Abstract
Dental pulp cells can differentiate toward an odontoblastic phenotype to produce reparative dentin beneath caries lesions. However, the mechanisms involved in pulp cell differentiation under pro-inflammatory stimuli have not been well-explored. Thus, we hypothesized that the pro-inflammatory cytokine tumor necrosis factor-α (TNF-α) could be a mediator involved in dental pulp cell differentiation toward an odontoblastic phenotype. We observed that TNF-α-challenged pulp cells exhibited increased mineralization and early and increased expression of dentin phosphoprotein (DPP), dentin sialoprotein (DSP), dentin matrix protein-1, and osteocalcin during a phase of reduced matrix metalloproteinase (MMP) expression. We investigated whether these events were related and found that p38, a mitogen-activated protein kinase, differentially regulated MMP-1 and DSP/DPP expression and mediated mineralization upon TNF-α treatment. These findings indicate that TNF-α stimulates differentiation of dental pulp cells toward an odontoblastic phenotype via p38, while negatively regulating MMP-1 expression.
Keywords: dental pulp cells, tumor necrosis factor-α, dentin sialophosphoprotein, matrix metalloproteinase-1, p38 MAPK
Introduction
Dentinogenesis is a process requiring the elaboration of a collagenous extracellular matrix (ECM), which undergoes mineralization. Dental pulp cells can contribute to this process, and dentin ECM proteins actively promote and control mineralization of collagen fibers and apatite crystal growth during conversion of predentin to dentin (Butler et al., 2003). One ECM protein of notable importance is dentin sialophosphoprotein (DSPP), which undergoes cleavage to DPP (dentin phosphoprotein) and DSP (dentin sialoprotein), is associated with dentin mineralization (Papagerakis et al., 2002), and is expressed by odontoblast-like cells underlying newly formed reparative dentin (Lee et al., 2006; Hwang et al., 2008).
The integrity of the collagenous dentin ECM is critical to the attachment of calcium-binding proteins for the initiation of apatite crystal formation (Butler and Ritchie, 1995). This ECM can be compromised by proteolytic degradation by collagenases (MMP-1, MMP-8, MMP-13) and gelatinases (MMP-2, MMP-9), members of the matrix metalloproteinase (MMP) family (Fanchon et al., 2004). Since inflammatory cytokines play a major role in regulating collagenase expression in dental pulp cells (Lin et al., 2001; Wisithphrom and Windsor, 2006), these agents could compromise the integrity of the predentin ECM, although their role in dentinogenesis has not been fully investigated.
Another potential regulator of dentinogenesis is p38, since it transduces signals from growth factors and stressful stimuli (Patil and Kirkwood, 2007; Schindler et al., 2007), and it is activated in response to growth factors to mediate alkaline phosphatase expression in dental pulp and osteoblastic cells (Wang et al., 2006; Rey et al., 2007). However, the role of p38 in the regulation of dentinogenesis by pro-inflammatory stimuli is not known.
Dental caries or trauma can result in an inflammatory response in the dental pulp, characterized by an accumulation of inflammatory cells, which release host inflammatory cytokines, including tumor necrosis factor-α (TNF-α) (Pezelj-Ribaric et al., 2002; Bletsa et al., 2004). These insults can also stimulate underlying progenitor pulp cells to differentiate into odontoblasts, capable of secreting dentin matrix proteins as part of reparative dentinogenesis (Smith et al., 1995; Smith, 2003; Sloan and Smith, 2007). However, since the mechanisms involved in dental pulp repair under pro-inflammatory stimuli are not completely understood, the aim of this study was to examine the reparative response in primary human dental pulp cells challenged with TNF-α.
Materials & Methods
Cell Culture
Teeth extracted for orthodontic reasons were collected after informed patient content was obtained. Human dental pulp and periodontal ligament (PDL) cells were isolated from teeth under IRB approval as previously described (Kapila et al., 1996; Wisithphrom and Windsor, 2006). Cells were grown in medium consisting of DMEM for pulp cells and α-MEM for periodontal ligament (PDL) cells, both supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, and 1% fungizone. Cells from the third through fifth passages were used for experimentation.
