Abstract
The mitogen-activated protein (MAP) kinase phosphatase (MKP) family plays an important function in regulating the pro-inflammatory cytokines by de-activating MAP kinases. MKP-1 is essential for the dephosphorylation of p38 MAP kinase that regulates expression of IL-6, TNF-α, and IL-1β. We hypothesized that MKP-1 regulates inflammatory bone loss in experimental periodontitis. Wild-type and Mkp-1−/− mice received A. actinomycetemcomitans LPS injection in the palatal region or PBS control 3 times/wk for 30 days. Mice were killed, and maxillae were assessed by microcomputed tomography, histological analysis, and TRAP staining for measurement of bone loss, extent of inflammation, and degree of osteoclastogenesis. Results indicated that, in LPS-injected Mkp-1−/− mice, significantly greater bone loss occurred with more inflammatory infiltrate and a significant increase in osteoclastogenesis compared with Mkp-1−/− control sites or either wild-type group. Analysis of these data indicates that MKP-1 plays a key role in the regulation of inflammatory bone loss.
Keywords: MKP-1, innate immunity, LPS, periodontal disease
Introduction
Bacterial constituents, including Gram-negative-derived lipopolysaccharides (LPS), initiate inflammatory bone loss, as exhibited in periodontal diseases. LPS can stimulate the expression of IL-1ß, TNF-α, IL-6, and RANKL by activating the innate immune response (Nakashima et al., 2000; Jiang et al., 2002; Kikuchi et al., 2003). Within periodontal tissues, TLR-2 and -4 expression is increased in severe disease states, suggesting that these receptors have an increased capacity to signal and influence downstream cytokine expression (Mori et al., 2003). TLR-4 signaling activates MyD88-dependent pathways to subsequent activation of IRAK, TRAF6, and, ultimately, nuclear factor kappa B (NF-κB), which is required for cytokine induction. Also, TRAF6-dependent pathways are required for recruitment of different adaptor proteins and/or activation of various MAPK cascades such as ERK-1 and -2 (An et al., 2002), JNK and p38 (Schumann et al., 1996), needed for cytokine mRNA transcription and mRNA stabilization. In addition to the important role of p38 MAPK in innate immune responses, p38 MAPK has been shown to be an important signaling link between innate and adaptive immune activation. Our group has recently shown the significance of p38 MAPK signaling in periodontal disease progression, where orally active p38 inhibitors reduced periopathogenic LPS-induced bone destruction in a rat model (Kirkwood et al., 2007; Rogers et al., 2007b).
Negative regulation of MAPK activity is mediated by the MAPK phosphatases (MKPs) that dephosphorylate MAPK proteins. Though there are several classes of phosphatases, a family of dual-specificity phosphatases has been shown to be important for MAPK regulation, due to their ability to dephosphorylate threonine and tyrosine residues, which are necessary for their activation (Keyse, 2000). The founding member of this class of phosphatases is MKP-1. Cloned initially as an early response gene in response to growth factors (Lau and Nathans, 1985), MKP-1 is localized into the nucleus, where it preferentially dephosphorylates activated p38 and JNK compared with ERK MAPK (Wu and Bennett, 2005). Consistent with this activity, Mkp-1 null mice have sustained p38 and JNK activity in response to stress (Nimah et al., 2005; Zhao et al., 2005, 2006; Chi et al., 2006). Importantly, MKP-1 has been shown to attenuate TNF-α and IL-6 after LPS stimulation (Chen et al., 2002; Shepherd et al., 2004; Nimah et al., 2005). Indeed, MKP-1 plays a crucial role in the regulation of pro-inflammatory cytokine production through the inactivation of p38 signaling (Salojin et al., 2006; Zhao et al., 2006). Thus, MKP-1 acts as a major negative regulator of p38 MAPK phosphorylation.
In vivo, Mkp-1 null mice have increased sensitivity to endotoxemia, septic shock, and increased alveolar infection in acute models of inflammation and infection (Hammer et al., 2006; Wang et al., 2008). To date, no studies have examined the role of MKP-1 in bone resorption. In this study, we hypothesized that MKP-1 negatively regulates inflammatory bone loss in experimental periodontitis.
