Abstract
Interleukin-17 (IL-17), the prototype cytokine produced by the Th17 subset of T-helper cells, plays a role in inflammatory responses, autoimmunity, and antimicrobial responses in a variety of infectious and inflammatory diseases. In view of the inflammatory nature and severity of aggressive periodontitis, we hypothesized that IL-17 might be detected in sera from patients with aggressive periodontitis. We used ELISA to measure IL-17 serum concentrations from 67 periodontally healthy (NP) individuals and from 53 patients with localized (LAgP) and 49 patients with generalized (GAgP) aggressive periodontitis. IL-17 was barely detectable in sera from periodontally healthy individuals (1.9 ± 2.0 pg/mL), but was present at significantly higher concentrations in sera from those with LAgP (7.6 ± 2.2 pg/mL) and GAgP (17.1 ± 2.3 pg/mL). Multivariate analyses demonstrated associations of IL-17 concentrations with periodontal attachment loss, but not with current smoking. Therefore, Th17 responses may be characteristic of AgP, and IL-17 may play a role in the pathogenesis of aggressive periodontitis.
Keywords: aggressive periodontitis, IL-17, cytokine
Introduction
Interleukin-17 (IL-17) is a pro-inflammatory cytokine and the prototype cytokine for a subset of T-helper (Th17) cells. IL-17 stimulates a variety of cell types to produce inflammatory mediators such as IL-1, IL-6, TNF-α, metalloproteinases, and chemokines (Beklen et al., 2007). Cytokines of the Th17 lineage are thought to contribute to the pathology noted in a variety of inflammatory and autoimmune diseases, such as rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease, Crohn’s disease, ulcerative colitis, asthma, and allergic diseases. Additionally, Th17 inflammatory responses are thought to provide protection against a variety of microbial infections (Tesmer et al., 2008).
The role of Th17 cells and their specific cytokines in periodontal disease is just beginning to be investigated, and their specific role in disease pathogenesis and host protection is not known (Gaffen and Hajishengallis, 2008). There are ample examples of a role for Th17 cytokines in protection against bacterial infections and, specifically, against periodontal infections. For example, it was reported that IL-17 is protective against Porphyromonas gingivalis-induced bone loss via mobilization of neutrophils in a murine model (Yu et al., 2007). However, clinical studies reported increased IL-17 levels associated with chronic periodontitis (Takahashi et al., 2005; Vernal et al., 2005; Ohyama et al., 2009). In addition, peripheral blood mononuclear cells from patients with gingivitis and periodontitis stimulated with outer membrane protein of P. gingivalis express IL-17 (Oda et al., 2003). IL-17 mRNA was shown to be expressed in gingival tissue, with higher levels in diseased tissues (Honda et al., 2008; Cardoso et al., 2009). In vitro, IL-17 stimulates HGF to produce IL-6, IL-8, and MMPs (Beklen et al., 2007; Mahanonda et al., 2008). Moreover, significant numbers of CD4+ T-cell clones isolated from gingival tissues of periodontitis patients have been found to express IL-17 (Ito et al., 2005). Murine models of periodontitis and other chronic diseases have demonstrated both protective aspects of Th17-induced immune responses due to neutrophil mobilization and destructive aspects due to lesion chronicity. Thus, clinical studies are useful to gain insight into the role of the Th17 lineage in periodontal disease pathology. Additionally, results from several studies, including recent data from our laboratories, indicate that autoimmune responses can be detected in sera, tissues, and gingival crevicular fluids from patients with chronic and aggressive periodontitis (Schenkein et al., 2003; Rajapakse and Dolby, 2004; De-Gennaro et al., 2006; Koutouzis et al., 2009).
