Abstract
Increasing evidence suggests an association between periodontal disease and adverse pregnancy outcomes. Although infection is considered as a risk factor for preterm delivery, the localization of oral bacteria or their antigens in chorioamnionitis placental tissue has never been demonstrated. This study was devised to test the hypothesis that periodontal pathogens may be present and affect human placenta in cases of chorioamnionitis. Using immunocytochemistry, we have identified the presence of Porphyromonas gingivalis antigens in placental tissues. The antigens were detected in the placental syncytio-trophoblasts, chorionic trophoblasts, decidual cells, and amniotic epithelial cells, as well as the vascular cells. There was a substantial increase in immunostaining intensity of the tissues sectioned from women with chorioamnionitis compared to those experiencing normal-term pregnancy, p < 0.019 (Mann-Whitney test). These results suggest that P. gingivalis may commonly colonize placental tissue, and that the presence of the organism may contribute to preterm delivery.
Keywords: P. gingivalis, placenta, preterm, chorioamnionitis, periodontitis
Introduction
Periodontal disease and its associated bacteria are thought to be involved in a significant number of systemic conditions, such as cardiovascular disease, diabetes mellitus, and osteoporosis (Garcia et al., 2001; Katz et al., 2001). Although some recent studies failed to demonstrate an association (Michalowicz et al., 2006; Vettore et al., 2008), other reports—including animal, and human epidemiological and interventional studies—have suggested that poor periodontal status may result in preterm delivery and low birthweight (Offenbacher et al., 2006; Goldenberg et al., 2008). The association between preterm labor and bacterial infection is well-established, and it has been reported that as many as 40-50% of cases involve an infection (Lamont, 2003; Klein and Gibbs, 2005). Furthermore, up to 80% of preterm deliveries at fewer than 30 wks of gestation have possible infection (Goldenberg et al., 2000), and there is evidence that these infections precede the development of the pregnancy complications (Gonçalves et al., 2002). Infection, therefore, represents an important and frequent contributor to complications of pregnancy. The route of infection can be either ascending from the urogenital tract, such as occurs with E. coli (Goldenberg et al., 2000), or transplacental, such as occurs with Listeria monocytogenes and Salmonella (Lecuit et al., 2004; Schloesser et al., 2004; Bakardjiev et al., 2005). Bacteria can invade the feto-placental tissue, causing tissue damage and premature labor. Alternatively, adverse outcomes can result from a complex interplay between the bacteria and the host cells and tissues that disrupts normal immune and inflammatory status.
P. gingivalis, a consensus pathogen in periodontal disease, has been detected in amniotic fluid from women identified as having threatened premature labor (preterm premature rupture of membranes without clinical infection or labor and preterm labor with intact membranes) (Leon et al., 2007), although a definitive role for P. gingivalis in preterm delivery has yet to be established. However, recurrent bacteremias with P. gingivalis and other oral bacteria are common after dental procedures and can occur in persons with periodontitis during simple mastication (Loesche, 1997; Geerts et al., 2002), which would allow P. gingivalis to reach the placental tissues by hematogenous spread.
To our knowledge, there are no human studies that have directly identified the presence of periodontal bacteria in placental tissue affected by chorioamnionitis. This missing link is essential in establishing the direct relationship between preterm births and periodontal disease. The purpose of the present study, therefore, is to utilize specific antibodies to demonstrate the presence of P. gingivalis in placental tissues.
Materials & Methods
The study was approved by the University of Florida Health Center IRB.
Tissue
Formalin-fixed paraffin-embedded sections were obtained from placental and fetal membranes from women who had normal-term pregnancies (n = 5) or preterm labor complicated by chorioamnionitis (n = 9) with fewer than 37 wks of gestation. The sections were obtained from the Department of Obstetrics and Gynecology, University of Florida College of Medicine, and Shands Medical Center, Gainesville, Florida.
Chorioamnionitis was diagnosed on the basis of fever (greater than 38°C) and maternal and fetal tachycardia in the absence of other localizing signs of infection.
Antibody Staining
P. gingivalis polyclonal antibodies were raised against formalinized cells of the type strain ATCC 33277 (Yilmaz et al., 2002). This antibody does not react with human cells or with other bacteria at dilutions of 1:500 or greater. Control antibodies were normal rabbit serum and serum raised to S. gordonii strain G9B (Lamont et al., 1988). Tissue sections were immunostained with antibodies at a 1:5000 dilution, followed by peroxidase-conjugated secondary antibody and enzyme substrate. Sections were counterstained with hematoxylin and visualized by light microscopy (Olympus BH2, Center Valley, PA, USA) according to the procedure for paraffin-embedded sections (Koenig and Chegini, 2000).
