Abstract
The long-term effectiveness of chlorhexidine as a matrix metalloproteinase (MMP) inhibitor may be compromised when water is incompletely removed during dentin bonding. This study challenged this anti-bond degradation strategy by testing the null hypothesis that wet-bonding with water or ethanol has no effect on the effectiveness of chlorhexidine in preventing hybrid layer degradation over an 18-month period. Acid-etched dentin was bonded under pulpal pressure simulation with Scotchbond MP and Single Bond 2, with water wet-bonding or with a hydrophobic adhesive with ethanol wet-bonding, with or without pre-treatment with chlorhexidine diacetate (CHD). Resin-dentin beams were prepared for bond strength and TEM evaluation after 24 hrs and after aging in artificial saliva for 9 and 18 mos. Bonds made to ethanol-saturated dentin did not change over time with preservation of hybrid layer integrity. Bonds made to CHD pre-treated acid-etched dentin with commercial adhesives with water wet-bonding were preserved after 9 mos but not after 18 mos, with severe hybrid layer degradation. The results led to rejection of the null hypothesis and highlight the concept of biomimetic water replacement from the collagen intrafibrillar compartments as the ultimate goal in extending the longevity of resin-dentin bonds.
Keywords: degradation, dentin bonding, ethanol, hybrid layer, hydrophobic resin
Introduction
Ethanol wet-bonding represents a new philosophical approach to dentin bonding with etch-and-rinse adhesives. This philosophy embraces the concept of water replacement from interfibrillar and intrafibrillar spaces by ethanol to create a comparatively hydrophobic, ethanol-suspended demineralized collagen matrix for infiltration by hydrophobic resin monomers (Nishitani et al., 2006; Pashley et al., 2007; Tay et al., 2007; Sadek et al., 2008; Hosaka et al., 2009). This approach has profoundly improved our understanding of the deficiencies associated with contemporary etch-and-rinse and self-etch adhesives, notably, their inability to replace free and loosely bound water from the intrafibrillar compartments (Cameron et al., 2007) of water-saturated collagen fibrils (Kim et al., 2010). The procedure also prevents phase separation of hydrophobic resin monomers in the presence of water (Tay et al., 1996; Wang et al., 2006; Ye et al., 2008), since the latter is completely replaced by ethanol prior to the application of these ethanol-soluble monomers (Becker et al., 2007; Sauro et al., 2009). Replacement of water by ethanol from the collagen intrafibrillar compartments also removes the hydrolytic medium for the functioning of the collagen-bound matrix metalloproteinases (MMPs) that are responsible for the degradation of resin-sparse collagen fibrils within the hybrid layer.
Ethanol wet-bonding differs conceptually from the use of chlorhexidine, a potent MMP inhibitor (Gendron et al., 1999), in preventing hybrid layer degradation (Pashley et al., 2004; Hebling et al., 2005; Carrilho et al., 2007a, 2007b; Breschi et al., 2009). In theory, degradation of the interface should not occur when water within the intrafibrillar and interfibrillar compartments of a collagen matrix is completely replaced by resin. Since the use of chlorhexidine with a water wet-bonding technique does not provide a mechanism for progressive removal of intrafibrillar water from the collagen matrix (Kim et al., 2010c), the chlorhexidine may eventually leach out of the hybrid layer because of its electrostatic binding characteristics (Blackburn et al., 2007), with water functioning as the desorption medium. Thus, the long-term effectiveness of chlorhexidine as a MMP inhibitor may be compromised when water is incompletely removed during water wet-bonding with etch-and-rinse adhesives. This study challenged the currently accepted anti-degradation strategy of using chlorhexidine in water wet-bonding by testing the null hypothesis that wet-bonding with water or ethanol has no effect on the effectiveness of chlorhexidine in preventing hybrid layer degradation over an 18-month period.
Materials & Methods
Dentin Bonding
Forty-two recently extracted human third molars were collected after patients’ informed consents were obtained under a protocol reviewed and approved by the Ethical Research Committee of the University of São Paulo. A flat mid-coronal dentin surface was prepared perpendicular to the longitudinal axis of each tooth by means of an Isomet saw (Buehler Ltd, Lake Bluff, IL, USA) under water-cooling. A second parallel cut was made 3 mm below the cement-enamel junction to expose and remove pulp chamber contents. The crown segment was attached with cyanoacrylate glue (Zapit, Dental Ventures of America, Anaheim Hills, CA, USA) to a Plexiglas platform assembly to deliver 20 cm of water pressure during bonding. The bonding surface was polished with 180-grit silicon carbide paper, etched with 37% H3PO4 (3M ESPE, St. Paul, MN, USA) for 15 sec, rinsed with water, and left moist prior to bonding procedures.