Treatment with Pharmacological Agents and siRNA Transfection
We treated 1 × 105 cells/well with 0.01-50 ng/mL of recombinant human TNF-α (R&D Systems, Minneapolis, MN, USA) in serum-free medium. For time-course experiments, we added 10 ng/mL of TNF-α to wells and conditioned medium, and collected cells after 3, 6, 12, 24, 48, and 96 hrs. For inhibitor experiments, cells were pre-treated with BMS345541 (5 µM), U0126, SB203580, or SP600125 (10 µM) for 1 hr, then medium was replaced with serum-free medium containing the inhibitor plus 10 ng/mL of TNF-α. Pharmacological inhibitors were dissolved in dimethyl sulfoxide (DMSO) to a final concentration that did not exceed 0.5% and was not cytotoxic to cells. For transfection, 20 or 60 pmol of p38 siRNA (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used in 2 x 105 cells/well. After transfection, cells were stimulated with TNF-α as described above. Medium and cells were collected 12, 24, or 48 hrs later.
Western Immunoblotting
Total protein content was measured by the Bradford Microassay (Bio-Rad, Hercules, CA, USA). We resolved 10-30 µg of total protein electrophoretically using 4-12% SDS-PAGE, then electroblotted it onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA) using standard methods. Primary antibodies were MMP-1 (1:500; Calbiochem, San Diego, CA, USA), MMP-2 (1:500; Chemicon, Temecula, CA, USA), MMP-13 (1:1000; Sigma, St. Louis, MO, USA), DSPP/DPP (1:250; Santa Cruz Biotechnology), DSPP/DSP (1:250; Santa Cruz Biotechnology), DMP-1 (1:500; Santa Cruz Biotechnology), osteocalcin (1:250; Abcam, Cambridge, MA, USA), phospho-p38 Thr180/Tyr182 (1:1000; Cell Signaling Technology, Danvers, MA, USA), p38 (1:2000; Cell Signaling Technology), and GAPDH (1:2000; Santa Cruz Biotechnology). Secondary antibodies were anti-mouse IgG, anti-goat IgG, or anti-rabbit IgG conjugated to horseradish peroxidase (Santa Cruz Biotechnology). Immunoblot bands were visualized by enhanced chemiluminescence with the West-Pico ECL detection system (Pierce Biotechnology, Rockford, IL, USA) and analyzed by densitometry with Image J 1.34s Software (National Institutes of Health, Bethesda, MD, USA). We used gels stained with Coomassie blue to verify equal loading of conditioned media samples as previously described (Tran and Neary, 2006; Duan et al., 2007).
The antibodies for DPP and DSP are known to recognize bands at 82 kDa and 42 kDa, respectively, according to the manufacturer. We have also explored the molecular weights of these proteins using the UniProt program (Universal protein resource; http://www.uniprot.org/uniprot/Q9NZW4) to calculate the molecular weights for these proteins from human samples, and they compute to the same. Although processing of human DSP has not yet been characterized, we note that the expected molecular weights for porcine, mouse, or rat DSP are, in the higher ranges, approximately 100-140 kDa. It is possible that our human samples represent differences in species for these proteins, or they may be fragments of DSPP (dentin sialophosphoprotein).
Immunocytochemistry
Standard immunocytochemistry was examined with a DSPP/DPP antibody (1:50). Incubation with a secondary anti-rabbit antibody (Biocare Medical, Concord, CA, USA) was followed by a streptavidin horseradish peroxidase reagent. 3,3′-diaminobenzidine was the enzyme substrate (Biocare Medical). Slides were counterstained with Mayer’s hematoxylin, dehydrated, mounted with permount, and examined by light microscopy. Rabbit IgG was used as the negative control.
Mineralization Assays
We examined mineralized nodule formation by culturing confluent dental pulp cells in DMEM containing 10 mM β-glycero-phosphate, 50 µg/mL ascorbic acid, 1% FBS, and 1% penicillin/streptomycin for 28 days, with changes of media every 3 days. Nodules were visualized by staining with a von Kossa 5% silver nitrate solution and exposure to bright light. Quantification of insoluble calcium in cell/matrix layers was performed with a calcium assay kit (Diagnostic Chemicals Limited, Oxford, CT, USA) (Addison et al., 2007).
