Abstract
The Oral HIV/AIDS Research Alliance is part of the AIDS Clinical Trials Group, the largest HIV clinical trial organization in the world, and it is funded by the National Institute of Dental and Craniofacial Research, in collaboration with the National Institute of Allergy and Infectious Diseases. The alliance’s main objective is to investigate the oral complications associated with HIV/AIDS as the epidemic is evolving—in particular, the effects of potent antiretrovirals on the development of oral mucosal lesions and associated fungal and viral pathogens. Furthermore, oral fluids are being explored for their potential monitoring and diagnostic role with respect to HIV disease and coinfections. This article presents an overview of the alliance, its scientific agenda, and an outline of the novel interventional and noninterventional clinical studies ongoing and developing within the AIDS Clinical Trials Group infrastructure in the United States and internationally.
Keywords: HIV/AIDS, OHARA, infectious diseases, AIDS Clinical Trials Group, oral cavity
Since the discovery of hairy leukoplakia in San Francisco in the 1980s, early in the AIDS epidemic (D. Greenspan et al., 1984; J. Greenspan et al., 1985), the oral cavity has been found to play an important role in the natural history of HIV infection and AIDS, with specific oral lesions serving as hallmarks for monitoring early signs of HIV/AIDS and disease progression. The occurrence of oral candidiasis and hairy leukoplakia has been shown to be strongly associated with a low CD4 cell count (Feigal et al., 1991; Katz et al., 1992; Glick et al., 1994; Shiboski et al., 1994; Shiboski et al., 1996; Patton et al., 1999; Shiboski et al., 2001) and a higher plasma viral load (Patton et al., 1999; D. Greenspan et al., 2000). The prevalence of most oral lesions has been found to be significantly lower since the advent of combination antiretroviral therapy (ART; Arribas et al., 2000; Ceballos-Salobrena et al., 2000; Patton et al., 2000; Tappuni and Fleming, 2001; Ramírez-Amador et al., 2003; D. Greenspan et al., 2004; Nicolatou-Galitis et al., 2004; Umadevi et al., 2007); however, other conditions have been found to be more common, such as oral warts (D. Greenspan et al., 2001; King et al., 2002; Cameron et al., 2005) and salivary gland disease (D. Greenspan et al., 2001). In a retrospective study of 1280 HIV-infected patients visiting an oral medicine clinic from 1990 to 1999, D. Greenspan et al. (2001) found a significantly higher proportion of individuals with oral warts among those on highly active ART (23%) than among those who were not on therapy (5%). Similarly, a study comparing HIV-infected adults who had oral warts with those who did not (n = 56 and 168, respectively) revealed that the risk of oral warts was associated with a ≥ 1 log10 decrease in HIV-1 RNA levels in the 6 months before diagnosis of oral warts (odds ratio, 2.35; 95% confidence interval, 1.08-5.11) (King et al., 2002).
The apparent increased incidence of oral warts among those on ART and the association of the presence of oral warts with reductions in plasma HIV-1 RNA load are intriguing and merit further investigation. Furthermore, many unanswered research questions regarding HIV/AIDS-related oral manifestations remain as the HIV/AIDS epidemic is changing and as new antiretrovirals become available. To address the latter gap, the National Institute of Dental and Craniofacial Research, with the National Institute of Allergy and Infectious Diseases, published a funding opportunity announcement in December 2004. The overarching objective of this announcement was to improve clinical diagnosis and management of comorbidities of AIDS-related oral complications, and it called for the development of novel interventional and noninterventional clinical studies in the United States and internationally to achieve this goal.
In response to the funding opportunity announcement, the Oral HIV/AIDS Research Alliance (OHARA) was created in 2006 within the AIDS Clinical Trials Group (ACTG), with a main objective to investigate the oral complications associated with HIV/AIDS as the epidemic evolves—in particular, to explore the effects of new-generation antiretrovirals on the development of oral mucosal lesions and associated fungal and viral pathogens. Furthermore, oral fluids are being explored for their potential monitoring and diagnostic role with respect to HIV disease and coinfections. Another goal is to develop a comprehensive oral infection and mucosal disease database with HIV and opportunistic virologic and mycologic correlates. The HIV/AIDS oral specimen bank being developed will provide valuable material for future investigations.
