Abstract
The protein compositions, or the proteomes, found in human salivary and plasma fluids are compared. From recent experimental work by many laboratories, a catalogue of 2290 proteins found in whole saliva has been compiled. This list of salivary proteins is compared with the 2698 proteins found in plasma. Approximately 27% of the whole-saliva proteins are found in plasma. However, despite this apparent low degree of overlap, the distribution found across Gene Ontological categories, such as molecular function, biological processes, and cellular components, shows significant similarities. Moreover, nearly 40% of the proteins that have been suggested to be candidate markers for diseases such as cancer, cardiovascular disease, and stroke can be found in whole saliva. These comparisons and correlations should encourage researchers to consider the use of saliva to discover new protein markers of disease and as a diagnostic non-proximal fluid to detect early signs of disease throughout the body.
Keywords: proteins, saliva, plasma, proteomics, mass spectrometry
Introduction
The human body is composed of a plethora of different fluids such as blood, urine, saliva, tears, and sweat, and all contain a wide variety of proteins. These fluids circulate in the body, fill an organ or body cavity, are secreted or excreted from the body, and play vital roles in the overall health and well-being of an individual. Body fluids provide a unique window into the biological processes and functioning of the human body. Some fluids, such as blood, urine, and cerebrospinal fluid (CSF), have found widespread clinical applications for the monitoring of human health and the diagnosis of diseases. Advances in the field of proteomics have opened new doors and are likely to revolutionize the way diseases will be diagnosed in the future. Mining human body fluids to discover disease biomarkers is challenging, but it is hoped that changes in the proteome may be observed even before clinical symptoms become obvious.
Whole saliva is the fluid that bathes the mouth and oral cavity. Whole saliva is made up of several salivary and non-salivary components. It contains secretions from the salivary glands (parotid, submandibular, and sublingual glands, and minor salivary glands) and non-salivary components such as the gingival crevicular fluid, nasal and bronchial secretions, serum and blood derivatives from wounds, desquamated epithelial linings, food components, and micro-organisms that reside in the oral cavity (Kaufman and Lamster, 2002). The salivary glands are composed of two types of epithelial cells: acinar and ductal. Saliva is generated in the acinar cells and stored in granules that are released upon secretory stimulation. The ductal cells line the salivary ducts and direct secreted saliva into the mouth.
Whole saliva is composed of water, peptides and proteins (including enzymes), hormones, sugars, lipids, electrolytes, and several other components. Saliva plays important roles in the oral cavity. It lubricates, hydrates, and bathes the oral cavity, aiding speech and mastication. It forms a barrier on teeth, protects against demineralization, and aids remineralization. Saliva is required for chewing and food bolus preparation, contains enzymes needed for digestion, and mediates the sense of taste. Saliva helps wound healing and protects teeth and the oral cavity from attack by micro-organisms.
Human Salivary Proteome
Efforts to characterize and catalogue proteins from saliva have been expended by many researchers over the past many years. A thorough discussion of the various experimental strategies used to elucidate the salivary proteome is beyond the scope of this review. However, it is clear that each overall proteomics platform using protein/peptide separation and mass spectrometry (MS)-based protein identification has its inherent advantages and disadvantages.
Many of the abundant proteins in human saliva from healthy individuals have been individually studied and characterized previously. These include amylases, proline-rich proteins, statherin, histatin, mucin, and cystatins (Oppenheim et al., 2007). Several MS-based identification efforts have also focused on individual abundant salivary proteins, such as proline-rich proteins (Leymarie et al., 2002; Messana et al., 2004), histatins (Castagnola et al., 2004), and cystatins (Hardt et al., 2005b).
Some of the initial attempts toward large-scale proteomic analysis of whole saliva and glandular secretions used 2D-gel electrophoresis (2-DE) and MS. For example, our group used 2-DE to separate salivary proteins and used MS to identify the proteins; from 105 gel spots, 64 non-redundant proteins were identified in whole saliva (Hu et al., 2005). The total number of proteins identified by 2-DE and MS was less than 200 (Guo et al., 2006). Several groups have since performed liquid chromatography-tandem mass spectrometry (LC-MS/MS) experiments and have added considerably to the salivary protein list. The use of membranes to separate proteins based on molecular weight prior to LC-MS/MS analysis yielded 266 salivary proteins (Hu et al., 2005). Xie and co-workers used free-flow electrophoresis (FFE) separation techniques and identified 437 proteins in whole saliva (Xie et al., 2005; Nissum et al., 2007). Using a combination of offline strong cation exchange (SCX) chromatography combined with LC-MS/MS, Wilmarth et al. identified 102 whole salivary proteins (Wilmarth et al., 2005). Capillary isoelectric focusing (CIEF)-MS/MS was applied by Balgley and co-workers to separate and identify 1381 salivary proteins (Xie et al., 2005). They subsequently extended the list and identified low-abundance salivary proteins by utilizing capillary isotachophoresis/capillary zone electrophoresis (CITP/CZE) and reported a total of 1479 proteins in whole saliva (Fang et al., 2007).
Although many groups have been involved in the identification of whole saliva proteins, large-scale proteome analysis of the ductal fluids has not been as widespread. Hardt and co-workers studied proteins in parotid saliva by 2-DE and MS and reported 17 proteins (Hardt et al., 2005a). Walz and co-workers utilized 2-DE and observed differences in protein migration patterns between parotid and submandibular/sublingual (SM/SL) saliva and also identified 39 proteins from ductal fluids (Walz et al., 2006). More recently, three groups (Scripps Research Institute/University of Rochester, University of California, Los Angeles/University of Southern California, and University of California, San Francisco) supported by the National Institutes of Health (NIH) National Institute of Dental and Craniofacial Research (NIDCR) carried out a comprehensive proteomic analysis to catalogue proteins in ductal fluids. The three groups used different multidimensional separation methods to separate and identify proteins in parotid and SM/SL fluids. In total, 1116 proteins were identified in ductal fluids—914 in parotid and 917 in SM/SL fluids (http://www.hspp.ucla.edu/) (Denny et al., 2008).