Statistical Analysis
Experiments were performed in triplicate. To compare the levels of MMP expressed after TNF-α stimulation over time, we derived quantitative densitometric data as fold-change relative to baseline control levels. Percentages of inhibition of MMP-1 and MMP- 13 by pharmacological inhibitors were calculated based on densitometric analysis of the bands for treatment groups with inhibitors plus TNF-α compared with TNF-α stimulation alone. One-way ANOVA and post hoc comparisons by Tukey (α = 0.05) were performed.
Results
TNF-α Induces Mineralization and Early Expression of DSP, DPP, DMP-1, and Osteocalcin in Dental Pulp Cells
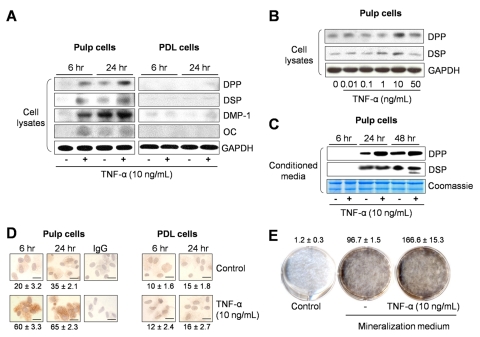
To examine the reparative responses of dental pulp cells to pro-inflammatory stimuli, we treated cells with different doses of TNF-α for different times. Pulp cells exhibited early expression of mineralization-associated proteins, including DPP, DSP, dentin matrix protein-1 (DMP-1), and osteocalcin, 6 and 24 hrs after TNF-α stimulation (Fig. 1A). Furthermore, this response was dose-dependent, and maximum amounts of DSP and DPP were produced with a 10 ng/mL dose of TNF-α (Fig. 1B). Extracellular DPP and DSP could be detected in higher amounts in conditioned media from TNF-α-treated pulp cells compared with non-treated controls (Fig. 1C). In contrast, PDL cells did not produce osteocalcin,but produced low levels of DPP, DSP, and DMP-1 upon TNF-α stimulation (Fig. 1A), suggesting an inherently different response to TNF-α stimulation by these 2 cell types.
Figure 1.
Expression of mineralization-associated proteins and mineralized nodule formation after treatment with TNF-α. (A) Dental pulp and PDL cells were cultured for 6 and 24 hrs in the absence or presence of 10 ng/mL of TNF-α, and expression of DPP (87 kDa), DSP (42 kDa), DMP-1 (57 kDa), and osteocalcin (OC) (11 kDa) in cell lysates was evaluated. GAPDH (37 kDa) was used as loading control. (B) Dose-response effects of TNF-α on DPP and DSP expression were assessed at 24 hrs in dental pulp cells. (C) Extracellular levels of DPP and DSP were evaluated 6, 24, and 48 hrs in the presence or absence of TNF-α. Coomassie blue staining showed equal loading for all lanes. (D) Immunostaining for DSPP/DPP expression in dental pulp and PDL cells with or without TNF-α (10 ng/mL) treatment. The number of DSPP/DPP-positive cells was counted in 5 representative areas and expressed as a percentage of total number of cells in the field of view. Percentages of positive cells are given below the corresponding images (bar = 10 µm). (E) Mineralized nodule formation was visualized by von Kossa staining. Calcium content was quantified with the use of a calcium assay kit, and data were normalized by total protein concentration (in µg calcium/mg). Values shown above the images depict mean and standard deviation from 3 different cell donors.
Since DPP is an important protein in dentin mineralization (Butler et al., 2003; Qin et al., 2004), its expression was further examined by immunocytochemistry. The number of DSPP/DPP-positive pulp cells was higher for TNF-α-treated cells compared with controls, 6 and 24 hrs after stimulation (Fig. 1D). In contrast, DSPP/DPP staining in PDL cells was minimal and comparable with that in controls at 6 and 24 hrs following stimulation.IgG-negative controls did not show positive staining.
Last, TNF-α stimulated mineralization in pulp cells beyond that stimulated in controls (Fig. 1E). Thus, TNF-α induced an odontoblastic phenotype in dental pulp cells, characterized by early and increased expression of DPP, DSP, DMP-1, and osteocalcin, not observed in PDL cells, and increased mineralization, suggesting that TNF-α could initiate an early reparative dentin response.
