electrode tip would reside approximately in layer 4A/B of V1 [37, 38]. Each electrode base is electrically isolated from its neighboring electrodes with glass and the rest of the electrode, with the exception of the iridium-oxide tip, is insulated with a 2-μm coat of parylene-C. Each electrode tip is metalized with Sputtered Iridium Oxide Film (SIROF) for better charge injection capacity. The electrodes have been manufactured to have an exposed tip length of 60±40 μm, resulting in an active geometrical surface area of 500–4000 μm2 (Personal communication, Blackrock Microsystems, Salt Lake City, UT). Electrode impedances ranged from 40 kΩ to 80 kΩ before implantation as measured with a 1 kHz sine-wave constant current signal (Cerebus, Blackrock Microsystems, Salt Lake City, UT). Median impedances were used for analysis after outliers (i.e. impedances greater than 2 MΩ) were discarded.
2.5. Data collection
Multi-unit activity (MUA), local field potentials (LFP), and behavioural data were recorded using a 128-channel data acquisition system (Cerebus, Blackrock Microsystems, Salt Lake City, UT). The continuous signal was sampled at 30 kHz and band-pass filtered with cutoff frequencies of 0.3 Hz and 7500 Hz. This signal was digitally low-pass filtered at 250 Hz and was used for LFP analysis. It was digitally high-pass filtered at 250 Hz, and a threshold value of −3.5 RMS times the noise was used to obtain MUAs.
2.6. Microstimulation
For microstimulation experiments, we used a system capable of delivering electrical currents to 96 electrodes (RX7, Tucker-Davis Technologies, Alachua, FL). The stimulator battery had a compliance voltage of ±24 V (NC48, Tucker-Davis Technologies, Alachua, FL). The stimulators were capable of delivering currents up to 100 μA to each electrode.
Constant-current pulses were delivered to V1 through the individual electrodes on the UEA. Each pulse consisted of a cathodic-first symmetric biphasic square wave with no interphase interval and a phase width of 200 μsec. Pulses were delivered at 200 Hz for a duration of 200 ms. Current amplitudes ranged from 0 to 96 μA. At these stimulation levels, 1.0–19.2 nC/phase was delivered to the tissue through each electrode. The charge density for each electrode ranged from 50–960 μC/cm2 for an electrode tip geometrical surface area of 2000 μm2. Similar stimulation parameters evoked phosphenes in a human patient [5] and behavioural responses in macaque monkeys [9].
Systematic microstimulation was performed on a subset of 37 electrodes on Array1 and 45 electrodes on Array2 in NHP1. Initially, electrodes that were chosen for microstimulation were evenly distributed across the arrays. Later, more electrodes were selected for microstimulation based on their ability to record neural signals. Since these electrodes were not part of the initial subset, the total number of electrodes that were stimulated is different for each array.
2.7. Receptive Field Mapping
For RF mapping, the display was calibrated with a photometer (L203, Macam Photometrics LTD, Tranent, East Lothian, England) so that the white intensity level was set to 84.2 cd/m2 and the background level was set to 41.9 cd/m2. RF properties of each electrode were measured by flashing a small (0.45×0.45 visual degrees) white square at random locations on the screen against a gray background. The stimulus was flashed every 100 ms for a trial duration of 1000 or 2000 ms. RF maps from MUA (MUA-RF) were generated by reverse correlation of the visual stimuli with action potential event times [39] for 21 temporal offsets (0, 5, 10, …, 100 ms). The amplitude of the RF response for each offset was calculated by dividing the value of the peak response by the mean of the background. The five largest RF responses of the 21 temporal offsets were averaged to create the RF map. From this RF map, responses with amplitudes greater than 10 are defined as large. RF maps were also generated from stimulus evoked LFPs by forward correlation of the stimulus presentations to the response of the LFP signal. Specifically, for each location at which a stimulus was presented, the average evoked LFP response was integrated from 0 to 200 ms after stimulus onset. The LFP-RF was defined as the distribution of the integrated LFP responses for all spatial locations at which stimuli were presented.
2.8. Analysis of Visuotopic Organization
The conformal nature of the visuotopic organization of V1 was estimated. A non-linear, least-mean-square method was used to minimize the distances between the actual RF centers and the coordinate transforms of each array into visual space. The coordinate transformation allowed for five degrees of freedom: magnification along both sides of the array, rotation of the array, and translation along both the horizontal and vertical axes [30]. The degree of conformality was estimated as the average length of the vector connecting each RF center to the transformed location of the electrode on which the RF was measured.
3. Results
We examined a number of physiological and device performance factors that could influence the development of a high-resolution cortically-based visual prosthesis.
3.1. Validation of primate behaviour and functional status of implant sites
To ensure that the NHPs could correctly report visual percepts, and to verify that their visual systems remained functional post-implantation, they were trained on a forced-choice photic detection task prior to implantation. The NHPs were required to indicate whether they were able to perceive photic stimuli at various luminance intensities. Threshold values that were obtained from the NHPs were compared to those obtained from human subjects. The visual detection thresholds for five human subjects for a stimulus located in the right visual hemisphere at an eccentricity of two degrees along the horizontal meridian ranged from 520 to 1750 μcd/m2 (946±493 μcd/m2, mean±std). Using the same task and stimuli, the thresholds obtained for NHP1 and NHP2 were 960 μcd/m2 and 1480 μcd/m2, respectively, indicating that the NHPs were able to perform the detection task at a level comparable to human subjects. For NHP1, luminance thresholds were also obtained for visual stimuli that were evenly distributed across a circular region spanning 24 degrees of visual space (figure 1b). Threshold values were in agreement with the spatial density distribution of rods and cones on the human [40] and macaque [41] retinas.
