Abstract
Higher 24-hour resin-dentin bond strengths are created when ethanol is used to replace water during wet bonding. This in vitro study examined if ethanol-wet-bonding can increase the durability of resin-dentin bonds over longer times. Five increasingly hydrophilic experimental resin blends were bonded to acid-etched dentin saturated with water or ethanol. Following composite build-ups, the teeth were reduced into beams for 24-hour microtensile bond strength evaluation, and for water-aging at 37°C for 3, 6, or 12 months before additional bond strength measurements. Although most bonds made to water-saturated dentin did not change over time, those made to ethanol-saturated dentin exhibited higher bond strengths, and none of them fell over time. Decreased collagen fibrillar diameter and increased interfibrillar spacing were seen in hybrid layers created with ethanol-wet-bonding. Increases in bond strength and durability in ethanol-wet-bonding may be due to higher resin uptake and better resin sealing of the collagen matrix, thereby minimizing endogenous collagenolytic activities.
Keywords: dentin bonding, durability, hydrophilicity, ethanol
Introduction
Compared with the stability of resin-enamel bonds (Loguercio et al., 2008), the durability of resin-dentin bonds is relatively poor (see Carrilho et al., 2005, for review). A 30% to 40% decrease in bond strength has often been observed after 3-6 mos of in vitro aging. Ultrastructural studies have also demonstrated spontaneous degradation of the dentin matrix over time (DeMunck et al., 2003; Armstrong et al., 2004; Pashley et al., 2004; Garcia-Godoy et al., 2007), in the absence of bacteria.
Several mechanisms are thought to be responsible for the poor durability of resin-dentin bonds. The comonomer blends used in contemporary adhesives produce very hydrophilic polymers that absorb 5% to 12% water (Ito et al., 2005; Malacarne et al., 2006), resulting in plasticization that lowers their mechanical properties. The second mechanism is that acid-etching exposes and activates matrix metalloproteinases (MMPs) in the dentin matrix that can slowly cleave collagen peptides (Pashley et al., 2004; Mazzoni et al., 2006, 2007; Nishitani et al., 2006a; Tay et al., 2006). Resin-infiltration into acid-etched dentin is frequently incomplete and leaves some collagen fibrils exposed and surrounded by water, which is required for MMPs to hydrolyze collagen. Thus, procedures that improve resin infiltration by coating each collagen fibril with resin may prevent collagen-bound MMPs from access to water and make resin-dentin bonds more durable.
Hydrophilicity of adhesive resins was recently found to be the determinant of the 24-hour microtensile bond strength of resins to water-saturated acid-etched dentin (Nishitani et al., 2006b). Hydrophobic resins gave very low bond strengths because they were not very miscible with water. When acid-etched dentin was saturated with 100% ethanol instead of water, the bond strengths of both hydrophilic and hydrophobic resins increased significantly. Although “ethanol-wet-bonding” looks promising (Pashley et al., 2007; Tay et al., 2007; Sadek et al., 2008), it involves an extra step of replacing rinsing water with 100% ethanol. Thus, more evidence is needed to justify this extra step.
In the present study, 5 experimental primers/adhesives prepared from comonomer blends with different hydrophobic/hydrophilic characteristics were applied to acid-etched dentin saturated with water or ethanol. The objective was to determine if bonds made to acid-etched ethanol-saturated dentin are more durable than those made to water-saturated dentin. The null hypothesis tested was that there are no differences in the durability of the 5 experimental adhesives bonded to water- vs. ethanol-saturated dentin over 12 mos in vitro.
Materials & Methods
Teeth
Third molars were collected after the donors’ informed consent was obtained under a protocol approved by the IRB of the Medical College of Georgia. A flat surface was prepared with a slow-speed IsoMet saw (Buehler Ltd., Lake Bluff, IL, USA) under water cooling to expose mid-coronal dentin. The dentin surface was polished with 320-grit SiC paper to create a standardized smear layer. The crown segments were randomly allocated to 10 groups (N = 20). All teeth were acid-etched with 37 mass% H3PO4 gel for 15 sec, rinsed with water, and then inverted on wet lint-free tissues until bonded. There were 2 matrix solvents (water vs. ethanol) and 5 experimental solvated resins (Table 1), yielding 10 groups. Within each group, there were 4 time periods (24-hour, 3, 6, and 12 mos). Each tooth yielded 16 beams. Four beams from each tooth were assigned to each time period. Twenty beams, derived from 5 separate teeth, were designated to each time period (Loguercio et al., 2005).
