Abstract
Phosphoinositide-dependent kinase (PDK1) plays a central role in signal transduction mediated by phosphatidylinositol 3-kinases (PI3K) and regulates cellular functions in neutrophils. Neutrophils from individuals diagnosed with localized aggressive periodontitis (LAP) present an in vivo phenotype with depressed chemotaxis. The aim of this study was to test the hypothesis that PDK1 regulates chemotaxis in neutrophils and is responsible for the abnormal neutrophil chemotaxis LAP. Neutrophil chemotaxis was significantly suppressed by the PDK1 inhibitor staurosporine. When cells were transfected with PDK1 siRNA, there was a significant reduction in chemotaxis, while superoxide generation was not significantly affected. In primary neutrophils from persons with LAP, PDK1 expression and activation levels were significantly reduced, and this reduction was associated with the reduced phosphorylation of Akt (Thr308) and chemotaxis. Analysis of these data demonstrates that PDK1 is essential for the chemotactic migration of neutrophils, and in the absence of PDK1, neutrophil chemotaxis is impaired.
Keywords: PDK1, PI3K, chemotaxis, neutrophil, periodontitis, LAP
Introduction
Neutrophils play an important role in combating infection by phagocytosis and the killing of bacteria (Malech and Gallin, 1987). Coordinated activation and recruitment of neutrophils through chemotaxis are essential and are the crucial step in the inflammatory response (Ockene et al., 1980; Weiss et al., 1981; Babior, 1984; Baggiolini et al., 1994). Neutrophil abnormalities are associated with pathological conditions and lead to recurrent microbial infections (Lekstrom-Himes and Gallin, 2000). Localized Aggressive Periodontitis (LAP) is a destructive periodontal disease characterized by defective neutrophil chemotaxis (Cianciola et al., 1977; Perez et al., 1991; Dennison and Van Dyke, 1997), which persists after treatment of the clinical condition (Dennison and Van Dyke, 1997). Cell shape change has been associated with chemotaxis, and it has been shown that the polarization of LAP PMN exhibits reduced orientation toward a chemotactic gradient, elevated percentages of non-polar cells, and reduced chemotactic migration (MacFarlane et al., 1987). Second-messenger-mediated signaling pathways are altered in LAP neutrophils, leading to changes in other responses vital to host defense, including increased superoxide generation (Tyagi et al., 1992; Dennison and Van Dyke, 1997; Pouliot et al., 2000). Evidence at the signaling pathways of chemotactic regulation in neutrophils is lacking, and it is of interest to explain the mechanism of disparate chemotaxis in LAP neutrophils.
The receptor-controlled mechanisms that dictate neutrophil chemotaxis in response to specific stimuli begin rapidly following engagement of the receptor by its ligand. Activation of phosphoinositide 3-kinase (PI3K) leads to the accumulation of PIP3 along the leading edge of migrating neutrophils and is required for cells to undergo chemotaxis. The class Ia and Ib isoforms of PI3K specifically regulate chemotaxis in neutrophils (Hawkins et al., 2006). In particular, one member of the class Ia group (PI3Kδ) and the class Ib member (PI3Kγ) play roles (Heit et al., 2008) involving the activity of 3-phosphoinositide-dependent kinase 1 (PDK1) (Stephens et al., 2002). PDK1 is a pivotal molecule in cellular signaling through the PI3K/Akt pathway (Choi and Jeong, 2005). PDK1 regulates the directional migration of vascular endothelial cells, indicating that one of the mechanisms by which PDK1 controls motility is the regulation of cell orientation (Primo et al., 2007). In addition to its involvement in chemotaxis, activation and phosphorylation of Akt are also mediated by PDK1, while Akt phosphorylation at Thr308 induces apoptosis (Sato et al., 2002). The role of PDK1 in chemotactic migration of human neutrophils has not been studied. The aim of this study was to test the hypothesis that PDK1 regulates chemotaxis in neutrophils and is responsible for the abnormal neutrophil chemotaxis in LAP.
Materials & Methods
Neutrophil Isolation and Chemotaxis
The study protocol was approved by the Boston University Institutional Review Board (IRB); the study participants signed written consent prior to their recruitment into the study. Neutrophils were obtained from healthy donors or individuals diagnosed with LAP. The LAP diagnosis was based on the classification by the American Academy of Periodontology (Armitage, 1999). Neutrophils were isolated according to a discontinuous gradient system as previously reported (Kalmar et al., 1988). Cell preparations were 99% neutrophils with > 95% viability. Chemotaxis was measured by the Transwell method, with a 24-well microchemotaxis chamber with a 5-µm pore-sized filter (Costar, Corning, NY, USA) separating the upper and lower chambers. Isolated neutrophils were incubated with or without inhibitors for 15 min at 4°C, and the cells were placed into the upper chambers (1 × 106 per well). In the lower chambers, fMLP (10 nM) or IL-8 was added as a chemoattractant. Neutrophils were allowed to migrate for 120 min at 37°C at 5% CO2, then collected and counted by microscopy. All experiments were performed at least 3 times.
