Abstract
The continuous growth of rodent incisors requires the presence of stem cells capable of generating ameloblasts and odontoblasts. While epithelial stem cells giving rise to ameloblasts have been well-characterized, cells giving rise to the odontoblasts in incisors have not been fully characterized. The goal of this study was to gain insight into the potential population in dental pulps of unerupted and erupted incisors that give rise to odontoblasts. We show that pulps from unerupted incisors contain a significant mesenchymal-stem-cell (MSC)-like population (cells expressing CD90+/CD45-, CD117+/CD45-, Sca-1+/CD45-) and few CD45+ cells. Our in vitro studies showed that these cells displayed extensive osteo-dentinogenic potential, but were unable to differentiate into chondrocytes and adipocytes. Dental pulps from erupted incisors displayed increased percentages of CD45+ and decreased percentages of cells expressing markers of an MSC-like population. Despite these differences, pulps from erupted incisors also displayed extensive osteo-dentinogenic potential and inability to differentiate into chondrocytes and adipocytes. These results provide evidence that continuous generation of odontoblasts and dentin on the labial and lingual sides of unerupted and erupted incisors is supported by a progenitor population and not multipotent MSCs in the dental pulp.
Keywords: murine incisor, dental pulp, dentin, odontoblasts, progenitor
Introduction
Rodent incisors grow continuously throughout the life of the animal. The continuous growth of the incisors requires the presence of stem and/or progenitor cells capable of generating the ameloblasts and odontoblasts that give rise to enamel and dentin. There has been significant progress in the identification of epithelial stem cells residing in the cervical loops of the incisor and the signaling network that regulates their survival, proliferation, and differentiation (Harada et al., 1999; Harada and Ohshima, 2004; Thesleff et al., 2007; Tummers and Thesleff, 2009). In cervical loops, slowly dividing epithelial stem cells reside in the Notch1-expressing stellate reticulum, surrounded by a single layer of basal epithelium expressing lunatic fringe (Harada et al., 1999; Harada and Ohshima, 2004). In the labial cervical loop, the epithelial stem cells proliferate actively, give rise to transit-amplifying progeny, and differentiate into ameloblasts secreting enamel, unlike the lingual cervical loop, which contains fewer proliferating stem cells and does not give rise to ameloblasts or enamel (Harada et al., 1999; Harada and Ohshima, 2004; Thesleff et al., 2007; Tummers and Thesleff, 2009). These differences in the labial and lingual cervical loops result in the asymmetric distribution of enamel and provide a mechanism for the wear at the tips.
Elegant series of experiments have indicated that survival, proliferation, and differentiation of epithelial stem cells in the cervical loops are dependent on and regulated by signals from the surrounding dental mesenchyme. FGF signaling originating from dental mesenchyme plays essential roles in proliferation and fate decisions of the epithelial stem cells by regulating Notch signaling (Harada et al., 1999, 2002; Harada and Ohshima, 2004; Wang et al., 2007). In addition, BMP4 and Activin, two members of the TGFβ family of signaling, modulate the expression of Fgf3 in the dental mesenchyme and, thus, regulate proliferation and differentiation of epithelial stem cells (Wang et al., 2004a, 2007). Furthermore, genetic studies indicated roles for the Follistatin and Sprouty families of proteins in asymmetric patterning of the enamel in the murine incisors (Wang et al., 2004b; Klein et al., 2008; Boran et al., 2009).
The continuous growth of rodent incisors is also dependent on continuous generation of odontoblasts and dentin on the labial and lingual sides of the incisors. It has been suggested that these odontoblasts arise from stem cells residing in the incisor dental pulp.
Stem cells of mesenchymal origin were originally isolated from bone-marrow-derived stromal cells (BMSCs) (Bianco et al., 2008). Bone-marrow-derived multipotent mesenchymal stem cells (MSCs), also referred to as skeletal stem cells (Bianco et al., 2008), have been described as non-hematopoietic, colony-forming fibroblasts that, when transplanted into an animal, formed bone, cartilage, hematopoietic marrow, fat cells, and the stroma that supports blood formation (Bianco et al., 2008).
The search for identification of “MSC-like populations” in hard tissues has led to their discovery in post-natal human teeth (Huang et al., 2009) and pulps of various teeth in animal models, including continuously growing rodent incisors (Yu et al., 2006; Yang et al., 2007a,b, 2009; Sasaki et al., 2008; Ma et al., 2009; Patel et al., 2009; Alge et al., 2010). However, the MSC-like population in the incisors has still not been fully characterized. Thus, in the present study, we examined and compared the dentinogenic potential, the expression of various surface antigens shown to be expressed by MSC-like populations, and the multipotency of dental pulps of unerupted (P57 mice) and erupted (P18–21) murine incisors.