Table 1.
Composition and Hoy’s Solubility Parameters for the Solvated Comonomersa and for Demineralized Dentin (collagen)
| Hoy’s Solubility Parameters (MPa)−1 |
|||||
|---|---|---|---|---|---|
| Compositiona | δd | δp | δh | δt | |
| Solvated 1 | 35 wt% E-BisADM; 14.38% TEGDMA | 13.8 | 10.7 | 13.3 | 22.0 |
| Solvated 2 | 35 wt% BisGMA; 14.38% TEGDMA | 14.3 | 11.8 | 13.3 | 22.8 |
| Solvated 3 | 35 wt% BisGMA; 14.38% HEMA | 14.2 | 12.1 | 14.3 | 23.5 |
| Solvated 4 | 20 wt% BisGMA; 15% TCDM; 14.38% TEGDMA | 14.6 | 12.0 | 13.5 | 23.2 |
| Solvated 5 | 20 wt% BisGMA; 15% BisMP; 14.38% HEMA | 13.9 | 12.3 | 15.5 | 24.2 |
| Collagen | 30% water, 70% peptides | 11.8 | 15.3 | 22.5 | 30.1 |
| Collagen | 27% water, 10% ethanol, 63% peptides | 11.9 | 14.8 | 22.2 | 29.2 |
| Collagen | 17% water, 20% ethanol, 63% peptides | 11.9 | 13.7 | 20.2 | 27.1 |
| Collagen | 7% water, 30% ethanol, 63% peptides | 12.0 | 12.5 | 18.1 | 25.1 |
| Collagen | 0% water, 0% ethanol, 70% peptides | 11.7 | 12.1 | 14.8 | 22.5 |
All solvated comonomer blends contained 0.5% EDMAB, 0.125% CQ, and 50 mass% ethanol. All Hoy’s solubility parameters were calculated with commercially available software (Computer Chemistry Consultancy, www.compchemcons.com). δd—Hoy’s solubility parameter for dispersive forces; δp—Hoy’s solubility parameter for polar forces; δh—Hoy’s solubility parameter for hydrogen-bonding forces; δt—Hoy’s solubility parameter for total cohesive forces, equivalent to Hildebrand’s solubility parameter.
Abbreviations: BisGMA = 2,2-bis[4-(2-hydroxy-3-methacryloyloxypropoxy)]-phenyl propane; BisMP = Bis[2-(methacryloyloxy) ethyl] phosphate; CQ = camphorquinone; E-BisADM = ethoxylated Bisphenol A diglycidyl methacrylate; EDMAB = 2-ethyl dimethyl-4-aminobenzoate; HEMA = 2-hydroxyethyl methacrylate; TCDM = di(hydroxyethylmethacrylate)ester of 5-(2,5,-dioxotetrahydrofurfuryl)-3-methyl-3-cyclohexane-1,2′-dicarboxylic acid; TEGDMA = triethylene-glycol dimethacrylate.
Primer/Adhesives
The 5 neat resin blends (Table 1) were formulated by Bisco Inc. (Schaumburg, IL , USA). We created primers from the 5 experimental blends by mixing 50 mass% resin with 50 mass% ethanol. These primers were applied to water- or ethanol-saturated acid-etched dentin. The solubility parameters of these blends are shown in Fig. 1. Solubility parameter theory was first used by Asmussen and his colleagues (Asmussen et al., 1991; Asmussen and Uno, 1993). They found that the higher the Hildebrand solubility parameter (δt) of primers, the thicker were hybrid layers. This concept was expanded by Miller et al. (1998), using Hansen’s triple solubility parameters to calculate the relative contributions of dispersion cohesive forces (δd), polar cohesive forces (δp), and hydrogen bonding forces (δh) to the total cohesive forces of adhesives (δt). Their other major contribution was the calculation of the solubility parameters for dentin collagen. They summed the molar attraction constants of the functional groups of all amino acids in collagen (Miller et al., 1998; Chappelow et al., 2000). Since Hoy’s triple solubility parameters are more widely used than Hansen’s (Pashley et al., 2007), we used them to show how solubility parameters vary with the degree of dehydration of collagen, and how they can estimate the miscibility of various solvated resins with dentin collagen.