We hereby present an overview of OHARA, its scientific agenda, and an outline of the novel interventional and noninterventional clinical studies ongoing and developing within the ACTG infrastructure in the United States and internationally. This is especially relevant to the Sixth World Workshop on Oral Health and Disease in AIDS because OHARA’s scientific agenda was derived in part from the proceedings of the Fifth World Workshop, held in Phuket, Thailand, in 2004 (Challacombe et al., 2006).
OHARA Infrastructure Within the ACTG
OHARA is part of the ACTG network, which is the largest HIV/AIDS clinical trial organization in the world and which plays a major role in defining the standards of care for treatment of HIV infection and opportunistic diseases related to HIV/AIDS (ACTG, 2011). The ACTG’s mission is to develop and conduct scientifically rigorous translational research and therapeutic clinical trials in the United States and internationally—specifically, (1) to investigate the viral and immune pathogenesis of HIV-1 infection and its complications; (2) to evaluate novel therapeutic agents and the most effective approaches and strategies for the use of existing agents to treat HIV-1 infection; and (3) to evaluate interventions and strategies to treat and prevent HIV-related opportunistic infections, coinfections, complications of therapies, and other HIV-1-related comorbidities. The ACTG network comprises a leadership group that oversees, through an executive committee, the network laboratories, a coordinating and operations center, statistical and data management centers, scientific and resource committees, and 54 clinical trial units and clinical research sites.
The OHARA infrastructure comprises an epidemiology research unit at the University of California–San Francisco, a medical mycology unit and repository at Case Western Reserve University, and a virology unit and repository at the University of North Carolina. These centers were initially selected by the National Institute of Dental and Craniofacial Research, the National Institute of Allergy and Infectious Diseases, and the ACTG because of their complementary expertise in conducting large epidemiologic studies on the natural history of HIV/AIDS oral diseases in the United States and Africa and in deciphering the oral pathogenesis of fungal and viral infections in the setting of HIV/AIDS disease. In the joint National Institute of Allergy and Infectious Diseases–National Institute of Dental and Craniofacial Research funding opportunity announcement, the ACTG leadership established oral candidiasis, oral manifestations of Kaposi sarcoma, and oral manifestations of the human papilloma virus and herpes group virus infections as the areas of greatest clinical importance and relevance to the ACTG scientific agenda. The herpes group virus of interest included cytomegalovirus, Kaposi sarcoma herpesvirus, Epstein-Barr virus, and herpes simplex virus.
The OHARA Scientific Committee is a subcommittee of the ACTG Optimization of Coinfection and Comorbidity Management Scientific Committee (Fig.). OHARA investigators include dentists specialized in oral medicine, physicians (including infectious disease specialists), virologists, mycologists, immunologists, epidemiologists, and statisticians. The OHARA Scientific Committee includes a steering committee composed of the principal investigators of the 3 units (C. Shiboski at University of California San Francisco, M. Ghannoum at Case, and J. Webster-Cyriaque and D. Dittmer at the University of North Carolina), the director of the AIDS and Immunosuppression Program at the National Institute of Dental and Craniofacial Research (I. Rodriguez-Chavez), representatives from the ACTG Optimization of Coinfection and Comorbidity Management Scientific Committee and the Statistical Data Analysis Center, and a clinical trial specialist and committee coordinator from the operations center. In addition to the steering committee members, the OHARA Scientific Committee comprises a medical officer from the Division of AIDS at the National Institute of Allergy and Infectious Diseases, representatives from each ACTG scientific committee, the data management center, the 3 units, the international committee, the ACTG Community Advisory Board, and clinical research site field representatives. In addition, OHARA has a representative on each ACTG scientific committee. This infrastructure provides optimal integration, communication, and work effectiveness within the ACTG.
Fig.