Human Plasma Proteome
Efforts to characterize the human plasma proteome have been more extensive than for the salivary proteome. Pooled plasma proteome data from 8 groups have been compiled into a Peptide Atlas that lists 960 plasma proteins derived from 6929 peptides (Deutsch et al., 2005). Anderson et al. combined plasma proteins previously identified by 2-DE and MS (Pieper et al., 2003), by SCX/LC-MS/MS (Adkins et al., 2005), and by LC-MS/MS (Tirumalai et al., 2006) to report a list of 1175 proteins (Anderson et al., 2005). The international Human Proteome Organization (HUPO) initiated a more concerted Plasma Proteome Project (HUPO PPP) (Omenn et al., 2005) that merged data from the same sample pool and emerging from 35 laboratories to report a list of 3020 high-confidence proteins. More recently, Mann and co-workers reported an ultra-high-confidence list of 697 plasma proteins with a false discovery rate of 0.29% and a confidence of at least 99%. An average of 14 peptides per protein was reported, with a median of 6 peptides per protein (Schenk et al., 2008). Proteins reported by the HUPO PPP, David Clemmer’s group at Indiana University, PeptideAtlas (Deutsch et al., 2005), and Leigh Anderson’s group have been compiled into a list of 12,787 proteins from 86,831 peptides (Saha et al., 2008).
Comparison of Salivary and Plasma Proteomes
Blood products (i.e., serum and plasma) are by far the most popular and well-accepted choice for clinical testing, because blood is the circulating fluid that surrounds all tissue and organs and potentially collects by-products from diseased areas, e.g., tumors. Changes in the concentrations of specific plasma proteins have been associated with disease processes, leading to well-accepted clinical applications.
However, whole saliva is a potentially attractive fluid for disease biomarker discovery and diagnostic efforts, because it is readily available from most individuals, can be easily collected and stored and processed, and the collection procedure is non-invasive. Therefore, it is instructive to compare and contrast the protein compositions of salivary and plasma fluids, as determined by numerous laboratories worldwide. Significant overlap in protein content between saliva and plasma may suggest that saliva could be used as a diagnostic alternative to blood tests.
To compare the salivary and plasma proteomes, we started with the HUPO-initiated core dataset of 3020 distinct plasma proteins (with a minimum of 2 unique peptides identified per protein) (Adamski et al., 2005; Omenn et al., 2005). The initial salivary proteome datasets were recently compiled and have been described previously (Yan et al., 2009). Protein identifications from ductal saliva, i.e., parotid/SMSL, were the result of the NIDCR-supported consortium (Denny et al., 2008). The whole-saliva (WS) proteome was contributed by datasets from four research groups: the University of Minnesota, Research Triangle Institute, Calibrant Biosystems/University of Maryland, and the University of California, Los Angeles (UCLA) (Yan et al., 2009).
This initial WS dataset was then augmented with a recent study by Griffin’s lab (Bandhakavi et al., 2009) and newly acquired WS data from our lab. Using a three-dimensional peptide fractionation strategy, the Minnesota lab compiled a list of 2340 proteins in whole saliva (with 60%, or 1395 proteins, identified at the 2-peptide or greater level) (Bandhakavi et al., 2009). Compared with the previously published WS proteome (Yan et al., 2009) (and supplemented by additional data from our lab), the data from the Minnesota group (Bandhakavi et al., 2009) added approximately 497 new WS protein identifications. (It is not clear, however, why 472 proteins in the previously reported WS protein list were not found in the Minnesota dataset.) Presumably, because of the extensive sample fractionation used, the additional WS proteins represent the less abundant species.
The heterogeneous protein identifications for both saliva and plasma were integrated and standardized to the IPI database (IPI version 3.69, February 2010 release date). The integration process started at the peptide level and resolved a non-redundant minimal set of protein identifications, defined such that within a group of proteins containing the sequences with 100% identity to a set of peptides, one of them was selected to represent the group of proteins. All single-peptide-based identifications were excluded.
As before (Yan et al., 2009), we compared the WS proteome with the ductal parotid/SM/SL saliva proteome. Similarly, to examine the common nature of saliva and blood, we compared the saliva proteins with the plasma proteome. As shown in Fig. 1, at the protein level, 72% of the 1205 parotid/SM/SL proteins are found in WS; the previous comparison study showed only a 60% overlap of parotid/SM/SL found in WS, primarily because of the expansion of the WS proteome dataset from the Griffin study, since the number of WS proteins increased from 1444 to 2290. Of these 2290 WS proteins, approximately 27% are found in plasma.
Figure 1.
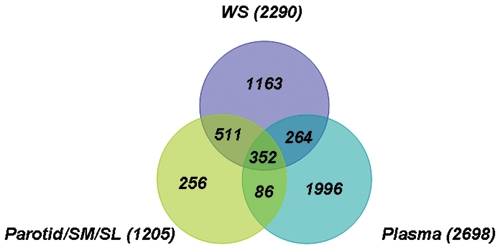
Venn diagram showing the overlapping protein identifications among plasma, whole saliva, and parotid/SM/SL.
Other differences in protein composition and function can be viewed from the comparison of the salivary and plasma proteomes. Human plasma/serum is dominated by immunoglobulins and albumins that make up 60-80% of the total weight (Bjorhall et al., 2005). The most abundant 22 proteins in plasma represent 99% of the total protein content of plasma. These abundant proteins saturate gels and columns for protein separation and display, and make the identification of lower-abundance proteins challenging. Because of the wide dynamic range presented by plasma biofluids [greater than 1010 (Issaaq et al., 2007)], depletion of abundant plasma proteins is critical for improving the prospects of the identification of lower-abundance plasma proteins (Whiteaker et al., 2007). However, for WS, the top 20 most abundant proteins represent only approximately 40% of the saliva protein content (Loo et al., unpublished observations). Some of the most abundant plasma proteins are found to be of moderate-to-high relative abundance in saliva (Fig. 2, right), but only a few high-abundance proteins in saliva are found to be of equally high relative abundance in plasma (Fig. 2, left). [Relative abundance is based on the relative sequence coverage represented by the peptide fragments identified by the LC-MS/MS measurements (Denny et al., 2008).] This situation makes it a far easier task to use salivary fluids for potential biomarker discovery.