The teeth were randomly divided into 3 groups (n = 14) according to the technique/adhesive: (1) water wet-bonding/Scotchbond Multi-Purpose (3M ESPE), a 3-step etch-and-rinse adhesive; (2) water wet-bonding/Single Bond 2 (3M ESPE), a 2-step etch-and-rinse adhesive; and (3) ethanol wet-bonding [wet dentin surface was treated with a series of increasing ethanol concentrations (50%, 70%, 80%, 95%) and 3 100% ethanol applications for 30 sec each]/an experimental 3-step hydrophobic adhesive. Each group was divided into 2 subgroups (n = 7). One subgroup was pre-treated with chlorhexidine diacetate (CHD; Sigma-Aldrich, St. Louis, MO, USA) prior to adhesive application, while the other subgroup was not. For the CHD subgroups, the acid-etched dentin was pre-treated with a 2 wt% aqueous CHD solution for 60 sec and blot-dried. The CHD-treated collagen matrix was re-wetted with water for water wet-bonding or re-wetted with water and progressively dehydrated with ethanol for ethanol wet-bonding.
Scotchbond Multi-Purpose and Single Bond 2 were applied following the manufacturer’s instructions (Table). A comonomer resin blend comprised of 70 wt% Bis-GMA (Esstech, Essington, PA, USA), 28.75 wt% TEGDMA (Esstech), 0.25 wt% camphorquinone (Sigma-Aldrich), and 1 wt% ethyl N,N-dimethyl-4-aminobenzoate (Sigma-Aldrich) was used to formulate the adhesive component of the experimental hydrophobic adhesive. We prepared the primer component by diluting the neat comonomer blend with 50 wt% absolute ethanol. Two consecutive coats of the hydrophobic primer were applied to the ethanol-saturated dentin. Excess ethanol solvent was evaporated with a gentle air stream for 10 sec. A layer of the neat comonomer adhesive was then applied, spread thin with moisture-free air, and light-cured for 20 sec by means of a halogen light-curing unit with an output intensity of 600 mW/cm2. For all groups, composite build-ups were constructed with a light-cured resin composite (Filtek Z250, 3M ESPE) in five 1-mm-thick increments.
Table.
Microtensile Bond Strengths to Acid-etched Dentin When Experimental and Commercial Etch-and-Rinse Adhesives Were Applied with or without Chlorhexidine Treatment after 24 hrs, 9 or 18 mos of Aging in Artificial Saliva (PF = premature failure)
| Adhesive/Technique | Chlorhexidine Treatment | PF | 24-hourBond Strength (MPa) | 9-monthBond Strength (MPa) | % Reduction between 24-hour and 9-month Bond Strength | 18-monthBond Strength (MPa) | % of Reduction between 24-hour and 18-month Bond Strength |
|---|---|---|---|---|---|---|---|
| Exp | No | 4 | 45.8 (7.2)Aa [50] | 44.4 (6.9)Aa [52] | 3.0 | 44.2 (7.8)Aa [47] | 3.3 |
| ethanol wet-bonding | Yes | 5 | 46.8 (5.1)Aa [54] | 44.6 (5.6)Aa [50] | 4.7 | 43.6 (5.5)ABa [55] | 7.0 |
| MP | No | 8 | 44.2 (3.5)Aa [60] | 37.4 (3.5)Ab [53] | 15.5 | 32.6 (7.1)BCb [56] | 26.2 |
| water wet-bonding | Yes | 10 | 41.3 (8.1)Aa [57] | 37.4 (5.6)Aa [54] | 9.5 | 30.5 (8.0)Cb [55] | 26.1 |
| SB | No | 9 | 42.3 (7.4)Aa [53] | 34.4 (4.9)Ab [55] | 18.9 | 31.5 (4.3)BCb [56] | 25.7 |
| water wet-bonding | Yes | 9 | 42.6 (5.2)Aa [56] | 38.2 (4.7)Aa [57] | 10.4 | 28.8 (8.3)Cb [60] | 32.4 |
Values are means (± SD) in MPa and [number of specimens tested]. Groups identified with different upper-case superscript letters (analysis in columns) and lower-case letters (analysis in lines) represent statistically significant differences (p < 0.05).