Prior to array implantation, luminance thresholds were measured for the stimuli presented in the approximate location of the visual space representing the region of cortex in which the arrays were later implanted. These values ranged from 250 to 550 μcd/m2 and 250 to 715 μcd/m2 for NHP1 and NHP2, respectively. At greater than 290 days post-implantation and after two months of periodic microstimulation using currents up to 96 μA, the photic thresholds for the visual field locations represented by the cortex where the arrays were implanted were measured and ranged from 410 to 890 μcd/m2. For the same spatial location in the opposite visual field, represented by un-implanted V1, thresholds ranged from 390 to 1080 μcd/m2 (figure 1b). These values confirm that cortical function had not been impaired by the implantation and chronic presence of the UEA.
3.2. Electrode impedances
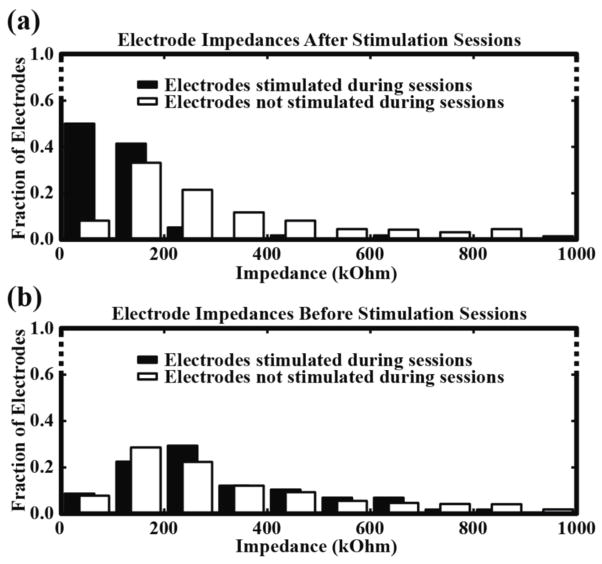
Electrode impedances were measured multiple times each week over the course of eight months. In NHP1, the median pre-implantation impedances were 46 kΩ for Array1 and 67.4 kΩ for Array2 and the first post-implantation impedances, taken three weeks after the surgery, were 192 kΩ for Array1 and 396 kΩ for Array2. In NHP2, the median pre-implantation impedances were 44 kΩ for Array1 and 46 kΩ for Array2 and the first post-implantation measurements, taken three weeks after the surgery, were 170 kΩ for Array1 and 113 kΩ for Array2. Over time, we observed a gradual drop in impedances down to near pre-implantation values. In NHP1, the median impedances taken eight months after surgery were 105 kΩ for Array1 and 121.5 kΩ for Array2. This drop in impedances occurred on both stimulated and non-stimulated electrodes and was not significantly different between the two groups (p>0.34, Kolmogorov-Smirnov). Since NHP2 damaged its connectors shortly after surgery, impedances were not measured over a long enough period of time to observe a similar drop. Electrode impedances greater than 2 MΩ were not included in these analyses. We also measured electrode impedances before and after each stimulation session. After stimulation sessions, a significant drop (p<0.01, Kolmogorov-Smirnov) in impedance was observed for all electrodes that were stimulated (figure 2a). Impedances on the stimulated electrodes increased by the next stimulation session and their distribution was not significantly different (p>0.36, Kolmogorov-Smirnov) when compared to the distribution of the non-stimulated electrodes (figure 2b).
Figure 2.
Stimulated electrodes show a consistent drop in impedance after stimulation sessions. (a) Distribution of impedances after each stimulation session for electrodes that were stimulated (black) and those that were not stimulated (white). A significant decrease in impedance was observed for electrodes that were stimulated when compared to electrodes that were not stimulated. Data was taken from eight sessions and consisted of 58 stimulated and 710 non-stimulated electrodes. (b) Post-stimulation impedances increased by the next stimulation session and the distribution of impedances was not significantly different when compared to impedances from electrodes that were never stimulated.
3.3. MUA and LFP recording and mapping
Throughout seven months of experimentation, full-bandwidth data were recorded on every electrode for both arrays in NHP1. On Array1 of NHP1, when using a threshold value of −3.5 RMS times the noise, MUA was detected on every electrode. Twenty-eight electrodes recorded large MUA, defined as mean waveforms with a peak-to-peak amplitude greater than 125 μV, across 19 recording sessions spanning 6 months (figure 3a). The activity on these channels was not consistent over time, i.e. large MUA was observed to appear and disappear across sessions for individual electrodes. The region of the array with large MUA was consistent over time. RFs were mapped using MUA. Twenty-five electrodes exhibited large MUA-RF responses (Methods 2.7). Sixteen of these responses came from electrodes that recorded large MUA. Electrodes exhibiting large MUA-RFs were generally clustered in the upper-middle region of the array where large MUA was recorded (figure 3b).
Figure 3.