Western Blotting
Neutrophils (5 × 106) underwent rapid lysis by the addition of 6XSDS sample buffer to the reaction mixture [2% (w/v) SDS, 58.3 mM Tris-HCl (pH 6.8), 6% (v/v) glycerol, 5% (v/v) 2-ME, 0.02% (w/v) bromophenol blue, 1% (v/v) protease inhibitor cocktail, 1 mM PMSF]. Aliquots were separated by SDS-PAGE on 10% or 13% (v/v) polyacrylamide gels and transferred electrophoretically to PVDF membranes immediately with Tris-borate buffer [25 mM Tris, 192 mM glycine, 20% (v/v) methanol (pH 8.4)] at 100 V for 1 hr (4°C). Membranes were blocked for 1 hr at room temperature with 5% skimmed milk in TBS (pH 7.6). The blocking buffer was removed, and the membranes were incubated with the appropriate primary antibodies [1:1000 dilution for PDK1, Phospho-PDK1 Ser241, Akt, Phospho-Akt Thr308, Ser473 (Cell Signaling, Danvers, MA, USA)] overnight at 4°C in 20 mM Tris-HCl [250 mM NaCl, 0.1% (v/v) Tween 20, 1% (w/v) NaN3 (pH 7.6)]. The membranes were washed with TBS-T [20 mM Tris-HCl (pH 7.6) containing 150 mM NaCl, 0.1% (v/v) Tween 20] and incubated with the secondary antibodies (goat anti-rabbit IgG-HRP conjugate; 1:4000 dilution; Cell Signaling) in TBS-T for 1 hr at room temperature. The activity of HRP was visualized in an ECL detection system (Pierce, Rockford, IL, USA), followed by autoradiography. The immunodetection system and the bound antibody were removed from the blot with re-probing buffer [62.5 mM Tris-HCl (pH 6.7), 2% SDS, 100 mM 2-ME], followed by staining with an anti-β-actin antibody (1:2000 dilution) to confirm the loading of equal amounts of protein. The band densities were measured by imaging densitometry (Bio-Rad, Hercules, CA, USA).
RNA Preparation and Quantitative Polymerase Chain-reaction (real-time PCR)
Total RNA was extracted with Trizol (Invitrogen, Carlsbad, CA, USA) and quantified at 260-280 nm. Reverse transcription of 50 ng of total RNA and real-time PCR were performed with TaqMan probes, and sense and antisense primers of PDK1 and β-actin (ABI7000; Applied Biosystems, Foster City, CA, USA). PCR conditions were: 50°C, 2 min; 95°C, 10 min; 92°C, 15 sec; and 60°C, 1 min for 40 cycles. The sequence of Human PDK1 (TaqMan Gene Expression assay, FAM/Non-Quencher Probe, #:Hs00928925_g1) was ggaaacgagtatcttatatttcagaagatcattaagttggaatatgactttccagaaaaattcttccctaaggcaagagacctcgtggagaaacttttggttttagatgcca. Human β-actin was the endogenous control (VIC/TAMRA Probe, #4310881E).
PDK1 Inhibition and Silencing
To study the direct involvement of PDK1 in neutrophil chemotaxis, we first treated neutrophils with staurosporine (stauro-sporine-derived inhibitor-UCN-01; 0.01-1000 nM) for 15 min at 4°C, and chemotaxis was assessed. Then, to test whether the impact of staurosporine could be reproduced by silencing the PDK1 at the gene level, we used siRNA. HL-60 cells (ATCC, Manassas, VA, USA) were induced to differentiate into neutrophil-like cells with DMSO for 3 days and transfected with the Cell Line Nucleofector Kit V, program S-11 (Lonza, Walkersville, MD, USA) with either PDK1-specific siRNA (CACGCCTAACAGGACGTATTA) or non-target siRNA. After 48 hrs, chemotaxis was assessed.
Superoxide Measurement
We monitored superoxide release spectrophotometrically at 37°C by measuring the superoxide dismutase-inhibitable reduction of ferricytochrome C at 550 nm (Cohen and Chovaniec, 1978). Neutrophils or neutrophil-like HL-60 cells (0.5 x 105/well) were stimulated by fMLP, and the absorbance was recorded in a kinetic microplate reader at 550 nm (Molecular Devices, Sunnyvale, CA, USA). Superoxide generation was monitored as a linear rate with respect to both time and cell number and is expressed as nmole O2–/106 cells/min.