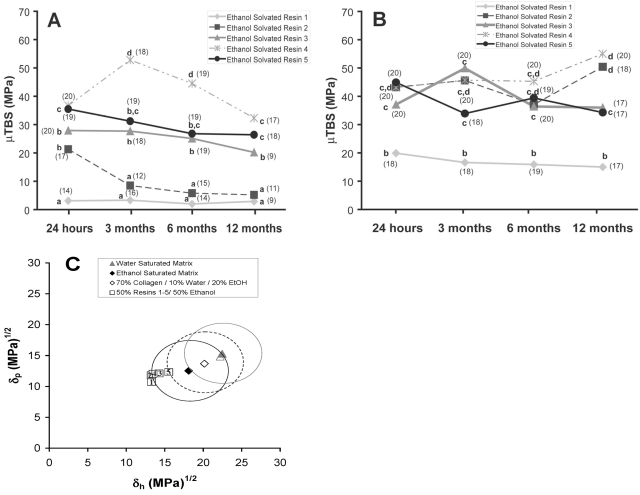
Figure 1.
Bond strengths and miscibility of resins to water- vs. ethanol-saturated dentin. (A) Plot of microtensile bond strength (µTBS) vs. incubation time for the 5 experimental resin blends listed in Table 1. The blends were dissolved in 50% ethanol and applied to acid-etched dentin wet with water. Means identified by different lower-case letters are significantly different (p < 0.05). (B) Plot of µTBS vs. storage time. The blends were dissolved in 50% ethanol and applied to acid-etched dentin saturated with 100% ethanol. Means identified by different lower-case letters are significantly different (p < 0.05). (C) Plot of Hoy’s solubility parameters for polar cohesive forces (δp) vs. those for hydrogen bonding cohesive forces (δh) of the 5 solvated resin blends relative to “miscibility circles” created by drawing a circle with a radius of 5 (MPa)1/2 around those same δ values for water- or ethanol-saturated dentin matrix (see Table 1 for specific values). The dotted circle indicates the direction in which the “miscibility circle” for water-saturated dentin would move as ethanol in the solvated resins removes water from the matrix beginning with water-saturated dentin (solid circle, far right) and then becomes half-saturated dentin matrix (dotted middle circle) ending with ethanol-saturated dentin (far left solid circle).
We achieved ethanol saturation of the interfibrillar spaces by inverting the water-moist dentin surfaces in 2 mL of 100% ethanol for 1 min and never allowing the ethanol-saturated dentin to evaporate to dryness, thereby avoiding surface tension forces. Two applications of primer were made and gently agitated with a microbrush for 10 sec. After resin-infiltration, the ethanol solvent was evaporated from the primed surface by means of a gentle stream of air. A layer of the same neat resin blend (i.e., without ethanol-solvation) was then applied as an adhesive and light-cured for 60 sec with 600 mW/cm2 of light energy. Resin composite build-ups were made with 2-mm increments of Z100 (3M ESPE, St. Paul, MN, USA) that were light-cured for 40 sec.
Preparation of Bonded Specimens for Long-term Incubation
After storage in water at 37°C for 24 hrs, the bonded teeth were longitudinally sectioned into 0.9-mm-thick slabs by means of an IsoMet saw (Buehler Ltd., Lake Bluff, IL, USA). The middle 4 slabs were cut into 0.9-mm-wide beams to yield multiple 0.9 x 0.9 mm composite-dentin beams. This model represents a form of accelerated aging (Shono et al., 1999). The beams from each tooth were separated into the 4 time periods and incubated in 37°C distilled water containing 0.02 mass% sodium azide as an antimicrobial agent.
Microtensile Testing
At each time period, the bonded beams were mounted on a microtensile testing jig with cyanoacrylate glue and pulled to failure at 0.6 mm/min in a Vitrodyne universal tester. We divided the load in N at failure by the cross-sectional area to calculate the microtensile bond strength (MPa). We summed the means of 4 beams from each tooth with the means of the other 4 teeth to obtain a mean ± SD for each time period in each of the 10 groups. Pre-test failures were noted, but not included in the data analysis.
Statistics
A three-way ANOVA design with the general linear model was used for examining the effects of substrate solvent, resin hydrophilicity, and aging time on microtensile bond strength. Due to significant interactions between and among factors, a least-squares means (LSM) analysis was used. LSM are the expected value of group means that one expects for a balanced design involving the group variable, with all covariates at the mean value. The variances in the LSM values are given in standard error of the mean (SEM) instead of standard deviation (SD). Multiple comparisons of the LSM were performed by the Holm-Sidak method. Statistical significance was set in advance at α = 0.05.