Across multiple recording sessions, the largest MUA was mostly localized to the upper-middle region of the array. This region correlated with the region where robust MUA-RFs were observed. (a) Largest MUA for each electrode compiled from 19 recording sessions spanning 6 months. Waveforms were extracted from full-bandwidth (30 kS/sec), high-pass filtered (250 Hz) neural recordings using a 100 μV threshold crossing. The mean waveform for each electrode was then calculated from these extracted waveforms. Mean waveforms with a peak-to-peak amplitude greater than 125 μV are shown in bold red. Firing rate in Hertz is shown in the upper left corner of each box. (b) RFs generated using reverse correlation. Each box represents a region of visual space vertically from −4.7 to −8.2 visual degrees and horizontally from 2.1 to 9.6 visual degrees with the fixation point near the upper left corner. Data came from an experimental session five months post-implantation in NHP 1 Array 1.
Over three months of experimentation, visually-evoked LFP were recorded on most electrodes on Array1 of NHP1. Fifty-two of these electrodes exhibited large responses, defined as average LFP waveforms with a peak-to-peak amplitude greater than 75 μV. For this array, a phase reversal in the amplitude of the responses was observed across the electrodes. Approximately half of the visually evoked responses manifested a prominent negative-going average waveform, and the other half showed a prominent positive-going waveform (figure 4a). Every electrode on this array that recorded evoked responses also exhibited an LFP-RF. Moreover, the positive-going and negative-going evoked response waveforms yielded significantly different RF diameters (p<0.05, Kolmogorov-Smirnov) for the positive (0.9±0.2 visual degrees, mean±std) and the negative (0.6±0.1 visual degrees, mean±std) RFs (figure 4d). LFPs, but not MUA, were also recorded on Array2. Similar to the findings on Array1, any electrode that exhibited an evoked LFP response to visual stimuli also yielded an LFP-RF. However, all of the evoked responses on this array were negative-going (figure 4c).
3.4. MUA and LFP Visuotopy
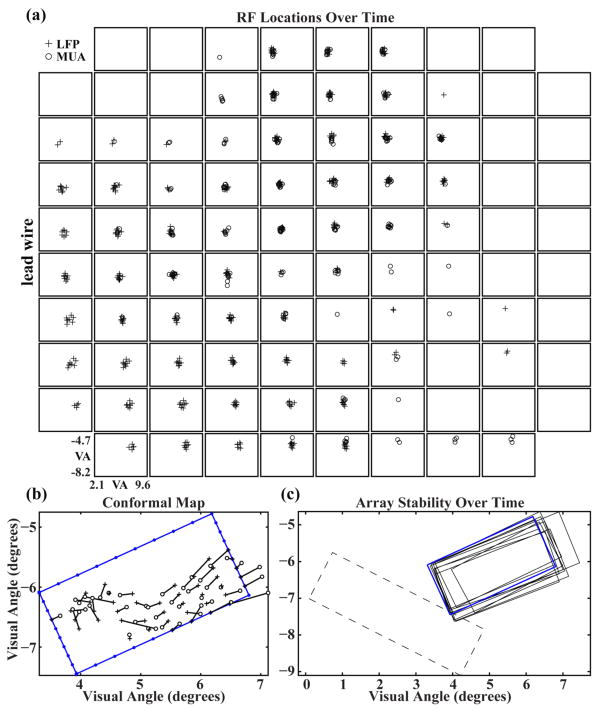
The spatial location of both MUA-RFs and LFP-RFs were measured during ten recording sessions over a 97-day period. The mean spatial separation for all RF centers during this time was 0.2±0.1 (mean±std) visual degrees. The mean spatial separation between MUA-RF and LFP-RF centers, for those electrodes and experimental sessions on which both were simultaneously recorded, was 0.3±0.2 (mean±std) visual degrees (figure 5a). The global visuotopic organization of RFs across the array was further analyzed by applying a least-mean-squared-error fit to a conformal transformation of the electrode array geometry to the locations of the RF centers associated with their respective electrodes (Warren et al, 2001). The least-mean-squared-error fit minimizes the length of vectors connecting each transformed electrode location and its respective RF center. This calculation was performed independently for MUA-RF and LFP-RF centers. As calculated from LFP-RF centers, Array1 spanned approximately 1.5 by 3.0 degrees of visual field (figure 5b), while Array2 spanned 1.5 by 4 degrees of visual field. Repeated calculations of the global visuotopic organization were made and found to be consistent across the 97 day time period (figure 5c). There was some movement of the array outline across time, limited to around 1/3 of a degree of visual angle, but there was no long-term trend apparent in this movement. The mean vector length is an estimate of the difference between the conformal representation of the array in visual space and the observed RF locations and can be used as a method of quantifying visuotopic scatter. The mean vector length was 1.97±0.34 and 0.94±0.18 visual degrees (mean±std) for LPF and MUA calculated RF centers, respectively, and are comparable to the values previously obtained from cats [30].
Figure 5.