- Apply a single coat of Primer to moist acid-etched dentin. Dry gently for 5 sec.
- Apply one coat of Adhesive to the primed dentin. Avoid excessive air-thinning of the adhesive. Light-cure for 10 sec.
- Apply 2 to 3 consecutive coats of adhesive to moist acid-etched dentin for 15 sec with gentle agitation.
- Gently air-thin for 5 sec to evaporate solvent. Light-cure for 10 sec.
Testing Procedures
Restored crown segments were stored in artificial saliva [CaCl2 (0.7), MgCl2·6H2O (0.2), KH2PO4 (4.0), KCl (30), NaN3 (0.3), HEPES buffer (20), in mM/L] (Pashley et al., 2004) at 37°C for 24 hrs under 20 cm of simulated pulpal pressure and removed from Plexiglass platforms only before being sectioned. They were vertically sectioned into 0.9-mm-thick serial slabs with the Isomet saw under water cooling. The central slab of each crown segment was used for ultrastructural examination. Adjacent slabs were sectioned into 0.9 x 0.9 mm beams, according to the microtensile “non-trimming” technique. Intact beams and central slabs were randomly divided into 3 similarly sized subgroups. One subgroup was tested or examined immediately, while the others were stored in artificial saliva at 37°C for 9 or 18 mos before being tested. The storage medium was changed weekly.
Each resin-dentin beam was stressed to failure under tension by means of a Geraldeli’s testing device mounted on a universal testing machine (Model 5565, Instron Corp., Canton, MA, USA) at a crosshead speed of 1 mm/min. Premature failures were excluded from the statistical analysis, since they were few and were evenly distributed among different groups (Table). Since the values were normally distributed (Kolmogorov-Smirnov test) and homoscedastic (Levene test), the data were analyzed with a repeated-measures three-way ANOVA design (bonding technique/adhesive, CHD pre-treatment, and storage time as the repeated-measured variable). Post hoc multiple comparisons were performed with the Tukey test, with statistical significance set at α = 0.05.
Transmission Electron Microscopy (TEM)
For each group, we randomly selected central slabs after 24 hrs, 9 or 18 mos to examine for signs of the degradation within the resin-dentin interface according to a TEM protocol reported by Tay et al. (1999). Briefly, untested specimen slabs were fixed initially in glutaraldehyde, completely demineralized with 0.5 M EDTA, post-fixed in 1% OsO4, dehydrated in an ascending ethanol series (50-100%), immersed in propylene oxide as the transitional medium, and embedded in epoxy resin. The 90- to 100-nm-thick sections were stained with methanolic uranyl acetate for 1 min and aqueous lead citrate for 5 min and examined with a JEM-1230 TEM (JEOL, Tokyo, Japan) at 110 kV.
Results
Bond Strength
Tensile bond strength results of the 3 factors (bonding technique/adhesive, CHD pre-treatment, and storage time), and the percentage reduction in bond strength after 9 and 18 mos are summarized in the Table. Repeated-measures three-way ANOVA showed that there were statistically significant differences for the factor ‘bonding technique/adhesive’ (p < 0.05) and the factor ‘storage time’ (p < 0.001). The interaction between these two factors was also significant (p < 0.001), indicating that the reduction in bond strength during storage was dependent upon the bonding technique/adhesive used.
The ethanol wet-bonding technique applied with a hydrophobic adhesive exhibited bond strength values similar to those of the commercial adhesives after 24 hrs (p > 0.05). Pre-treatment with CHD did not significantly affect adhesion in any group (p > 0.05). Bond strength did not significantly decline after aging for the groups in which the ethanol wet-bonding technique was applied, regardless of the adjunctive use of CHD (p > 0.05). Pre-treatment with CHD before the water wet-bonding did not result in a significant drop in bond strength in the commercial adhesive groups after 9 mos. However, significant bond strength reductions were observed after 18 mos (p < 0.01). Conversely, in the absence of CHD pre-treatment, significant drops in bond strength (p < 0.001) were detected in water wet-bonding groups as early as 9 mos (MP = 15.5% and SB = 18.9%).