Materials & Methods
Cells and Materials
The murine macrophage cell line RAW264.7 (ATCC #TIB-71) was cultured in DMEM supplemented with 100 IU/mL penicillin, 100 µg/mL streptomycin, and 10% heat-inactivated fetal bovine serum and maintained in a humidified atmosphere at 37°C and 5% CO2. LPS from Aggregatibacter actinomycetemcomitans (formerly known as Actinobacillus actinomycetemcomitans) was extracted from A. actinomycetemcomitans strain Y4 (serotype B) by the hot phenol-water method as described (Rogers, 2007b). A. actinomycetemcomitans LPS used in the present study was recently characterized as part of other studies from our lab group (Rogers et al., 2007b). The ELISA kit for IL-6 expression in cell culture supernatant was obtained from R&D Systems (Minneapolis, MN, USA). The expression vector containing MKP-1 cDNA in pSRII-Flag, along with the empty vector as a control, was obtained from Dr. Y. Liu (The Ohio State University).
Immunoblot and ELISA Analysis
RAW cells (5 x 104) were grown for 24 hrs in each well of 6-well plates and stimulated with A. actinomycetemcomitans LPS at indicated time-points. Whole-cell lysates were used for immunoblot analysis. We detected primary antibodies (total and phospho-p38α and MKP-1) by using HRP-conjugated secondary anti-body and a chemiluminescence system (SuperSignal West Pico Chemiluminescent Substrate, Pierce, Rockford, IL, USA). Digital images and quantitation of the membranes were analyzed via a chemiluminescent documentation system (ChemiDoc XRS, Bio-Rad, Hercules, CA, USA). For IL-6 production experiments, RAW cells were transfected with pSRII control or pSRII-MKP-1 by means of Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) and then stimulated for 24 hrs with A. actinomycetemcomitans LPS. IL-6 ELISA was performed per the manufacturer’s instructions with cell culture supernatant.
Animals
Mkp-1+/+ and Mkp-1−/− mice of a mixed genetic background were obtained through a material transfer agreement with Bristol-Myers-Squibb (New York, NY, USA) and housed under specific pathogen-free conditions with food and tap water ad libitum. Ten animals were used per group. Once weekly, we weighed the animals to ensure proper growth and nutrition. Genotype protocols were used as previously established (Zhao et al., 2006). The University Committee on the Use and Care of Animals at the University of Michigan approved all animal protocols.
Inflammatory Bone Loss Model
To initiate alveolar bone loss, we micro-injected A. actinomycetemcomitans LPS (20 µg per injection in 2 µL PBS total volume) directly into the palatal interproximal gingiva between the first and second molars on the left side. The same volume of PBS was injected into the same region on the right side. LPS and PBS injections were repeated 3 times each wk for 4 wks, when all animals were killed by carbon dioxide asphyxiation. Maxillae were hemisected, and posterior block sections were immersed in 10% buffered formalin fixative solution for up to 48 hrs.
Micro-computed Tomography
Non-demineralized rat maxillae were scanned in 70% ethanol by a cone-beam µCT system (GE Healthcare BioSciences; Chalfont St. Giles, UK). Each scan was reconstructed at a mesh size of 18 microns x 18 microns x 18 microns, and three-dimensional digitized images were generated for each specimen. Using GEHC MicroView software (version Viz+ 2.0 build 0029), we rotated the images into a standard orientation and threshold, to distinguish between mineralized and non-mineralized tissue. For each specimen, we generated a grayscale voxel value histogram to determine an optimal threshold value. Linear measurements on bone loss were taken from the cemento-enamel junction (CEJ) to the alveolar bone crest (ABC).
Loss of bone volume was assessed by means of 3-D isoform displays as previously described (Kirkwood et al., 2007; Rogers et al., 2007a,b). Briefly, after proper image orientation, the region of interest (ROI) was determined. The width of the ROI was dictated by the height of contour of the molars at the CEJ. Height of the ROI was measured from molar cusp tips to root apices. Depth was equal to the bucco-lingual size of the teeth plus 100 voxels (1.8 mm3). After establishing the threshold, we calculated the bone volume fraction as the percent of bone within the ROI.