RF centers are stable over time and occupy distinct regions of visual space for different electrodes across the array. The location of the array in visual space remains stable over time. (a) MUA-RF and LFP-RF centers for each electrode taken from ten recording sessions spanning 97 days. The spatial variance for all MUA and LFP centers is 0.2 ± 0.1 (mean±std) visual degrees. (b) Least-mean-squared-error fit of a conformal transformation of the electrode array geometry to the locations of the RF centers associated with their respective electrodes. Data was collected 148 days after implantation in NHP 1 on Array 1. The blue rectangular outline with small, filled circles represents the electrode array as mapped into visual space with each symbol indicating the location of an electrode along the periphery of the array. The large filled diamond and filled square on the left corners of this outline represent electrode sites at the most anterolateral and anteromedial corners of the array, respectively. Crosses represent the conformal geometry of the electrode array transformed into a visual space representation. Each cross is connected to an open circle that represents the mapped RF center of that electrode. (c) Solid outlines represent Array 1 position in visual space over 97 days of recording. Outlines were generated from LFP-RF data using the method described in (b). The blue outline represents the fit from the data shown in (b). The dashed outline shows the position of Array 2 for a single recording session.
3.5. Microstimulation
For NHP1, systematic stimulation via 37 electrodes of Array1 and 45 electrodes of Array2 was performed. By varying the stimulation current, psychometric curves were obtained for all electrodes that elicited a consistent behavioural response (figure 6a). Prior to the connector failure in NHP2, one electrode in Array1 was stimulated and elicited consistent behavioural responses. For NHP1, 5 of the 37 stimulated electrodes on Array1 and 3 of the 45 stimulated electrodes on Array 2 elicited consistent behavioural responses from which psychometric curves were generated. These electrodes were clustered in the upper-middle region of the Array1. Threshold values for all electrodes for which psychophysical curves could be generated ranged from 18 to 76 μA (46±19 μA mean±std) (figure 6b). Out of the 37 systematically stimulated electrodes in Array1, 32 did not elicit consistent behavioural responses when systematically stimulated over multiple days using currents up to 96 μA (figure 6b). Similar results are shown for stimulating electrodes on Array2 (figure 6c).
After repeated stimulation via individual electrodes during a session, we qualitatively observed a decrease in the ability of the NHP to detect phosphenes. When an electrode was stimulated above its threshold level repeatedly over a short period of time (i.e. minutes), increasingly higher levels of stimulation were required for the NHP to indicate detection. As a workaround to this, we increased the time between repeated stimulation of the same electrode by introducing photic stimuli interleaved with an occasional electrical stimulus.
4. Discussion
In this work, we investigated the functional consequences of chronic implantation and periodic electrical stimulation via a fixed-geometry intracortical microelectrode array in NHP V1. Measurement of photic detection thresholds demonstrated that the NHPs’ visual systems did not have deficits in visual or visually guided motor behaviour. Further, they have been successfully trained to perform the forced-choice photic and electric threshold tasks. NHP behaviour and attention can likely account for much of the variance observed across threshold detection data sets.
V1 of a behaving NHP was confirmed to be visuotopically organized, but only in a global sense. It was further demonstrated that maps of this organization using an array of penetrating microelectrodes was stable over the three months of experimentation. Although individual RFs did not follow a perfectly conformal map, when examining the global visuotopic organization across a large number of RFs the visuotopy agrees with previous findings [42]. As some electrodes did not provide an RF for all ten recording sessions, the variance seen in global organization could be simply one of regression error. Other sources of variance in the visuotopic organization could be due to microsaccades and the limited resolution of the eye tracking system (0.25–0.5 visual degrees).
After several months of being implanted and undergoing multiple microstimulation sessions, electrodes were still able to record MUA and LFP signals. This demonstrated that arrays could be subjected to physiologically effective (and higher) levels of microstimulation in vivo without functional degradation. Photic thresholds values were similar before and after array implantation and microstimulation, confirming that the neural tissue was not functionally impaired.
Below we discuss some of the challenges involved with electrical microstimulation of macaque V1.
4.1. Quality of signals recorded
Large amplitude MUA was recorded on a small number of electrodes throughout seven months of recording. The same sparse action potential activity has been observed in arrays implanted in A1 and the parabelt cortex of macaque [43], while large well-isolated action potentials have consistently been recorded on similar arrays implanted in the primary motor cortex of macaques [22] and cats [44]. The cells in the middle layers of sensory cortices, where the electrode tips are targeted, are relatively small compared to the large pyramidal projection cells in the primary motor cortex. Such a difference in size may be a significant factor influencing the differential in the quality of recordings in sensory versus motor cortices. In acute or sub-chronic recording experiments with single microelectrodes, the electrode can be positioned to optimize the neuronal activity [45]. In the experiments reported herein, however, fixed geometry electrode arrays were used as the basis for a visual prosthesis and such flexibility in positioning electrodes was not available. Thus, only a few electrodes may have been close enough to neuronal somata to record large MUA. However, LFPs were recorded on nearly all electrodes. LFPs are thought to be sampling neuronal signals over a larger electrode recording radius (several hundred μm) compared to MUA (~100 μm or less) [46, 47, 48], which may explain why more electrodes recorded evoked LFP than MUA signals. Additionally, similar to reports by others, large MUA recordings were inconsistent on individual electrodes from recording session to recording session, while LFPs were consistently recorded during all sessions [29, 49].
4.2. Factors influencing microstimulation efficacy
Our ability to evoke phosphenes was limited during these experiments. Stimulation evoked consistent behavioural responses on 5 out of the 37 electrodes and 3 out of 45 electrodes for the two arrays implanted in NHP1. Only one electrode on the arrays in NHP2 generated phosphenes, but these arrays were not systematically tested before their connectors were damaged. Our data suggests that the ability to reliably evoke phosphenes and thus elicit consistent behavioural responses may be influenced by several factors: changes in electrode impedance, the location of the electrodes tips in cortical lamina and relative to cell bodies, and the inability of the NHP to generalize from the training on photic stimuli to responding to phosphenes generated by microstimulation.