Statistical Analysis
Results are presented as the mean ± SEM. Unpaired two-tailed Student’s t test was used for analysis of relative density and fold induction. P values less than 0.05 were considered as statistically significant.
Results
Decreased PDK1 Expression and Activity Associated with Suppressed Chemotaxis in vivo
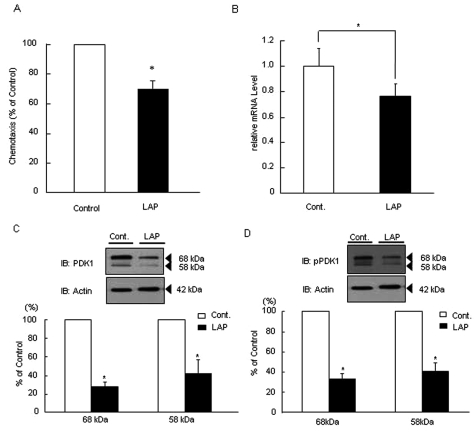
Twenty-two healthy individuals [11 males, 11 females; mean age 31.8 yrs (25-57)] and 19 persons with LAP [(nine males, 10 females; mean age 29.1 yrs (18-52)] participated in this study. Neutrophils from persons with LAP demonstrated 30% reduction in chemotaxis compared with age-, race-, and gender-matched control individuals (Fig. 1A). PDK1 mRNA expression in LAP neutrophils was significantly suppressed compared with that in healthy cells (P < 0.05) (Fig. 1B). PDK1 protein was observed as double bands (upper band, 68 kDa; lower band, 58 kDa). LAP neutrophils had significantly lower PDK1 protein expression (70%) compared with healthy cells (P < 0.05; Fig. 1C). The basal phospho-PDK1 (Ser241) protein expression was also significantly reduced in LAP neutrophils compared with healthy cells (~70%; Fig. 1D).
Figure 1.
SDecreased PDK1 expression and activity correlate with suppressed chemotaxis in vivo. (A) Chemotaxis was measured by the transwell insert method with a 24-well microchemotaxis chamber in which a 5-µm-pore-sized filter separates the upper and lower chambers. To investigate neutrophil chemotaxis from healthy individuals and those with LAP, we placed neutrophils into the upper chamber at 1 × 106 per well in PBS. In the lower chambers, PBS with fMLP (10 nM) was added as chemoattractant. Neutrophils were allowed to migrate toward the soluble attractants in the lower chambers for 120 min at 37°C in a humidified atmosphere (5% CO2). Migrating cells were counted by microscopy. Neutrophil chemotaxis from individuals with LAP was suppressed about 30% (70.5 ± 4.8 neutrophils compared with 97.5 ± 5.8 neutrophils from healthy individuals; P < 0.05. The values are means ± SEM of 3 independent experiments from different individuals). (B) Total PDK1 mRNA expression in neutrophils from individuals with LAP was significantly suppressed compared with that in periodontal-disease-free control individuals (Cont.: total PDK1 mRNA expression in neutrophils from healthy individuals, *P < 0.05; n = 10 persons per group). (C) Total PDK1 protein expression was decreased in neutrophils from individuals with LAP. Total PDK1 protein expression in neutrophils from individuals with LAP was suppressed about 70% (Cont.: total PDK1 protein expression in neutrophils from healthy individuals = 100%; *P < 0.05; n = 5 persons per group). (D) Phosphorylation at Ser241 of PDK1 was decreased in neutrophils from individuals with LAP. The relative intensity of PDK1 phosphorylation normalized to β-actin. Phospho-PDK1 protein (Ser241) expression in neutrophils from individuals with LAP was decreased about 60%.
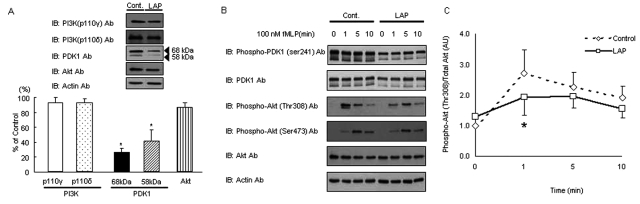
To investigate the specificity of decreased PDK1 in neutrophils with reduced chemotaxis, we analyzed the immediate upstream (PI3K; p110γ and p110δ) and downstream (Akt) molecules, which regulate PDK1 expression in human cells (Fig. 2A). There was no difference in the total expression of PI3K (p110γ or p110δ) and Akt in neutrophils. To investigate phosphorylation events of PDK1 as a measure of PDK1 function, we stimulated neutrophils with fMLP (100 nM) at different time-points and analyzed whole-cell lysates using antibodies to phospho-PDK1 (Ser241), total PDK1, phospho-Akt (Thr308), phospho-Akt (Ser473), and total Akt. In resting cells, LAP neutrophils had significantly reduced phospho-PDK1 and total PDK1 expression (Fig. 2B). The total Akt expression was similar at rest, and there was no detectable phospho-Akt expression. Phosphorylation of PDK1 at Ser241 did not change. Phos-phorylation of Akt (Thr308), however, was reduced in neutrophils with depressed chemotaxis, while Akt phosphorylation at Ser473 did not show any variation, suggesting that PDK1-induced regulation of chemotaxis in neutrophils is mediated through the phosphorylation of Akt at Thr308 (Fig. 2C).