Transmission Electron Microscopy (TEM)
The outer slabs of 5 bonded teeth from each group (24-hour) were processed for TEM, according to a method described previously (Tay et al., 2002). Undemineralized, epoxy-resin-embedded, 90- to 100-nm-thick sections were stained in 1 mass% phosphotungstic acid and 2 mass% uranyl acetate and examined with a TEM (JEM-1230, JEOL, Tokyo, Japan) operated at 80 kV. Images were analyzed with software (Scion Corp., Frederick, MD, USA) for the diameter of collagen fibrils.
Results
Water-saturated Dentin
Solvated resins 1-5 applied to acid-etched, water-saturated dentin produced mixed results (Fig. 1A). The bond strengths created by resin 1 were the lowest of all 5 resins and did not significantly change from 24 hrs to 12 mos (Table 2). The 24-hour bond strength of resin 2 was significantly higher than that of resin 1, but fell to low values over 3-12 mos. Bond strengths of solvated resin 4 increased at 3 and 6 mos, but by 12 mos returned to 24-hour values. The 24-hour bond strengths of resin 5 were similar to those produced by resin 4, but, unlike resin 4, the bond strength of resin 5 did not change significantly over time.
Table 2.
Microtensile Bond Strengthsa of Experimental Resins 1-5 Bonded to Water- or Ethanol-saturated Acid-etched Dentin
| Water-wet Dentin | ||||
|---|---|---|---|---|
| Solvated Resins |
24 hrs |
3 mos |
6 mos |
12 mos |
| 1 | 3.14 ± 2.23 (14/20)a | 3.28 ± 2.79 (16/20)a | 2.02 ± 3.74 (14/20)a | 2.91 ± 2.79 (9/20)a |
| 2 | 21.28 ± 2.79 (17/20)b | 8.50 ± 3.16 (12/20)a | 5.79 ± 3.16 (15/20)a | 5.21 ± 2.79 (11/20)a |
| 3 | 27.93 ± 2.23 (20/20)b | 27.65 ± 2.79 (18/20)b | 25.07 ± 2.64 (19/20)b | 20.15 ± 2.64 (9/20)b |
| 4 | 36.73 ± 2.23 (19/20)c | 52.81 ± 2.79 (18/20)d | 44.51 ± 2.64 (19/20)d | 32.33 ± 2.64 (17/20)c |
| 5 |
35.53 ± 2.41 (20/20)c |
31.15 ± 2.41 (19/20)b,c |
26.76 ± 2.79 (19/20)b,c |
26.38 ± 2.64 (18/20)c |
| Ethanol-wet Dentin | ||||
| Solvated Resins |
24 hrs |
3 mos |
6 mos |
12 mos |
| 1 | 19.95 ± 4.24 (18/20)b | 16.58 ± 4.04 (18/20)b | 15.88 ± 4.24 (19/20)b | 14.97 ± 4.24 (17/20)b |
| 2 | 43.70 ± 3.97 (20/20)c,d | 45.62 ± 3.87 (20/20)c,d | 37.15 ± 4.24 (19/20)c | 50.35 ± 4.24 (20/20)c |
| 3 | 37.07 ± 3.46 (20/20)c | 36.08 ± 4.24 (20/20)c | 36.36 ± 4.46 (18/20)c | 35.95 ± 4.46 (18/20)c |
| 4 | 43.15 ± 3.87 (20/20)c,d | 49.88 ± 4.04 (20/20)c,d | 45.26 ± 4.24 (20/20)c,d | 54.96 ± 4.24 (20/20)d |
| 5 | 44.88 ± 3.87 (20/20)c,d | 33.90 ± 4.04 (18/20)c | 39.42 ± 4.24 (19/20)c,d | 34.26 ± 4.24 (17/20)c |
Values are least-squares mean ± SEM in MPa. Values identified with different superscript letters are significantly different at p < 0.05. All teeth were acid-etched with 37% phosphoric acid for 15 sec, rinsed, and maintained wet by inversion on moist lint-free tissues. Numbers in parentheses are the numbers of specimens tested of the 20 in each group.
Ethanol-saturated Dentin
When bond strengths of ethanol-solvated comonomers made to ethanol-saturated dentin were followed over 12 mos, there were no significant changes among the solvated resins (Table 2). Bond strengths of solvated resins 2-4 on ethanol-saturated dentin were significantly higher than those of resin 1 at all time periods.