Changes in electrode impedances
After array implantation, impedances increased, which presumably was due to the addition of tissue impedances and/or fibrous tissue encapsulation promoted by the foreign body response [50, 51, 52, 53]. Over the course of the experiments, the impedance of all electrodes gradually dropped toward the pre-implantation levels. This drop has also been reported by others [22, 24, 44]. After each microstimulation session, the impedance of stimulated electrodes dropped significantly. This post-stimulation impedance drop has been previously reported and termed “rejuvenation”. It is thought to be due to removal of the cellular and acellular debris around the electrode site by a hydrous monolayer produced at iridium electrodes during pulsing [51, 54]. When the impedance of an electrode drops during the microstimulation with a constant-current pulse, the voltage across the electrode will drop accordingly. The acute impedance drop, and concomitant change in voltage, may have influenced the ability to microstimulate to effect. However, the impedances remained within an acceptable range throughout the experiments, so that microstimulation does not appear to have functionally impaired the electrode tips.
Electrode tip location
Electrodes that evoked consistent behavioural responses to microstimulation were grouped in the upper-middle region of Array1 in NHP1. This is the same region where the electrodes recorded large MUA. This observation suggests that the distance from an electrode tip to neuronal somata may be an important factor influencing our ability to effectively evoke behavioural responses to microstimulation.
Previous findings demonstrate a complex temporal pattern in visually-evoked LFP responses as a function of the laminar placement of the recording electrode tip [37, 55]. We observed that many of the visually evoked LFP responses manifested a positive-going waveform on contiguous electrodes, and that many other evoked responses manifested a negative-going waveform on a different set of contiguous electrodes. It has also been shown that the visual spread of RFs, generated with similar techniques used in this study, was related to the cortical lamina from which the recordings were made [48]. This study showed that this visual spread is smallest in layer 4C and increases in shallower and deeper layers. We observed that the sizes of LFP-RFs on electrodes recording negative-going waveforms were smaller than the sizes of the LFP-RFs on electrodes recording positive-going waveforms. Further, it has been shown that microstimulation detection thresholds vary as a function of the cortical lamina in which the electrode tip is placed [37, 56]. These values tended to decrease with depth and the lowest threshold values were reported in layer 2/3 [37] or layer 5/6 [56]. The electrodes on Array1 from NHP1 that elicited consistent behavioural responses were in the same region of the array as the electrodes which recorded negative-going LFPs and smaller LFP-RFs. Together, these findings suggest that the plane of the electrodes tips of Array1 in NHP1 was not parallel to the plane of the cortical lamina, so that the electrode tips straddle a sink-source current boundary. The electrodes which recorded negative-going LFP waveforms and small LFP-RFs were likely located in or close to layer 4C and in a layer with low thresholds, and were therefore more likely to elicit a behavioural response with microstimulation, while the electrodes which recorded positive-going LFP waveforms were likely located across a sink-source boundary in a layer with relatively high thresholds.
Our data is consistent with the results of others described above and suggests that the ability to evoke phosphenes and therefore elicit behavioural responses may be influenced by the proximity of the electrode tips to neuronal somata and their placement in cortical lamina.
Limitations of NHP experimentation
In previous human studies, phosphenes have been described as round Gaussian spots, clouds of smaller dots, and points of light with different colours and shapes [1, 5]. We trained the NHPs to indicate detection of round monochromatic Gaussian photic stimuli of a single size. Because the NHPs were only trained to respond to this simple, round Gaussian stimulus, and microstimulation may have generated more complex visual perceptions, it is possible that the NHPs were simply confused by some of the microstimulation-evoked percepts and therefore did not respond. Increasing the level of microstimulation has also been shown to change the number of perceived phosphenes from one to two [5]. We found that RF center locations of MUA and LFP signals of the same electrode did not perfectly overlap, so that it is unclear whether phosphenotopy will follow the visuotopic organization of the MUA-RFs or LFP-RFs. It is possible that at high stimulation levels two phosphenes, one located at the MUA-RF and another at the LFP-RF, were generated. This could also cause confusion in the NHPs and result in a lack of response.
The factors described above may account for the fact that only 8 out of 82 systematically stimulated electrodes elicited behavioural responses. These results can be compared to the other studies of electrical stimulation via chronically implanted electrodes. In an NHP study, 12 of the 37 stimulated electrodes (144 total implanted electrodes) resulted in behavioural responses [9]. In a human patient who could easily understand and articulate responses to complex visual percepts, 34 of the 36 stimulated electrodes (38 implanted electrodes) elicited subjective visual perceptions [5].
5. Conclusion
We demonstrated that microstimulation of the primary visual cortex via a high-density array of penetrating microelectrodes is capable of evoking visual perceptions in NHPs. Continuous long-term microstimulation of neural tissue has been shown to produce neuronal cell loss within a small radius (~60–150 μm) of the stimulating electrodes [57]. Further, we acknowledge that any implanted system will evoke some degree of foreign body response. However, in our experiments, any such cell loss and foreign body response did not appear to functionally impair the NHP visual system or the electrode array. This was shown by 1) the similar photic threshold values before and after the experiments and 2) the ability to record large amplitude MUA and LFPs with spatially distinct RFs for different electrodes throughout the experiments. Electrode impedances dropped acutely in response to microstimulation, and, over time, the impedances of all electrodes in the array dropped toward the pre-implantation values. Impedance values, however, never indicated that a functional electrode failed during the experiments. While some further animal experimentation will be needed to determine factors such as optimal laminar placement of electrode tips and optimal stimulation parameters, these results support the move into human experiments where verbal feedback on the evoked visual perceptions will greatly accelerate our understanding of how the parameters of cortical microstimulation relate to subjective visual perception.