Figure 2.
Down-regulated PDK1, but not PI3K and Akt protein synthesis, caused reduced phosphorylation of Akt Thr308 in LAP neutrophils. (A) PDK1, Phospho-PDK1 (Ser241), PI3K (p110γ), PI3K (p110δ), and Total Akt protein expression were analyzed by Western blotting. Western blots revealed that there was no difference in the total expression of PI3K (p110γ), PI3K (p110δ), and Total Akt in neutrophils at rest between LAP and control individuals. However, total PDK1 protein expression in neutrophils from individuals with LAP was down-regulated ˜70% (Cont.: PI3K (p110γ), PI3K (p110δ), total PDK1, and total Akt protein expression in neutrophils from healthy individuals = 100%; *P < 0.05; at least n = 3 persons per group). (B) In response to fMLP (100 nM, 1 min) stimulation, phosphorylation at Thr308 of Akt was reduced in neutrophils from individuals with LAP. Neutrophils from individuals with LAP and control individuals were stimulated with fMLP (100 nM) for the indicated periods of time. Whole-cell lysates were analyzed by Western blotting with antibodies to phospho-PDK1 (Ser241), total PDK1, phospho-Akt (Thr308), phospho- Akt (Ser473), and total Akt. In response to fMLP (100 nM, 1 min) stimulation, phosphorylation of Akt (Thr308) was reduced in LAP neutrophils, while Akt phosphorylation at Ser473 was unchanged. Phosphorylation of PDK1 at Ser241 did not change regardless of the fMLP stimulation time. Western blots shown are representative of 4 experiments. (C) Quantitative analysis of Western blots demonstrated that LAP PMN have less activity (phosphorylation) of Akt (Thr 308) in parallel with the decreased phosphorylation of PDK1 (*P < 0.05 compared with control cells). Results are expressed as mean ± SEM of at least three persons in each group. IB, immunoblot.
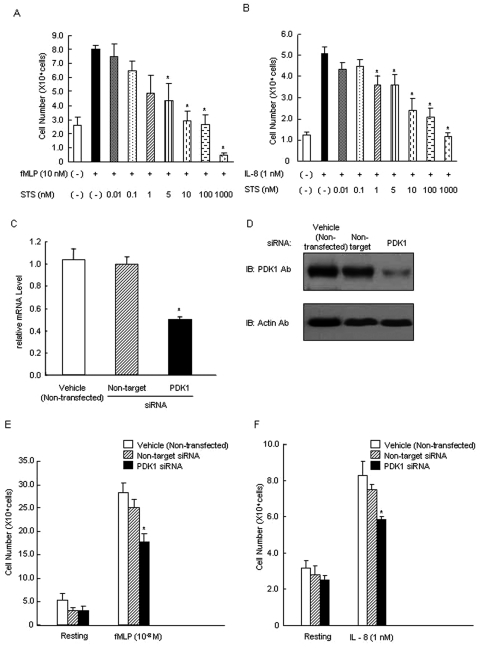
Inhibition of PDK1 Blocks Neutrophil Chemotaxis
Staurosporine dose-dependently suppressed neutrophil chemotaxis in response to fMLP (Fig. 3A) or IL-8 (Fig. 3B), suggesting that PDK1 is involved in intracellular signaling during neutrophil chemotaxis. To rule out other signaling pathways chemically suppressed by staurosporine, we depleted PDK1 with specific siRNA in neutrophils. Since primary neutrophils cannot be reproducibly cultured for extended periods, and therefore transfected, HL-60 cells were differentiated into neutrophil-like cells to accommodate the siRNA transfection. PDK1 siRNA-transfected HL-60 cells showed a 50% reduction in PDK1 mRNA compared with non-specific siRNA transfection (Fig. 3C), followed by a reduction in protein levels of PDK1 (68 kDa; Fig. 3D). PDK1 siRNA-transfected HL-60 cells exhibited decreased fMLP or IL-8-induced chemotaxis, confirming that PDK1 is essential in neutrophil chemotaxis (Figs. 3E, F, P < 0.05).
Figure 3.