Data Analysis
Staining intensity was rated according to the following scale: no visible staining = 0, faint staining = 1+, moderate staining = 2+, and strong staining = 3+. Extensiveness was graded semi-quantitatively as 0%, < 10%, 10-25%, 25-50%, 50-75%, 75-90%, and > 90% of positively stained cells, per high-power field. To compare all of the available data, we assigned an overall score to each case by multiplying the intensity score by the mean percentage of cells stained (extensiveness). This method has been used and verified by others (Luo et al., 2002; Patel et al., 2008). The overall score was used as the basis for statistical comparison (Table). The Mann-Whitney test was used for the statistical analysis.
Table.
Immunohistochemical Staining Grading for P. gingivalis (overall score = intensity × extensiveness; adapted after Patel et al., 2008)
| Intensity |
Extensiveness |
||
|---|---|---|---|
| Score | Interpretationa | Score (assigned) | Interpretationa |
| 0 | No staining | 0% (0)b | No visible areas |
| 1 | Faint staining | <10% (10) | Very few stained |
| 2 | Moderate staining | 10-25% (17.5) | Few staining |
| 3 | Strong staining | 25-50% (37.5) | Less than half |
| 50-75% (62.5) | More than half | ||
| 75-90% (82.5) | Majority staining | ||
| >90% (90) | All cells staining | ||
The intensity and extensiveness were determined per high-power field.
Values in brackets are the assigned average of the extensiveness percentage that was used for the overall score calculation.
Results
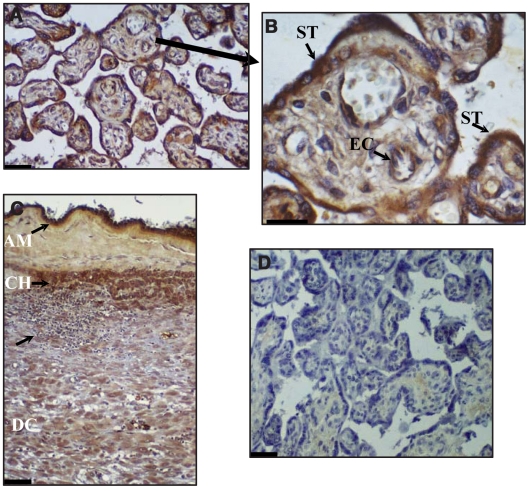
Immunostaining by P. gingivalis antiserum specifically was associated with placental syncytio-trophoblasts, chorionic trophoblasts, decidual cells, and amniotic epithelial cells, as well as with vascular cells (Fig. 1). There was a substantial increase in immunostaining intensity of the tissues sectioned from women with chorioamnionitis as compared with those experiencing normal-term pregnancy (Fig. 2).
Figure 1.
Immunohistochemical localization of P. gingivalis in human placenta (A, and higher magnification in B) and fetal membrane (C) from women with chorioamnionitis. Bacteria are labeled with polyclonal antibodies to P. gingivalis and appear red-brown after immuno-enzymatic color development. Note the presence of immunoreactive P. gingivalis in placental syncytio-trophoblasts (ST) and placental arterial endothelial cells (EC), as well as in amniotic epithelial cells (AM), chorion (CH), and decidual cells (DC). The lowest arrow in (C) shows the presence of a focal inflammatory response with a large inflammatory cell infiltrate, some of which also immunostained for P. gingivalis. (D) Pre-immune rabbit serum as a control did not show any red-brown-staining bacteria. Magnifications: A, C, and D, 60X; B, 150X.
Figure 2.
Immunohistochemical localization of P. gingivalis from women with chorioamnionitis (A,B). Normal placenta (C) and control section (D). Bacteria are labeled with polyclonal antibodies to P. gingivalis and appear red-brown after immuno-enzymatic color development. (D) Pre-immune rabbit serum as a control did not show any red-brown-staining bacteria. Magnifications: 150X.
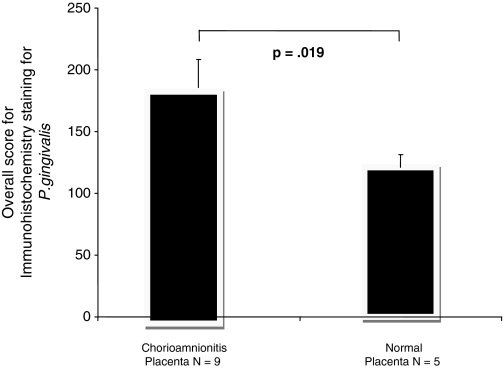
After the staining scores of both groups were compared, the chorioamnionitis group expressed P. gingivalis antigens almost 30% more intensely than the normal placenta groups. This difference was statistically significant (Fig. 3, p < 0.019).
Figure 3.
Comparison between P. gingivalis expression in placental tissues affected by chorioamnionitis (n = 9), and normal placenta (n = 5). The overall score = intensity × extensiveness. Adapted after Patel et al., 2008. The error bars represent SD.