Aggressive periodontitis (AgP), a disease typified by onset in teenagers and young adults, affects approximately 0.5% of the US population (Löe and Brown, 1991). It is most common in the African-American population in the US, with a disease prevalence of approximately 2%, but is also found in other racial and ethnic groups. It is the most severe form of post-pubertal periodontitis and can lead to significant oral inflammation and premature tooth loss in the most severe of cases. AgP may provide an interesting model for examination of the role of Th17 in periodontal disease. There is abundant literature implicating aberrant PMN function as a key pathogenic mechanism in this form of periodontitis (Schenkein and Van Dyke, 1994). Although Th17 pathways are most commonly associated with protection against bacterial-induced inflammation via recruitment of phagocytes, pathways involving Th17 leading to enhanced periodontal inflammation and to increased bone resorption have also been proposed (Ruddy et al., 2004; Takahashi et al., 2005; Herman et al., 2008; Yu and Gaffen, 2008).
We hypothesized that, as seen in other inflammatory conditions such as Crohn’s disease and inflammatory bowel disease, IL-17 may play a role in periodontal disease pathogenesis, and that we could detect IL-17 in sera from patients affected with AgP.
Materials & Methods
Clinical Methods
This study was approved by the office of Research Subjects Protection for the Conduct of Human Research of Virginia Commonwealth University. Participants in this study were ascertained, and provided informed consent, between 1983 and 2005 through the Virginia Commonwealth University School of Dentistry clinics. The participants were randomly selected from those in our database who met the criteria described below and for whom we had stored serum samples. All participants were systemically healthy as determined by history, and data on smoking history and racial category were determined by self-report. The periodontal examination included determination of pocket depth (PD), attachment loss (ALOSS), plaque index (PI) (Silness and Löe, 1964), gingival index (GI) (Löe and Silness, 1963), and bleeding upon probing (BI) (Mühlemann and Son, 1971). Measurements of PD and ALOSS were recorded to the nearest 1 mm, with measures between 1-mm probe markings rounded down to the nearest mm. Measurements were performed at 4 sites per tooth (mesiobuccal, mid-buccal, distobuccal, and mid-lingual). At the time of the examination, a blood sample was taken and processed for serum, which was then stored at -70°C until utilized.
The diagnostic groupings were defined as follows: Periodontally healthy individuals (NP) had no evidence of ALOSS other than facial recession and had no periodontal pockets greater than 3 mm. Generalized Aggressive Periodontitis (GAgP) patients had a history of disease onset prior to age 35 and presented with at least 8 teeth with 5 mm or more attachment loss at interproximal sites; at least 3 of the affected teeth were not first molars and incisors. Persons with Localized Aggressive Periodontitis (LAgP) had a history of disease onset prior to age 30, with at least 5-mm interproximal attachment loss limited to first molars and incisors and no more than 2 additional teeth.
Laboratory Methods
IL-17 serum concentrations were assessed by ELISA with the use of commercial kits (Quantikine Human IL-17 Immunoassay, cat # D1700, R&D Systems, Minneapolis, MN, USA) following the manufacturer’s instructions.
Statistical Analyses
Differences between and among the study groups (periodontal diagnostic categories) were assessed by ANOVA followed by Tukey’s HSD post hoc test. We used multivariable ANCOVA to adjust for periodontal diagnostic categories and demographic variables.
Results
Serum IL-17 concentrations were measured in 67 periodontally healthy individuals (NP), 53 patients with localized aggressive periodontitis (LAgP), and 49 patients with generalized aggressive periodontitis (GAgP). Demographic and clinical variables for the 3 groups and analyses of intergroup differences are shown in Table 1. GAgP patients were significantly older than those in the other two groups, with greater mean pocket depth, attachment loss, bleeding upon probing, gingival index, and plaque index. Significantly more patients were Black in the LAgP group than in the other two groups (98%), but there were no differences between the groups with respect to sex. These data are consistent with the disease definitions and previously described characteristics of these conditions.
Table 1.