TRAP Staining
Formalin-fixed specimens were decalcified in a 10% EDTA solution for 2 wks at 4°C. The EDTA solution was changed 3 times per wk. The maxillae were paraffin-embedded, and sagittal sections of 5 µm were prepared. Some slides were stained with hematoxylin and eosin (H&E) for descriptive histology. For enumeration of osteoclasts, tartrate-resistant acid phosphatase (TRAP) staining was performed from all the groups by means of a Leukocyte Acid Phosphatase kit (Sigma, St. Louis, MO, USA). Active osteoclasts were defined as multinucleated (≥3) TRAP-positive cells in contact with the bone surface. Slides from approximately the same sagittal sections were used for the enumeration of osteoclasts (as defined previously). Images were captured by means of a Nikon microscope (Nikon Eclipse 80i) and digital camera (Nikon CCD camera).
Statistical Analysis
Ten animals were used per experiment (n = 10). We used the D’Agostino-Pearson test of normality to determine normality assumption data, either parametric or non-parametric. With normality assumptions satisfied, we could use parametric tests. Data were analyzed by Student’s t test or one-way analysis of variance and post hoc Bonferroni tests where indicated. P values < 0.05 were considered significant.
Results
MKP-1 Decreases LPS-induced p38 MAPK Phosphorylation and IL-6 Expression
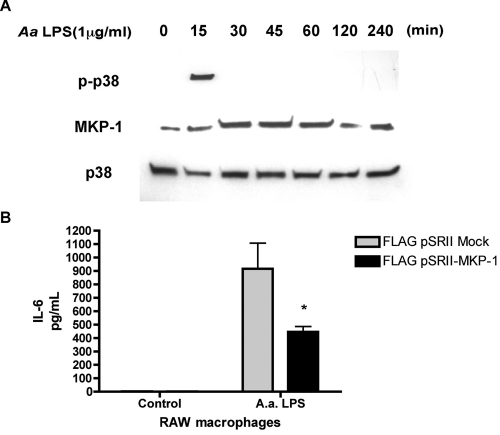
RAW macrophages stimulated with A. actinomycetemcomitans LPS RAW (Fig. 1A) showed a rapid induction of phosphorylated p38 in 15 min, but quickly attenuated as MKP-1 levels became elevated by 30 min. These data are consistent with previous studies indicating that MKP-1 expression increases when phospho-p38 MAPK is reciprocally repressed during the same time course. Over-expression of MKP-1 in an expression construct decreased A. actinomycetemcomitans LPS-induced IL-6 production in transfected RAW macrophages compared with empty vector transfected control LPS-stimulated cells (Fig. 1B).
Figure 1.
MKP-1 activation coincides with phospho-p38 inactivation and decreases IL-6 production. (A) A. actinomycetemcomitans LPS stimulated a time-dependent increase in MKP-1 expression in RAW macrophage cells. RAW 264.7 cells were stimulated with A. actinomycetemcomitans LPS for the indicated times. Cell lysates were then collected for Western analysis of P-p38, p38, and MKP-1 protein. (B) IL-6 attenuation by overexpression of MKP-1 in macrophages. RAW264.7 macrophages were transfected with FLAG-pSRII (empty vector control) or pSRII- MKP-1 and stimulated 24 hrs later with A. actinomycetemcomitans LPS. After 24 hrs, cell culture supernatants were assayed for IL-6 via ELISA. Results from 3 independent experiments are expressed as mean ± SE (*p < 0.05).