Transmission Electron Microscopy (TEM)
Although voids were evident within hybrid layers created with the hydrophobic adhesive applied on ethanol-saturated dentin after 18 mos (Figs. 1B, 1D), the collagen matrix within the hybrid layers did not degrade, regardless of whether CHD was included in the absolute ethanol used for the final rinse (Fig.1A – no CHD; Fig.1C – with CHD). Wide interfibrillar spaces (ca. 50 nm) were clearly observed (Figs. 1B, 1D) that produced a higher resin/collagen ratio within the hybrid layer.
Figure 1.
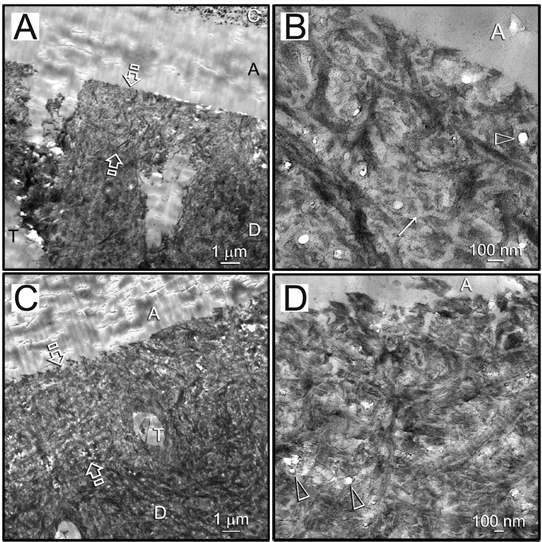
Representative TEM images of 18-month-aged hybrid layers created by ethanol wet-bonding in the absence/presence of chlorhexidine diacetate (CHD) and coaxing the ethanol-saturated matrix with an experimental hydrophobic adhesive. Although voids were evident within the hybrid layers, the collagen matrix within did not degrade, regardless of whether CHD was included in the absolute ethanol used for the final rinse. C, composite; A, adhesive. Between open arrows, hybrid layer. D, dentin; T, dentinal tubule. (A) Low magnification of the resin-dentin interface in the subgroup without CHD. (B) High magnification of (A) showing wide interfibrillar spaces (ca. 50 nm; arrow) within the hybrid layer that were probably caused by shrinkage of interfibrillar proteoglycans (Mazzoni et al., 2008) in absolute ethanol. This provided a higher resin/collagen ratio within the hybrid layer. Open arrowhead: voids within the hybrid layer. (C) Low magnification of the resin-dentin interface in the CHD subgroup. (D) High magnification of (C). Similar voids were present within the hybrid layer (open arrowheads).
Resin-dentin interfaces bonded with Scotchbond Multi-Purpose after 18 months’ storage showed partial and totally degraded hybrid layers in the subgroup without CHD (Figs. 2A, 2B). Loss of fibrillar integrity and denatured collagen components were frequently observed in the remnant hybrid layers (Fig. 2C). Similar degradation features were observed in resin-dentin interfaces bonded with Single Bond 2 without CHD after 18 mos of aging (Figs. 3A, 3B). Interestingly, in specimens with partially degraded hybrid layers, the degradation ended rather abruptly, without transition from a partially degraded region to the intact hybrid layer. Breakdown of intact collagen fibrils into microfibrillar strands could be observed within the degraded hybrid layer (Fig. 3C). Intact hybrid layers were seen in 75% of the specimens from the CHD subgroup bonded by the commercial adhesives after 18 mos of aging in artificial saliva (Figs. 2D, 3D).
Figure 2.
