Abstract
The purpose of this study was to identify risk factors to predict caries progression in toddlers in primary-healthcare settings for the cost-effective targeting of preventive and referral strategies. We examined 329 children (26 ± 6 mos old) twice, one year apart, in Indiana, USA. A 107-item structured interview was used to collect information from the primary caregiver and child on factors/beliefs/perceptions/behaviors that could affect caries development, transmission of bacteria, medical-dental health, and access to care. Bacterial levels, gingivitis, dental plaque, and caries experience were assessed. Multiple-variable logistic regression models of caries progression toward cavitation included family caries experience, transmission-related behaviors, dietary factors, health beliefs, and lower income, but differed in selected predictors/predictive power by race/ethnicity. Addition of clinical variables did not significantly improve the prediction.
Keywords: longitudinal study, toddler, dental caries, risk assessment
Introduction
Targeted health care has become paramount in an environment of increasing healthcare costs and resource constraints. The management of dental caries, the most common chronic disease of childhood (US Department of Health and Human Services, 2000), is no exception. This is particularly true with the increase in caries prevalence among 2- to 5-year-olds (Beltrán-Aguilar et al., 2005; Dye et al., 2007), and the marked disparity and lack of uniformity in dental caries distribution among US children (Beltrán-Aguilar et al., 2005). In addition, the current system has a limited capacity to provide early access to dental services, particularly to young children (Mouradian et al., 2000). Therefore, it is important to identify the “highest of the high” risk to concentrate limited financial and manpower resources (Anderson, 2002). Because children have easier access to medical than to dental care, partnerships with the medical community may help cost-effectively target preventive and/or referral strategies (Hale, 2003; Keels et al., 2008). Therefore, the purpose of this study was to identify risk factors that could be later validated to predict caries progression in toddlers in primary-healthcare settings. With a large number of possible risk factors to be examined, the risk factors were classified into the following areas for evaluation: demographics/access to care, medical history, dental history, dental habits, dietary habits, protective factors, and dental beliefs.
Materials & Methods
This longitudinal, Institutional Review Board-approved study was conducted in Indianapolis and Connersville, IN, USA. The questionnaire (Appendix) was developed after a review of the literature, peer feedback, and field testing with 25 primary caregivers (PCG) similar to the target population. “PCG” was defined as the individual consistently responsible for the child’s housing, health, and safety.
The English-/Spanish-speaking population was recruited from pre-natal/pediatric clinics utilizing the Indiana University Primary Care Practice-Based Research Network, and media advertisements. Three hundred ninety-nine PCGs and their children met the following inclusion criteria at baseline: provision of written informed consent; completion of the risk questionnaire; allowing a dental examination and dental plaque sample to be collected from the child, and the PCG providing a saliva sample; the child being 18-36 mos of age and generally healthy; and being available for the two study visits.
Of the 399 PCGs, 396 participated at baseline and provided a paraffin-stimulated saliva sample to determine mutans streptococci levels (CRT® Bacteria, Ivoclar Vivadent AG, Schaan, Liechtenstein). They were interviewed by a trained examiner using a structured 105-item questionnaire. Children received a dental examination conducted by a calibrated dentist, which included a visual assessment of the quantity of dental plaque and the presence/absence of gingivitis (American Academy of Pediatric Dentistry Caries Assessment Tool, 2006). Dental plaque samples were collected by means of a sterile microbrush (Dentanova, Huddinge, Sweden), the end of which was aseptically cut, placed in 0.5 mL of transport fluid containing Tryptic Soy Broth (Difco, Detroit, MI, USA) with 10% glycerol (v/v), and frozen prior to analyses. Study teeth (those at greater risk of early childhood caries-ECC: maxillary incisors and occlusal surfaces of primary molars) were cleaned with a toothbrush, air-dried, and assessed under light, without magnification, according to the International Caries Detection and Assessment System (ICDAS) criteria (Pitts, 2004; ICDAS, 2005; Ismail et al., 2007). No radiographs were taken. One of two blinded and calibrated examiners performed a quantitative light-induced fluorescence examination (QLF/clin 007, Inspektor Research Systems B.V., Amsterdam, The Netherlands), grading the image for the presence/absence of caries (loss of fluorescence in an area compatible with caries). PCGs were informed of conditions requiring treatment, and all received identical written advice concerning diet, oral hygiene, and the use of fluorides.