Acknowledgments
This work was supported by NIH R01EY019363 to B Greger. Supported in part by an Unrestricted Grant from Research to Prevent Blindness, Inc., New York, NY, to the Department of Ophthalmology & Visual Sciences, University of Utah. The authors thank the staff of the CMC at the University of Utah for all their assistance in conducting the study.
References
- 1.Brindley GS, Lewin WS. The visual sensations produced by electrical stimulation of the medial occipital cortex. J Physiol. 1968;194:54–5P. [PubMed] [Google Scholar]
- 2.Dobelle WH, Mladejovsky MG. Phosphenes produced by electrical stimulation of human occipital cortex, and their application to the development of a prosthesis for the blind. J Physiol. 1974;243:553–76. doi: 10.1113/jphysiol.1974.sp010766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pollen DA. Some perceptual effects of electrical stimulation of the visual cortex in man. The Nervous System. 1975;2 [Google Scholar]
- 4.Kotler S. Vision Quest Wired Magazine. 10.09.2002. [Google Scholar]
- 5.Schmidt EM, Bak MJ, Hambrecht FT, Kufta CV, O’Rourke DK, Vallabhanath P. Feasibility of a visual prosthesis for the blind based on intracortical microstimulation of the visual cortex. Brain. 1996;119 (Pt 2):507–22. doi: 10.1093/brain/119.2.507. [DOI] [PubMed] [Google Scholar]
- 6.Bak M, Girvin JP, Hambrecht FT, Kufta CV, Loeb GE, Schmidt EM. Visual sensations produced by intracortical microstimulation of the human occipital cortex. Med Biol Eng Comput. 1990;28:257–9. doi: 10.1007/BF02442682. [DOI] [PubMed] [Google Scholar]
- 7.DeYoe EA. An investigation in the awake macaque of the threshold for the detection of electrical currents applied to striate cortex: Psychophysical properties and laminar differences. Rochester University; 1976. [Google Scholar]
- 8.Bartlett JR, DeYoe EA, Doty RW, Lee BB, Lewine JD, Negrao N, Overman WH., Jr Psychophysics of electrical stimulation of striate cortex in macaques. J Neurophysiol. 2005;94:3430–42. doi: 10.1152/jn.00406.2005. [DOI] [PubMed] [Google Scholar]
- 9.Bradley DC, Troyk PR, Berg JA, Bak M, Cogan S, Erickson R, Kufta C, Mascaro M, McCreery D, Schmidt EM, Towle VL, Xu H. Visuotopic mapping through a multichannel stimulating implant in primate V1. J Neurophysiol. 2005;93:1659–70. doi: 10.1152/jn.01213.2003. [DOI] [PubMed] [Google Scholar]
- 10.Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164–71. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- 11.Kim SP, Simeral JD, Hochberg LR, Donoghue JP, Black MJ. Neural control of computer cursor velocity by decoding motor cortical spiking activity in humans with tetraplegia. J Neural Eng. 2008;5:455–76. doi: 10.1088/1741-2560/5/4/010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ojakangas CL, Shaikhouni A, Friehs GM, Caplan AH, Serruya MD, Saleh M, Morris DS, Donoghue JP. Decoding movement intent from human premotor cortex neurons for neural prosthetic applications. J Clin Neurophysiol. 2006;23:577–84. doi: 10.1097/01.wnp.0000233323.87127.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Truccolo W, Friehs GM, Donoghue JP, Hochberg LR. Primary motor cortex tuning to intended movement kinematics in humans with tetraplegia. J Neurosci. 2008;28:1163–78. doi: 10.1523/JNEUROSCI.4415-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vargas-Irwin CE, Shakhnarovich G, Yadollahpour P, Mislow JM, Black MJ, Donoghue JP. Decoding complete reach and grasp actions from local primary motor cortex populations. J Neurosci. 2010;30:9659–69. doi: 10.1523/JNEUROSCI.5443-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhuang J, Truccolo W, Vargas-Irwin C, Donoghue JP. Decoding 3-D reach and grasp kinematics from high-frequency local field potentials in primate primary motor cortex. IEEE Trans Biomed Eng. 2010;57:1774–84. doi: 10.1109/TBME.2010.2047015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis TS, Torab K, House P, Greger B. A minimally invasive approach to long-term head fixation in behaving nonhuman primates. J Neurosci Methods. 2009;181:106–10. doi: 10.1016/j.jneumeth.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rousche PJ, Normann RA. A method for pneumatically inserting an array of penetrating electrodes into cortical tissue. Ann Biomed Eng. 1992;20:413–22. doi: 10.1007/BF02368133. [DOI] [PubMed] [Google Scholar]
- 18.Kaas J, Collins C. The primate visual system. 2004;1:265–269. [Google Scholar]
- 19.Hatsopoulos N, Mukand J, Polykoff G, Friehs G, Donoghue J. Cortically controlled brain-machine interface. Conf Proc IEEE Eng Med Biol Soc. 2005;7:7660–7663. doi: 10.1109/IEMBS.2005.1616286. [DOI] [PubMed] [Google Scholar]