Materials & Methods
Cell Cultures
Pulps from unerupted and erupted mandibular incisors and bone marrow from P5-P7 suckling and P18–21 weanling CD1 mice were isolated and prepared for primary cultures as previously described (Balic et al., 2010).
Induction and Detection of Differentiated Tissues
Mineralization was induced in all primary cultures by the addition of media containing αMEM, 10% FCS, 40 U/mL penicillin, 40 µg/mL streptomycin, 50 µg/mL ascorbic acid, and 4 mM β-glycerol phosphate to confluent cultures (around day 7), which were then assayed and quantified as described previously (Balic et al., 2010).
Adipogenesis was induced in primary cultures by the addition of various adipogenic media (0.5 µM rosiglitazone and 1 µM insulin; 1 mM dexamethasone, 0.5 mM IBMX, and 1 mM insulin; and 0.5 mM IBMX, 0.5 mM hydrocortisone, and 60 mM indomethacin) to confluent cultures (Balic et al., 2010) and assayed by Oil Red O (ORO) staining (Balic et al., 2010).
Chondrogenesis was examined by micromass cultures and media containing a 60:40 ratio of F12:DMEM, 10% fetal calf serum, 2 mM glutamine, 200 µg/mL ascorbic acid, 100 U/mL penicillin, 100 µg/mL streptomycin, and 0.25 µg/mL Fungizone, with or without the addition of 10 ng/ml: of TGF-β3, and examined with Alcian Blue staining (Balic et al., 2010).
RNA Extraction and Analysis
Total RNA was isolated in TRIzol reagent and processed for RT-PCR with specific primers for GAPDH, osteocalcin (OC) bone sialoprotein (BSP), dentin matrix protein 1 (DMP1), Type I collagen (Col1a1), dentin sialophosphoprotein (DSPP) peroxisome proliferation-activated receptor gamma 2 (PPARg2), fatty acid binding protein 4 (FABP4, also known as aP2), and Type II collagen (Col2a1) (Balic et al., 2010).
Flow Cytometric Analysis (FACS)
Flow cytometry was done on a BD FacsCalibur cytometer, and data were processed with Cell Quest software and various commercially available anti-mouse antibodies, including CD45.2-Biotin (104), CD117-APC (2B8), Sca1-PE (D7), and CD90/Thy1.2-FITC (53–2.1), as previously described (Balic et al., 2010). Between 20,000 and 100,000 cells were used for analysis.
Immunocytochemistry
Cultured cells were processed for immunocytochemistry according to an established protocol. Antibodies used were anti-DSP (LF-153, a kind gift from Dr. Larry Fisher) 1:200 diluted in 0.1% Triton in phosphate-buffered saline (PBS) and secondary Alexa Fluor® 568 goat anti-rabbit antibody. Hoechst 33342 dye was used to stain the nuclei. Endothelial cells in the blood vessels were analyzed with a 1:100 dilution of CD31 antibody (Balic et al., 2010).
Results
Characterization of Dental Pulp Cells from Unerupted (P5–7) Incisors
Freshly isolated DP of the unerupted mandibular incisors from P5–7 mice contained approximately 4.5 x 105 ± 0.2 cells per tooth (n = 3), a very low representation of cells with hematopoietic phenotypes (CD45+), very few blood vessels, a high percentage of CD90+/CD45- and CD117+/CD45-, and a low percentage of Sca-1+/CD45- cells (Fig. 1). Freshly isolated bone marrow cells from P5–7 long bones contained a high percentage of CD45+ and Sca1+/CD45-, CD90+/CD45-, and CD117+/CD45- cells (Fig. 1b). The selection of CD90, CD117, and Sca-1 as markers for an MSC-like population in the present study was based on their expression in murine MSCs (Bobis et al., 2006; Eslaminejad et al., 2007; Nadri et al., 2007; Popp et al., 2009; Nemeth et al., 2010), while STRO-1 antibody was excluded due to controversial specificity for mouse tissue (Kemoun et al., 2007).
Figure 1a.