From the original study population, 329 children (83%) were examined a second time 12 ± 3 mos later. Reasons for attrition were: no response to communications (43), moved (five), unavailable for appointments (seven), failed appointment (11), and deceased (one). At the second appointment, all baseline procedures were repeated with the exception of collecting the PCG’s saliva sample, and the questionnaire was increased to 107 items. Ten percent of the individuals had the procedures repeated after each visit to determine intra-examiner reliability and stability of questionnaire responses.
For microbiological analyses, transport tubes were thawed, vortexed, and sonicated (Trahan et al., 1992). After serial 10-fold dilutions, the bacteria were double-plated in Mitis Salivarius Agar (Difco, Detroit, MI, USA) supplemented with sucrose for total streptococci counts and counted with an automatic colony-counter unit (ProtoCOL Systems, Frederick, MD, USA), and in Mitis Salivarius agar supplemented with sucrose and bacitracin (Sigma, St. Louis, MO, USA) (Gold et al., 1973) for mutans streptococci counts based on morphology. The plates were incubated at 37°C in 5% CO2 for 48 hrs.
The primary outcome for predictive modeling was the presence of at least one new lesion (ICDAS score of 3 or higher), one new filling, and/or progression of a lesion from a score of 3 or 4 to ≥ 5 between the two examinations.
Predictors included baseline questionnaire responses and dental examination results. Questionnaire items were categorized as: demographics/access to care, medical history, dental history, dental habits, dietary habits, protective factors, and dental beliefs. The dental examination provided plaque and gingivitis classifications, proportion of mutans streptococci in relation to total streptococci counts in plaque, and baseline ICDAS and QLF scores. Evaluating a large number of predictors can produce a multiple testing problem; however, the use of multivariable prediction models reduces the overstatement of conclusions in the final models.
Logistic regressions were performed individually with each predictor. Multiple-variable models were developed in stages. Once predictors were divided into categories, a reverse-elimination procedure removed predictors with p > 0.30. Remaining predictors were combined into a single model, and reverse-elimination was repeated, retaining predictors with p < 0.05 in the final model. The area under the receiver operating characteristic (ROC) curve (AUC) was calculated to assess the overall predictive ability of the final model. The use of the ROC AUC is one way of measuring the accuracy of caries risk assessment, and is a common way to measure the prognostic ability of risk indicators. Baseline gingivitis, dental plaque, and ICDAS and QLF scores were examined for significance. The importance of examining these predictors last is that they involve a dental examination, which would add to the complexity of using the prediction model in non-dental settings.
Agreement for categorical questionnaire items was assessed by 2-way contingency tables and kappa statistics. Intraclass correlation coefficients and plots were examined for the assessment of repeatability for continuous variables.
Results
Most PCGs were mothers, but also included eight fathers, two grandmothers, and one other. The toddlers (52% females, 48% males) were 26 ± 6 mos old at baseline. The ethnic/racial distribution of PCG/children was (rounded%): Hispanic (all races), 18%/20%; African-American-Non-Hispanic, 46%/50%; Caucasian-Non-Hispanic, 36%/29%; and Other-Non-Hispanic, 1%/<1%. Race/ethnicity was self-reported. Toddlers had 0.5 ± 2.0 surfaces (range, 0-16) with ICDAS score 3 or higher or a filling at baseline (mean dfs). Within race/ethnic group, baseline dfs were 1.5 ± 3.6, 0.4 ± 1.3, and 0.1 ± 0.5 in Hispanic, African-American-Non-Hispanic, and Caucasian-Non-Hispanic individuals, respectively. By the second examination, 75 (23%) toddlers had at least one of the following: new lesion with an ICDAS score of 3 or higher, new filling, and/or lesion progression (score of 3 or 4 increasing to ≥ 5). The numbers were 19 (29%), 33 (20%), 22 (23%) and 1 (100%) within Hispanic, African-American-Non-Hispanic, Caucasian-Non-Hispanic, and Other-Non-Hispanic subjects, respectively. The baseline and incremental caries data for each ethnic group are presented in the Appendix Table. Repeatability of the ICDAS severity scores (weighted kappa = 0.68), QLF Yes/No call (kappa for the two examiners = 0.62 and 0.56), and presence of mutans streptococci (kappa = 0.65) were acceptable. Because the ICDAS index includes earlier stages of caries severity and 6 lesion codes for use in each surface, the index is more prone to variability than traditional criteria with lesion cavitation as the only outcome variable. Repeatability of the plaque and gingivitis assessments was poor (kappa < 0.20). Repeatability of the survey responses varied widely (kappa ranged from 0.45 to 0.95). Children not returning for follow-up (n = 67, 19.4%) were significantly (p < 0.05) different from those who continued, but no sensitivity analysis was done, since this was a screening study.