Latent TNF-α-mediated Secretion of MMPs by Dental Pulp vs. PDL Cells
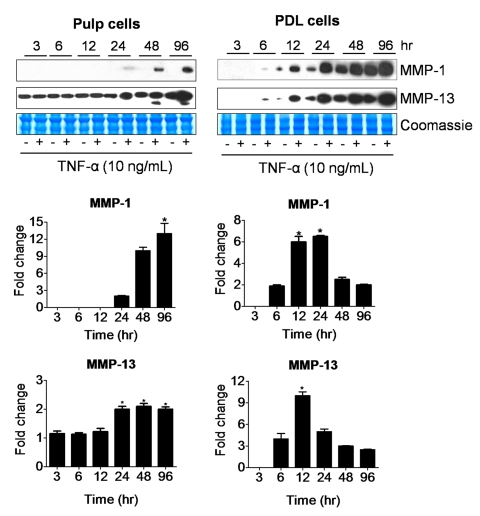
Since MMPs may alter the predentin ECM and ultimately affect early reparative dentinogenesis, MMPs were examined in the context of TNF-α stimulation of dental pulp cells. In response to increasing doses of TNF-α, both pulp and PDL cells secreted increasing levels of MMP-1, MMP-2, and MMP-13 (Fig. 2, Appendix Fig. 1). MMP-8 and MMP-9 were not detected. However, over time, fold-changes in MMP-1 and MMP-13 relative to unstimulated controls increased latently in pulp cells, such that these relative increases were present at later time-points. In contrast, PDL cells showed fold-increases in MMP-1 and MMP-13 as early as 6 hrs, which peaked at 12 hrs (Fig. 2). In general, conditioned media showed higher levels of MMPs than cell extracts (data not shown), and therefore conditioned media was used for comparisons. Thus, compared with dentinogenesis-related proteins, MMPs were expressed at later time-points in pulp cells in response to TNF-α stimulation. This suggests that pro-inflammatory stimuli may elicit a reparative dentin response manifested by increased expression of dentin-associated proteins during a phase of reduced MMP expression that favors increased dentinogenesis.
Figure 2.
Time-course effects of TNF-α on MMP-1 (55 kDa) and MMP-13 (60 kDa) secretion by dental pulp and PDL cells. Western immunoblots are representative of conditioned medium samples from cells treated with 10 ng/mL of TNF-α for 3, 6, 12, 24, 48, and 96 hrs. Coomassie blue staining shows equal loading for all lanes. Graphs represent fold-change in expression of MMP-1 and MMP-13 adjusted to baseline levels for dental pulp and PDL cells. Graphs depict mean and standard deviation from 3 different cell donors; *p < 0.05.
NFκB, MEK-1/2, and JNK Signaling Up-regulate MMP-1 and MMP-13 Expression, whereas p38 MAPK Signaling Down-regulates MMP-1 Expression
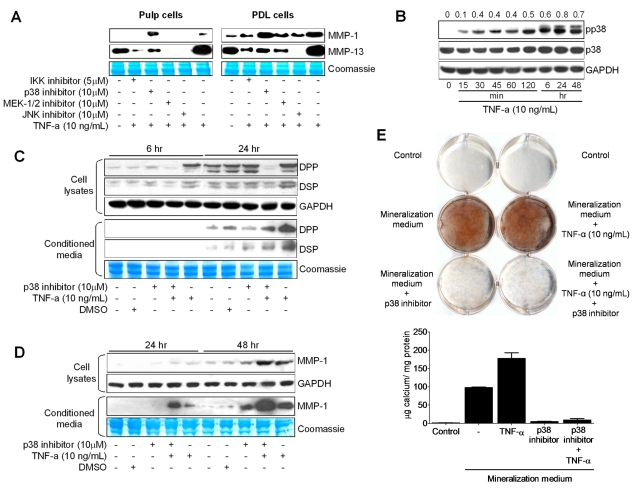
In pulp cells, TNF-α stimulates latent MMP expression; however, the signaling mechanisms that regulate this process are not known. To determine which pathway regulates this process, we treated cells with nuclear factor kappa B (NFκB) and mitogen-activated protein kinase (MAPK) inhibitors, since these pathways regulate MMP expression in different tissues (Vincenti and Brinckerhoff, 2007). Pre-treatment of pulp and PDL cells with an inhibitor of NFκB kinase (BMS345541), for examination of the NFκB pathway, and a MAPK kinase inhibitor (U0126) or c-Jun N-terminal kinase inhibitor (SP600125), for examination of the MAPK pathway, followed by stimulation with TNF-α, prevented MMP-1 and MMP-13 expression in these cells. In contrast, inhibition of p38 (SB203580) stimulated MMP-1 expression in both pulp and PDL cells, whereas it diminished MMP-13 expression (Fig. 3A). Analysis of these data suggests that under TNF-α stimulation, NFκB, MEK-1/2, and JNK signaling pathways mediate up-regulation of MMP-1 and MMP-13 expression, whereas p38 mediates MMP-1 down-regulation in these cells.