LPS-induced Bone Loss is Significantly Increased in Mkp-1−/− Mice
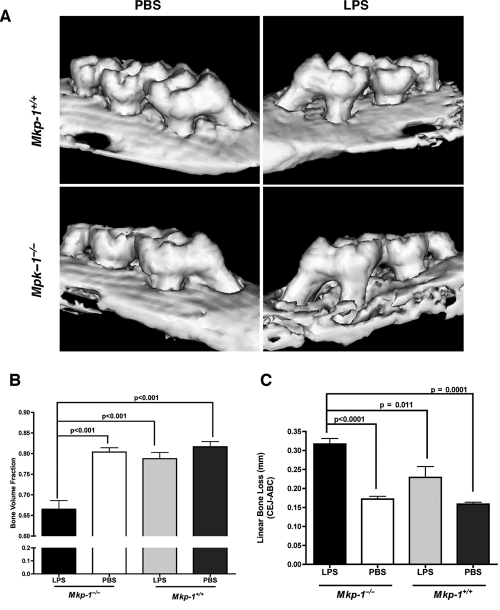
To determine the overall contribution of MKP-1 in an inflammatory bone loss model, we subjected age-matched Mkp-1+/+ and Mkp-1−/− mice to LPS-induced alveolar bone loss. Representative µCT images (Fig. 2A) revealed that Mkp-1−/− mice exhibited visibly more bone loss at LPS injection sites compared with the PBS control sites or LPS- or PBS-injected sites in Mkp-1+/+ mice. Linear and volumetric quantitative analyses of alveolar bone loss showed significantly less bone volume (Fig. 2B; p < 0.01) and significantly more destruction of the interproximal bone on Mkp-1−/− mice at the site of LPS injection in comparison with PBS control, and also in comparison with either LPS or PBS sites of Mkp-1+/+ mice (Fig. 2C; p < 0.001).
Figure 2.
MKP-1 protects from LPS-induced bone loss in experimental periodontitis. (A) Mouse maxillary images from µCT. A. actinomycetemcomitans LPS-induced bone loss. The Bone Volume Fraction (B) and the Linear Bone Loss (C) were analyzed by MicroView software (GE Healthcare). Statistical analysis was performed by Student’s t test. Results are expressed as mean ± SE (n = 10 per group).
Increased Inflammatory Infiltrate in Mkp-1−/− Mice
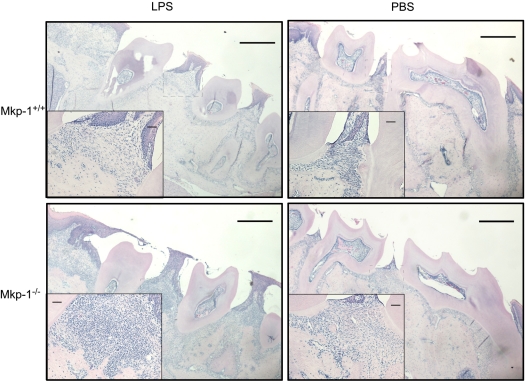
Histological analysis was performed on the interproximal tissue at the sites of LPS and control injection. Representative H&E-stained sections (Fig. 3) showed that LPS-injected Mkp-1−/− mice had a considerable inflammatory cell infiltrate, predominantly leukocytes, compared with either LPS-injected Mkp-1+/+ mice and PBS-injected sites of either genotype.
Figure 3.
Histological analysis of experimental periodontitis in wild-type and Mkp-1-deficient mice. Representative H&E images from LPS (A,C) or PBS (B,D) contralateral control sites. Boxed area from interproximal areas enlarged to show inflammatory infiltrate. Bars indicate scale; lower-magnification image represents 1 mm, and higher-magnification insert is 10 µM (n = 10 per group).
Enhanced Osteoclastogenesis Occurs in Mkp-1−/− Mice
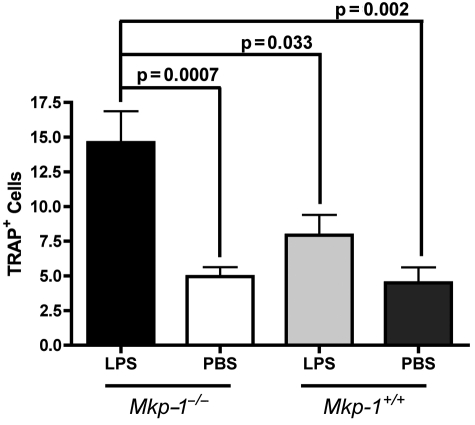
The number of osteoclasts present on tissue sections was determined after TRAP staining. TRAP-positive multinucleated cells were visually enumerated between maxillary first and second molars. LPS injections in Mkp-1−/− mice resulted in significantly greater numbers of TRAP+ cells in comparison with the PBS-injected site in Mkp-1−/− mice, as well as in comparison with wild-type controls (Fig. 4).