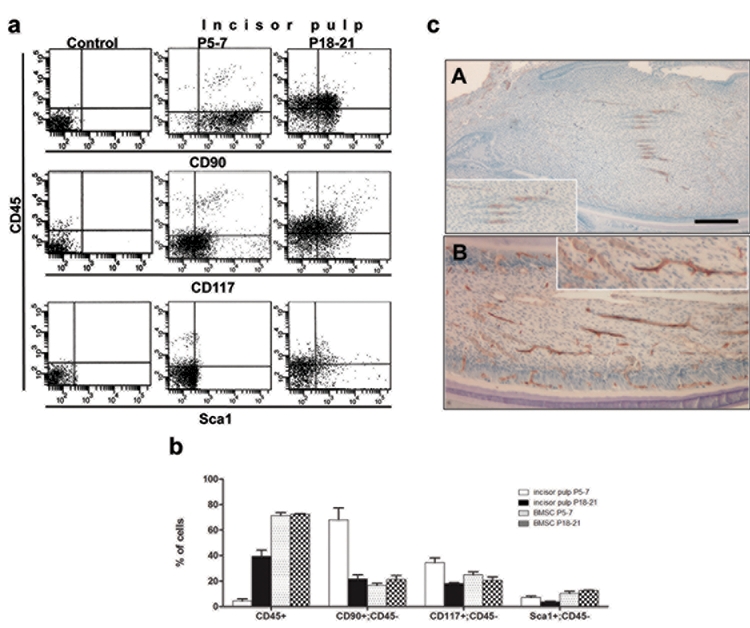
Characterization of the expression of cell-surface markers. Flow cytometric analysis of the expression of CD45 (y axis) and CD90, CD117, and Sca-1 (X-axes) cell-surface markers in freshly isolated pulps from unerupted (P5–7) and erupted (P18–21) incisors. Approximately 0.5 - 1 x 106 cells was incubated with pre-titrated antibodies (1:50–1:800), in the presence of rat Ig (when necessary), washed, and re-suspended in 300 µL of staining medium containing 1 µg/mL of PI (propidium iodide). Between 20,000 and 100,000 cells were used for analysis. Unstained dental pulp cells were used as a control. Figure 1b. Characterization of the expression of cell-surface markers. Histogram showing the percentages of cells isolated from various tissues expressing various markers. Values represent mean ± SE of 3 independent experiments. Figure 1c. Vascularization in dental pulp. Sections of incisors from P5–7 (A) and P18–21 (B) mice processed for CD31 immunohistochemistry. Note the increases in the numbers of blood vessels surrounded by CD31+ endothelial cells in pulps from P18–21 as compared with P5–7 mice. Insets in (A) and (B) represent higher magnification of blood vessels showing CD31+ in the endothelial lining of blood vessels. Scale bar = 100 µm.
When placed in primary cultures, DPs from unerupted incisors proliferated rapidly and reached confluence at around day 7 (Table). Mineralization was detected 3 days after the addition of mineralization-inducing media and increased thereafter (Figs. 2a, 2b). At days 14 and 21, the entire culture dish was covered by mineralized tissue (Figs. 2a, 2b).
Table.
Number of Viable Attached Cells in Dental Pulp and BMSC Cultures
| Dental Pulp |
Bone Marrow |
||||
|---|---|---|---|---|---|
| P5–7 | P18–21 | P5–7 | P18–21 | ||
| Day of culture | 1 | 280,000 ± 4400 | 270,000 ± 4500 | 793,000 ± 47,000 | 774,000 ± 48,000 |
| 3 | 1,351,000 ± 23,000 | 1,290,000 ± 15,000 | 1,910,000 ± 54,000 | 1,825,000 ± 13,000 | |
| 7 | 33,000,000 ± 707,000 | 33,250,000 ± 630,000 | 36,700,000 ± 450,000 | 35,700,000 ± 335,000 | |
Cultures at days 1, 3, and 7 were trypsinized, and live cells were counted by hemocytometry. Data represent mean ± SE of a total number of cells in 35-mm wells from 3 independent experiments.
Figure 2a.