Figure 3.
Effects of NFκB and MAPK inhibitors on TNF-α-mediated MMP-1 and MMP-13 expression in dental pulp (A, left) and PDL (A, right) cells. Cells were pre-treated with BMS345541 (IKK phosphorylation inhibitor; 5 µM; 1.27 mg/mL), U0126 (MEK-1/2 inhibitor; 10 µM; 4.03 mg/mL), SB203580 (p38 inhibitor; 10 µM; 3.77 mg/mL), and SP600125 (JNK inhibitor; 10 µM; 2.02 mg/mL) for 1 hr and stimulated with 10 ng/mL of TNF-α for 24 hrs. Western immunoblots show basal levels of MMP-1 and MMP-13, after stimulation with the inhibitors plus TNF-α or TNF-α alone. Coomassie blue staining shows equal loading for all lanes. (B) Time-course experiments showing the effects of TNF-α on p38 (43 kDa) phosphorylation levels after treatment of dental pulp cells with TNF-α (10 ng/mL) for the indicated periods. Ratios of phosphorylated to total p38 levels were calculated by densitometric analysis and are shown above the panels. The effects of p38 MAPK inhibition on TNF-α-mediated DPP, DSP (C), and MMP-1 (D) expression in dental pulp cells were evaluated by Western immunoblotting. Cells were pre-treated with 10 µM of p38 inhibitor (SB203580) for 1 hr and then treated with TNF-α (10 ng/mL) plus p38 inhibitor (10 µM) for 6, 24, and 48 hrs. (E) Effects of p38 inhibition on mineralized nodule formation were assessed as in Fig. 1. Graphs depict mean and standard deviation from 3 different cell donors; *p < 0.05.
p38 Inversely Regulates MMP-1 and DPP/DSP in Dental Pulp Cells under TNF-α Challenge
Since pulp cells exhibited early expression of mineralization-associated proteins and a latent MMP response upon treatment with TNF-α, and considering the fact that p38 negatively regulated MMP-1 expression, we hypothesized that p38 could be the mediator involved in early DSP/DPP expression induced by TNF-α treatment. TNF-α treatment activated the p38 pathway in dental pulp cells via phosphorylation of p38 over time, whereas total p38 levels remained unchanged (Fig. 3B).
For further examination of the role of p38 in this mechanism, cells were pre-treated with a p38 inhibitor for 1 hr, then stimulated with TNF-α for 6, 24, or 48 hrs. p38 inhibition blocked expression of DPP and DSP at 6 and 24 hrs after stimulation, most notably in cell lysates (Fig. 3C). In contrast, inhibition of p38 plus treatment with TNF-α stimulated MMP-1 expression to higher levels than TNF-α treatment alone (Fig. 3D). Inhibition of p38 also blocked the increased mineralization induced by TNF-α (Fig. 3E).
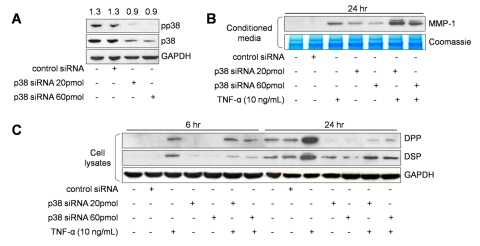
Regulation of DSP and DPP via p38 was confirmed by p38 gene suppression with siRNA (Fig. 4A). DPP and DSP were maximally down-regulated when p38 was inhibited. Furthermore, simultaneous suppression of p38 and stimulation with TNF-α resulted in diminished levels of DPP and DSP compared with TNF-α stimulation alone (Fig. 4C). In contrast, inhibition of p38 plus treatment with TNF-α stimulated MMP-1 expression to higher levels than TNF-α treatment alone, confirming the negative regulation of MMP-1 by p38 (Fig. 4B). Thus, p38 inversely mediated MMP-1 and DSP/DPP expression in dental pulp cells upon TNF-α treatment.