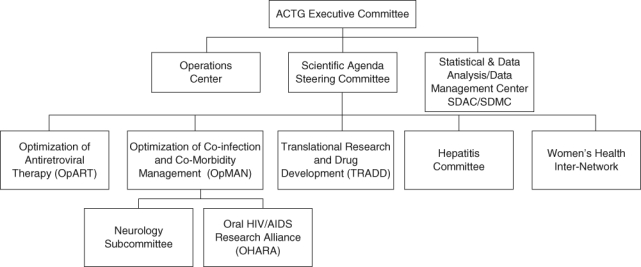
The Oral HIV/AIDS Research Alliance (OHARA) within the infrastructure of the AIDS Clinical Trial Group (ACTG). OHARA is funded by the National Institutes of Health and National Institute of Allergy and Infectious Diseases through the ACTG network.
The OHARA Scientific Agenda
The OHARA scientific agenda was developed and presented at the first ACTG annual meeting attended by OHARA investigators, in December 2006, and finalized at the first OHARA investigators meeting, in June 2007. The research objectives included in the agenda were derived from group discussions among the OHARA investigators from the 3 units and were inspired in part from the proceedings of the Fifth World Workshop on Oral Health and Disease in AIDS, held in Phuket, Thailand, in 2004 (Challacombe et al., 2006). The objectives are organized into themes to facilitate the assessment of their suitability with ACTG studies, as part of which they may be conducted. The scientific agenda is periodically updated during the annual OHARA investigators meeting. The themes and objectives are as follows:
Theme 1: Use of Oral Fluid for Diagnostics and Monitoring Purposes
To study the relationship between HIV-1 viral load in plasma and oral fluids
To study the association between the presence of HIV-associated opportunistic viruses in saliva (e.g., human papilloma virus, Epstein-Barr virus, Kaposi sarcoma herpesvirus, cytomegalovirus, herpes simplex virus) and immune reconstitution and HIV suppression (CD4 cell count and plasma HIV RNA load)
Theme 2: Oral Mucosal Diseases
- To explore the scope of HIV-related oral manifestations in the era of new-generation ART, including
- — Whether an oral immune reconstitution inflammatory syndrome (IRIS) phenomenon exists and, if so, to characterize it
- — The association between immune reconstitution and HIV suppression and specific HIV-related oral disease, including oral warts and necrotizing ulcerative gingivitis, periodontitis, and stomatitis
To evaluate the use of oral candidiasis as a marker of HIV disease progression in resource-limited settings and its possible association with HIV coinfections, including tuberculosis and cryptococcal meningitis
To optimize the treatment of oral candidiasis in resource-limited countries using inexpensive topical agents such as gentian violet
To optimize the prevention and management of human papilloma virus–associated oral warts
Theme 3: Oral Lymphoid Tissue-Associated Diseases
- To explore the replication of HIV in oral mucosal lymphoid tissue and its possible correlation with
- — The replication of other opportunistic viruses (Epstein-Barr virus, Kaposi sarcoma herpesvirus, human papilloma virus) in mucosal lymphoid tissue
- — HIV replication in gut mucosal lymphoid tissue
Theme 4: Salivary Gland Disease
To evaluate HIV-related salivary gland disease CD8 T-cell lymphocytosis associated with parotid gland enlargement and ART
OHARA Activities to Date and Protocols
Case Definitions and Training Modules
Many OHARA protocols have shared end points, which include oral mucosal diseases known to be associated with HIV/AIDS. The OHARA epidemiology/clinical team has updated existing diagnostic criteria of the oral manifestations of HIV—those published in 1992 (J. Greenspan et al., 1992) and 1993 (often referred to as EC-Clearinghouse [or ECC] criteria; see “Classification and Diagnostic Criteria,” 1993), which most studies of HIV oral diseases have used in the past decade. The proposed case definitions are designed to be used in large-scale epidemiologic studies and clinical trials in the United States and in resource-poor settings, where diagnoses may be made by nondental health care providers (Shiboski et al., 2009). To standardize the measurement of oral mucosal outcomes associated with HIV/AIDS across clinical specialties within the ACTG infrastructure, the OHARA epidemiology/clinical team has developed extensive training modules, based on slide and video presentations adapted for distance learning, as well as training manuals.