Water-saturated vs. Ethanol-saturated Dentin
Bond strengths of solvated resins 1-3 in ethanol-saturated dentin were significantly (p < 0.05, Fig. 1A vs. 1B) higher than those of their water-saturated controls. Bonds made with solvated resin 4 increased slightly over time, but bonds made with resin 5 fell slightly (Fig. 1B, Table 2). Plots of the Hoy’s δp vs. δh values of ethanol-solvated resins 1-5 are plotted next to those for water vs. ethanol-saturated dentin (Fig. 1C). Note that the solvated resins fall outside the “miscibility circle” of water-saturated dentin (Tay et al., 2007), but within or along the periphery of the “miscibility circle” of ethanol-saturated dentin. The dotted circles indicate how the “miscibility circle” of water-saturated dentin may change during the infiltration of ethanol-solvated resins.
Transmission Electron Microscopy
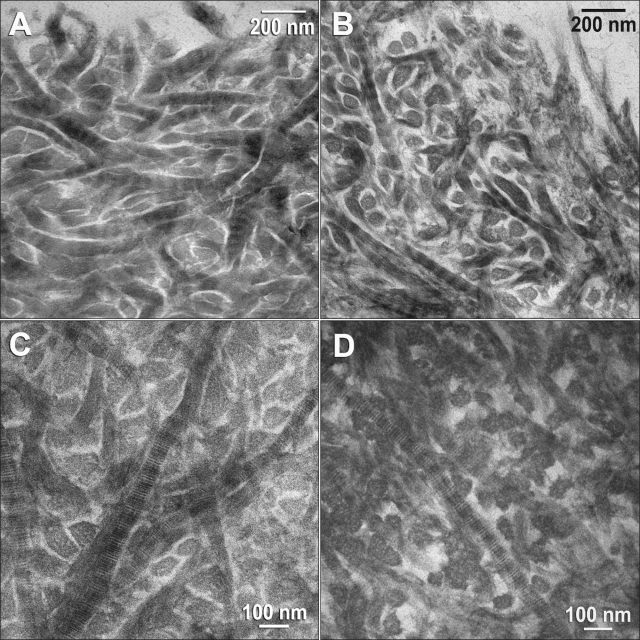
Images from the surfaces of hybrid layers of solvated resin 2 bonded to water- or ethanol-saturated dentin are shown, respectively, in Figs. 2A and 2B. Images from the middle portions of hybrid layers of solvated resin 3 bonded to water- vs. ethanol-saturated dentin are shown, respectively, in Figs. 2C and 2D. Results from the other solvated resins were similar (not shown). The diameters of the collagen fibrils in ethanol-saturated dentin (65.7 ± 5.3 nm) were significantly (p < 0.05) smaller than those of water-saturated dentin (87.4 ± 6.3 nm). This resulted in wider interfibrillar spaces in the hybrid layers created in ethanol-saturated dentin compared with water-saturated hybrid layers (Tay et al., 2007).
Figure 2.
Transmission electron microscopy images of acid-etched dentin bonded with (A) solvated resin 2 to water-saturated dentin (top of hybrid layer), (B) solvated resin 2 to ethanol-saturated dentin (top of hybrid layer), (C) solvated resin 3 to water-saturated dentin (middle portion of hybrid layer), and (D) solvated resin 3 to ethanol-saturated dentin (middle portion of hybrid layer).
Discussion
The results of this study showed a slow decline in the microtensile bond strength of resin 2 applied to water-saturated dentin over 1 yr, compared with the constancy of bond strengths of solvated resins 1, 3, 4, and 5. However, the bond strength of resin 1 was so low that they could not have fallen any lower. When resin 4 was bonded to water-saturated dentin, there was a large, significant increase in bond strength between the 24-hour mean and the three-month mean, which was not seen in resin 5, the other hydrophilic resin. We have no explanation for that result. When these same resins were bonded to ethanol-saturated dentin, the bond strengths of solvated resins 1, 2, and 3 increased significantly, while those of solvated resins 4 and 5 were similar. This confirms the 24-hour bond strength results reported previously (Nishitani et al., 2006b), but over 12 mos. Importantly, at the end of 12 mos, solvated resins 1, 2, 3, and 4 bonded to ethanol-saturated dentin were significantly (p < 0.05) higher than those same resins bonded to water-saturated dentin. This led to partial rejection of the test’s null hypothesis, that there would be no differences in the durability of the 5 experimental adhesives bonded to water- vs. ethanol-saturated dentin over 12 mos in vitro.