Figure 4.
p38 MAPK siRNA effects on TNF-α-mediated DPP/DSP and MMP-1 expression. Efficacy of p38 siRNA inhibition on total p38 protein expression was quantified after GAPDH normalization, and values are shown above the panels (A). The effects of p38 MAPK siRNA (20 or 60 pmol) on TNF-α-mediated DPP and DSP (B), and MMP-1 (C) expression in dental pulp cells were evaluated. Coomassie blue staining shows equal loading for all lanes. Graphs depict mean and standard deviation from 3 different cell donors; *p < 0.05.
Given these data, we propose a model whereby TNF-α mediates expression of DSPP via activation of p38 and negatively regulates MMP-1 expression downstream of p38 (Appendix Fig. 2).
Discussion
The mechanisms involved in differentiation of dental pulp cells into odontoblasts remain poorly understood. Although there are no specific odontoblastic markers, osteocalcin, osteonectin, alkaline phosphatase, bone sialoprotein, and DSPP have been used as indicators of odontoblastic differentiation (Gronthos et al., 2000; Liu et al., 2006; Wei et al., 2007). However, undifferentiated dental pulp cells also exhibit alkaline phosphatase activity (Lindroos et al., 2008) and DSPP expression (Kitagawa et al., 2007; Nomiyama et al., 2007; Pääkkönen et al., 2008). Furthermore, dental pulp cells adjacent to reparative dentin stain more intensely for DSP and DPP than undifferentiated cells within the dental pulp tissue, indicating an up-regulation of these proteins during reparative dentinogenesis (Lee et al., 2006). Also, dental pulp cells implanted subcutaneously into mice synthesize a mineralized tissue that resembles dentin and stains positively for DSP, demonstrating the in vivo plasticity of pulp cells in differentiating toward an odontoblastic phenotype (Gronthos et al., 2002). Our results agree with these findings, since dental pulp cells expressed DSP, DPP, DMP-1 and osteocalcin and exhibited increased mineralization in response to TNF-α, suggesting that inflammatory mediators induced expression of mineralization-associated proteins and differentiation of dental pulp cells into odontoblasts as part of a reparative dentinogenesis response.
The roles of TNF-α and p38 in dental pulp cell differentiation are not known. In long-term culture, osteonectin and bone sialoprotein expression were inhibited upon treatment with TNF-α in dental pulp cells (Shiba et al., 1998; Min et al., 2006). However, none of these studies focused on early time events, dentin-mineralization-associated protein expression, or mineralized nodule formation. At early time-points, we observed that TNF-α-challenged pulp cells exhibited increased expression of DSP, DPP, DMP-1, and osteocalcin—proteins that are closely related to reparative dentin formation.
Accumulation of collagen and secretion of dentin-mineralization-associated proteins are time-related events during dentin matrix mineralization. Further evidence supporting the concept that a stable collagenous matrix is critical to dentin mineralization comes from studies showing that inhibiting MMP-2 and MMP-9 alters dentin remodeling (Fanchon et al., 2004). Similarly, an inverse correlation exists between collagenase expression and differentiation of osteoblast-like PDL cells (Hayami et al., 2007, 2008), and this is related to the stability of the collagenous matrix. Our results agree with these findings, since treatment of undifferentiated pulp cells with TNF-α induced DSP and DPP expression during a phase of reduced collagenase (MMP-1) expression. The latent expression of collagenases observed in dental pulp cells may be important to maintaining a stable collagenous matrix for mineralization.
This study demonstrated that dental pulp cells exhibit increased mineralization, increased expression of mineralization-associated proteins, and latent secretion of MMPs in response to TNF-α via p38 regulation, suggesting a role for TNF-α in reparative dentin formation.
Supplementary Material
Footnotes
This study is supported by an NIH GrantNIH-R01-13725 to YLK and by a CAPES Foundation Fellowship to FWGPS (0668/07-9).