Figure 2.

Array views of the human salivary and plasma proteomes. The view on the left is sorted according to the salivary proteins with highest percentage sequence coverage (indicated by the red bars), and the view on the right is sorted by the plasma proteins with highest sequence coverage. Proteins with low sequence coverage are indicated by the green bars.
The WS proteome was compared with the plasma proteome with regard to their theoretical molecular weight and isoelectric point (pI). The salivary proteome contains a larger proportion (14.5%) of low-molecular-weight proteins (< 20 kDa), in contrast to only 7% for the plasma proteome. The highest fraction of proteins found in WS range in size between 20 and 40 kDa (26%), whereas the 40- to 60-kDa range is the largest fraction for plasma (18%). In total, 65% of the saliva proteins have a molecular weight less than 60 kDa, compared with 36% of the plasma proteins. With regard to the proteins found in common between saliva and plasma, the molecular weight distributions are similar to the distributions of the salivary proteome with a tendency toward the low-molecular-weight end, except in the highest MW range (≥ 200 kDa).
A pI comparison of the saliva and plasma proteomes revealed that saliva contains more proteins in the lower acidic end (pI ≤ 5; 10.5% for saliva compared with 6.7% for plasma) and higher basic end (≥ 11) of the pI scale, with an average protein pI of 7.03 and 7.13 for saliva and plasma, respectively.
The salivary and plasma proteomes were also compared based on their gene ontology (GO) in terms of cellular component, molecular process, and biological function (Fig. 3). In general, the salivary and plasma proteomes showed similar distributions in all 3 major GO categories. However, some exceptions were found: Compared with plasma, saliva is over-represented in the catalytic function, but is under-represented in the transcription regulator function. The distributions of the salivary proteins are enhanced in protein metabolic and catabolic processes compared with plasma, which may reflect its major physiologic function in food digestion.
Figure 3.
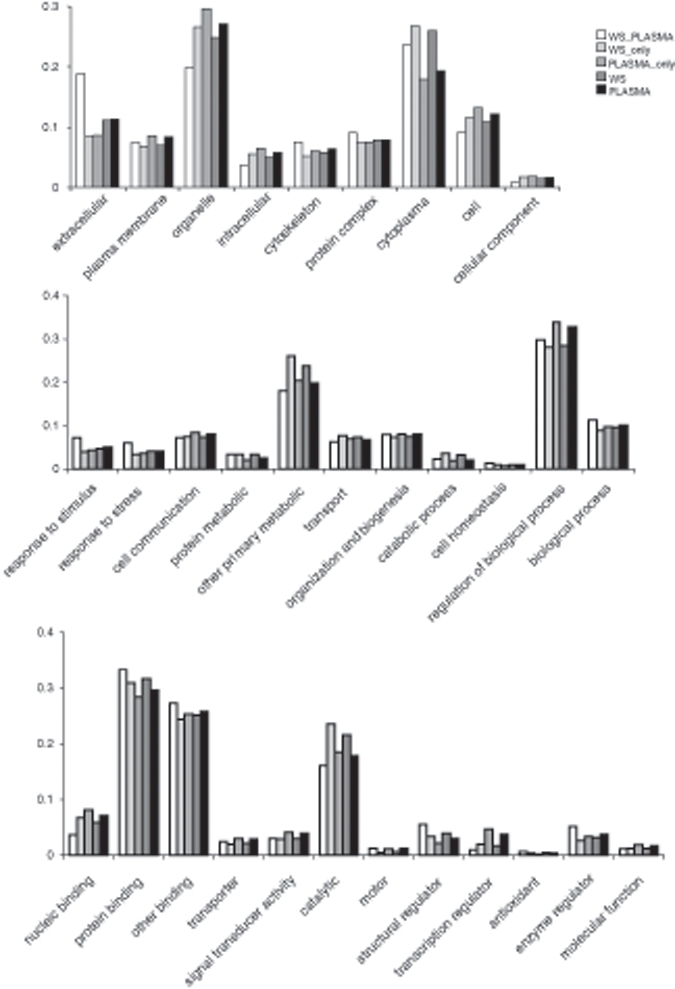
GO distributions (cellular component, top; biological process, middle; molecular function, bottom) of proteins in whole saliva (WS), plasma (Plasma), proteins unique to only whole saliva (WS_only) and plasma (Plasma_only), and proteins found in both whole saliva and plasma (WS_Plasma).
Peptidase and hydrolase activities are enriched in whole saliva, which may be consistent for the saliva proteome’s overrepresentation of lower-molecular-weight proteins, or cleaved products. Gene ontology studies showed that whole saliva proteins associate with a total of 5006 biological functions; 439 functions map to hydrolase and peptidase activities. In contrast, 362 out of 5430 biological functions found in plasma are involved in hydrolase and peptidase activities. Compared with plasma, saliva is significantly overrepresented in hydrolase and peptidase activities (p < 0.001).
However, as discussed previously (Yan et al., 2009), compared with the total human proteome, the salivary and plasma proteomes are over-represented in the extracellular component, an indication of secretion. The salivary and plasma proteins are also over-represented in the cytoplasmic and cytoskeleton components. In contrast, intracellular components are under-represented in saliva and plasma. With regard to biological processes, compared with the human proteome, saliva and plasma are over-represented in the categories of response-to-stimulus, response-to-stress, and cell organization and biogenesis, but are under-represented in cell communication and other primarily metabolic processes. The over-representation of proteins involved in response-to-stimulus and response-to-stress processes presumably reflects the functions of these fluids in the body’s defense system. In the GO molecular functional categories, the salivary and plasma proteomes are significantly over-represented in protein binding but are under-represented in nucleic acid binding, transporter activity, and signal transducer activity.