- 20.Linderman MD, Gilja V, Santhanam G, Afshar A, Ryu S, Meng TH, Shenoy KV. Neural recording stability of chronic electrode arrays in freely behaving primates. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference; 2006. pp. 4387–91. [DOI] [PubMed] [Google Scholar]
- 21.Velliste M, Perel S, Spalding MC, Whitford AS, Schwartz AB. Cortical control of a prosthetic arm for self-feeding. Nature. 2008;453:1098–101. doi: 10.1038/nature06996. [DOI] [PubMed] [Google Scholar]
- 22.Baker J, Bishop W, Kellis S, Levy T, House P, Greger B. Multi-scale recordings for neuroprosthetic control of finger movements. Conf Proc IEEE Eng Med Biol Soc.; 2009; 2009. pp. 4573–7. [DOI] [PubMed] [Google Scholar]
- 23.Kim SJ, Manyam SC, Warren DJ, Normann RA. Electrophysiological mapping of cat primary auditory cortex with multielectrode arrays. Ann Biomed Eng. 2006;34:300–9. doi: 10.1007/s10439-005-9037-9. [DOI] [PubMed] [Google Scholar]
- 24.Rousche PJ, Normann RA. Chronic recording capability of the Utah Intracortical Electrode Array in cat sensory cortex. J Neurosci Methods. 1998;82:1–15. doi: 10.1016/s0165-0270(98)00031-4. [DOI] [PubMed] [Google Scholar]
- 25.Hillman T, Badi AN, Normann RA, Kertesz T, Shelton C. Cochlear nerve stimulation with a 3-dimensional penetrating electrode array. Otol Neurotol. 2003;24:764–8. doi: 10.1097/00129492-200309000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Kim SJ, Badi AN, Normann RA. Selective activation of cat primary auditory cortex by way of direct intraneural auditory nerve stimulation. Laryngoscope. 2007;117:1053–62. doi: 10.1097/MLG.0b013e31804714a7. [DOI] [PubMed] [Google Scholar]
- 27.Middlebrooks JC, Snyder RL. Intraneural stimulation for auditory prosthesis: modiolar trunk and intracranial stimulation sites. Hear Res. 2008;242:52–63. doi: 10.1016/j.heares.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 28.McDonnall D, Clark GA, Normann RA. Selective motor unit recruitment via intrafascicular multielectrode stimulation. Can J Physiol Pharmacol. 2004;82:599–609. doi: 10.1139/y04-047. [DOI] [PubMed] [Google Scholar]
- 29.Maynard EM, Fernandez E, Normann RA. A technique to prevent dural adhesions to chronically implanted microelectrode arrays. J Neurosci Methods. 2000;97:93–101. doi: 10.1016/s0165-0270(00)00159-x. [DOI] [PubMed] [Google Scholar]
- 30.Warren DJ, Fernandez E, Normann RA. High-resolution two-dimensional spatial mapping of cat striate cortex using a 100-microelectrode array. Neuroscience Annual Meeting. 2001;105:19–31. doi: 10.1016/s0306-4522(01)00174-9. [DOI] [PubMed] [Google Scholar]
- 31.House PA, MacDonald JD, Tresco PA, Normann RA. Acute microelectrode array implantation into human neocortex: preliminary technique and histological considerations. Neurosurg Focus. 2006;20:E4. doi: 10.3171/foc.2006.20.5.5. [DOI] [PubMed] [Google Scholar]
- 32.Keller CJ, Truccolo W, Gale JT, Eskandar E, Thesen T, Carlson C, Devinsky O, Kuzniecky R, Doyle WK, Madsen JR, Schomer DL, Mehta AD, Brown EN, Hochberg LR, Ulbert I, Halgren E, Cash SS. Heterogeneous neuronal firing patterns during interictal epileptiform discharges in the human cortex. Brain. 2010;133:1668–81. doi: 10.1093/brain/awq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schevon CA, Goodman RR, McKhann G, Jr, Emerson RG. Propagation of Epileptiform Activity on a Submillimeter Scale. J Clin Neurophysiol. 2010 doi: 10.1097/WNP.0b013e3181fdf8a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schevon CA, Ng SK, Cappell J, Goodman RR, McKhann G, Jr, Waziri A, Branner A, Sosunov A, Schroeder CE, Emerson RG. Microphysiology of epileptiform activity in human neocortex. J Clin Neurophysiol. 2008;25:321–30. doi: 10.1097/WNP.0b013e31818e8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schevon CA, Trevelyan AJ, Schroeder CE, Goodman RR, McKhann G, Jr, Emerson RG. Spatial characterization of interictal high frequency oscillations in epileptic neocortex. Brain. 2009;132:3047–59. doi: 10.1093/brain/awp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waziri A, Schevon CA, Cappell J, Emerson RG, McKhann GM, 2nd, Goodman RR. Initial surgical experience with a dense cortical microarray in epileptic patients undergoing craniotomy for subdural electrode implantation. Neurosurgery. 2009;64:540–5. doi: 10.1227/01.NEU.0000337575.63861.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeYoe EA, Lewine JD, Doty RW. Laminar variation in threshold for detection of electrical excitation of striate cortex by macaques. J Neurophysiol. 2005;94:3443–50. doi: 10.1152/jn.00407.2005. [DOI] [PubMed] [Google Scholar]