Laboratory Activity
OHARA has established a dedicated repository for oral fluid and tissue at the University of North Carolina and a fungal repository at Case Western Reserve University. The University of North Carolina laboratories have developed and validated several assays that will be used in OHARA protocols, including a multiplex assay for simultaneous detection and quantitation of human papilloma virus types from throat wash specimens (Andrews et al., 2009). They have developed automated high-throughput assay for simultaneous detection and quantitation of all herpesviruses (cytomegalovirus, Kaposi sarcoma herpesvirus, Epstein-Barr virus, and others) from throat wash specimens, as well as saliva, plasma, and tissue specimens (Jacobson et al., 2009). An assay for detection and quantification of low–copy number HIV RNA in throat wash specimens has also been developed.
The mycology unit has conducted preclinical studies of inexpensive topical antifungals, identifying gentian violet as an effective fungicidal at low concentration (Traboulsi et al., 2008). In addition, in vitro evaluation of a new slow-release miconazole-based buccal tablet was undertaken by the mycology unit in preparation for clinical protocol development to assess the safety and efficacy of such formulations (Isham and Ghannoum, 2010).
Protocols
OHARA is currently engaged in 8 protocols that are at different stages of development. The Table summarizes study designs and objectives. Some protocols are stand-alone studies not part of ACTG parent studies (a5254 and a5265), whereas others are conducted as part of existing or developing ACTG studies (a5240, a5253, a5263, a5264). Three protocols are collaborative studies with the AIDS Malignancy Consortium (AMC052, AMC066, and AMC067), and one protocol will coenroll participants with another ACTG protocol (a5272).
Table.
Oral HIV/AIDS Research Alliance Protocols: February 2011
| Protocol | Study Design; AIDS Clinical Trials Group Parent Study Aim | Oral HIV/AIDS Research Alliance Objective | Status |
|---|---|---|---|
| a5240 and AMC052 | Phase II study to evaluate the immunogenicity and safety of a quadrivalent human papilloma virus vaccine in HIV-infected females (N = 402) and males (N = 110; AMC052) | To explore human papilloma virus shedding and strain variation in the oral cavity before and after administration of quadrivalent human papilloma virus vaccine among 75 women participating in a5240 and 110 men participating in AMC052 | 75 baseline oral fluid samples collected in a5240 and 110 baseline samples in AMC052 |
| a5253 | Exploratory, observational, cross-sectional study designed to construct a standardized diagnostic evaluation that improves diagnosis of pulmonary tuberculosis as compared to standard-of-care tuberculosis screening in HIV-infected males and females not currently receiving antiretroviral therapy (N = 800) | To explore the value of oral candidiasis as a predictor of tuberculosis disease | Closed to accrual; enrollment completed as of November 29, 2010 |
| a5254 | No AIDS Clinical Trials Group parent study; stand-alone Oral HIV/AIDS Research Alliance protocol: cross-sectional observational study (N = 360) | Assess the accuracy of clinical diagnoses of HIV-related oral mucosal disease made by clinical research site examiners compared with diagnoses made by oral medicine trained specialists Describe the relationship between HIV-1 viral load in plasma and saliva Assess the relationship between Candida colony-forming unit levels in the oral cavity and oral candidiasis |
Enrollment as of February 8, 2011: N = 177 |
| a5265 | No AIDS Clinical Trials Group parent study. Stand-alone Oral HIV/AIDS Research Alliance protocol: 13-week phase III open-label randomized assessment-blinded clinical trial to compare the safety and efficacy of gentian violet oral solution to that of nystatin oral suspension for the treatment of oropharyngeal candidiasis in HIV-1-infected participants in non-US settings (N = 494) |