Inhibition of PDK1 suppressed chemotaxis in neutrophils. Staurosporine suppressed fMLP-induced (A) or IL-8-induced (B) chemotaxis in neutrophils from healthy individuals (n = 5 persons per group). Chemotaxis was measured by the transwell insert method with a 24-well chemotaxis chamber in which a 5-µm-pore-sized filter separates the upper and lower chambers. To investigate staurosporine inhibition on fMLP- or IL-8-induced chemotaxis, we pre-incubated neutrophils with the various indicated concentrations of staurosporine. In the lower chambers, PBS with fMLP (100 nM) or IL-8 (10 nM) was added as chemo-attractant. Neutrophils were allowed to migrate toward the soluble attractants in the lower chambers for 120 min at 37°C in a humidified atmosphere (5% CO2). Migrating cells were collected and counted by microscopy. Values are mean ± SEM of 3 independent experiments. Asterisk indicates statistically significant compared with vehicle with fMLP or IL-8 (*P < 0.05). PDK1-specific siRNA decreased total PDK1 mRNA and protein expression in HL60 cells. HL60 cells were induced to differentiate (to dHL-60) with DMSO for 3 days to achieve the expression of the neutrophilic phenotype, followed by transfection with PDK1-specific siRNA and non-target siRNA as a control for 48 hrs. HL60 cells were analyzed by Western blotting and real-time RT-PCR. (C) PDK1 siRNA-transfected HL60 cells showed ˜50% reduction in PDK1 mRNA levels compared with vehicle (non-transfected) and non-target siRNA-transfected cells. Values are mean ± SEM of 3 independent experiments. Asterisk indicates statistically significant compared with vehicle (non-transfected) and non-target (*P < 0.05). (D) PDK1 siRNA-transfected HL-60 cells showed a detectable reduction in PDK1 protein levels compared with non-target siRNA-transfected. PDK1 was detected as a single 68-kDa band in differentiated HL60 cells. The viability during the transfection process was not affected (data not shown) compared with non-transfected cells. Results are representative of at least 3 independent experiments. (E) PDK1 siRNA-transfected HL60 cells exhibited decreased fMLP-induced chemotaxis. (F) PDK1 siRNA-transfected HL60 cells exhibited decreased IL-8-induced chemotaxis. Chemotaxis was measured by the transwell insert method with a 24-well chemotaxis chamber in which a 5-µm-pore-sized filter separates the upper and lower chambers. HL60 cells were allowed to migrate toward the soluble attractants in the lower chambers for 120 min at 37°C in a humidified atmosphere (5% CO2). Migrating cells were collected and counted. Values are mean ± SEM of 3 independent experiments. Asterisk indicates statistically significant compared with non-transfected HL60 cells or transfected with non-target HL60 cells on fMLP- or IL-8-induced chemotaxis (*P < 0.05).
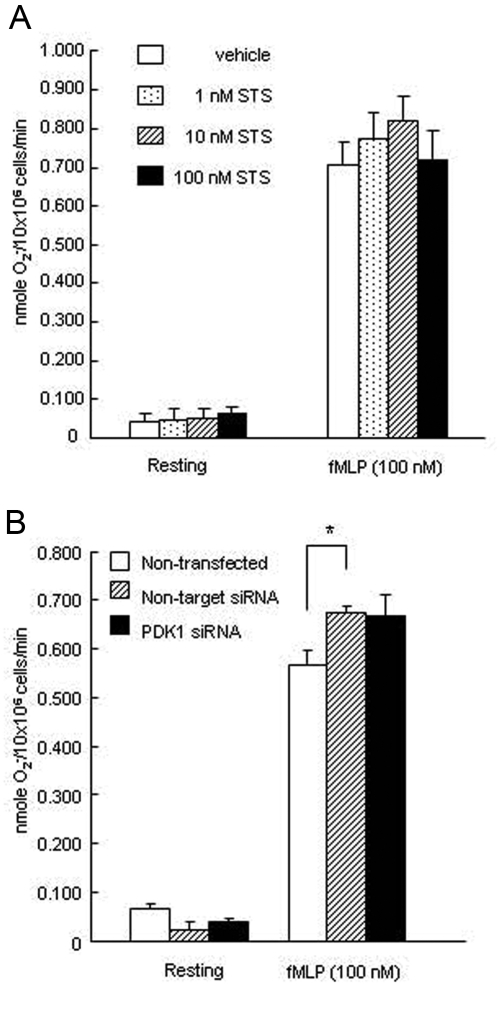
PDK1-specific siRNA Does Not Alter Superoxide Generation by Neutrophils
To investigate whether other functions in addition to chemotaxis are also affected by PDK1 inhibition, we evaluated superoxide generation by neutrophils when PDK1 was silenced. Superoxide release was not affected by PDK1 knockdown (Figs. 4A, 4B), confirming the specificity of PDK1 in neutrophil chemotaxis.