The functional role of salivary fluids includes lubrication, antimicrobial processes, protection of mucosal integrity, and digestion of food. Proteins in saliva that participate in one or more of these functions include mucins, amylases, defensins, cystatins, histatins, proline-rich proteins, statherin, lactoperoxidase, lysozyme, lactoferrin, and immunoglobulins. The functions of these proteins can be redundant and overlapping. Except for immunoglobulins, these proteins with known salivary functions were commonly, but not always, absent in plasma. For example, statherin and histatin protein families are specific to saliva. The number of isoforms and the abundance of mucin, cystatin, and proline-rich protein families in plasma were significantly lower in plasma than in the WS and parotid/SM/SL proteomes.
Glycosylation of salivary proteins is believed to play a role in salivary protective functions. Characterizations of the glycosylated proteins in saliva and plasma have reported over 70 proteins in saliva (Ramachandran et al., 2006, 2008; Larsen et al., 2007; Sondej et al., 2009) and over 300 in plasma (Liu et al., 2005) as glycosylated proteins. The result of the annotation information extracted from the UniProt knowledge base indicates that potentially more glycosylated proteins exist in saliva and plasma. Indeed, the glycosylation profiles generated from lectin blots indicated that the majority of salivary proteins are potentially glycosylated (Sondej et al., 2009).
Previous estimates established that immunoglobulins contribute from 5 to 15% of the total number of salivary proteins. In the present study, 7% of the total salivary proteins identified were immunoglobulins, and 58% of these were found in plasma. [Compared with the previous report (Yan et al., 2009), the total number of salivary proteins has increased from 1939 to 2692; however, the number of immunoglobulins did not increase. Rather, the number of immunoglobulins identified in saliva was slightly less than previously reported, which is attributed to an increase in the number of unique peptides used for the identification of immunoglobulins and how the immunoglobulin identifications were clustered as a result of these additional peptides.] Our study reveals that there is a high correlation between the abundance of the overlapping immunoglobulins in saliva and plasma, suggesting that these overlapping immunoglobulins could result from leakage from plasma. The precise mechanism of leakage of plasma proteins and whether specific plasma proteins are selectively transported into the salivary system are unknown. However, leakage of plasma into saliva through intracellular or extracellular routes, including outflow of gingival crevicular fluid, is likely (Yan et al., 2009).
This observation can have relevant translational and clinical diagnostic implications, in that we observed significant linearity of immunoglobulin distributions between the plasma and saliva compartments (isotypes, subtypes), suggesting that any antibody, including an autoantibody, would be expected to be detectable in both plasma and saliva, with saliva concentration being reflective and linear to the plasma concentration. This likely is the rationale for the saliva HIV antibody detection test (OraSure, OraQuick Rapid Saliva HIV test). This observation further suggests that other autoantibodies with diagnostic value in plasma/blood are likely to be detectable and can be diagnostic when monitored in saliva.
However, despite the seemingly low overlap of proteins identified in the WS and plasma proteomes (only 616 proteins, or 14%, shared among the 4372 total unique proteins in the two combined proteomes), their gene ontology distributions are remarkably similar (Fig. 3). This perhaps suggests the potential clinical role of salivary fluids for health screening and disease detection.
Use of Saliva in Biomarker Discovery Studies
Many studies have been reported in the past few years to explore the potential use of saliva to discover biomarkers for diseases localized in the oral cavity or in the head/neck region. Some diseases for which saliva has been used as a medium for biomarker discovery include oral cancer squamous cell carcinoma (OSCC) (Negri et al., 1988; Meyer and Zechel, 2001; St John et al., 2004; Rhodus et al., 2005; Zhong et al., 2005; Nagler et al., 2006; Hu et al., 2007b, 2008; Pickering et al., 2007), head and neck squamous cell carcinoma (HNSCC) (Franzmann et al., 2003, 2007; Drake et al., 2005), glossodynia or burning mouth syndrome (Loeb et al., 2008), and Sjögren’s syndrome (Takei et al., 1995; Grisius et al., 1997; Tishler et al., 1999; Castro et al., 2003; Haghighat and al-Hashimi, 2003; Sfrisco et al., 2003; Ryu et al., 2006; Yavuz et al., 2006; Hu et al., 2007a).
In addition, saliva has shown promise for use in the detection of many non-oral (i.e., non-proximal) systemic diseases. In patients with breast cancer, elevated levels of C-erb2 and CA15–3 have been detected in saliva of cancer patients vs. control individuals (Streckfus et al., 1999, 2000a,b). High-throughput SELDI-MS analysis of saliva from breast cancer patients showed promise (Streckfus et al., 2006). Antibodies to HIV have been found in saliva of HIV-positive patients (Malamud, 1997). Castagnola et al. found hypophosphorylation of the salivary peptidome, particularly on the proteins statherin, histatin, and salivary acidic proline-rich protein in individuals with autism (Castagnola et al., 2008). Increased levels of transaldolase and phosphoglycerate mutase I were found from samples of saliva in a study from 22 individuals with fibromyalgia (FM); no validated tests for the diagnosis and the prognostic stratification of FM are currently available (Bazzichi et al., 2009).
While the biological and mechanistic rationale for non-oral systemic diseases to be reflected diagnostically in saliva is currently unclear, efforts are in progress to elucidate the systemic connectivity. Using animal models, we have shown that, upon development of systemic non-oral diseases (e.g., melanoma or lung cancer), there are disease-specific biomarker changes in saliva (Gao et al., 2009). Our working hypothesis is that tumors are known to ectopically produce hormones, lymphokines, and cytokines that can exert systemic effects (alpha gonadotropins in lung cancer and tumor necrosis factor). These tumor-secreted mediators, upon reaching the salivary gland, can alter gene expression and translation of ectopic proteins that can serve as surrogate biomarkers in saliva.