To compare the safety and efficacy of gentian violet oral solution to that of nystatin oral suspension for the treatment of oropharyngeal candidiasis in HIV-1-infected participants in non-US settings | Projected to open April 2011 |
| a5272 | Frequency of oral human papilloma virus shedding and oral warts after initiation of antiretroviral therapy; observational 48-week study (N = 500) Coenrollment with a5257, a phase III prospective randomized open-label trial comparing 3 nonnucleoside analogue reverse transcriptase inhibitor–sparing antiretroviral regimens administered to ART-naïve participants who are followed at regular time intervals, including 4, 8, 16 and 24 weeks; a suitable source of participants (N = 1800) for coenrollment in the proposed study a5272 |
To assess the persistence or new occurrence of type-specific oral human papilloma virus DNA shedding within 24 weeks after ART initiation To evaluate the association between the development of oral warts and (1) CD4+ T-cell count changes and (2) plasma HIV RNA load at 4-, 16-, and 24-week follow-ups after ART initiation |
Enrollment as of February 4, 2011: N = 238 |
| a5263/AMC066a | A randomized comparison of 3 chemotherapy regimens as an adjunct to antiretroviral therapy for treatment of advanced AIDS–Kaposi sarcoma in resource-limited settings (N = 706) | To determine whether Kaposi sarcoma herpesvirus load in saliva changes after completion of ART alone or in combination chemotherapy with ART To determine whether Kaposi sarcoma herpesvirus load in saliva at baseline predicts Kaposi sarcoma response to ART alone or in combination chemotherapy with ART |
In development |
| a5264/AMC067a | A randomized comparison of antiretroviral therapy versus antiretroviral therapy with adjunctive chemotherapy for treatment of limited-stage AIDS–Kaposi sarcoma (N = 468) | To determine whether Kaposi sarcoma herpesvirus load in saliva changes after completion of ART alone or in combination chemotherapy with ART To determine whether Kaposi sarcoma herpesvirus load in saliva at baseline predicts Kaposi sarcoma response to ART alone or combination chemotherapy with ART |
In development |
ART, antiretroviral therapy.
AMC052, AMC066, and AMC067 are protocols conducted in collaboration with the AIDS Malignancy Consortium.
OHARA Yearly Performance Appraisal
An External Scientific Expert Group comprising a virologist, a mycologist, an epidemiologist, and a salivary biomarker scientist was convened to review and assess the OHARA scientific agenda as part of the OHARA investigators meeting held in San Francisco in November 2008. The group will attend subsequent annual meetings, and it is charged with providing scientific and clinical expert opinion to the National Institute of Dental and Craniofacial Research as part of the activities conducted for the yearly performance appraisal of OHARA. The National Institute of Dental and Craniofacial Research, in collaboration with the National Institute of Allergy and Infectious Diseases, assesses the group’s opinion and provides final recommendations to OHARA for each performance period and for future directions.
Conclusion
Since the onset of the HIV/AIDS epidemic, the oral cavity has played a central role in helping to define the natural history of HIV/AIDS, and in the future, specific oral lesions (e.g., candidiasis) may be used as potential surrogate markers for the initiation of ART or prophylactic regimens to prevent HIV coinfections. The development of an array of salivary assays for diagnostic and monitoring purposes shows promise for an even greater role of the oral cavity in the management of patients with HIV disease. OHARA is a successful multicenter and multidisciplinary project that provides a unique context to address public health needs regarding oral HIV/AIDS research.
Footnotes
This work was supported by the National Institutes of Health, National Institute of Allergy and Infectious Diseases, and National Institute of Dental and Craniofacial Research (U01 AI 68636). We thank Isaac Rofriguez-Chavez for his contribution to the manuscript.