Figure 4.
Increased number of osteoclasts observed in LPS-challenged Mkp-1−/− mice. TRAP+ cells were enumerated, and values represent the mean ± SE for all specimens from each group (n = 10 per group). Statistical analysis was performed by Student’s t test.
Discussion
Activation of the innate immune system is critical for lymphocyte activation and other immune cells to help clear infectious micro-organisms. However, exuberant production of pro-inflammatory cytokines leads to severe pathology, including periodontal bone loss, rheumatoid arthritis, and autoimmune diseases (Assuma et al., 1998; Graves, 1999; Delima et al., 2001, 2002; Graves and Cochran, 2003). To help prevent the deleterious effects of TLR activation, several signaling mechanisms have evolved. These mechanisms include the down-regulation of surface TLR receptor expression, transcriptional induction of negative regulators such as IL-1 receptor-associated kinase (IRAK-M), suppressor of cytokine signaling-1 (SOCS1), and SH2-containing inositol phosphatase (SHIP), and production of anti-inflammatory cytokines including IL-10 and TGF-ß (Liew et al., 2005). In this report, we addressed the role of MKP-1, an important negative regulator of MAPK activation, in LPS-induced bone loss. We observed that genetic deletion of MKP-1 resulted in increased LPS-induced bone resorption.
Previous studies have shown that MKP-1 expression was necessary to attenuate IL-6 expression (Zhao et al., 2006). In the present study, analysis of the data provided in macrophages supports the role of MKP-1 in negative regulation of A. actinomycetemcomitans LPS-induced p38 activation and IL-6 production. These data mimic the effects of anti-inflammatory agents, such as corticosteroids, that selectively induce MKP-1 expression (Zhao et al., 2005). Analysis of these data, collectively, suggests that TLR engagement leads to MAPK pathway activation, producing pro-inflammatory cytokines, and that MKP-1 plays a crucial role in decreasing inflammatory cytokine biosynthesis.
In the LPS-induced inflammatory intra-oral bone loss model, A. actinomycetemcomitans LPS is used to induce bone loss in long-term experimental conditions (Rogers et al., 2007a). This model is distinct from previously reported acute inflammatory models that showed the crucial role of MKP-1, in view of the fact that the dose of LPS was five-fold less and given over a four-week period vs. via a single administration (Zhao et al., 2006). In these short-term models, there is unequivocal support for a central role of MKP-1 in the restraint of innate immune responses and the prevention of septic shock syndrome during pathogenic microbial infection. Similarly, analysis of the data presented here suggests a similar protective response in a more chronic model of inflammation and bone loss. After 4 wks of LPS challenge, Mkp-1−/− mice exhibited significantly more bone loss than did their wild-type littermates. These same mice also exhibited an increased amount of inflammatory infiltrate in the periodontal areas injected with LPS, consistent with the role of MKP-1 in regulating innate immune cytokine expression. Previous short-term studies reported higher TNF-α, IL-6, and IL-10 in Mkp-1−/− macrophages, but attenuated levels of IL-12, a classic Th1 cytokine. It is possible to conclude that MKP-1 is an essential phosphatase to prevent or decrease LPS-induced inflammatory response following short- and long-term LPS exposure. Although the histological appearance is very similar to human periodontitis, this model is not initiated by host response to a subgingival biofilm. Results obtained here may be relevant to human periodontitis, where increased levels of p38 and JNK MAPKs have been observed with an increase in clinical inflammation and attachment loss (our unpublished observations). Further studies are needed to address this issue.