Finally, it should be noted that 27% of the salivary proteome overlapped with the plasma proteome (Fig. 1). While this suggests that circulatory biomarkers may be detectable in saliva, there is a greater implication in reference to saliva’s diagnostic potential. Approximately 73% of the salivary proteins are not present in plasma, providing unique opportunities to discover and harness salivary proteomic markers that are uniquely present in saliva. The successful validation of these saliva-unique disease biomarkers will provide the strongest rationale for the use of saliva for translational and clinical applications for non-oral systemic diseases.
Conclusions and Outlook
In this present examination, we have attempted to construct a comprehensive catalogue of the human salivary proteome by integrating protein identifications from both whole and ductal salivary fluids. The salivary proteome was analyzed and compared among whole and ductal saliva as well as with the human plasma proteome. These analyses should greatly facilitate the characterization of these two human body fluid proteomes and should facilitate the discovery and development of diagnostic disease biomarkers.
Saliva is an excellent body fluid for use in proteomic studies for biomarkers. Efforts by our group and several other laboratories are ongoing to understand the salivary proteome, using varied proteomics and mass spectrometric techniques. Saliva has proven to be of great utility for discovering biomarkers for local oral diseases, such as oral squamous cell carcinoma and Sjögren’s syndrome. The question remains whether it can be harnessed for biomarker discovery for systemic diseases as well. Comparisons between the proteomes of plasma and saliva have indicated that a number of proteins found in saliva are present in blood.
The usefulness of saliva for the diagnosis of systemic diseases needs to be explored further and in greater depth. To date, the application of salivary proteins for the discovery of non-oral-cavity-related diseases has not been widespread. However, a recent report by Nagalla and co-workers may encourage others to consider using saliva as a non-proximal sampling fluid (Rao et al., 2009). From a study group of 40 individuals (10 with previously diagnosed type 2 diabetes on specific therapy, 10 with impaired glucose tolerance, 10 with both impaired fasting glucose and impaired glucose tolerance, and 10 clinically healthy individuals as controls), 65 salivary proteins demonstrated a greater than two-fold difference in abundance between control and type 2 diabetes samples. Not surprisingly, a majority of the differentially abundant proteins belong to pathways regulating metabolism and immune response. Moreover, the analysis of pre-diabetic samples demonstrated a trend of relative increase in their abundance with progression from the pre-diabetic to the diabetic state.
A report from the Plasma Proteome Institute lists 177 candidate biomarker proteins with reported associations to cardiovascular disease (CVD) and stroke that could be targeted for a “directed-proteomics” approach (Anderson, 2005). Slightly over 40% of these candidate CVD/stroke markers are listed in the WS proteome, and approximately 28% are found in both the plasma and WS proteomes. Similarly, from the list of 1058 proteins reported as potential cancer biomarkers (Anderson, 2010), 34% are found in the whole saliva proteome, and 12% are found in both WS and plasma proteomes.
Studies such as these should open new doors to the use of the salivary proteome as the basis for new, non-invasive tests for disease screening, detection, and monitoring. Because of the dynamic range issue associated with plasma (Issaaq et al., 2007), saliva-based testing may prove fruitful. Moreover, newer methods developed for plasma-based sampling may be applied for saliva. The Clinical Proteomic Technologies for Cancer initiative of the National Cancer Institute (NCI-CPTC) has led the development of multiplexed multiple-reaction monitoring (MRM) coupled with isotope dilution MS for pre-clinical verification and quantification of candidate protein biomarkers (Addona et al., 2009). The MRM assay may be sufficiently reproducible across laboratories and may be sufficiently sensitive to monitor low-abundance proteins in biofluids.
Dr. Lawrence Tabak, director of the NIH NIDCR, believes that the elucidation of the components of salivary fluid “could one day lead to miniature labs-on-a-chip used to monitor an individual’s health and detect changes that could signal the beginnings of disease” (Tabak, 2004). Scientists have long recognized that saliva serves as a mirror of health, in that it contains the full repertoire of proteins, hormones, antibodies, and other analytes frequently measured in blood tests. The completion of the salivary proteome, a comprehensive catalogue of all proteins found in the saliva of healthy people, will help to establish saliva as a scientifically validated diagnostic non-proximal fluid to detect early signs of disease throughout the body.
Footnotes
Support was provided by the National Institutes of Health (U01DE016275), a Ruth L. Kirschstein National Service Award (GM07185, to PR), and a UCLA Fundamental Clinical Research Training Grant (T32 DE007296, to PR).