References
- AIDS Clinical Trial Group (2011). https://actgnetwork.org/ Accessed February 8, 2011
- Andrews E, Seaman WT, Webster-Cyriaque J. (2009). Oropharyngeal carcinoma in non-smokers and non-drinkers: a role for HPV. Oral Oncol 45:486-491 [DOI] [PubMed] [Google Scholar]
- Arribas J, Hernandez-Albujar S, Gonzalez-Garcia J, Pena J, Gonzalez A, Canedo T, et al. (2000). Impact of protease inhibitor therapy on HIV-related oropharyngeal candidiasis. AIDS 14:979-985 [DOI] [PubMed] [Google Scholar]
- Cameron JE, Mercante D, O’Brien M, Gaffga AM, Leigh JE, Fidel PL, Jr, et al. (2005). The impact of highly active antiretroviral therapy and immunodeficiency on human papillomavirus infection of the oral cavity of human immunodeficiency virus-seropositive adults. Sex Transm Dis 32:703-709 [DOI] [PubMed] [Google Scholar]
- Ceballos-Salobrena A, Gaitan-Cepeda L, Ceballos-Garcia L, Lezama-Del Valle D. (2000). Oral Lesions in HIV/AIDS patients undergoing highly active antiretroviral treatment including protease inhibitors: a new face of oral AIDS? AIDS Patient Care STDS 14:627-635 [DOI] [PubMed] [Google Scholar]
- Challacombe S, Coogan M, Williams D, Greenspan J. (2006). Overview and research agenda arising from the 5th World Workshop on Oral AIDS and Disease in AIDS. Adv Dent Res 19:5-9 [DOI] [PubMed] [Google Scholar]
- Classification and diagnostic criteria for oral lesions in HIV infection: EC-Clearinghouse on Oral Problems Related to HIV Infection and WHO Collaborating Centre on Oral Manifestations of the Immunodeficiency Virus (1993). J Oral Pathol Med 22:289-291 [PubMed] [Google Scholar]
- Feigal DW, Katz MH, Greenspan D, Westenhouse J, Winkelstein W, Jr, Lang W, et al. (1991). The prevalence of oral lesions in HIV-infected homosexual and bisexual men: three San Francisco epidemiological cohorts. AIDS 5:519-525 [DOI] [PubMed] [Google Scholar]
- Glick M, Muzyka BC, Lurie D, Salkin LM. (1994). Oral manifestations associated with HIV-related disease as markers for immune suppression and AIDS. Oral Surg Oral Med Oral Pathol 77:344-349 [DOI] [PubMed] [Google Scholar]
- Greenspan D, Canchola AJ, MacPhail LA, Cheikh B, Greenspan JS. (2001). Effect of highly active antiretroviral therapy on frequency of oral warts. Lancet 357:1411-1412 [DOI] [PubMed] [Google Scholar]
- Greenspan D, Gange S, Phelan J, Navazesh M, Alves M, MacPhail L, et al. (2004). Incidence of oral lesions in HIV-1-infected women: reduction with HAART. J Dent Res 83:145-150 [DOI] [PubMed] [Google Scholar]
- Greenspan D, Greenspan JS, Conant M, Petersen V, Silverman S, Jr, De Souza Y. (1984). Oral “hairy” leucoplakia in male homosexuals: evidence of association with both papillomavirus and a herpes-group virus. Lancet 2:831-834 [DOI] [PubMed] [Google Scholar]
- Greenspan D, Komaroff E, Redford M, Phelan JA, Navazesh M, Alves ME, et al. (2000). Oral mucosal lesions and HIV viral load in the Women’s Interagency HIV Study (WIHS). J Acquir Immune Defic Syndr 25:44-50 [DOI] [PubMed] [Google Scholar]
- Greenspan JS, Barr CE, Sciubba JJ, Winkler JR. (1992). Oral manifestations of HIV infection: definitions, diagnostic criteria, and principles of therapy. The USA Oral AIDS Collaborative Group Oral Surg Oral Med Oral Pathol 73:142-144 [DOI] [PubMed] [Google Scholar]
- Greenspan J, Greenspan D, Lennette E. (1985). Replication of Epstein-Barr virus within the epithelial cells of oral “hairy” leukoplakia, an AIDS-associated lesion. N Engl J Med 313:1564-1571 [DOI] [PubMed] [Google Scholar]
- Isham N, Ghannoum MA. (2010). Antifungal activity of miconazole against recent Candida strains. Mycoses 53:434-437 [DOI] [PubMed] [Google Scholar]