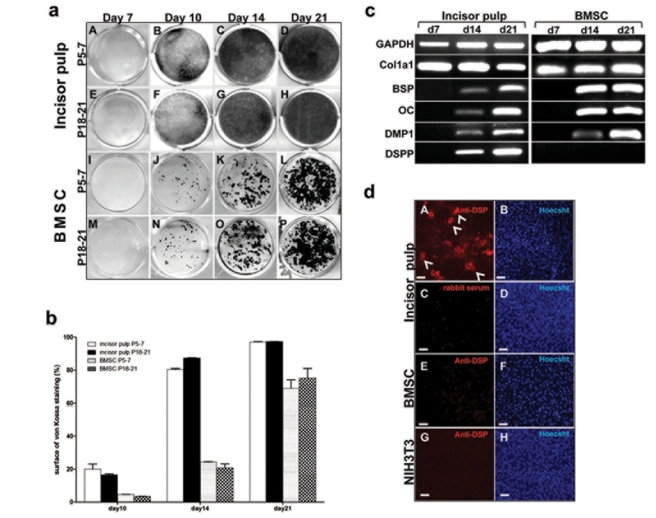
Mineralization potential of primary cultures derived from dental pulps, and BMSCs from P5–7 and P18–21 mice. von Kossa staining of primary cultures derived from dental pulp (A-H) and bone marrow (I-P) of (P5–7) (A-D and I-L) and (P18–21) (E-H and M-P) mice at various time-points. Cells derived from dental pulps were plated at a density of 520 cells/mm2. BMSCs were prepared from femurs and tibiae and plated at a density of 5200 cells/mm2. All cultures were grown first in media containing DMEM, 20% FBS, 40 U/mL of penicillin, and 40 µg/mL of streptomycin, 0.1 mg/mL of Fungizone, and 2 mM glutamine at 37°C and 5% CO2. Three days later, medium was changed to medium containing 10% FBS. Mineralization was induced in all cultures by the addition of media containing αMEM, 10% FCS, 40 U/mL penicillin, 40 µg/mL streptomycin, 50 µg/mL ascorbic acid, and 4 mM β-glycerol phosphate to confluent cultures (around day 7). Note that in all cultures the first sign of mineralization was at day 10, with increases thereafter. Note the sheet of mineralized tissue in cultures derived from incisor pulps and patchy distribution of mineralized nodules in BMSC cultures. Figure 2b. Quantification of von Kossa staining in cultures from dental pulp cells and bone marrow at different time-points. von Kossa staining was performed at days 10, 14, and 21. Area of the culture plate covered with stained mineralized tissue was quantified by Photoshop and ImageJ programs. Bars represent the percentage of area of von Kossa staining within the culture dish. Values represent mean ± SE from duplicate plates from 3 independent experiments. Figure 2c. RT-PCR analysis of the expression of selected known markers for early (Col1a1) and late stages of mineralization and odontoblast differentiation (BSP, DMP1, OC, and DSPP) in dental pulp and BMSC cultures from P5–7 mice at various time-points At day 7, prior to the addition of mineralization media, high levels of Col1a1 were detected in pulp and BMSC cultures. BSP, DMP1, and OC were not detected at day 7 and were detected at low levels at day 14 with increases at day 21. Note that DSPP expression was not detected in BMSCs cultures at any time-points. Figure 2d. Epifluorescence images of 14-day-old primary dental pulp (A-D), BMSCs (E, F), and NIH3T3 (G, H) cultures stained with anti-DSP (A, E, G), rabbit serum (C), and Hoechst 33342 dye (B, D, F, H). Cultures were fixed with 4% paraformaldehyde, blocked with 3% milk, and incubated with a 1:200 dilution of anti-DSP antibody and a 1:800 dilution of secondary Alexa Fluor 568 goat anti-rabbit antibody. Prior to being imaged, cultures were incubated with Hoechst 33342 dye (final concentration, 1 µg/mL) for detection of the nucleus. Cultures were examined and photographed by fluorescence microscopy and appropriate filters. Note that DSP expression in primary dental pulp cultures was detected in some (examples indicated by arrowheads), but not all, mineralized nodules (A). Note the lack of DSP expression in BMSC cultures (E) and NIH3T3 cells (G). Note the lack of staining in dental pulp culture incubated with rabbit serum (C). Scale bar = 100 µm.
RT-PCR analysis showed expression of markers of mineralization, including DSPP, in these cultures (Fig. 2c). Immunocytochemical analysis with anti-DSP antibody showed expression of DSP in some, but not all, mineralized nodules in these cultures (Fig. 2dA), indicating that cultures contained both dentin-like and bone-like matrices, consistent with our previous observations (Braut et al., 2003; Balic et al., 2010).
Under these conditions, the non-hematopoietic attached cells from bone marrow proliferated and reached an optimal density at day 7 (Table). BMSC cultures from P5–7 and P18–21 mice displayed patchy distributions of the mineralized nodules (Fig. 2a) and did not express DSPP and DSP (Fig. 2c).