Compared with LPS-challenged wild-type mice, Mkp-1−/− mice had a much higher level of inflammatory infiltrate. Similar data have been reported following the systemic administration of LPS in lungs and liver tissue, and are consistent with the increased expression of monocyte chemoattractant protein (MCP)-1/CCL2 following LPS challenge in Mkp-1−/− mice (Zhao et al., 2006; Liu et al., 2007). The increased infiltrate into the periodontal micro-environment can probably be attributed to the loss of MKP-1 regulation in the inflammatory cells in terms of cytokine expression and the overall severity of the inflammatory process leading to greater osteoclast recruitment, differentiation, and, ultimately, bone loss in this model.
In summary, these studies indicate the importance of MKP-1 in the development of immune responses that contribute to LPS-induced alveolar bone loss. Through regulation of MAPK pathways, where p38 MAPK and MKP-1 are critical positive and negative regulators of TLR-induced cytokine expression, the loss of MKP-1 disrupts this balance, permitting sustained MAPK activation and immune cytokine production in periodontal disease progression.
Footnotes
This investigation was supported by USPHS Research Grant 1R01DE018290 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD 20892, USA, and by 3P20 RR017696 from the National Center for Research Resources.
References
- An H, Yu Y, Zhang M, Xu H, Qi R, Yan X, et al. (2002). Involvement of ERK, p38 and NF-kappaB signal transduction in regulation of TLR2, TLR4 and TLR9 gene expression induced by lipopolysaccharide in mouse dendritic cells. Immunology 106:38-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assuma R, Oates T, Cochran D, Amar S, Graves DT. (1998). IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol 160:403-409 [PubMed] [Google Scholar]
- Chen P, Li J, Barnes J, Kokkonen GC, Lee JC, Liu Y. (2002). Restraint of proinflammatory cytokine biosynthesis by mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J Immunol 169:6408-6416 [DOI] [PubMed] [Google Scholar]
- Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, et al. (2006). Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci USA 103:2274-2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delima AJ, Oates T, Assuma R, Schwartz Z, Cochran D, Amar S, et al. (2001). Soluble antagonists to interleukin-1 (IL-1) and tumor necrosis factor (TNF) inhibits loss of tissue attachment in experimental periodontitis. J Clin Periodontol 28:233-240 [DOI] [PubMed] [Google Scholar]
- Delima AJ, Karatzas S, Amar S, Graves DT. (2002). Inflammation and tissue loss caused by periodontal pathogens is reduced by interleukin-1 antagonists. J Infect Dis 186:511-516 [DOI] [PubMed] [Google Scholar]
- Graves DT. (1999). The potential role of chemokines and inflammatory cytokines in periodontal disease progression. Clin Infect Dis 28:482-490 [DOI] [PubMed] [Google Scholar]
- Graves DT, Cochran D. (2003). The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol 74:391-401 [DOI] [PubMed] [Google Scholar]
- Hammer M, Mages J, Dietrich H, Servatius A, Howells N, Cato AC, et al. (2006). Dual specificity phosphatase 1 (DUSP1) regulates a subset of LPS-induced genes and protects mice from lethal endotoxin shock. J Exp Med 203:15-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Mehta CK, Hsu TY, Alsulaimani FF. (2002). Bacteria induce osteoclastogenesis via an osteoblast-independent pathway. Infect Immun 70:3143-3148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyse SM. (2000). Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr Opin Cell Biol 12:186-192 [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Yoshikai Y, Miyoshi J, Katsuki M, Musikacharoen T, Mitani A, et al. (2003). Cot/Tpl2 is essential for RANKL induction by lipid A in osteoblasts. J Dent Res 82:546-550 [DOI] [PubMed] [Google Scholar]