The “miscibility circle” of water-saturated dentin matrix represents the Hoy’s solubility parameters of the matrix just prior to addition of the first coating of solvated resins 1-5. Within seconds, the ethanol in the primer would remove much of the water in the collagen. Similarly, the comonomers would tend to coat the collagen and mask it so that subsequent coatings would interact with monomer-coated collagen instead of collagen (Vaidyanathan et al., 2001, 2003, 2005). All of these changes would tend to shift the “miscibility circle” of water-saturated dentin to the left, toward the ethanol-saturated collagen circle.
While bonds made to water-saturated dentin by resin 2 fell over 12 mos, and bonds made with resin 4 fell from 3 to 12 mos, bonds made to ethanol-saturated dentin with those same resins did not fall over time. With bonding to water-wet dentin, there is always the danger that overly wet matrices will cause phase changes in the adhesive during its initial application. In contrast, with bonding to ethanol-wet dentin, solvated resins 1-5 cannot exhibit any phase changes, because they are both solvated by ethanol.
Several possible mechanisms may be responsible for the improved durability of resin-dentin bonds made to acid-etched ethanol-saturated dentin. Our TEM observations—that the interfibrillar spaces of ethanol-saturated dentin are larger than those of water-saturated dentin for the same resins—indicate that there is more resin surrounding each collagen fibril. This may be responsible for the stronger resin-dentin bond strengths observed in ethanol-wet-bonding compared with water-wet-bonding (Carvalho et al., 2003; Nishitani et al., 2006b).
When dentin is acid-etched, the etchant dissolves apatite crystallites from within and between collagen fibrils (Van Meerbeek et al., 1996). Numerous authors have reported that the interfibrillar spaces between collagen fibrils contain a hydrogel composed of proteoglycans (Goldberg and Takagi, 1993; Oyarzún et al., 2000; Embery et al., 2001; Pereira et al., 2007). It has been speculated that the presence of this hydrogel may interfere with comonomer infiltration during bonding. However, ethanol removes the water from these spaces, causing the hydrogel to collapse (Scott and Thomlinson, 1998). Ethanol also shrinks the diameter of collagen fibrils (Tay et al., 2007) more than it shrinks the volume of the matrix, thereby enlarging the interfibrillar spaces and allowing for more resin infiltration.
Several authors have suggested that it may be impossible for solvated resins to displace all water from collagen fibrils and the interfibrillar spaces (Guo et al., 2007). This results in a layer of residual water over the collagen fibrils that prevents intimate contact between resins and collagen fibrils. When ethanol is used to replace water in the collagen microfibrils, the spaces between the microfibrils are filled with ethanol, which is a much better solvent for comonomers than water. This may permit adhesive monomers to actually “dock” with molecular cavities along the surfaces of collagen tripeptides that make up the microfibrils (Vaidyanathan et al., 2001, 2003, 2005). If adhesive monomers can truly coat collagen microfibrils and prevent access of MMPs to water, they may block the action of intrinsic collagenases known to be bound to collagen (Martin-De Las Heras et al., 2000; Mazzoni et al., 2007; Sulkala et al., 2007), because, as hydrolases, they require the presence of water (Pashley et al., 2004; Carrilho et al., 2005). If hydrophobic resins are used in ethanol-wet-bonding, they may both provide both superior sealing of collagen and absorb too little water (Ito et al., 2005; Malacarne et al., 2006) to permit collagenases to hydrolyze collagen peptides. If, in contrast, ethanol-wet-bonding with hydrophilic resins provides durable resin-dentin bonds, it may indicate that comonomers have infiltrated MMPs bound to collagen, as well as the collagen itself, thereby inactivating them. Clearly, more research is required on this topic to sort out the many possible reasons why ethanol-wet-bonding increases the durability of resin-dentin bonds.
Acknowledgments
The authors are grateful to Michelle Barnes for outstanding secretarial support
Footnotes
This work was supported by grant R01 DE014911 from the National Institute of Dental and Craniofacial Research (P.I. DHP).