Next we examined the adipogenic and chondrogenic potentials of DPs from unerupted incisors. Under the various adipogenic conditions used in our study, BMSC cultures differentiated into a significant number of mature adipocytes, as evidenced by ORO staining and the expression of PPARg2 and FABP4 after 14 days, which increased after 3 wks (Figs. 3aA-3aC, 3b). This is consistent with previous observations (Muruganandan et al., 2009). The lack of detectable ORO staining and the lack of expression of PPARg2 and FABP4 in 3- to 7-week-old cultures from unerupted incisors grown under various adipogenic-inducing media indicated the inability of these cells to undergo adipogenesis (Figs. 3aE-3aG, 3b, and unpublished observations).
Figure 3.
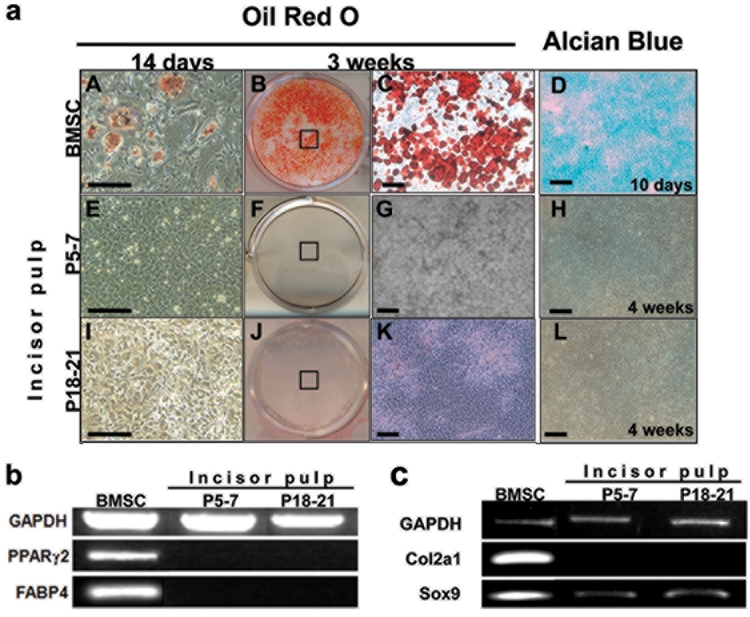
Adipogenic and chondrogenic differentiation in cultures derived from dental pulp and BMSCs. (a) Images of cultures at various time-points from BMSCs (A-D) and dental pulp from unerupted (P5–7) (E-H) and erupted (P18–21) incisors (I-L) stained with Oil Red O after induction of adipogenesis by media containing 0.5 µM rosiglitazone and 1 µM insulin (A-C, E-G, I-K), and stained with Alcian Blue after induction of chondrogenesis (D, H, L). A, E, I are bright-field images of 14-day-old cultures after Oil Red O staining. Note the staining in a few cells (examples indicated by arrowheads) in BMSCs. Staining was not present in cultures from dental pulps. C, G, K are bright-field images of the areas of culture indicated in boxes in B, F, J, showing lipid vacuoles stained with Oil Red. D, H, L are representative bright-field images of micromass cultures from BMSCs (D) and pulps from unerupted (P5–7) (H) and erupted (P18-P21) (L) incisors stained with Alcian Blue. Micromass cultures derived from BMSCs (D) display cartilage nodules stained with Alcian Blue after 10 days. Chondrogenesis and Alcian Blue-stained nodules were not detected in micromass cultures derived from dental pulps from unerupted (P5–7) (H) or erupted (P18–21) incisors (L) after 4 wks. Scale bars = 100 µm. Figure 3b. RT-PCR analysis of expression of FABP4 and PPARγ2, markers of adipogenesis in these cultures. Note the high levels of PPARγ2 and FABP4 expression in the BMSCs cultures after 7 wks. The expression of PPARγ2 and FABP4 was not detected in dental pulp cells derived from erupted and unerupted incisors after 7 wks. Figure 3c. RT-PCR analysis of expression of markers of chondrogenesis in micromass cultures. Col2a1 was expressed at high levels in micromass cultures established from BMSCs (14 days), but not in cultures derived from dental pulps from unerupted and erupted incisors (4 wks).