References
- Adamski M, Blackwell T, Menon R, Martens L, Hermjakob H, Taylor C, et al. (2005). Data management and preliminary data analysis in the pilot phase of the HUPO Plasma Proteome Project. Proteomics 5:3246-3261 [DOI] [PubMed] [Google Scholar]
- Addona TA, Abbatiello SE, Schilling B, Skates SJ, Mani DR, Bunk DM, et al. (2009). Multi-site assessment of the precision and reproducibility of multiple reaction monitoring-based measurements of proteins in plasma. Nat Biotechnol 27:633-641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkins JN, Varnum SM, Auberry KJ, Moore RJ, Angell NH, Smith RD, et al. (2005). Toward a human blood serum proteome: analysis by multidimensional separation coupled with mass spectrometry. Mol Cell Proteomics 1:947-955 [DOI] [PubMed] [Google Scholar]
- Anderson L. (2005). Candidate-based proteomics in the search for biomarkers of cardiovascular disease. J Physiol 563(Pt 1):23-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson NL. (2010). The clinical plasma proteome: a survey of clinical assays for proteins in plasma and serum. Clin Chem 56:177-185 [DOI] [PubMed] [Google Scholar]
- Anderson NL, Polanski M, Pieper R, Gatlin T, Tirumalai RS, Conrads T, et al. (2005). The human plasma proteome: a nonredundant list developed by combination of four separate sources. Mol Cell Proteomics 3:311-326 [DOI] [PubMed] [Google Scholar]
- Bandhakavi S, Stone MD, Onsongo G, Van Riper SK, Griffin TJ. (2009). A dynamic range compression and three-dimensional peptide fractionation analysis platform expands proteome coverage and the diagnostic potential of whole saliva. J Proteome Res 8:5590-5600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzichi L, Ciregia F, Giusti L, Baldini C, Giannaccini G, Giacomelli C, et al. (2009). Detection of potential markers of primary fibromyalgia syndrome in human saliva. Proteomics Clin Appl 3:1296-1304 [DOI] [PubMed] [Google Scholar]
- Bjorhall K, Miliotis T, Davidsson P. (2005). Comparison of different depletion strategies for improved resolution in proteomic analysis of human serum samples. Proteomics 5:307-317 [DOI] [PubMed] [Google Scholar]
- Castagnola M, Inzitari R, Rossetti DV, Olmi C, Cabras T, Piras V, et al. (2004). A cascade of 24 histatins (histatin 3 fragments) in human saliva. J Biol Chem 279:41436-41443 [DOI] [PubMed] [Google Scholar]
- Castagnola M, Messana I, Inzitari R, Fanali C, Cabras T, Morelli A, et al. (2008). Hypo-phosphorylation of salivary peptidome as a clue to the molecular pathogenesis of autism spectrum disorders. J Proteome Res 7:5327-5332 [DOI] [PubMed] [Google Scholar]
- Castro J, Jimenez-Alonso J, Sabio JM, Rivera-Civico F, Martin-Armada M, Rodriguez MA, et al. (2003). Salivary and serum beta2-microglobulin and gamma-glutamyl-transferase in patients with primary Sjögren syndrome and Sjögren syndrome secondary to systemic lupus erythematosus. Clin Chim Acta 334:225-231 [DOI] [PubMed] [Google Scholar]
- Denny P, Hagen FK, Hardt M, Liao L, Yan W, Arellanno M, et al. (2008). The proteomes of human parotid and submandibular/sublingual gland salivas collected as the ductal secretions. J Proteome Res 7:1994-2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch EW, Eng JK, Zhang H, King NL, Nesvizhskii AI, Lin B, et al. (2005). Human plasma peptide atlas. Proteomics 5:3497-3500 [DOI] [PubMed] [Google Scholar]
- Drake RR, Cazare LH, Semmes OJ, Wadsworth JT. (2005). Serum, salivary and tissue proteomics for discovery of biomarkers for head and neck cancer. Expert Rev Mol Diagn 5:93-100 [DOI] [PubMed] [Google Scholar]
- Fang X, Yang L, Wang W, Song T, Lee CS, DeVoe DL, et al. (2007). Comparison of electrokinetic-based multidimensional separations coupled with electrospray ionization-tandem mass spectrometry for characterization of human salivary proteins. Anal Chem 79:5785-5792 [DOI] [PubMed] [Google Scholar]
- Franzmann EJ, Schroeder GL, Goodwin WJ, Weed DT, Fisher P, Lokeshwar VB. (2003). Expression of tumor markers hyaluronic acid and hyaluronidase (HYAL1) in head and neck tumors. Int J Cancer 106:438-445 [DOI] [PubMed] [Google Scholar]
- Franzmann EJ, Reategui EP, Pedroso F, Pernas FG, Karakullukcu BM, Carraway KL, et al. (2007). Soluble CD44 is a potential marker for the early detection of head and neck cancer. Cancer Epidemiol Biomarkers Prev 16:1348-1355 [DOI] [PubMed] [Google Scholar]
- Gao K, Zhou H, Zhang L, Lee JW, Zhou Q, Hu S, et al. (2009). Systemic disease-induced salivary biomarker profiles in mouse models of melanoma and non-small cell lung cancer. PLoS ONE 4:e5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisius MM, Bermudez DK, Fox PC. (1997). Salivary and serum interleukin 6 in primary Sjögren’s syndrome. J Rheumatol 24:1089-1091 [PubMed] [Google Scholar]
- Guo T, Rudnick PA, Wang W, Lee CS, DeVoe DL, Balgley BM. (2006). Characterization of the human salivary proteome by capillary isoelectric focusing/nanoreversed-phase liquid chromatography coupled with ESI-tandem MS. J Proteome Res 5:1469-1478 [DOI] [PubMed] [Google Scholar]
- Haghighat N, al-Hashimi I. (2003). The status of lactoferrin and total iron binding capacity of human parotid saliva in Sjögren’s syndrome. Clin Exp Rheumatol 21:485-488 [PubMed] [Google Scholar]