References
- Armstrong SR, Vargas MA, Chung I, Pashley DH, Campbell JA, Laffoon JE, et al. (2004). Resin-dentin interfacial ultrastructure and microtensile dentin bond strength after five-year water storage. Oper Dent 29:705-712 [PubMed] [Google Scholar]
- Asmussen E, Uno S. (1993). Solubility parameters, fractional polarities, and bond strengths of some intermediary resins used in dentin bonding. J Dent Res 72:558-565 [DOI] [PubMed] [Google Scholar]
- Asmussen E, Hansen EK, Peutzfeldt A. (1991). Influence of the solubility parameter of intermediary resins on the effectiveness of the Gluma bonding system. J Dent Res 70:1290-1293 [DOI] [PubMed] [Google Scholar]
- Carrilho MRO, Carvalho RM, Tay FR, Yiu C, Pashley DH. (2005). Durability of resin-dentin bonds related to water and oil storage. Am J Dent 18:315-319 [PubMed] [Google Scholar]
- Carvalho RM, Mendonça JS, Santiago SL, Silveira RR, Garcia FCP, Tay FR, et al. (2003). Effects of HEMA/solvent combinations on bond strength to dentin. J Dent Res 82:597-601 [DOI] [PubMed] [Google Scholar]
- Chappelow CC, Power MD, Bowles CQ, Miller RC, Pinzino CS, Eick JD. (2000). Novel priming and cross-linking systems for use with isocyanoatomethacrylate dental adhesives. Dent Mater 16:396-405 [DOI] [PubMed] [Google Scholar]
- DeMunck J, Van Meerbeek B, Yoshida Y, Inoue S, Vargas M, Suzuki K, et al. (2003). Four-year water degradation of total-etch adhesives bonded to dentin. J Dent Res 82:136-140 [DOI] [PubMed] [Google Scholar]
- Embery G, Hall R, Waddington R, Septier D, Goldberg M. (2001). Proteoglycans in dentinogenesis. Crit Rev Oral Biol Med 12:331-349 [DOI] [PubMed] [Google Scholar]
- Garcia-Godoy F, Tay FR, Pashley DH, Feilzer A, Tjäderhane L, Pashley EL. (2007). Degradation of resin-bonded human dentin after 3 years of storage. Am J Dent 19:109-113 [PubMed] [Google Scholar]
- Goldberg M, Takagi M. (1993). Dentine proteoglycans: composition, ultrastructure and functions. Histochem J 25:781-806 [PubMed] [Google Scholar]
- Guo X, Spencer P, Wang Y, Ye Q, Yao X, Williams K. (2007). Effects of a solubility enhancer on penetration of hydrophobic component in model adhesives into wet demineralized dentin. Dent Mater 23:1473-1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Hashimoto M, Wadgaonkar B, Svizero N, Carvalho RM, Yiu C, et al. (2005). Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials 26:6449-6459 [DOI] [PubMed] [Google Scholar]
- Loguercio AD, Barroso LP, Grande RHM, Reis A. (2005). Comparison of intra- and intertooth resin-dentin bond strength variability. J Adhes Dent 7:151-158 [PubMed] [Google Scholar]
- Loguercio AD, Moura SK, Pellizzaro A, Dal-Bianco K, Patzlaff RT, Grande RHM, et al. (2008). Durability of enamel bonding using two-step, self-etch systems on ground and unground enamel. Oper Dent 33:79-88 [DOI] [PubMed] [Google Scholar]
- Malacarne J, Carvalho RM, deGoes MF, Svizero N, Pashley DH, Tay FR, et al. (2006). Water sorption/solubility of dental adhesive resins. Dent Mater 22:973-980 [DOI] [PubMed] [Google Scholar]
- Martin-De Las Heras S, Valenzuela A, Overall CM. (2000). The matrix metalloproteinases gelatinase A in human dentine. Arch Oral Biol 45:757-765 [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Pashley DH, Nishitani Y, Breschi L, Tjäderhane L, Toledano M, et al. (2006). Reactivation of inactivated endogenous proteolytic activities in phosphoric acid-etched dentin by etch-and-rinse adhesives. Biomaterials 27:4470-4476 [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Mannello F, Tay FR, Tonti GAM, Papa S, Mazzotti G, et al. (2007). Zymographic analysis and characterization of MMP-2 and -9 forms in human sound dentin. J Dent Res 86:436-440; erratum, J Dent Res 86:792, 2007 [DOI] [PubMed] [Google Scholar]