Chondrogenic differentiation was analyzed in a micromass culture system with and without the addition of TGF-β3. Extensive chondrogenic differentiation assayed by Alcian Blue staining and the expression of C012a1 and Sox9 was detected in micromass cultures derived from BMSCs after 10 days (Figs. 3aD, 3c) and is consistent with previous observations (Mina et al., 1994; Koelling and Miosge, 2009; Balic et al., 2010). However, chondrogenic differentiation was not detected in DP cultures from unerupted incisors (Figs. 3aH, 3c). Addition of media supplemented with TGF-β3 enhanced the chondrogenesis in BMSC micromass cultures, but did not induce chondrogenic differentiation by DP from unerupted incisors (unpublished observations). These observations together showed the inability of DP from unerupted incisors to differentiate into adipocytes and chondrocytes.
Characterization of Dental Pulp Cells from Erupted (P18-P21) Incisors
Our recent studies in murine molars showed distinct differences in the expression of cell-surface markers, cellularity, and the differentiation potentials of DPs from erupted and unerupted molars (Balic et al., 2010). To examine the changes associated with eruption in incisors, we next characterized the DPs from erupted mandibular incisors from P18-P21. DPs isolated from erupted mandibular incisors contained approximately 4.7 x 105 ± 0.1 cells per tooth (n = 3), indicating no significant changes in the cellularity of DPs in erupted and unerupted incisors. DPs from erupted incisors contained significantly higher percentages of CD45+ cells, more blood vessels, lower percentages of CD90+/CD45- and CD117+/CD45- cells, and no significant changes in the percentages of Sca1+/CD45- cells (Fig. 1). These observations indicated that eruption of the incisors was associated with increases in vascularity and decreases in CD90+ and CD117+ expression. The percentages of various markers in freshly isolated bone marrow cells from P18–21 mice were similar to those in P5–7 mice (Fig. 1b).
DPs from erupted incisors proliferated and reached cell numbers similar to those in cultures from unerupted incisors at day 7 (Table). The onset and the extent of mineralization, as well as expression of DSPP and DSP in these cultures, were similar to those from unerupted incisors (Figs. 2E-2H, unpublished observations). Chondrogenic and adipogenic assays showed the inability of DP from erupted incisors to differentiate into adipocytes and chondrocytes (Fig. 3).
Discussion
The results of the present study showed that pulps from unerupted incisors contained few blood vessels, little CD45+, and a high representation of CD90+/CD45- and CD117+/CD45- cells. Primary cultures derived from pulps of unerupted incisors exhibited extensive and rapid in vitro mineralization and the inability to differentiate into adipocytes and chondrocytes. The behavior of the cells isolated from unerupted murine incisors was similar to that of those in unerupted molars (Balic et al., 2010). Our further analysis showed that pulps from erupted incisors displayed increased vascularization and a significantly higher percentage of CD45+, but lower percentages of CD90+ and CD117+ cells. Despite these changes, the cellularity and differentiation potentials of primary cultures from DPs of erupted incisors were similar to those of unerupted incisors. Thus, the behavior of cells isolated from erupted incisors was different from that of those in erupted molars and similar to that of those in unerupted molars (Balic et al., 2010). DPs of the erupted molars exhibited reductions in the osteo-dentinogenic potential and contained a small fraction of cells capable of differentiation into adipocytes and chondrocytes that was infrequent and dependent on a long induction period.
Based on these findings, we propose that DPs of both unerupted and erupted incisors are enriched in a progenitor population and may not contain stem cells and/or an MSC-like population. The progenitor population in the incisor pulps includes the dental papilla underlying the inner enamel epithelium and the mesenchyme surrounding the cervical loops on the labial and lingual sides expressing Fgf3 and Fgf10. In the continuously erupting incisors, this population is sustained throughout the life of the animal and is engaged in the formation of odontoblasts and dentin that is necessary for keeping up with the wear at the incisor edges and in supporting the epithelial stem cells in the cervical loops. Several studies have shown redundant and essential roles for FGF3 and FGF10 in regulating the proliferation of mesenchymal cells in dental papilla, and the self-renewal and differentiation of the epithelial stem cells in the cervical loops of incisors (Harada et al., 2002; Wang et al., 2007). It is also known that ameloblast differentiation from inner enamel epithelium is dependent on the formation of predentin produced by functional odontoblasts derived from nearby dental papilla cells (Thesleff and Åberg, 1997).