Figure 4.
PDK1 is not involved in superoxide generation. (A) PDK1 inhibitor (Staurosporine) did not alter superoxide generation in neutrophils from individuals with LAP upon fMLP stimulation. Isolated neutrophils from individuals with LAP and control individuals were stimulated with fMLP (1.0 µM) or PBS for 5 min. Superoxide release was measured as the superoxide dismutase-inhibitable reduction of cytochrome c and expressed as described in Materials & Methods. (n = 5 persons per group). (B) PDK1 silencing did not alter superoxide generation in neutrophil-like HL 60 cells. Values are mean ± SEM of 3 independent experiments. Asterisk indicates statistical significance compared with non-transfected HL 60 cells (*P < 0.05).
Discussion
Reduced chemotaxis is a well-established neutrophil abnormality in LAP (Van Dyke et al., 1980; Daniel et al., 1993). We demonstrated that PDK1 plays a central role in neutrophil chemotaxis. A similar decrease in chemotactic response to fMLP or IL-8 suggests that PDK1 involvement was not dependent on the receptor-mediated activation through different chemoattractants. We have also shown that PDK1 is not involved in superoxide generation, suggesting that its role is specific and directly related to neutrophil chemotaxis. Compared with healthy cells, LAP neutrophils demonstrated reduced expression and activity of PDK1. Reduced PDK1 was not correlated with any defect in upstream PI3K expression, while downstream phosphorylation of Akt at Thr308 paired with defective PDK1, suggesting that Akt may be the mechanism of PDK1-mediated neutrophil chemotaxis. Thus, this study demonstrates, for the first time, that PDK1 is an essential regulator of neutrophil chemotaxis in neutrophils, and its in vivo reduction leads to impaired chemotaxis.
PDK1 is downstream of PI3K (Alessi et al., 1997); it phosphorylates and activates a group of related protein kinases belonging to the AGC family (Vanhaesebroeck and Alessi, 2000), including Akt and PKC (Brazil and Hemmings, 2001). PDK1 also contains a PH domain that binds PI3P with high affinity and has a crucial role in Akt activation (Anderson et al., 1998; McManus et al., 2004). PI3K (p110γ and p110δ) plays a central role in neutrophil function (Sasaki et al., 2000; Sadhu et al., 2003; Ferreira et al., 2006; Ferguson et al., 2007), and PI3K (p110γ) has been proposed to be a major isoform involved in chemotaxis (Sasaki et al., 2000; Ferguson et al., 2007). Recent studies have demonstrated a role for PI3K (p110δ) in mediating the directionality of neutrophil chemotaxis, although the pathway by which PI3K (p110δ) is activated by chemotactic receptors remains unknown (Sadhu et al., 2003; Ferreira et al., 2006). PI3K may accelerate the initial polarization and chemotaxis to fMLP, but may play no further role in maintaining the directionality and speed of chemotaxis (Heit et al., 2008). Therefore, to investigate the upstream regulation of PDK1 in neutrophils with depressed chemotactic migration, we assessed the expression of PI3K in LAP neutrophils. There was no difference in the total expression of PI3K (p110γ) or PI3K (p110δ), suggesting that PI3K is not defective in this specific neutrophil phenotype where chemotaxis is impaired, and confirming the role of PDK1.
Akt is a key kinase regulating cellular functions downstream of PDK1. It was demonstrated that the inhibition of Akt markedly inhibited neutrophil chemotaxis (Chen et al., 2003). In LAP neutrophils, Akt expression was not changed. To understand the mechanism of PDK1-mediated neutrophil chemotaxis, we quantified the phosphorylation of Akt at two key regulatory sites, Thr308 (activation loop of the catalytic domain) and Ser473 (COOH-terminal domain). Previous publications suggested that phosphorylation of Akt at Thr308 was catalyzed by the ubiquitously expressed PDK1, while the kinase responsible for phosphorylation of Akt at Ser473 is PDK2 (Stokoe et al., 1997; Vanhaesebroeck and Alessi, 2000). Akt phosphorylation was normal at Ser473, while Thr308 phosphorylation was significantly reduced in LAP neutrophils. This is consistent with the previously reported work showing increased phosphorylation of Akt at Thr308 in PDK1-overexpressing cells, with no change in Ser473 phosphorylation (Primo et al., 2007). Analysis of these data suggests that Thr308 phosphorylation determines the activation state of Akt for the stimulation of directional migration near the entrance to the active site, the activation loop, and a second phosphorylation site, which correspond to Thr308 in the activation loop (Toker and Newton, 2000).