Demographic and Clinical Variables
| NPa (n = 67) | LAgPb (n = 53) | GAgPc (n = 49) | P-value (ANOVA) | |
|---|---|---|---|---|
| Age (± SEM) | 22.9 ± 0.9 | 20.4 ± 1.0 | 31.2 ± 1.1 | < 0.0001 |
| Race (% Black) | 84.0 | 98.0 | 52.0 | < 0.0001 |
| Sex (% F) | 56.7 | 66.0 | 68.8 | NS |
| PDd (± SEM) | 2.0 ± 0.1 | 2.8 ± 0.1 | 3.7 ± 0.1 | < 0.0001 |
| ALOSSe (± SEM) | 0.1 ± 0.1 | 0.9 ± 0.1 | 2.8 ± 0.1 | < 0.0001 |
| GIf (± SEM) | 0.7 ± 0.1 | 1.1 ± 0.1 | 1.5 ± 0.1 | < 0.0001 |
| BIg (± SEM) | 0.2 ± 0.0 | 0.4 ± 0.0 | 0.5 ± 0.0 | < 0.0001 |
| PIh (± SEM) | 0.6 ± 0.05 | 0.9 ± 0.1 | 1.3 ± 0.1 | < 0.0001 |
NP: periodontally healthy individuals.
LAgP: localized aggressive periodontitis.
GAgP: generalized aggressive periodontitis.
PD: pocket depth.
ALOSS: attachment loss.
GI: gingival index.
BI: bleeding index.
PI: plaque index.
We measured serum IL-17 concentrations for the three groups (Fig. 1). These values were all significantly different from each other. The highest concentrations were found in the GAgP group (17.1 ± 2.3 pg/mL), while the serum levels in the LAgP and NP groups were 7.6 ± 2.2 pg/mL and 1.9 ± 2.0 pg/mL, respectively. We plotted IL-17 concentrations as a function of attachment loss for the LAgP, GAgP, and NP participants (Fig. 2). It is noteworthy that a subset of the AgP patients demonstrated increased IL-17 concentrations compared with levels in NP individuals for both forms of disease.
Figure 1.
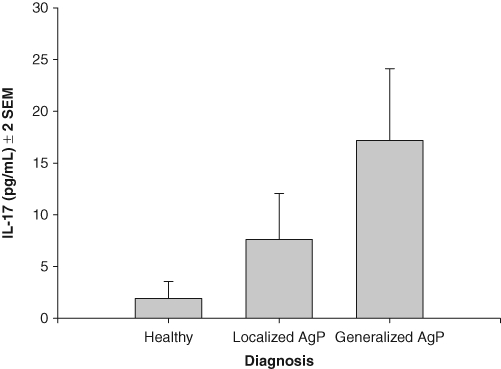
Mean IL-17 concentrations (± 2 standard errors of the mean) in sera from periodontally healthy individuals (NP, n = 67) and in patients with localized aggressive periodontitis (LAgP, n = 53) and generalized aggressive periodontitis (GAgP, n = 49). The clinical groups are significantly different by ANOVA (p < 0.0001) and different from each other by Tukey’s HSD (p < 0.05).
Figure 2.
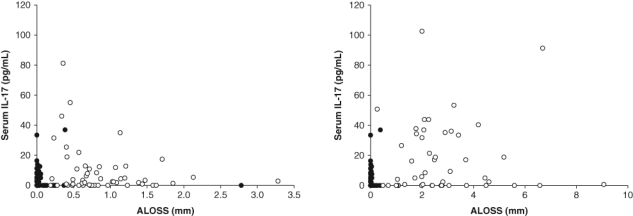
Plot of serum IL-17 concentrations as a function of attachment loss (ALOSS) in localized aggressive periodontitis (LAgP) (left panel) and generalized aggressive periodontitis (GAgP) (right panel). Closed circles denote periodontally healthy control individuals (n = 67), while open circles denote LAgP (n = 53) patients (left panel) and GAgP (n = 49) patients (right panel).