- Hardt M, Thomas LR, Dixon SE, Newport G, Agabian N, Prokobhol A, et al. (2005a). Toward defining the human parotid gland salivary proteome and peptidome: identification and characterization using 2D SDS-PAGE, ultrafiltration, HPLC and mass spectrometry. Biochemistry 44:2885-2899 [DOI] [PubMed] [Google Scholar]
- Hardt M, Witkowska HE, Webb S, Thomas LR, Dixon SE, Hall SC, et al. (2005b). Assessing the effects of diurnal variation on the composition of human parotid saliva: quantitative analysis of native peptides using iTRAQ reagents. Anal Chem 77:4947-4954 [DOI] [PubMed] [Google Scholar]
- Hu S, Xie Y, Ramachandran P, Ogorzalek Loo RR, Li Y, Loo JA, et al. (2005). Large-scale identification of proteins in human salivary proteome by liquid chromatography/mass spectrometry and two-dimensional gel electrophoresis-mass spectrometry. Proteomics 5:1714-1728 [DOI] [PubMed] [Google Scholar]
- Hu S, Wang J, Meijer J, Leong S, Xie Y, Yu T, et al. (2007a). Salivary proteomic and genomic biomarkers for primary Sjögren’s syndome. Arthritis Rheum 56:3588-3600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Yu T, Xie Y, Yang Y, Li Y, Zhou X, et al. (2007b). Discovery of oral fluid biomarkers for human oral cancer by mass spectrometry. Cancer Genomics Proteomics 4:55-64 [PubMed] [Google Scholar]
- Hu S, Arellano M, Boontheung P, Wang J, Zhou H, Jiang J, et al. (2008). Salivary proteomics for oral cancer biomarker discovery. Clin Cancer Res 14:6246-6252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issaaq HJ, Xiao Z, Veenstra TD. (2007). Serum and plasma proteomics. Chem Rev 107:3601-3620 [DOI] [PubMed] [Google Scholar]
- Kaufman E, Lamster IB. (2002). The diagnostic application of saliva—a review. Crit Rev Oral Biol Med 13:197-212 [DOI] [PubMed] [Google Scholar]
- Larsen MR, Jensen SS, Jakobsen LA, Heegaard NH. (2007). Exploring the sialome using titanium dioxide chromatography and mass spectrometry. Mol Cell Proteomics 6:1778-1787 [DOI] [PubMed] [Google Scholar]
- Leymarie N, Berg EA, McComb ME, O’Connor PB, Grogan J, Oppenheim FG, et al. (2002). Tandem mass spectrometry for structural characterization of proline rich proteins: application to salivary PRP-3. Anal Chem 74:4124-4132 [DOI] [PubMed] [Google Scholar]
- Liu T, Qian WJ, Gritsenko MA, Camp DG, 2nd, Monroe ME, Moore RJ, et al. (2005). Human plasma N-glycoproteome analysis by immuno-affinity subtraction, hydrazide chemistry and mass spectrometry. J Proteome Res 4:2070-2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb LM, Naffah-Mazzacoratti MG, Porcionatto MA, Martins JR, Kouyoumdjian M, Weckx LM, et al. (2008). Chrondroitin sulfate and kallikrein in saliva: markers for glossodynia. Int Immunopharmacol 8:1056-1058 [DOI] [PubMed] [Google Scholar]
- Malamud D. (1997). Oral diagnostic testing for detecting human immuno-deficiency virus-1 antibodies: a technology whose time has come. Am J Med 102:9-14 [DOI] [PubMed] [Google Scholar]
- Messana I, Cabras T, Inzitari R, Lupi A, Zuppi C, Olmi C, et al. (2004). Characterization of the human salivary basic proline-rich protein complex by a proteomic approach. J Proteome Res 3:792-800 [DOI] [PubMed] [Google Scholar]
- Meyer P, Zechel T. (2001). Quantitative studies of lysozymes and phosphohexose isomerase in mixed saliva in oral squamous epithelial carcinoma. HNO 49:626-629 [DOI] [PubMed] [Google Scholar]
- Nagler R, Bahar G, Shpitzer T, Feinmesser R. (2006). Concomitant analysis of salivary tumor markers—a new diagnostic tool for oral cancer. Clin Cancer Res 12:3979-3984 [DOI] [PubMed] [Google Scholar]
- Negri L, Pacchioni D, Calabrese F, Giacomasso S, Mastromatteo V, Fazio M. (1988). Serum and salivary CEA and GICA levels in oral cavity tumors. Int J Bio Markers 3:107-112 [PubMed] [Google Scholar]
- Nissum M, Kuhfuss S, Hauptmann M, Obermaier C, Sukop U, Wildgruber R, et al. (2007). Two-dimensional separation of human plasma proteins using iterative free-flow electrophoresis. Proteomics 7:4218-4227 [DOI] [PubMed] [Google Scholar]
- Omenn GS, States DJ, Adamski M, Blackwell TW, Menon R, Hermjakob H, et al. (2005). Overview of the HUPO plasma proteome project: results from the pilot phase with 35 collaborating laboratories and multiple analytical groups, generating a core dataset of 3020 proteins and a publicly-available database. Proteomics 5:3226-3245 [DOI] [PubMed] [Google Scholar]
- Oppenheim FG, Salih E, Siqueira WL, Zhang W, Helmerhorst E. (2007). Salivary proteome and its genetic polymorphisms. Ann NY Acad Sci 1098:22-50 [DOI] [PubMed] [Google Scholar]
- Pickering V, Jordan RC, Schmidt BL. (2007). Elevated salivary endothelin levels in oral cancer patients—a pilot study. Oral Oncol 43:37-41 [DOI] [PubMed] [Google Scholar]
- Pieper R, Gatlin CL, Mukusky AJ, Russo PS, Schatz CR, Miller SS, et al. (2003). The human serum proteome: display of nearly 3700 chromatographically separated protein spots on two-dimensional electrophoresis gels and identification of 325 distinct proteins. Proteomics 3:1345-1364 [DOI] [PubMed] [Google Scholar]
- Ramachandran P, Boontheung P, Xie Y, Sondej M, Wong DT, Loo JA. (2006). Identification of N-linked glycoproteins in human saliva by glycoprotein capture and mass spectrometry. J Proteome Res 5:1493-1503 [DOI] [PubMed] [Google Scholar]
- Ramachandran P, Boontheung P, Pang E, Yan W, Wong DT, Loo JA. (2008). Comparison of N-linked glycoproteins in human whole saliva, parotid, submandibular and sublingual glandular secretions identified using hydrazide chemistry and mass spectrometry. Clin Proteomics 4:80-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PV, Reddy AP, Lu X, Dasari S, Krishnaprasad A, Biggs E, et al. (2009). Proteomic identification of salivary biomarkers of type-2 diabetes. J Proteome Res 8:239-245 [DOI] [PubMed] [Google Scholar]