Analysis of our data demonstrated that, regardless of the agonist, PDK1 inhibition by staurosporine leads to the suppression of chemotaxis. While the impact of staurosporine is reportedly specific on PDK1 (Komander et al., 2003), to rule out other signaling pathways that may be suppressed by chemical inhibition, we used PDK1 depletion with specific siRNA silencing. Silencing PDK1 at the gene level led to a 30% reduction in neutrophil chemotaxis, further confirming the involvement of PDK1 in neutrophil chemotactic migration. The dysregulation of chemotaxis of LAP neutrophils affected microbial clearance and neutrophil-mediated tissue injury. It follows that the present findings provide further evidence for the key role of neutrophil signal transduction in the pathogenesis of LAP.
In this study, we provide the first evidence, in vitro and in vivo, that PDK1 activation is an absolute requirement for neutrophil chemotaxis, but is not involved in superoxide generation. This action is specific, as shown by the lack of impact on other cell functions, and independent of agonist. The results suggest that PDK1 plays a crucial role in the regulation of chemotaxis in LAP neutrophils and represents a novel pathway for pharmacological intervention.
Acknowledgments
We thank Amanda Blackwood for laboratory assistance.
Footnotes
These studies were supported by USPHS grants DE16191 and RR00533.
References
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, et al. (1997). Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol 7:261-269 [DOI] [PubMed] [Google Scholar]
- Anderson KE, Coadwell J, Stephens LR, Hawkins PT. (1998). Translocation of PDK-1 to the plasma membrane is important in allowing PDK-1 to activate protein kinase B. Curr Biol 8:684-691 [DOI] [PubMed] [Google Scholar]
- Armitage GC. (1999). Development of a classification system for periodontal disease and conditions. Ann Periodontol 4:1-6 [DOI] [PubMed] [Google Scholar]
- Babior BM. (1984). Oxidants from phagocytes: agents of defense and destruction. Blood 64:959-966 [PubMed] [Google Scholar]
- Baggiolini M, Dewald B, Moser B. (1994). Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol 55:97-179 [PubMed] [Google Scholar]
- Brazil DP, Hemmings BA. (2001). Ten years of protein kinase B signalling: a hard Akt to follow [review]. Trends Biochem Sci 26:657-664 [DOI] [PubMed] [Google Scholar]
- Cianciola LJ, Genco RJ, Patters MR, McKenna J, van Oss CJ. (1977). Defective polymorphonuclear leukocyte function in a human periodontal disease. Nature 265:445-447 [DOI] [PubMed] [Google Scholar]
- Chen Q, Powell DW, Rane MJ, Singh S, Butt W, Klein JB, et al. (2003). Akt phosphorylates p47phox and mediates respiratory burst activity in human neutrophils. J Immunol 170:5302-5308 [DOI] [PubMed] [Google Scholar]
- Choi YS, Jeong S. (2005). PI3-kinase and PDK-1 regulate HDAC1-mediated transcriptional repression of transcription factor NF-kappaB. Mol Cells 20:241-246 [PubMed] [Google Scholar]
- Cohen HJ, Chovaniec ME. (1978). Superoxide generation by digitonin-stimulated guinea pig granulocytes. A basis for a continuous assay for monitoring superoxide production and for the study of the activation of the generating system. J Clin Invest 61:1081-1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel MA, McDonald G, Offenbacher S, Van Dyke TE. (1993). Defective chemotaxis and calcium response in localized juvenile periodontitis neutrophils. J Periodontol 64:617-621 [DOI] [PubMed] [Google Scholar]
- Dennison DK, Van Dyke TE. (1997). The acute inflammatory response and the role of phagocytic cells in periodontal health and disease. Periodontol 2000 14:54-78 [DOI] [PubMed] [Google Scholar]
- Ferguson GJ, Milne L, Kulkarni S, Sasaki T, Walker S, Andrews S, et al. (2007). PI(3)Kγ has an important context-dependent role in neutrophil chemokinesis. Nat Cell Biol 9:86-91 [DOI] [PubMed] [Google Scholar]
- Ferreira AM, Isaacs H, Hayflick JS, Rogers KA, Sandig M. (2006). The p110delta isoform of PI3K differentially regulates beta1 and beta2 integrin-mediated monocyte adhesion and spreading and modulates diapedesis. Microcirculation 13:439-456 [DOI] [PubMed] [Google Scholar]
- Hawkins PT, Anderson KE, Davidson K, Stephens LR. (2006). Signalling through Class I PI3Ks in mammalian cells. Biochem Soc Trans 34(Pt 5):647-662 [DOI] [PubMed] [Google Scholar]