All normal placentas (N = 5) expressed some degree of reactivity with P. gingivalis antibodies. The most commonly stained areas in both normal and infected placentas were trophoblasts (60% of all positively stained cells), decidual cells (20%), and vasculature cells (20%). No staining was observed with S. gordonii or normal rabbit antibodies.
Discussion
In the present study, we have demonstrated the presence of P. gingivalis antigens on trophoblasts, decidual cells, amniotic epithelial cells, and vascular cells, suggesting a possible route of transmission from the vasculature to placental tissue.
Although the staining was present in both normal placentas and placentas affected by chorioamnionitis, the extent to which the latter were positive was 30% higher compared with the normal. The difference in staining between the two groups was statistically significant. As far as we know, this is the first report that documents increased expression of P. gingivalis antigens in human placental cells affected by chorioamnionitis.
P. gingivalis is a Gram-negative anaerobe that can invade and survive within epithelial cells (Lamont and Jenkinson, 1998). Infection by P. gingivalis disrupts cytokine expression and increases cell proliferation while suppressing apoptosis (Mao et al., 2007). All of these properties could potentially disrupt homeostasis in the placental tissues. It is also potentially important that, during the first 10-12 wks of gestation, the placenta is in a state of physiological hypoxia (James et al., 2006), which would facilitate growth of anaerobes such as P. gingivalis.
Preterm delivery of low-birthweight infants is a major public health concern that contributes to infant mortality and short- and long-term morbidity. Preterm delivery occurs in about 12% of births, although rates are higher (more than 20%) among poor and minority pregnant women. Despite dramatic advances in reproductive and neonatal medicine in general, the rate of preterm delivery is not decreasing in either developed or developing countries. While several risk factors for preterm delivery have been identified, their predictive power is limited, and there are no effective treatments to prolong pregnancy once preterm labor commences.
Our present findings show that P. gingivalis antigens are more frequent and more intense in the chorioamnionitis placenta compared with the normal placenta. This is in accordance with some epidemiological studies that have associated severe periodontal disease with subsequently increased bacterial load and preterm delivery.
We chose to focus on P. gingivalis, since this organism is the predominant pathogen in severe cases of periodontal disease, is epidemiologically associated with preterm delivery, and expresses appropriate virulence properties in animal models. However, it is possible, and indeed likely, that other species—such as F. nucleatum, A. actinomycetemcomitans, P. intermedia. T. forsythia, and T. denticola—are present (Barak et al., 2007). Other relevant factors are immune system competency, as well as the physical integrity of placental tissues and their ability to resist bacterial intrusion. It is important to note that the reactivity to P. gingivalis antibodies does not confirm the presence of whole bacteria, but rather their antigens.
In conclusion, accumulating evidence implicates a role for periodontal pathogens such as P. gingivalis in pregnancy complications, including preterm delivery. To date, this evidence is based on epidemiological studies and the pathogenic attributes of the organism in animal placentas following infection and in human amniotic fluid (Lin et al., 2003; Leon et al., 2007). Analysis of our data confirms the results of another study (Barak et al., 2007) showing that P. gingivalis antigens can be present in the normal asymptomatic human placenta. The significance of this finding remains to be determined, although asymptomatic tissue colonization of bacteria is well-documented. The hypothesis is that P. gingivalis bacteremia spreads to the placenta, and infection becomes common. Disease would then occur when there was a change in the overall host-microbe balance, much as in periodontal disease.
Disease outcome following P. gingivalis colonization of the placenta may therefore be dependent on many factors, including bacterial genotype and load, host physiology, genetics, and environmental factors.
Footnotes
This study was supported by NIDCRgrant DE11111 and by an MIPPG grant from the University of Florida, College of Dentistry.