Univariate analyses of IL-17 concentrations as a function of demographic and clinical variables were then carried out. It was found that pocket depth (p = 0.0051, r2 = 0.046), attachment loss (p = 0.0076, r2 = 0.0042), and age (p = 0.0075, r2 = 0.042) were all significantly, but weakly, associated with IL-17 concentrations, while GI, BI, PI, and sex were not. An analysis invoking a regression model that adjusted for the significant variables attachment loss, pocket depth, and age revealed that the diagnostic category remained strongly associated with IL-17 concentrations (p = 0.0006) (Table 2).
Table 2.
Multivariate Regression Analyses of Associations of Study Variables with Serum IL-17 Levels in the Entire Study Population and in the Subpopulation with Available Data on Current Smoking
| Model without Smoking |
Model Including Smoking |
|||
|---|---|---|---|---|
| F Ratio | P-value | F Ratio | P-value | |
| Diagnosis | 7.76 | 0.0006 | 5.09 | 0.0091 |
| Smoker | - | - | 0.94 | 0.3946 |
| Age | 2.56 | 0.1116 | 0.02 | 0.8985 |
| Pocket depth | 0.85 | 0.3587 | 1.30 | 0.2575 |
| Attachment loss | 2.99 | 0.0858 | 5.14 | 0.0271 |
To explore the possible associations of IL-17 concentrations with smoking, we identified a subset of 94 individuals for whom data on smoking habits at the time of diagnosis and blood sampling were available. We first noted that, for this subset, the overall relationship between diagnostic category and IL-17 serum concentrations was similar to that noted for the entire study cohort (NP = 2.0 ± 3.7 pg/mL, LAgP = 7.4 ± 4.6 pg/mL, GAgP = 17.1 ± 2.1 pg/mL, p = 0.0045 by ANOVA). In an adjusted analysis including current smoking as a covariate, periodontal diagnosis (p = 0.0091) and attachment loss (p = 0.0271), but not smoking (p = 0.39), were significantly associated with serum IL-17 concentrations (Table 2).
Discussion
This is the first study reporting elevated levels of IL-17 in the systemic circulation of patients with periodontitis. IL-17 was present in sera from a significant proportion of systemically healthy patients with AgP, while there were very low levels found in periodontally healthy control individuals. Although the source of IL-17 in AgP sera is not known, it is possible that at least some of the cytokine is produced locally in the periodontal tissues. This is likely, in view of previously published data demonstrating elevated levels of IL-17 (Johnson et al., 2004; Takahashi et al., 2005; Lester et al., 2007), IL-17 mRNA (Takahashi et al., 2005; Honda et al., 2008; Cardoso et al., 2009), IL-23 (Lester et al., 2007; Cardoso et al., 2009; Ohyama et al., 2009), as well as associated cytokines TGF-b, IL-1b, and IL-6 mRNA (Cardoso et al., 2009) that are simultaneously present in tissues from chronic periodontitis patients. However, the presence of such cytokines has not been demonstrated in tissues from patients with AgP. Furthermore, IL-17 has been detected in gingival crevicular fluid from periodontitis patients, with higher levels observed in chronic periodontitis lesions (Vernal et al., 2005) compared with healthy sites. These findings are consistent with the hypothesis that locally produced IL-17 finds its way into the systemic circulation in AgP.
An alternative hypothesis explaining elevated systemic levels of IL-17 in AgP is that there may be an altered systemic response to oral bacterial antigens in such patients. However, studies have indicated that stimulation of peripheral blood leukocytes from individuals with GAgP failed to produce higher IL-17 levels compared with matched control individuals (Borch et al., 2009), suggesting a lack of heterogeneity in responsiveness as a function of periodontal status. An additional possibility is that, despite medical histories to the contrary, some AgP patients have subclinical systemic inflammatory conditions associated with periodontal infection that contribute to serum IL-17 levels.