- Kirkwood KL, Li F, Rogers JE, Otremba J, Coatney DD, Kreider JM, et al. (2007). A p38alpha selective mitogen-activated protein kinase inhibitor prevents periodontal bone loss. J Pharmacol Exp Ther 320:56-63 [DOI] [PubMed] [Google Scholar]
- Lau LF, Nathans D. (1985). Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. EMBO J 4:3145-3151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew FY, Xu D, Brint EK, O’Neill LA. (2005). Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol 5:446-458 [DOI] [PubMed] [Google Scholar]
- Liu Y, Shepherd EG, Nelin LD. (2007). MAPK phosphatases—regulating the immune response. Nat Rev Immunol 7:202-212 [DOI] [PubMed] [Google Scholar]
- Mori Y, Yoshimura A, Ukai T, Lien E, Espevik T, Hara Y. (2003). Immunohistochemical localization of Toll-like receptors 2 and 4 in gingival tissue from patients with periodontitis. Oral Microbiol Immunol 18:54-58 [DOI] [PubMed] [Google Scholar]
- Nakashima T, Kobayashi Y, Yamasaki S, Kawakami A, Eguchi K, Sasaki H, et al. (2000). Protein expression and functional difference of membrane-bound and soluble receptor activator of NF-kappaB ligand: modulation of the expression by osteotropic factors and cytokines. Biochem Biophys Res Commun 275:768-775 [DOI] [PubMed] [Google Scholar]
- Nimah M, Zhao B, Denenberg AG, Bueno O, Molkentin J, Wong HR, et al. (2005). Contribution of MKP-1 regulation of p38 to endotoxin tolerance. Shock 23:80-87 [DOI] [PubMed] [Google Scholar]
- Rogers JE, Li F, Coatney DD, Rossa C, Bronson P, Krieder JM, et al. (2007a). Actinobacillus actinomycetemcomitans lipopolysaccharide-mediated experimental bone loss model for aggressive periodontitis. J Periodontol 78:550-558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JE, Li F, Coatney DD, Otremba J, Kriegl JM, Protter TA, et al. (2007b). A p38 mitogen-activated protein kinase inhibitor arrests active alveolar bone loss in a rat periodontitis model. J Periodontol 78:1992-1998 [DOI] [PubMed] [Google Scholar]
- Salojin KV, Owusu IB, Millerchip KA, Potter M, Platt KA, Oravecz T. (2006). Essential role of MAPK phosphatase-1 in the negative control of innate immune responses. J Immunol 176:1899-1907 [DOI] [PubMed] [Google Scholar]
- Schumann RR, Pfeil D, Lamping N, Kirschning C, Scherzinger G, Schlag P, et al. (1996). Lipopolysaccharide induces the rapid tyrosine phosphorylation of the mitogen-activated protein kinases erk-1 and p38 in cultured human vascular endothelial cells requiring the presence of soluble CD14. Blood 87:2805-2814 [PubMed] [Google Scholar]
- Shepherd EG, Zhao Q, Welty SE, Hansen TN, Smith CV, Liu Y. (2004). The function of mitogen-activated protein kinase phosphatase-1 in peptidoglycan-stimulated macrophages. J Biol Chem 279:54023-54031 [DOI] [PubMed] [Google Scholar]
- Wang X, Nelin LD, Kuhlman JR, Meng X, Welty SE, Liu Y. (2008). The role of MAP kinase phosphatase-1 in the protective mechanism of dexamethasone against endotoxemia. Life Sci 83:671-680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JJ, Bennett AM. (2005). Essential role for mitogen-activated protein (MAP) kinase phosphatase-1 in stress-responsive MAP kinase and cell survival signaling. J Biol Chem 280:16461-16466 [DOI] [PubMed] [Google Scholar]
- Zhao Q, Shepherd EG, Manson ME, Nelin LD, Sorokin A, Liu Y. (2005). The role of mitogen-activated protein kinase phosphatase-1 in the response of alveolar macrophages to lipopolysaccharide: attenuation of proinflammatory cytokine biosynthesis via feedback control of p38. J Biol Chem 280:8101-8108 [DOI] [PubMed] [Google Scholar]
- Zhao Q, Wang X, Nelin LD, Yao Y, Matta R, Manson ME, et al. (2006). MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J Exp Med 203:131-140 [DOI] [PMC free article] [PubMed] [Google Scholar]