- Jacobson MA, Ditmer DP, Sinclair E, Martin JN, Deeks SG, Hunt P, et al. (2009). Human herpesvirus replication and abnormal CD8+ T cell activation and low CD4+ T cell counts in antiretroviral-suppressed HIV-infected patients. PLoS One 4:e5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz MH, Greenspan D, Westenhouse J, Hessol NA, Buchbinder SP, Lifson AR, et al. (1992). Progression to AIDS in HIV-infected homosexual and bisexual men with hairy leukoplakia and oral candidiasis. AIDS 6:95-100 [DOI] [PubMed] [Google Scholar]
- King MD, Reznik DA, O’Daniels CM, Larsen NM, Osterholt D, Blumberg HM. (2002). Human papillomavirus-associated oral warts among human immunodeficiency virus-seropositive patients in the era of highly active antiretroviral therapy: an emerging infection. Clin Infect Dis 34:641-648 [DOI] [PubMed] [Google Scholar]
- Nicolatou-Galitis O, Velegraki A, Paikos S, Economopoulou P, Stefaniotis T, Papanikolaou I, et al. (2004). Effect of PI-HAART on the prevalence of oral lesions in HIV-I infected patients: a Greek study. Oral Dis 10:145-150 [DOI] [PubMed] [Google Scholar]
- Patton LL, McKaig RG, Eron JJ, Jr, Lawrence HP, Strauss RP. (1999). Oral hairy leukoplakia and oral candidiasis as predictors of HIV viral load. AIDS 13:2174-2176 [DOI] [PubMed] [Google Scholar]
- Patton LL, McKaig R, Strauss R, Rogers D, Eron JJ., Jr (2000). Changing prevalence of oral manifestations of human immuno-deficiency virus in the era of protease inhibitor therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 89:299-304 [DOI] [PubMed] [Google Scholar]
- Ramírez-Amador V, Esquivel-Pedraza L, Sierra-Madero J, Anaya-Saavedra G, González-Ramírez I, Ponce-de-León S. (2003). The changing clinical spectrum of human immunodeficiency virus (HIV)-related oral lesions in 1,000 consecutive patients: a 12-year study in a referral center in Mexico. Medicine (Baltimore) 82:39-50 [DOI] [PubMed] [Google Scholar]
- Shiboski CH, Hilton JF, Greenspan D, Westenhouse JL, Derish P, Vranizan K, et al. (1994). HIV-related oral manifestations in two cohorts of women in San Francisco. J Acquir Immune Defic Syndr 7:964-971 [PubMed] [Google Scholar]
- Shiboski CH, Hilton JF, Neuhaus JM, Canchola A, Greenspan D. (1996). Human immunodeficiency virus-related oral manifestations and gender: a longitudinal analysis. Arch Intern Med 156:2249-2254 [DOI] [PubMed] [Google Scholar]
- Shiboski CH, Patton LL, Webster-Cyriaque JY, Greenspan D, Traboulsi RS, Ghannoum M, et al. (2009). The Oral HIV/AIDS Research Alliance: updated case definitions of oral disease endpoints. J Oral Pathol Med 38:481-488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiboski CH, Wilson CM, Greenspan D, Hilton J, Greenspan JS, Moscicki AB. (2001). HIV-related oral manifestations among adolescents in a multicenter cohort study. J Adolesc Health 29 (suppl):109S-114S [DOI] [PubMed] [Google Scholar]
- Tappuni AR, Fleming GJ. (2001). The effect of antiretroviral therapy on the prevalence of oral manifestations in HIV-infected patients: a UK study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 92:623-628 [DOI] [PubMed] [Google Scholar]
- Traboulsi RS, Mukherjee PK, Ghannoum MA. (2008). In vitro activity of inexpensive topical alternatives against Candida spp isolated from the oral cavity of HIV-infected patients. Int J Antimicrob Agents 31: 272-276 [DOI] [PubMed] [Google Scholar]
- Umadevi KM, Ranganathan K, Pavithra S, Hemalatha R, Saraswathi TR, Kumarasamy N, et al. (2007). Oral lesions among persons with HIV disease with and without highly active antiretroviral therapy in southern India. J Oral Pathol Med 36:136-141 [DOI] [PubMed] [Google Scholar]