References
- Bakardjiev AI, Stacy BA, Portnoy DA. (2005). Growth of Listeria monocytogenes in the guinea pig placenta and role of cell-to-cell spread in fetal infection. J Infect Dis 191:1889-1897 [DOI] [PubMed] [Google Scholar]
- Barak S, Oettinger-Barak O, Machtei EE, Sprecher H, Ohel G. (2007). Evidence of periopathogenic microorganisms in placentas of women with preeclampsia. J Periodontol 78:670-676 [DOI] [PubMed] [Google Scholar]
- Garcia RI, Henshaw M, Krall EA. (2001). Relationship between periodontal disease and systemic health. Periodontol 2000 25:21-36 [DOI] [PubMed] [Google Scholar]
- Geerts SO, Nys M, De Mol P, Charpentier J, Albert A, Legrand V, et al. (2002). Systemic release of endotoxins induced by gentle mastication: association with periodontitis severity. J Periodontol 73:73-78 [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Hauth JC, Andrews WW. (2000). Intrauterine infection and preterm delivery. N Engl J Med 342:1500-1507 [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R. (2008). Epidemiology and causes of preterm birth. Lancet 371:75-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves LF, Chaiworapongsa T, Romero R. (2002). Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev 8:3-13 [DOI] [PubMed] [Google Scholar]
- James JL, Stone PR, Chamley LW. (2006). The regulation of trophoblast differentiation by oxygen in the first trimester of pregnancy. Hum Reprod Update 12:137-144 [DOI] [PubMed] [Google Scholar]
- Katz J, Heft M, Porter S, Ruskin J. (2001). Inflammation, periodontitis and coronary heart disease. The Lancet 358:1998. [DOI] [PubMed] [Google Scholar]
- Klein LL, Gibbs RS. (2005). Infection and preterm birth. Obstet Gynecol Clin North Am 32:397-410 [DOI] [PubMed] [Google Scholar]
- Koenig JM, Chegini N. (2000). Enhanced expression of Fas-associated proteins in decidual and trophoblastic tissues in pregnancy-induced hypertension. Am J Reprod Immunol 44:347-349 [DOI] [PubMed] [Google Scholar]
- Lamont RF. (2003). Infection in the prediction and antibiotics in the prevention of spontaneous preterm labour and preterm birth. BJOG 110 (Suppl 20):71-75 [DOI] [PubMed] [Google Scholar]
- Lamont RJ, Jenkinson HF. (1998). Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev 62:1244-1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont RJ, Rosan B, Murphy GM, Baker CT. (1988). Streptococcus sanguis surface antigens and their interactions with saliva. Infect Immun 56:64-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit M, Nelson DM, Smith SD, Khun H, Huerre M, Vacher-Lavenu MC, et al. (2004). Targeting and crossing of the human maternofetal barrier by Listeria monocytogenes: role of internalin interaction with trophoblast E-cadherin. Proc Natl Acad Sci USA 101:6152-6157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon R, Silva N, Ovalle A, Chaparro A, Ahumada A, Gajardo M, et al. (2007). Detection of Porphyromonas gingivalis in the amniotic fluid in pregnant women with a diagnosis of threatened premature labor. J Periodontol 78:1249-1255 [DOI] [PubMed] [Google Scholar]
- Lin D, Smith MA, Champagne C, Elter J, Beck J, Offenbacher S. (2003). Porphyromonas gingivalis infection during pregnancy increases maternal tumor necrosis factor alpha, suppresses maternal interleukin-10, and enhances fetal growth restriction and resorption in mice. Infect Immun 71:5156-5162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche WJ. (1997). Association of the oral flora with important medical diseases. Curr Opin Periodontol 4:21-28 [PubMed] [Google Scholar]
- Luo J, Zha S, Gage WR, Dunn TA, Hicks JL, Bennett CJ, et al. (2002). Alpha-methylacyl-CoA racemase: a new molecular marker for prostate cancer. Cancer Res 62:2220-2226 [PubMed] [Google Scholar]
- Mao S, Park Y, Hasegawa Y, Tribble GD, James CE, Handfield M, et al. (2007). Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis. Cell Microbiol 9:1997-2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalowicz BS, Hodges JS, DiAngelis AJ, Lupo VR, Novak MJ, Ferguson JE, et al. (2006). Treatment of periodontal disease and the risk of preterm birth. N Engl J Med 355:1885-1894 [DOI] [PubMed] [Google Scholar]
- Offenbacher S, Boggess KA, Murtha AP, Jared HL, Lieff S, McKaig RG, et al. (2006). Progressive periodontal disease and risk of very preterm delivery. Obstet Gynecol 107:29-36; erratum in Obstet Gynecol 107: 1171, 2006 [DOI] [PubMed] [Google Scholar]
- Patel M, Lu L, Zander DS, Sreerama L, Coco D, Moreb JS. (2008). ALDH1A1 and ALDH3A1 expression in lung cancers: correlation with histologic type and potential precursors. Lung Cancer 59:340-349 [DOI] [PubMed] [Google Scholar]
- Schloesser RL, Schaefer V, Groll AH. (2004). Fatal transplacental infection with non-typhoidal Salmonella. Scand J Infect Dis 36:773-774 [DOI] [PubMed] [Google Scholar]
- Vettore MV, Leal M, Leão AT, Monteiro da Silva A, Lamarca GA, Sheiham A. (2008). The relationship between periodontitis and preterm low birthweight. J Dent Res 87:73-78 [DOI] [PubMed] [Google Scholar]
- Yilmaz O, Watanabe K, Lamont RJ. (2002). Involvement of integrins in fimbriae-mediated binding and invasion by Porphyromonas gingivalis. Cell Microbiol 4:305-314 [DOI] [PubMed] [Google Scholar]