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Addison WN, Azari F, Sørensen ES, Kaartinen MT, McKee MD. (2007). Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up-regulating osteopontin, and inhibiting alkaline phosphatase activity. J Biol Chem 282:15872-15883 [DOI] [PubMed] [Google Scholar]
- Bletsa A, Heyeraas KJ, Haug SR, Berggreen E. (2004). IL-1 alpha and TNF-alpha expression in rat periapical lesions and dental pulp after unilateral sympathectomy. Neuroimmunomodulation 11:376-384 [DOI] [PubMed] [Google Scholar]
- Butler WT, Ritchie H. (1995). The nature and functional significance of dentin extracellular matrix proteins. Int J Dev Biol 39:169-179 [PubMed] [Google Scholar]
- Butler WT, Brunn JC, Qin C. (2003). Dentin extracellular matrix (ECM) proteins: comparison to bone ECM and contribution to dynamics of dentinogenesis. Connect Tissue Res 44(Suppl 1):171-178 [PubMed] [Google Scholar]
- Duan S, Yao Z, Hou D, Wu Z, Zhu WG, Wu M. (2007). Phosphorylation of Pirh2 by calmodulin-dependent kinase II impairs its ability to ubiquitinate p53. EMBO J 26:3062-3074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanchon S, Bourd K, Septier D, Everts V, Beertsen W, Menashi S, et al. (2004). Involvement of matrix metalloproteinases in the onset of dentin mineralization. Eur J Oral Sci 112:171-176 [DOI] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. (2000). Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA 97:13625-13630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. (2002). Stem cell properties of human dental pulp stem cells. J Dent Res 81:531-535 [DOI] [PubMed] [Google Scholar]
- Hayami T, Zhang Q, Kapila Y, Kapila S. (2007). Dexamethasone‘s enhancement of osteoblastic markers in human periodontal ligament cells is associated with inhibition of collagenase expression. Bone 40:93-104 [DOI] [PubMed] [Google Scholar]
- Hayami T, Kapila YL, Kapila S. (2008). MMP-1 (collagenase-1) and MMP-13 (collagenase-3) differentially regulate markers of osteoblastic differentiation in osteogenic cells. Matrix Biol 27:682-692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang YC, Hwang IN, Oh WM, Park JC, Lee DS, Son HH. (2008). Influence of TGF-beta1 on the expression of BSP, DSP, TGF-beta1 receptor I and Smad proteins during reparative dentinogenesis. J Mol Histol 39:153-160 [DOI] [PubMed] [Google Scholar]
- Kapila YL, Kapila S, Johnson PW. (1996). Fibronectin and fibronectin fragments modulate the expression of proteinases and proteinase inhibitors in human periodontal ligament cells. Matrix Biol 15:251-261 [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Ueda H, Iizuka S, Sakamoto K, Oka H, Kudo Y, et al. (2007). Immortalization and characterization of human dental pulp cells with odontoblastic differentiation. Arch Oral Biol 52:727-731 [DOI] [PubMed] [Google Scholar]
- Lee YL, Liu J, Clarkson BH, Lin CP, Godovikova V, Ritchie HH. (2006). Dentin-pulp complex responses to carious lesions. Caries Res 40:256-264 [DOI] [PubMed] [Google Scholar]
- Lin SK, Wang CC, Huang S, Lee JJ, Chiang CP, Lan WH, et al. (2001). Induction of dental pulp fibroblast matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 gene expression by interleukin-1alpha and tumor necrosis factor-alpha through a prostaglandin-dependent pathway. J Endod 27:185-189 [DOI] [PubMed] [Google Scholar]
- Lindroos B, Mäenpää K, Ylikomi T, Oja H, Suuronen R, Miettinen S. (2008). Characterisation of human dental stem cells and buccal mucosa fibroblasts. Biochem Biophys Res Commun 368:329-335 [DOI] [PubMed] [Google Scholar]
- Liu H, Gronthos S, Shi S. (2006). Dental pulp stem cells. Methods Enzymol 419:99-113 [DOI] [PubMed] [Google Scholar]