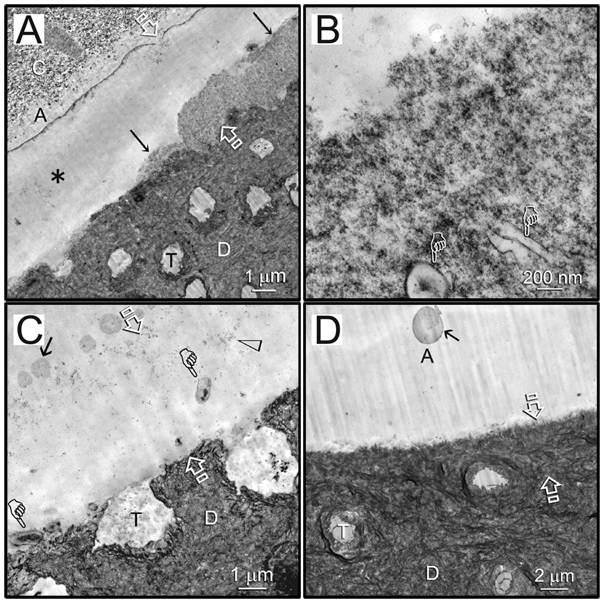
Representative TEM images of 18-month-aged hybrid layers created by water wet-bonding in the absence/presence of CHD and bonding with Scotchbond Multi-Purpose. C, composite; A, adhesive. Between open arrows: hybrid layer. D, dentin; T, dentinal tubule. (A) An example of partial degradation (arrows) of the hybrid layer in the subgroup without CHD. Regions with through-and-through disappearance of the hybrid layer from the composite side to the dentin side (asterisk) were supported by the laboratory-infiltrated epoxy resin. (B) High magnification of the region indicated by arrows in (A) showed loss of fibrillar integrity in the degraded hybrid layer. Pointers: lateral branches of the dentinal tubules. (C) High magnification of the through-and-through region in (A). That space contained denatured collagen components (open arrowhead) and possible microbial invasion (pointers). Arrow: polyalkenoic acid copolymer component of the adhesive. (D) An example of an intact hybrid layer that was sometimes seen in the CHD subgroup bonded by the same adhesive. Arrow: polyalkenoic acid copolymer.
Figure 3.
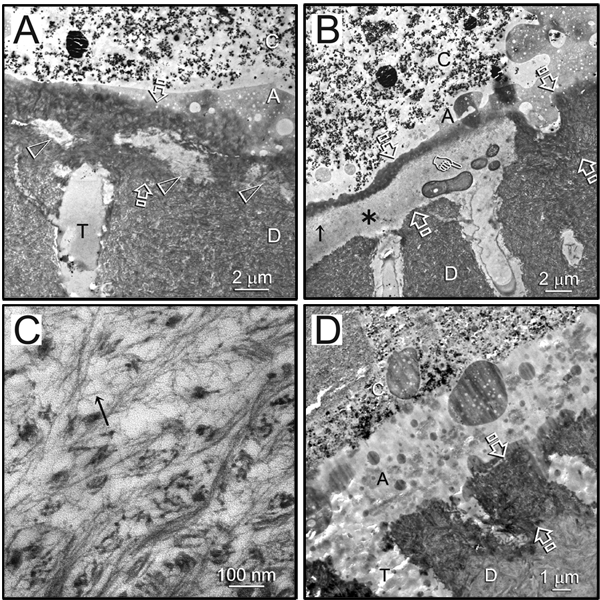
Representative TEM images of 18-month-aged hybrid layers created by water wet-bonding in the absence/presence of CHD and bonding with Single Bond 2. C, composite; A, adhesive. Between open arrows: hybrid layer. D, dentin; T, dentinal tubule. (A) An example of a hybrid layer with initial signs of collagen degradation (open arrowheads) in the subgroup without CHD. (B) An example of a hybrid layer with more severe degradation (asterisk) with possible microbial contamination (pointer) in the subgroup without CHD. (C) High magnification of a region indicated by a black arrow within the degraded hybrid layer in (B) (subgroup without CHD), showing the breakdown of intact collagen fibrils into microfibrillar strands (arrow). (D) An example of an intact hybrid layer that was seen in the CHD subgroup bonded by the same adhesive.
Discussion
The bond strength and TEM results indicated that the use of an ethanol wet-bonding approach with a hydrophobic adhesive is a philosophically viable approach for extending the durability of resin-dentin bonds, and that CHD pre-treatment had limited effect in preventing bond degradation after 9 mos. Thus, the null hypothesis—that wet-bonding with water or ethanol has no effect on the effectiveness of chlorhexidine in preventing hybrid layer degradation over an 18-month period—should be rejected.
Bonds made to water-saturated dentin by commercial etch-and-rinse adhesives deteriorated over time regardless of CHD pre-treatment. The CHD dissolved in ethanol should be more soluble in both the experimental and commercial comonomer blends than CHD dissolved in water. We speculate that this might allow more uptake of CHD dissolved in polymerized resin than is possible with CHD dissolved in water. The net effect may be a longer release of CHD from ethanol-dissolved CHD than from water solution of CHD, albeit at a lower concentration (YK Kim et al., 2010).