The observation of increased serum IL-17 in AgP is similar to increases previously demonstrated in Crohn’s disease and ulcerative colitis, in which it has been observed that there is increased expression of IL-17 in the intestinal mucosa and increased serum IL-17 concentrations (Fujino et al., 2003). Additionally, these authors observed that serum levels of IL-17 are incrementally higher during bouts of disease activity in Crohn’s disease, suggesting that IL-17 could be a serum marker of disease activity. Interestingly, it has also been observed that increased expression of IL-17 and RANKL mRNA are found in chronic periodontitis lesions from progressive periodontal lesions compared with quiescent lesions (Dutzan et al., 2009), leading to speculation that Th17 cytokines associated with bone resorption may be markers of, and mediators of, periodontal disease activity. It is noteworthy as well that Th17 has been shown to be a key Th cell subset associated with osteoclast generation (Sato et al., 2006).
It is reasonable to speculate that Th17 cells and IL-17 may play a role in the pathology of AgP. Th17 cells are thought to play a role in rheumatic and non-rheumatic inflammatory diseases and in some allergic and immune-mediated conditions. In these conditions, Th17 cells are inducible by inflammatory mediators such at IL-1β, IL-6, and IL-23 from dendritic cells, macrophages, or osteoblasts, either locally or outside the local lesion in draining lymph nodes. Secretion of Th17 cytokines such as IL-17 and IL-22 subsequently induces production of inflammatory cytokines, chemokines, and matrix metalloproteinases that contribute to pathology typical of the affected tissues (reviewed in Tesmer et al., 2008). The role of Th17 cytokines in the pathology noted in AgP is not known, but the intense inflammatory reaction to the oral microflora and rapid destruction of the periodontium in patients with these conditions may be a result of such responses.
It is likewise possible that the observation of IL-17 in serum from AgP is an indicator and mediator of the protective responses mounted in the periodontium against bacterial pathogens. In many Gram-negative infections, Th17 cells play an important role in protective antibacterial host responses (Yu et al., 2007; Tesmer et al., 2008; Yu and Gaffen, 2008). Studies in animal models have shown that IL-17 is a key cytokine involved in recruitment of PMNs to sites of bacterial colonization, and that blockage of IL-17 receptor signaling leads to an exacerbation of periodontal destruction. A significant proportion of patients with AgP are known to have aberrant PMN function, exhibiting defective in vitro PMN chemotactic responses and enhanced oxidative metabolic responses. One explanation for the elevated production of IL-17 noted in AgP is that it may represent a compensatory increase in cytokine production in response to these functional defects.
We are also intrigued by the association of Th17 with autoimmunity in conditions such as rheumatoid arthritis, systemic sclerosis, systemic lupus erythematosus, and multiple sclerosis (Tesmer et al., 2008). We have previously observed that GAgP patients demonstrate elevated levels of anti-phospholipid antibodies such as anti-phosphorylcholine and β2-glycoprotein-dependent anti-cardiolipin (aCL), and that GAgP patients with elevated aCL also demonstrate elevated systemic markers of vascular inflammation (Schenkein et al., 2003, 2007). The sources of such antibodies are unknown; they may be genuine autoantibodies, or they may be produced via molecular mimicry following exposure to cross-reactive periodontal bacterial pathogens (Chen et al., 2007, 2009). The involvement of such antibodies in the pathogenesis of AgP is also not known. However, their presence in these patients, combined with evidence that aggressive periodontitis is familial and likely to have a significant component of genetic risk, suggests the hypothesis that high levels of IL-17 in serum from AgP patients are indicative of autoimmune responses.
Of special concern with respect to AgP and other forms of periodontitis is the growing body of data indicating that they are strongly associated with, and may be a risk factor for, serious systemic conditions such as atherosclerosis, diabetes, adverse pregnancy outcomes (premature birth, fetal growth restriction, and pre-eclampsia), and others. It is possible that high levels of serum IL-17, derived initially from gingival tissues, may mediate inflammatory responses in tissues distant from the oral cavity in this relatively young cohort of patients.
Acknowledgments
The authors acknowledge the contributions of Kimberly Hollaway and Luanne Norvell for their expert patient management, and Haejin Han for excellent technical assistance.
Footnotes
This work was supported by USPHS Research Grants DE018125 and MD002256 from the National Institutes of Health.