- Heit B, Liu L, Colarusso P, Puri KD, Kubes P. (2008). PI3K accelerates, but is not required for, neutrophil chemotaxis to fMLP. J Cell Sci 121(Pt 2): 205-214 [DOI] [PubMed] [Google Scholar]
- Kalmar JR, Arnold RR, Warbington ML, Gardner MK. (1988). Superior leukocyte separation with a discontinuous one-step Ficoll-Hypaque gradient for the isolation of human neutrophils. J Immunol Methods 110:275-281 [DOI] [PubMed] [Google Scholar]
- Komander D, Kular GS, Bain J, Elliott M, Alessi DR, Van Aalten DM. (2003). Structural basis for UCN-01 (7-hydroxystaurosporine) specificity and PDK1 (3-phosphoinositide-dependent protein kinase-1) inhibition. Biochem J 375(Pt 2):255-262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekstrom-Himes JA, Gallin JI. (2000). Immunodeficiency diseases caused by defects in phagocytes. N Engl J Med 343:1703-1714 [DOI] [PubMed] [Google Scholar]
- MacFarlane GD, Herzberg MC, Nelson RD. (1987). Analysis of polarization and orientation of human polymorphonuclear leukocytes by computer-interfaced video microscopy. J Leukoc Biol 41:307-317 [DOI] [PubMed] [Google Scholar]
- Malech HL, Gallin JI. (1987). Current concepts: immunology. Neutrophils in human diseases. N Engl J Med 317:687-694 [DOI] [PubMed] [Google Scholar]
- McManus EJ, Collins BJ, Ashby PR, Prescott AR, Murray-Tait V, Armit LJ, et al. (2004). The in vivo role of PtdIns(3,4,5)P3 binding to PDK1 PH domain defined by knockin mutation. EMBO J 23:2071-2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockene IS, Shay MJ, Alpert JS, Weiner BH, Dalen JE. (1980). Unexplained chest pain in patients with normal coronary arteriograms: a follow-up study of functional status. N Engl J Med 303:1249-1252 [DOI] [PubMed] [Google Scholar]
- Perez HD, Kelly E, Elfman F, Armitage G, Winkler J. (1991). Defective polymorphonuclear leukocyte formyl peptide receptor(s) in juvenile periodontitis. J Clin Invest 87:971-976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouliot M, Clish CB, Petasis NA, Van Dyke TE, Serhan CN. (2000). Lipoxin A(4) analogues inhibit leukocyte recruitment to Porphyromonas gingivalis: a role for cyclooxygenase-2 and lipoxins in periodontal disease. Biochemistry 39:4761-4768 [DOI] [PubMed] [Google Scholar]
- Primo L, di Blasio L, Roca C, Droetto S, Piva R, Schaffhausen B, et al. (2007). Essential role of PDK1 in regulating endothelial cell migration. J Cell Biol 176:1035-1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadhu C, Masinovsky B, Dick K, Sowell CG, Staunton DE. (2003). Essential role of phosphoinositide 3-kinase delta in neutrophil directional movement. J Immunol 170:2647-2654 [DOI] [PubMed] [Google Scholar]
- Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, et al. (2000). Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science 287:1040-1046 [DOI] [PubMed] [Google Scholar]
- Sato S, Fujita N, Tsuruo T. (2002). Interference with PDK1-Akt survival signaling pathway by UCN-01 (7-hydroxystaurosporine). Oncogene 21:1727-1738 [DOI] [PubMed] [Google Scholar]
- Stephens L, Ellson C, Hawkins P. (2002). Roles of PI3Ks in leukocyte chemotaxis and phagocytosis [review]. Curr Opin Cell Biol 14:203-213 [DOI] [PubMed] [Google Scholar]
- Stokoe D, Stephens LR, Copeland T, Gaffney PR, Reese CB, Painter CF, et al. (1997). Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science 277:567-570 [DOI] [PubMed] [Google Scholar]
- Toker A, Newton AC. (2000). Cellular signaling: pivoting around PDK-1. Cell 103:185-188 [DOI] [PubMed] [Google Scholar]
- Tyagi SR, Uhlinger DJ, Lambeth JD, Champagne C, Van Dyke TE. (1992). Altered diacylglycerol level and metabolism in neutrophils from patients with localized juvenile periodontitis. Infect Immun 60:2481-2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke TE, Horoszewicz HU, Cianciola LJ, Genco RJ. (1980). Neutrophil chemotaxis dysfunction in human periodontitis. Infect Immun 27:124-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Alessi DR. (2000). The PI3K-PDK1 connection: more than just a road to PKB [review]. Biochem J 346(Pt 3):561-576 [PMC free article] [PubMed] [Google Scholar]
- Weiss SJ, Young J, LoBuglio AF, Slivka A, Nimeh NF. (1981). Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest 68:714-721 [DOI] [PMC free article] [PubMed] [Google Scholar]