- Miller RG, Bowles CQ, Chappelow CC, Eick JD. (1998). Application of solubility parameter theory to dentin-bonding systems and adhesive strength correlations. J Biomed Mater Res 41:237-243 [DOI] [PubMed] [Google Scholar]
- Nishitani Y, Yoshiyama M, Wadgaonkar B, Elrod D, Breschi L, Mannello F, et al. (2006a). Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. Eur J Oral Sci 114:160-166 [DOI] [PubMed] [Google Scholar]
- Nishitani Y, Yoshiyama M, Donnelly AM, Agee KA, Sword J, Tay FR, et al. (2006b). Effects of resin hydrophilicity on dentin bond strength. J Dent Res 85:1016-1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyarzún A, Rathkamp H, Dreyer E. (2000). Immunohistochemical and ultrastructural evaluation of the effects of phosphoric acid etching on dentin proteoglycans. Eur J Oral Sci 108:546-554 [DOI] [PubMed] [Google Scholar]
- Pashley DH, Tay FR, Hashimoto M, Breschi L, Carvalho RM, Ito S. (2004). Collagen degradation by host-derived enzymes during aging. J Dent Res 83:216-221 [DOI] [PubMed] [Google Scholar]
- Pashley DH, Tay FR, Carvalho RM, Rueggeberg FA, Agee KA, Carrilho M, et al. (2007). From dry bonding to water-wet bonding to ethanol-wet bonding. A review of the interactions between dentin matrix and solvated resins using a macromodel of the hybrid layer. Am J Dent 20:7-20 [PubMed] [Google Scholar]
- Pereira PNR, Bedran-de-Castro AKB, Durante WR, Yamauchi M. (2007). Removal of noncollagenous components affects dentin bonding. J Biomed Mater Res B: Appl Biomater 80:86-91 [DOI] [PubMed] [Google Scholar]
- Sadek FT, Pashley DH, Nishitani Y, Carrilho MR, Donnelly A, Ferrari M, et al. (2008). Application of the hydrophobic resin adhesive to acid-etched dentine with an alternative wet bonding technique. J Biomed Mater Res A 84:19-29 [DOI] [PubMed] [Google Scholar]
- Scott JE, Thomlinson AM. (1998). The structure of interfibrillar proteoglycan bridges (‘shape modules’) in extracellular matrices of fibrous connective tissues and their stability in various chemical environments. J Anat 192(Pt 3):391-405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shono Y, Terashita M, Shimada J, Kozono Y, Carvalho RM, Russell CM, et al. (1999). Durability of resin-dentin bonds. J Adhes Dent 1:211-218 [PubMed] [Google Scholar]
- Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjäderhane L. (2007). Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentine. Arch Oral Biol 52:121-127 [DOI] [PubMed] [Google Scholar]
- Tay FR, Pashley DH, Yoshiyama M. (2002). Two modes of nanoleakage in single-step adhesives. J Dent Res 81:472-476 [DOI] [PubMed] [Google Scholar]
- Tay FR, Pashley DH, Loushine RJ, Weller RN, Monticelli F, Osorio F. (2006). Self-etching adhesives increase collagenolytic activity in radicular dentin. J Endod 32:862-868 [DOI] [PubMed] [Google Scholar]
- Tay FR, Pashley DH, Kapur RR, Carrilho MRO, Hur YB, Garrett LV, et al. (2007). Bonding BisGMA to dentin—a proof of concept for hydrophobic dentin bonding. J Dent Res 86:1034-1039 [DOI] [PubMed] [Google Scholar]
- Van Meerbeek B, Conn LJ, Jr, Duke ES, Eick JD, Robinson SJ, Guerrero D. (1996). Correlative transmission electron microscopy examination of nondemineralized and demineralized resin-dentin interfaces formed by two dentin adhesive systems. J Dent Res 75:879-888 [DOI] [PubMed] [Google Scholar]
- Vaidyanathan J, Vaidyanathan TK, Yadav P, Linaras CE. (2001). Collagen-ligand interaction in dentinal adhesion: computer visualization and analysis. Biomaterials 22:2911-2920 [DOI] [PubMed] [Google Scholar]
- Vaidyanathan J, Kasinathan C, Vaidyanathan TK. (2003). Biomimetic recognition and immunochemical assay of ligand binding to collagen. J Adhes Dent 5:7-17 [PubMed] [Google Scholar]
- Vaidyanathan J, Vaidyanathan TK, Ramasubbu N, Ravichandran S. (2005). A computational and experimental analysis of ligand binding to type I collagen. Curr Comput-Aided Drug Des 1:397-422 [Google Scholar]