References
- Beklen A, Ainola M, Hukkanen M, Gurgan C, Sorsa T, Konttinen YT. (2007). MMPs, IL-1, and TNF are regulated by IL-17 in periodontitis. J Dent Res 86:347-351 [DOI] [PubMed] [Google Scholar]
- Borch TS, Lobner M, Bendtzen K, Holmstrup P, Nielsen CH. (2009). Decreased interleukin-2 responses to Fusobacterium nucleatum and Porphyromonas gingivalis in generalized aggressive periodontitis. J Periodontol 80:800-887 [DOI] [PubMed] [Google Scholar]
- Cardoso CR, Garlet GP, Crippa GE, Rosa AL, Junior WM, Rossi MA, et al. (2009). Evidence of the presence of T helper type 17 cells in chronic lesions of human periodontal disease. Oral Microbiol Immunol 24:1-6 [DOI] [PubMed] [Google Scholar]
- Chen YW, Iwai T, Umeda M, Nagasawa T, Huang Y, Takeuchi Y, et al. (2007). Elevated IgG titers to periodontal pathogens related to Buerger disease. Int J Cardiol 122:79-81 [DOI] [PubMed] [Google Scholar]
- Chen YW, Nagasawa T, Wara-Aswapati N, Ushida Y, Wang D, Takeuchi Y, et al. (2009). Association between periodontitis and anti-cardiolipin antibodies in Buerger disease. J Clin Periodontol 36:830-835 [DOI] [PubMed] [Google Scholar]
- De-Gennaro LA, Lopes JD, Mariano M. (2006). Autoantibodies directed to extracellular matrix components in patients with different clinical forms of periodontitis. J Periodontol 77:2025-2030 [DOI] [PubMed] [Google Scholar]
- Dutzan N, Gamonal J, Silva A, Sanz M, Vernal R. (2009). Over-expression of forkhead box P3 and its association with receptor activator of nuclear factor-kappa B ligand, interleukin (IL) -17, IL-10 and transforming growth factor-beta during the progression of chronic periodontitis. J Clin Periodontol 36:396-403 [DOI] [PubMed] [Google Scholar]
- Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, et al. (2003). Increased expression of interleukin 17 in inflammatory bowel disease. Gut 52:65-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen SL, Hajishengallis G. (2008). A new inflammatory cytokine on the block: re-thinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and IL-17. J Dent Res 87:817-828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman S, Kronke G, Schett G. (2008). Molecular mechanisms of inflammatory bone damage: emerging targets for therapy. Trends Mol Med 14:245-253 [DOI] [PubMed] [Google Scholar]
- Honda T, Aoki Y, Takahashi N, Maekawa T, Nakajima T, Ito H, et al. (2008). Elevated expression of IL-17 and IL-12 genes in chronic inflammatory periodontal disease. Clin Chim Acta 395:137-141 [DOI] [PubMed] [Google Scholar]
- Ito H, Honda T, Domon H, Oda T, Okui T, Amanuma R, et al. (2005). Gene expression analysis of the CD4+ T-cell clones derived from gingival tissues of periodontitis patients. Oral Microbiol Immunol 20:382-386 [DOI] [PubMed] [Google Scholar]
- Johnson RB, Wood N, Serio FG. (2004). Interleukin-11 and IL-17 and the pathogenesis of periodontal disease. J Periodontol 75:37-43 [DOI] [PubMed] [Google Scholar]
- Koutouzis T, Haber D, Shaddox L, Aukhil I, Wallet SM. (2009). Autoreactivity of serum immunoglobulin to periodontal tissue components: a pilot study. J Periodontol 80:625-633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester SR, Bain JL, Johnson RB, Serio FG. (2007). Gingival concentrations of interleukin-23 and -17 at healthy sites and at sites of clinical attachment loss. J Periodontol 78:1545-1550 [DOI] [PubMed] [Google Scholar]