- Min KS, Kwon YY, Lee HJ, Lee SK, Kang KH, Lee SK, et al. (2006). Effects of proinflammatory cytokines on the expression of mineralization markers and heme oxygenase-1 in human pulp cells. J Endod 32:39-43 [DOI] [PubMed] [Google Scholar]
- Nomiyama K, Kitamura C, Tsujisawa T, Nagayoshi M, Morotomi T, Terashita M, et al. (2007). Effects of lipopolysaccharide on newly established rat dental pulp-derived cell line with odontoblastic properties. J Endod 33:1187-1191 [DOI] [PubMed] [Google Scholar]
- Pääkkönen V, Vuoristo JT, Salo T, Tjäderhane L. (2008). Comparative gene expression profile analysis between native human odontoblasts and pulp tissue. Int Endod J 41:117-127 [DOI] [PubMed] [Google Scholar]
- Papagerakis P, Berdal A, Mesbah M, Peuchmaur M, Malaval L, Nydegger J, et al. (2002). Investigation of osteocalcin, osteonectin, and dentin sialophosphoprotein in developing human teeth. Bone 30:377-385 [DOI] [PubMed] [Google Scholar]
- Patil CS, Kirkwood KL. (2007). p38 MAPK signaling in oral-related diseases. J Dent Res 86:812-825 [DOI] [PubMed] [Google Scholar]
- Pezelj-Ribaric S, Anic I, Brekalo I, Miletic I, Hasan M, Simunovic-Soskic M. (2002). Detection of tumor necrosis factor alpha in normal and inflamed human dental pulps. Arch Med Res 33:482-484 [DOI] [PubMed] [Google Scholar]
- Qin C, Baba O, Butler WT. (2004). Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med 15:126-136 [DOI] [PubMed] [Google Scholar]
- Rey A, Manen D, Rizzoli R, Ferrari SL, Caverzasio J. (2007). Evidences for a role of p38 MAP kinase in the stimulation of alkaline phosphatase and matrix mineralization induced by parathyroid hormone in osteoblastic cells. Bone 41:59-67 [DOI] [PubMed] [Google Scholar]
- Schindler JF, Monahan JB, Smith WG. (2007). p38 pathway kinases as anti-inflammatory drug targets. J Dent Res 86:800-811 [DOI] [PubMed] [Google Scholar]
- Shiba H, Fujita T, Doi N, Nakamura S, Nakanishi K, Takemoto T, et al. (1998). Differential effects of various growth factors and cytokines on the syntheses of DNA, type I collagen, laminin, fibronectin, osteonectin/secreted protein, acidic and rich in cysteine (SPARC), and alkaline phosphatase by human pulp cells in culture. J Cell Physiol 174:194-205 [DOI] [PubMed] [Google Scholar]
- Sloan AJ, Smith AJ. (2007). Stem cells and the dental pulp: potential roles in dentine regeneration and repair. Oral Dis 13:151-157 [DOI] [PubMed] [Google Scholar]
- Smith AJ. (2003). Vitality of the dentin-pulp complex in health and disease: growth factors as key mediators. J Dent Educ 67:678-689 [PubMed] [Google Scholar]
- Smith AJ, Cassidy N, Perry H, Bègue-Kirn C, Ruch JV, Lesot H. (1995). Reactionary dentinogenesis. Int J Dev Biol 39:273-280 [PubMed] [Google Scholar]
- Tran MD, Neary JT. (2006). Purinergic signaling induces thrombospondin-1 expression in astrocytes. Proc Natl Acad Sci USA 103:9321-9326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincenti MP, Brinckerhoff CE. (2007). Signal transduction and cell-type specific regulation of matrix metalloproteinase gene expression: can MMPs be good for you? J Cell Physiol 213:355-364 [DOI] [PubMed] [Google Scholar]
- Wang FM, Hu T, Zhou X. (2006). p38 mitogen-activated protein kinase and alkaline phosphatase in human dental pulp cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 102:114-118 [DOI] [PubMed] [Google Scholar]
- Wei X, Ling J, Wu L, Liu L, Xiao Y. (2007). Expression of mineralization markers in dental pulp cells. J Endod 33:703-708 [DOI] [PubMed] [Google Scholar]
- Wisithphrom K, Windsor LJ. (2006). The effects of tumor necrosis factor-alpha, interleukin-1beta, interleukin-6, and transforming growth factor-beta1 on pulp fibroblast mediated collagen degradation. J Endod 32:853-861 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.