- Rhodus NL, Ho V, Miller CS, Myers S, Ondrey F. (2005). NF-kappaB dependent cytokine levels in saliva of patients with oral preneoplastic lesions and oral squamous cell carcinoma. Cancer Detect Prev 29:42-45 [DOI] [PubMed] [Google Scholar]
- Ryu OH, Atkinson JC, Hoehn GT, Illei GG, Hart TC. (2006). Identification of parotid salivary biomarkers in Sjögren’s syndrome by surface enhanced laser desorption/ionization time-of-flight mass spectrometry and two-dimensional gel electrophoresis. Rheumatology (Oxford) 45:1077-1086 [DOI] [PubMed] [Google Scholar]
- Saha S, Harrison SH, Shen C, Tang H, Radivojac P, Arnold RJ, et al. (2008). HIP2: an online database of human plasma proteins from healthy individuals. BMC Med Genomics 1:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Schoenhals GJ, de Souza G, Mann M. (2008). A high confidence, manually validated human blood plasma protein reference set. BMC Med Genomics 1:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfrisco P, Ostuni P, Botsios C, Andretta M, Oliviero F, Punzi L, et al. (2003). Serum and salivary neopterin and interferon-gamma in primary Sjögren’s syndrome. Correlation with clinical, laboratory and histopathological features. Scand J Rheumatol 32:74-78 [DOI] [PubMed] [Google Scholar]
- Sondej M, Denny PA, Xie Y, Ramachandran P, Si Y, Takashima J, et al. (2009). Glycoprofiling of the human salivary proteome. Clin Proteomics 5:52-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John MA, Li Y, Zhao X, Denny P, Ho CM, Montemagno C, et al. (2004). Interleukin 6 and interleukin 8 as potential biomarkers for oral cavity and oropharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 130:929-935 [DOI] [PubMed] [Google Scholar]
- Streckfus C, Bigler L, Dellinger T, Pfeifer M, Rose A, Thigpen JT. (1999). CA15–3 and c-erb-2 presence in saliva of women. Clin Oral Investig 3:138-143 [DOI] [PubMed] [Google Scholar]
- Streckfus C, Bigler L, Dellinger T, Dai X, Kingman A, Thigpen JT. (2000a). The presence of soluble c-erb-2 in saliva and serum among women with breast carcinoma: a preliminary study. Clin Cancer Res 6:2363-2370 [PubMed] [Google Scholar]
- Streckfus C, Bigler L, Tucci M, Thigpen JT. (2000b). A preliminary study of CA15–3, c-erb2, epidermal growth factor receptor, cathepsin-D and p53 in saliva among women with breast carcinoma. Cancer Investig 18:101-109 [DOI] [PubMed] [Google Scholar]
- Streckfus CF, Bigler LR, Zwick M. (2006). The use of surface enhanced laser desorption/ionization time of flight mass spectrometry to detect putative cancer markers in saliva: a feasibility study. J Oral Pathol Med 35:292-300 [DOI] [PubMed] [Google Scholar]
- Tabak LA. (2004). Dental, oral, and craniofacial research: the view from the NIDCR. J Dent Res 83:196-197 [DOI] [PubMed] [Google Scholar]
- Takei M, Azuhata T, Yoshimatu T, Shigihara S, Hashimoto S, Horie T, et al. (1995). Increased soluble CD23 molecules in serum/saliva and correlation with the stage of sialoectasis in patients with primary Sjögren’s syndrome. Clin Exp Rheumatol 13:711-715 [PubMed] [Google Scholar]
- Tirumalai RS, Chan KC, Prieto DA, Issaaq HJ, Conrads TP, Veenstra TD. (2003). Characterization of the low molecular weight human serum proteome. Mol Cell Proteomics 2:1096-1103 [DOI] [PubMed] [Google Scholar]
- Tishler M, Yaron I, Shirazi I, Yossipov Y, Yaron M. (1999). Increased salivary interleukin-6 levels in patients with primary Sjögren’s syndrome. Rheumatol Int 18:125-127 [DOI] [PubMed] [Google Scholar]
- Walz A, Stuhler K, Wattenberg A, Hawranke E, Meyer HE, Schmalz G, et al. (2006). Proteome analysis of glandular parotid and sub-mandibular saliva in comparison to whole human saliva by two-dimensional gel electrophoresis. Proteomics 6:1631-1639 [DOI] [PubMed] [Google Scholar]
- Whiteaker JR, Zhang H, Eng J, Fang R, Piening BD, Feng LC, et al. (2007). Head-to-head comparison of serum fractionation techniques. J Proteome Res 6:828-836 [DOI] [PubMed] [Google Scholar]
- Wilmarth PA, Riviere MA, Rustvold DL, Lauten JD, Madden TE, David LL. (2005). Two-dimensional liquid chromatography study of the human whole saliva proteome. J Proteome Res 3:1017-1023 [DOI] [PubMed] [Google Scholar]
- Xie H, Rhodus NL, Griffin RJ, Carlis JV, Griffin TJ. (2005). A catalogue of human saliva proteins identified by free flow electrophoresis based peptide separation and tandem mass spectrometry. Mol Cell Proteomics 4:1826-1830 [DOI] [PubMed] [Google Scholar]
- Yan W, Apweiler R, Balgley BM, Boontheung P, Bundy JL, Cargile BJ, et al. (2009). Systematic comparison of the human saliva and plasma proteomes. Proteomics Clin Appl 3:116-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavuz S, Toker E, Bicakcigil M, Mumcu G, Cakir S. (2006). Comparative analysis of autoantibodies against α-fodrin in serum, tear fluid, and saliva from patients with Sjögren’s syndrome. J Rheumatol 33:1289-1292 [PubMed] [Google Scholar]
- Zhong LP, Chen GF, Xu ZF, Zhang X, Ping FY, Zhao SF. (2005). Detection of telomerase activity in saliva from oral squamous cell carcinoma patients. Int J Oral Maxillofac Surg 34:566-570 [DOI] [PubMed] [Google Scholar]