- Löe H, Brown LJ. (1991). Early onset periodontitis in the United States of America. J Periodontol 62:608-616 [DOI] [PubMed] [Google Scholar]
- Löe H, Silness J. (1963). Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand 21:533-551 [DOI] [PubMed] [Google Scholar]
- Mahanonda R, Jitprasertwong P, Sa-Ard-Iam N, Rerkyen P, Charatkulangkun O, Jansisyanont P, et al. (2008). Effects of IL-17 on human gingival fibroblasts. J Dent Res 87:267-272 [DOI] [PubMed] [Google Scholar]
- Mühlemann HR, Son S. (1971). Gingival sulcus bleeding: a leading symptom in initial gingivitis. Helv Odontol Acta 15:107-113 [PubMed] [Google Scholar]
- Oda T, Yoshie H, Yamazaki K. (2003). Porphyromonas gingivalis antigen preferentially stimulates T cells to express IL-17 but not receptor activator of NF-kappaB ligand in vitro. Oral Microbiol Immunol 18:30-36 [DOI] [PubMed] [Google Scholar]
- Ohyama H, Kato-Kogoe N, Kuhara A, Nishimura F, Nakasho K, Yamanegi K, et al. (2009). The involvement of IL-23 and the Th17 pathway in periodontitis. J Dent Res 88:633-638 [DOI] [PubMed] [Google Scholar]
- Rajapakse PS, Dolby AE. (2004). Evidence for local production of antibodies to auto and non-self antigens in periodontal disease. Oral Dis 10:99-105 [DOI] [PubMed] [Google Scholar]
- Ruddy MJ, Shen F, Smith JB, Sharma A, Gaffen SL. (2004). Interleukin-17 regulates expression of the CXC chemokine LIX/CXCL5 in osteoblasts: implications for inflammation and neutrophil recruitment. J Leukoc Biol 76:135-144 [DOI] [PubMed] [Google Scholar]
- Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, et al. (2006). Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med 203:2673-2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkein HA, Van Dyke TE. (1994). Early-onset periodontitis: systemic aspects of etiology and pathogenesis. Periodontol 2000 6:7-25 [DOI] [PubMed] [Google Scholar]
- Schenkein HA, Berry CR, Burmeister JA, Brooks CN, Barbour SE, Best AM, et al. (2003). Anti-cardiolipin antibodies in sera from patients with periodontitis. J Dent Res 82:919-922 [DOI] [PubMed] [Google Scholar]
- Schenkein HA, Best AM, Brooks CN, Burmeister JA, Arrowood JA, Kontos MC, et al. (2007). Anti-cardiolipin and increased serum adhesion molecule levels in patients with aggressive periodontitis. J Periodontol 78:459-466 [DOI] [PubMed] [Google Scholar]
- Silness J, Löe H. (1964). Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand 22:121-135 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Azuma T, Motohira H, Kinane DF, Kitetsu S. (2005). The potential role of interleukin-17 in the immunopathology of periodontal disease. J Clin Periodontol 32:369-374 [DOI] [PubMed] [Google Scholar]
- Tesmer LA, Lundy SK, Sarkar S, Fox DA. (2008). Th17 cells in human disease. Immunol Rev 223:87-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernal R, Dutzan N, Chaparro A, Puente J, Valenzuela MA, Gamonal J. (2005). Levels of interleukin-17 in gingival crevicular fluid and in supernatants of cellular cultures of gingival tissue from patients with chronic periodontitis. J Clin Periodontol 32:383-389 [DOI] [PubMed] [Google Scholar]
- Yu JJ, Gaffen SL. (2008). Interleukin-17: a novel inflammatory cytokine that bridges innate and adaptive immunity. Front Biosci 13:170-177 [DOI] [PubMed] [Google Scholar]
- Yu JJ, Ruddy MJ, Wong GC, Sfintescu C, Baker PJ, Smith JB, et al. (2007). An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood 109:3794-3802 [DOI] [PMC free article] [PubMed] [Google Scholar]