Our observations in mice are different from those of studies which showed that DPs from adult rat incisors are capable of odontogenic, osteogenic, chondrogenic, neurogenic, adipogenic, and myogenic differentiation (Yu et al., 2006; Yang et al., 2007a,b, 2009; Sasaki et al., 2008; Ma et al., 2009; Patel et al., 2009; Alge et al., 2010). Yang and colleagues showed that the multipotent differentiation resides in the STRO-1+ population, constituting 0.5% of the rat pulp cells (Yang et al., 2007a,b, 2009). Therefore, it is possible that, in our study, the differentiation of a minor MSC-like population in the erupted incisor cells was masked by the significant number of osteo/dentinogenic progenitor cells. To test this possibility, we are conducting further experiments to characterize the differentiation potentials of pericytes from murine molars and incisors using in vivo transplantation assays.
Our observations suggest that DPs of murine incisors contain a large population of committed progenitors and may contain a minor (if any) population of stem cells. The progenitor population in the incisors is similar to that in the unerupted molars, which consisted of the remaining dental papilla surrounding the cervical loops in the crown, and supports the notion that, during active tooth morphogenesis, DPs are primarily enriched in progenitor cells committed to the osteo/dentinogenic lineage and may not contain an MSC-like population or stem cells. It is important to note that reparative dentinogenesis in post-natal teeth, which is dependent on stem cells, occurs after tooth eruption and in the absence of dental papilla.
Acknowledgments
We thank all the individuals who provided reagents, valuable input, and technical assistance in these studies, including Drs. Mark Kronenberg, Ivo Kalajzic, and Leonardo Aguila, and Mrs. Barbara Rodgers.
Footnotes
This work was supported by a grant from the National Institutes of Health to M.M. (DE016689).
References
- Alge DL, Zhou D, Adams LL, Wyss BK, Shadday MD, Woods EJ, et al. (2010). Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. J Tissue Eng Regen Med 4:73-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balic A, Aguila LH, Caimano MJ, Francone VP, Mina M. (2010). Characterization of stem and progenitor cells in dental pulps of the erupted and unerupted murine molars. Bone 46:1639-1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P, Robey PG, Simmons PJ. (2008). Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell 2:313-319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobis S, Jarocha D, Majka M. (2006). Mesenchymal stem cells: characteristics and clinical applications. Folia Histochem Cytobiol 44:215-230 [PubMed] [Google Scholar]
- Boran T, Peterkova R, Lesot H, Lyons DB, Peterka M, Klein OD. (2009). Temporal analysis of ectopic enamel production in incisors from sprouty mutant mice. J Exp Zool B Mol Dev Evol 312(B):473-485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braut A, Kollar EJ, Mina M. (2003). Analysis of the odontogenic and osteogenic potentials of dental pulp in vivo using a Col1a1–2.3-GFP transgene. Int J Dev Biol 47:281-292 [PubMed] [Google Scholar]
- Eslaminejad MB, Nadri S, Hosseini RH. (2007). Expression of Thy 1.2 surface antigen increases significantly during the murine mesenchymal stem cells cultivation period. Dev Growth Differ 49:351-364 [DOI] [PubMed] [Google Scholar]
- Harada H, Ohshima H. (2004). New perspectives on tooth development and the dental stem cell niche. Arch Histol Cytol 67:1-11 [DOI] [PubMed] [Google Scholar]
- Harada H, Kettunen P, Jung HS, Mustonen T, Wang YA, Thesleff I. (1999). Localization of putative stem cells in dental epithelium and their association with Notch and FGF signaling. J Cell Biol 147:105-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H, Toyono T, Toyoshima K, Yamasaki M, Itoh N, Kato S, et al. (2002). FGF10 maintains stem cell compartment in developing mouse incisors. Development 129:1533-1541 [DOI] [PubMed] [Google Scholar]
- Huang GT, Gronthos S, Shi S. (2009). Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res 88:792-806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemoun P, Laurencin-Dalicieux S, Rue J, Vaysse F, Romeas A, Arzate H, et al. (2007). Localization of STRO-1, BMP-2/-3/-7, BMP receptors and phosphorylated Smad-1 during the formation of mouse periodontium. Tissue Cell 39:257-266 [DOI] [PubMed] [Google Scholar]