It is important to stress that bonds made to ethanol-saturated dentin with the experimental hydrophobic adhesive were preserved regardless of CHD pre-treatment. Thus, preservation of hybrid layer integrity in the ethanol wet-bonding CHD subgroup is unlikely to be solely contributed by a higher concentration of retained CHD within the demineralized collagen matrix. When ethanol wet-bonding is meticulously performed with a 4-minute progressive ethanol substitution technique under simulated pulpal pressure, water is removed from the collagen intrafibrillar compartments (Kim et al., 2010b), resulting in higher intramolecular hydrogen bonding among the collagen molecules that causes shrinkage of the fibrils (Miles and Burjanadze, 2001; Miles et al., 2005; Pashley et al., 2007). As a consequence, the interfibrillar spaces of ethanol-saturated dentin are larger than those of water-saturated dentin (Tay et al., 2007). Since hydrophobic resins are used in this technique, their hydrolytic stability over time (Ito et al., 2005) further contributes to the preservation of hybrid layer integrity over the 18-month aging period.
In essence, collagen dehydration by ethanol wet-bonding followed by encapsulation of the collagen molecules by a water-free hydrophobic resin recapitulates the progressive dehydration of collagen fibrils by intrafibrillar apatite crystallites in biomineralization of hard tissues (Magne et al., 2001; Wehrli and Fernández-Seara, 2005). Incomplete water replacement by contemporary adhesives is the critical barrier to progress in contemporary dentin bonding (Kim et al., 2010b, 2010c). Conversely, removal of intrafibrillar water via apatite replacement conserves the integrity of the collagen matrix in mineralized tissues over a much longer time span (Collins et al., 2002) than is possible with hydrophilic dentin adhesives. This is due to molecular immobilization of MMPs and other enzymes with collagenolytic potential in an environment in which water exists only as structural water within the potential intrafibrillar water compartments of the collagen fibrils (Fullerton et al., 2006). In the presence of a more biomimetic and definitive intervention mechanism for “fossilizing” collagen, it is not difficult to perceive why CHD pre-treatment did not further contribute to preventing hybrid layer degradation in the present study.
The concept of enzyme immobilization by resin is not new and forms the basis of molecular imprinting of enzyme-template complexes by polymerized resinous materials (Jiang et al., 2007). In ethanol wet-bonding, residual water on collagen and associated bound non-collagenous proteins such as MMPs is removed with the use of ethanol. When comonomers dissolved in ethanol diffuse through interfibrillar spaces, they molecularly imprint those proteins after polymerization. The difference between ethanol wet-bonding of dental resins to collagen and MMPs and true “molecular imprinting” is that the template (collagen, MMPs, etc.) is not removed. That is, the adhesive dental resin presumably infiltrates into and around these peptides and occupies their active (catalytic) sites.
Previous studies have demonstrated that the use of chlorhexidine as an MMP inhibitor preserves the integrity of the hybrid layers created by commercial adhesives (Hebling et al., 2005; Carrilho et al., 2007b; Breschi et al., 2009). Nevertheless, increases in adhesive joint failures were observed in aged specimens pre-treated with chlorhexidine (Zhou et al., 2009). The observation that chlorhexidine can be released from chlorhexidine-diacetate-incorporated resin discs (Hiraishi et al., 2008) suggests that chlorhexidine can be released from resin-infiltrated dentin hybrid layers over time. This provides a possible rationale for the abrupt reduction in bond strengths between 9 and 18 mos for the CHD pre-treated Scotchbond Multi-Purpose and Single-Bond 2 groups. During the initial 9-month storage period, released chlorhexidine could continue to inhibit MMPs and protect collagen fibrils within the hybrid layers. However, with continuous release of chlorhexidine between 9 and 18 mos, the concentration of electrostatically bound chlorhexidine probably decreased over time, resulting in reduction of its MMP-inhibitory effect on collagen-bound MMPs. Thus, the present study challenges the use of electrostatically bound chlorhexidine as an anti-degradation strategy to maintain long-term hybrid layer integrity. This provides the rationale for our current investigations of methacrylate resin-based, light-curable MMP inhibitors in preventing bond degradation. Our work further highlights the concept of biomimetic water replacement from the internal compartments of collagen fibrils as the ultimate goal in improving the longevity of resin-dentin bonds created by contemporary etch-and-rinse adhesives.