- 38.Shushruth S, Ichida JM, Levitt JB, Angelucci A. Comparison of spatial summation properties of neurons in macaque V1 and V2. J Neurophysiol. 2009;102:2069–83. doi: 10.1152/jn.00512.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ringach D, Shapley R. Reverse correlation in neurophysiology. Cognitive Science. 2004;28:147–166. [Google Scholar]
- 40.Hecht E. Optics 2001 [Google Scholar]
- 41.Goodchild AK, Ghosh KK, Martin PR. Comparison of photoreceptor spatial density and ganglion cell morphology in the retina of human, macaque monkey, cat, and the marmoset Callithrix jacchus. J Comp Neurol. 1996;366:55–75. doi: 10.1002/(SICI)1096-9861(19960226)366:1<55::AID-CNE5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 42.Tootell RB, Switkes E, Silverman MS, Hamilton SL. Functional anatomy of macaque striate cortex. II. Retinotopic organization. J Neurosci. 1988;8:1531–68. doi: 10.1523/JNEUROSCI.08-05-01531.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith E, Greger B. Multiple, simultaneous recordings from auditory cortex in the awake, behaving macaque. Neuroscience Annual Meeting; 2010. [Google Scholar]
- 44.Parker RAVWR, House PA, Greger B. The effects of micro-stimulation on micro-electrode array performance - Implications for neural prosthetics. Neuroscience Annual Meeting; 2010. [Google Scholar]
- 45.Cham JG, Branchaud EA, Nenadic Z, Greger B, Andersen RA, Burdick JW. Semi-Chronic Motorized Microdrive and Control Algorithm for Autonomously Isolating and Maintaining Optimal Extracellular Action Potentials. J Neurophysiol. 2005;93:570–579. doi: 10.1152/jn.00369.2004. [DOI] [PubMed] [Google Scholar]
- 46.Buzsaki G. Large-scale recording of neuronal ensembles. Nat Neurosci. 2004;7:446–451. doi: 10.1038/nn1233. [DOI] [PubMed] [Google Scholar]
- 47.Katzner S, Nauhaus I, Benucci A, Bonin V, Ringach DL, Carandini M. Local origin of field potentials in visual cortex. Neuron. 2009;61:35–41. doi: 10.1016/j.neuron.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xing D, Yeh CI, Shapley RM. Spatial spread of the local field potential and its laminar variation in visual cortex. J Neurosci. 2009;29:11540–9. doi: 10.1523/JNEUROSCI.2573-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu X, McCreery DB, Carter RR, Bullara LA, Yuen TG, Agnew WF. Stability of the interface between neural tissue and chronically implanted intracortical microelectrodes. IEEE Trans Rehabil Eng. 1999;7:315–26. doi: 10.1109/86.788468. [DOI] [PubMed] [Google Scholar]
- 50.Newbold C, Richardson R, Huang CQ, Milojevic D, Cowan R, Shepherd R. An in vitro model for investigating impedance changes with cell growth and electrical stimulation: implications for cochlear implants. J Neural Eng. 2004;1:218–27. doi: 10.1088/1741-2560/1/4/005. [DOI] [PubMed] [Google Scholar]
- 51.Otto KJ, Johnson MD, Kipke DR. Voltage pulses change neural interface properties and improve unit recordings with chronically implanted microelectrodes. IEEE Trans Biomed Eng. 2006;53:333–40. doi: 10.1109/TBME.2005.862530. [DOI] [PubMed] [Google Scholar]
- 52.Williams JC, Hippensteel JA, Dilgen J, Shain W, Kipke DR. Complex impedance spectroscopy for monitoring tissue responses to inserted neural implants. J Neural Eng. 2007;4:410–23. doi: 10.1088/1741-2560/4/4/007. [DOI] [PubMed] [Google Scholar]
- 53.Lempka SF, Miocinovic S, Johnson MD, Vitek JL, Mclntyre CC. In vivo impedance spectroscopy of deep brain stimulation electrodes. J Neural Eng. 2009;6:046001. doi: 10.1088/1741-2560/6/4/046001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pickup PG, Birss VI. A model for anodic hydrous oxide growth at iridium. Journal of Electroanalytical Chemistry. 1987;220:83–100. [Google Scholar]
- 55.Maier A, Adams GK, Aura C, Leopold DA. Distinct superficial and deep laminar domains of activity in the visual cortex during rest and stimulation. Front Syst Neurosci. 2010;4 doi: 10.3389/fnsys.2010.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tehovnik EJ, Slocum WM, Carvey CE, Schiller PH. Phosphene induction and the generation of saccadic eye movements by striate cortex. J Neurophysiol. 2005;93:1–19. doi: 10.1152/jn.00736.2004. [DOI] [PubMed] [Google Scholar]
- 57.McCreery D, Pikov V, Troyk PR. Neuronal loss due to prolonged controlled-current stimulation with chronically implanted microelectrodes in the cat cerebral cortex. J Neural Eng. 2010;7:036005. doi: 10.1088/1741-2560/7/3/036005. [DOI] [PMC free article] [PubMed] [Google Scholar]