- Klein OD, Lyons DB, Balooch G, Marshall GW, Basson MA, Peterka M, et al. (2008). An FGF signaling loop sustains the generation of differentiated progeny from stem cells in mouse incisors. Development 135:377-385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelling S, Miosge N. (2009). Stem cell therapy for cartilage regeneration in osteoarthritis. Expert Opin Biol Ther 9:1399-1405 [DOI] [PubMed] [Google Scholar]
- Ma D, Ma Z, Zhang X, Wang W, Yang Z, Zhang M, et al. (2009). Effect of age and extrinsic microenvironment on the proliferation and osteogenic differentiation of rat dental pulp stem cells in vitro. J Endod 35:1546-1553 [DOI] [PubMed] [Google Scholar]
- Mina M, Upholt WB, Kollar EJ. (1994). Enhancement of avian mandibular chondrogenesis in vitro in the absence of epithelium. Arch Oral Biol 39:551-562 [DOI] [PubMed] [Google Scholar]
- Muruganandan S, Roman AA, Sinal CJ. (2009). Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci 66:236-253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadri S, Soleimani M, Hosseni RH, Massumi M, Atashi A, Izadpanah R. (2007). An efficient method for isolation of murine bone marrow mesenchymal stem cells. Int J Dev Biol 51:723-729 [DOI] [PubMed] [Google Scholar]
- Nemeth K, Mayer B, Mezey E. (2010). Modulation of bone marrow stromal cell functions in infectious diseases by Toll-like receptor ligands. J Mol Med 88:5-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M, Smith AJ, Sloan AJ, Smith G, Cooper PR. (2009). Phenotype and behaviour of dental pulp cells during expansion culture. Arch Oral Biol 54:898-908 [DOI] [PubMed] [Google Scholar]
- Popp FC, Renner P, Eggenhofer E, Slowik P, Geissler EK, Piso P, et al. (2009). Mesenchymal stem cells as immunomodulators after liver transplantation. Liver Transpl 15:1192-1198 [DOI] [PubMed] [Google Scholar]
- Sasaki R, Aoki S, Yamato M, Uchiyama H, Wada K, Okano T, et al. (2008). Neurosphere generation from dental pulp of adult rat incisor. Eur J Neurosci 27:538-548 [DOI] [PubMed] [Google Scholar]
- Thesleff I, Åberg T. (1997). Tooth morphogenesis and the differentiation of ameloblasts. Ciba Found Symp 205:3-12 [PubMed] [Google Scholar]
- Thesleff I, Wang XP, Suomalainen M. (2007). Regulation of epithelial stem cells in tooth regeneration. C R Biol 330:561-564 [DOI] [PubMed] [Google Scholar]
- Tummers M, Thesleff I. (2009). The importance of signal pathway modulation in all aspects of tooth development. J Exp Zool B Mol Dev Evol 312(B):309-319 [DOI] [PubMed] [Google Scholar]
- Wang XP, Suomalainen M, Jorgez CJ, Matzuk MM, Wankell M, Werner S, et al. (2004a). Modulation of activin/bone morphogenetic protein signaling by follistatin is required for the morphogenesis of mouse molar teeth. Dev Dyn 231:98-108 [DOI] [PubMed] [Google Scholar]
- Wang XP, Suomalainen M, Jorgez CJ, Matzuk MM, Werner S, Thesleff I. (2004b). Follistatin regulates enamel patterning in mouse incisors by asymmetrically inhibiting BMP signaling and ameloblast differentiation. Dev Cell 7:719-730 [DOI] [PubMed] [Google Scholar]
- Wang XP, Suomalainen M, Felszeghy S, Zelarayan LC, Alonso MT, Plikus MV, et al. (2007). An integrated gene regulatory network controls stem cell proliferation in teeth. PLoS Biol 5:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, van den Dolder J, Walboomers XF, Zhang W, Bian Z, Fan M, et al. (2007a). The odontogenic potential of STRO-1 sorted rat dental pulp stem cells in vitro. J Tissue Eng Regen Med 1:66-73 [DOI] [PubMed] [Google Scholar]
- Yang X, Zhang W, van den Dolder J, Walboomers XF, Bian Z, Fan M, et al. (2007b). Multilineage potential of STRO-1+ rat dental pulp cells in vitro. J Tissue Eng Regen Med 1:128-135 [DOI] [PubMed] [Google Scholar]
- Yang X, Walboomers XF, van den Beucken JJ, Bian Z, Fan M, Jansen JA. (2009). Hard tissue formation of STRO-1-selected rat dental pulp stem cells in vivo. Tissue Eng Part A 15:367-375 [DOI] [PubMed] [Google Scholar]
- Yu J, Deng Z, Shi J, Zhai H, Nie X, Zhuang H, et al. (2006). Differentiation of dental pulp stem cells into regular-shaped dentin-pulp complex induced by tooth germ cell conditioned medium. Tissue Eng 12:3097-3105 [DOI] [PubMed] [Google Scholar]