Footnotes
The microtensile bond strength part of the study was supported by FAPESP (process 07/55117-9). The TEM part of the study was supported by Grants R21 DE019213-01 (PI. Franklin R. Tay) and R01 DE015306-06 (PI. David H. Pashley) from the National Institute of Dental and Craniofacial Research. We thank Michelle Barnes for her secretarial support.
References
- Becker TD, Agee KA, Joyce AP, Rueggeberg FA, Borke JL, Waller JL, et al. (2007). Infiltration/evaporation-induced shrinkage of demineralized dentin by solvated model adhesives. J Biomed Mater Res B Appl Biomater 80:156-165 [DOI] [PubMed] [Google Scholar]
- Blackburn RS, Harvey A, Kettle LL, Manian AP, Payne JD, Russell SJ. (2007). Sorption of chlorhexidine on cellulose: mechanism of binding and molecular recognition. J Phys Chem B 111:8775-8784 [DOI] [PubMed] [Google Scholar]
- Breschi L, Cammelli F, Visintini E, Mazzoni A, Vita F, Carrilho M, et al. (2009). Influence of chlorhexidine concentration on the durability of etch-and-rinse dentin bonds: a 12-month in vitro study. J Adhes Dent 11:191-198 [PMC free article] [PubMed] [Google Scholar]
- Cameron IL, Short NJ, Fullerton GD. (2007). Verification of simple hydration/dehydration methods to characterize multiple water compartments on tendon type 1 collagen. Cell Biol Int 31:531-539 [DOI] [PubMed] [Google Scholar]
- Carrilho MR, Carvalho RM, de Goes MF, di Hipolito V, Geraldeli S, Tay FR, et al. (2007a). Chlorhexidine preserves dentin bond in vitro. J Dent Res 86:90-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrilho MR, Geraldeli S, Tay F, de Goes MF, Carvalho RM, Tjäderhane L, et al. (2007b). In vivo preservation of the hybrid layer by chlorhexidine. J Dent Res 86:529-533 [DOI] [PubMed] [Google Scholar]
- Collins MJ, Nielsen-Marsh CM, Hiller J, Smith CI, Roberts JP, Prigodich RV, et al. (2002). The survival of organic matter in bone: a review. Archaeometry 44:383-394 [Google Scholar]
- Fullerton GD, Nes E, Amurao M, Rahal A, Krasnosselskaia L, Cameron I. (2006). An NMR method to characterize multiple water compartments on mammalian collagen. Cell Biol Int 30:66-73 [DOI] [PubMed] [Google Scholar]
- Gendron R, Grenier D, Sorsa T, Mayrand D. (1999). Inhibition of the activities of matrix metalloproteinases 2, 8, and 9 by chlorhexidine. Clin Diagn Lab Immunol 6:437-439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebling J, Pashley DH, Tjäderhane L, Tay FR. (2005). Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J Dent Res 84:741-746 [DOI] [PubMed] [Google Scholar]
- Hiraishi N, Yiu CK, King NM, Tay FR, Pashley DH. (2008). Chlorhexidine release and water sorption characteristics of chlorhexidine-incorporated hydrophobic/hydrophilic resins. Dent Mater 24:1391-1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka K, Nishitani Y, Tagami J, Yoshiyama M, Brackett WW, Agee KA, et al. (2009). Durability of resin-dentin bonds to water- vs. ethanol-saturated dentin. J Dent Res 88:146-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Hashimoto M, Wadgaonkar B, Svizero N, Carvalho RM, Yiu C, et al. (2005). Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials 26:6449-6459 [DOI] [PubMed] [Google Scholar]
- Jiang X, Jiang N, Zhang H, Liu M. (2007). Small organic molecular imprinted materials: their preparation and application. Anal Bioanal Chem 389:355-368 [DOI] [PubMed] [Google Scholar]
- Kim J, Gu L, Breschi L, Tjäderhane L, Choi KK, Pashley DH, et al. (2010). Implication of ethanol wet bonding in hybrid layer remineralization.J Dent Res 89:575-580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Uchiyama T, Carrilho M, Agee KA, Mazzoni A, Breschi L, et al. (2010c). Chlorhexidine binding to mineralized versus demineralized dentin powder. Dent Mater 26:771-778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Mai S, Mazzoni A, Liu Y, Tezvergil-Mutluay A, Takahashi K, et al. (2010). Biomimetic remineralization as a progressive dehydration mechanism of collagen matrices—Implications in the aging of resin-dentin bonds. Acta Biomater 6:3729-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magne D, Weiss P, Bouler JM, Laboux O, Daculsi G. (2001). Study of the maturation of the organic (type I collagen) and mineral (nonstoichiometric apatite) constituents of a calcified tissue (dentin) as a function of location: a Fourier transform infrared microspectroscopic investigation. J Bone Miner Res 16:750-757 [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Pashley DH, Ruggeri A, Jr, Vita F, Falconi M, Di Lenarda R, et al. (2008). Adhesion to chondroitinase ABC treated dentin. J Biomed Mater Res B Appl Biomater 86:228-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles CA, Burjanadze TV. (2001). Thermal stability of collagen fibers in ethylene glycol. Biophys J 80:1480-1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles CA, Avery NC, Rodin VV, Bailey AJ. (2005). The increase in denaturation temperature following cross-linking of collagen is caused by dehydration of the fibres. J Mol Biol 346:551-556 [DOI] [PubMed] [Google Scholar]
- Nishitani Y, Yoshiyama M, Donnelly AM, Agee KA, Sword J, Tay FR, et al. (2006). Effects of resin hydrophilicity on dentin bond strength. J Dent Res 85:1016-1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, et al. (2004). Collagen degradation by host-derived enzymes during aging.J Dent Res 83:216-221 [DOI] [PubMed] [Google Scholar]
- Pashley DH, Tay FR, Carvalho RM, Rueggeberg FA, Agee KA, Carrilho M, et al. (2007). From dry bonding to water-wet bonding to ethanol-wet bonding. A review of the interactions between dentin matrix and solvated resins using a macromodel of the hybrid layer. Am J Dent 20:7-20 [PubMed] [Google Scholar]
- Sadek FT, Pashley DH, Nishitani Y, Carrilho MR, Donnelly A, Ferrari M, et al. (2008). Application of hydrophobic resin adhesives to acid-etched dentin with an alternative wet bonding technique. J Biomed Mater Res A 84:19-29 [DOI] [PubMed] [Google Scholar]
- Sauro S, Watson TF, Mannocci F, Miyake K, Huffman BP, Tay FR, et al. (2009). Two-photon laser confocal microscopy of micropermeability of resin-dentin bonds made with water or ethanol wet bonding. J Biomed Mater Res B Appl Biomater 90:327-337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay FR, Gwinnett JA, Wei SH. (1996). Micromorphological spectrum from overdrying to overwetting acid-conditioned dentin in water-free acetone-based, single-bottle primer/adhesives. Dent Mater 12: 236-244 [DOI] [PubMed] [Google Scholar]
- Tay FR, Moulding KM, Pashley DH. (1999). Distribution of nanofillers from a simplified-step adhesive in acid-conditioned dentin. J Adhes Dent 1:103-117 [PubMed] [Google Scholar]
- Tay FR, Pashley DH, Kapur RR, Carrilho MR, Hur YB, Garrett LV, et al. (2007). Bonding BisGMA to dentin—a proof of concept for hydrophobic dentin bonding. J Dent Res 86:1034-1039 [DOI] [PubMed] [Google Scholar]
- Wang Y, Spencer P, Hager C, Bohaty B. (2006). Comparison of interfacial characteristics of adhesive bonding to superficial versus deep dentine using SEM and staining techniques. J Dent 34:26-34 [DOI] [PubMed] [Google Scholar]
- Wehrli FW, Fernández-Seara MA. (2005). Nuclear magnetic resonance studies of bone water. Ann Biomed Eng 33:79-86 [DOI] [PubMed] [Google Scholar]
- Ye Q, Park JG, Topp E, Wang Y, Misra A, Spencer P. (2008). In vitro performance of nano-heterogeneous dentin adhesive. J Dent Res 87:829-833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Tan J, Chen L, Li D, Tan Y. (2009). The incorporation of chlorhexidine in a two-step self-etching adhesive preserves dentin bond in vitro. J Dent 37:807-812 [DOI] [PubMed] [Google Scholar]