Predictors of the primary outcome were first examined individually. Significance of predictors overall and across racial/ethnic groups is shown in Figs. 1-3. The final overall multiple-variable model (Table; AUC = 0.75) included: PCG has other children with fillings, PCG shares utensils with child, child does not snack on fresh fruit, child snacks on popcorn, ranked “worse” taking care of child’s medical health, did not respond “false” to ‘bad teeth are inherited from parents’, and lower income. None of the clinically assessed variables was significant. Addition of baseline ICDAS score was not significant in this model or in the analyses by racial/ethnic group.
Figure 1.

Odds ratios with 95% confidence intervals for demographic and oral bacteria/transmission for prediction of at least 1 new ICDAS score of 3 or higher, at least 1 new filling, and/or at least 1 previous lesion progressing from a 3 or 4 to a 5 or 6 for all children. AA = Non-Hispanic African-American. Hisp = Hispanic. Cauc = Non-Hispanic Caucasian. PCG = Primary Caregiver. “+” indicates a p-value less than 0.30 (the cut-off for inclusion in the multiple-variable models). “*” indicates a p-value less than 0.05.
Figure 3.

Odds ratios with 95% confidence intervals for dietary habits for prediction of at least 1 new ICDAS score of 3 or higher, at least 1 new filling, and/or at least 1 previous lesion progressing from a 3 or 4 to a 5 or 6 for all children. AA = Non-Hispanic African-American. Hisp = Hispanic. Cauc = Non-Hispanic Caucasian. PCG = Primary Caregiver. “+” indicates a p-value less than 0.30 (the cut-off for inclusion in the multiple-variable models). “*” indicates a p-value less than 0.05.
Table.
Multivariable Logistic Regression Models Overall and by Race/Ethnic Group
| Multivariable Logistic Regression Models | Odds Ratio (95% CI) |
|---|---|
| Overall (n = 329) | |
| PCG has other children with fillings | 2.73 (1.51-4.93) |
| PCG shares utensils with child | 2.02 (1.01-4.05) |
| Child does not snack on fresh fruit | 2.54 (1.25-5.13) |
| Child snacks on popcorn | 2.16 (1.19-3.91) |
| Ranked “worse” taking care of child’s medical health | 1.77 (1.23-2.55) |
| Did not respond “false” to ‘bad teeth are inherited from parents’ | 2.46 (1.38-4.39) |
| Lower income | 1.21 (1.06-1.37) |
| African-American Non-Hispanic (n = 166) | |
| PCG has other children with fillings | 3.24 (1.29-8.16) |
| Child does not snack on fresh fruit | 6.94 (2.17-22.22) |
| Child snacks on popcorn | 2.85 (1.07-7.57) |
| PCG not ‘very satisfied’ with child’s dentist | 3.73 (1.08-12.99) |
| Did not respond “false” to ‘bad teeth are inherited from parents’ | 3.01 (1.20-7.52) |
| PCG is afraid of going to the dentist | 4.02 (1.56-10.37) |
| Child born by vaginal birth | 5.66 (1.48-21.64) |
| Caucasian Non-Hispanic (n = 96) | |
| PCG snacks on unhealthy snacks | 6.52 (1.72-24.69) |
| Fewer adults with jobs in the child’s household | 3.64 (1.49-8.85) |
| Hispanic (n = 67) | |
| Child drinks regular soda between meals | 3.33 (1.07-10.41) |
Figure 2.
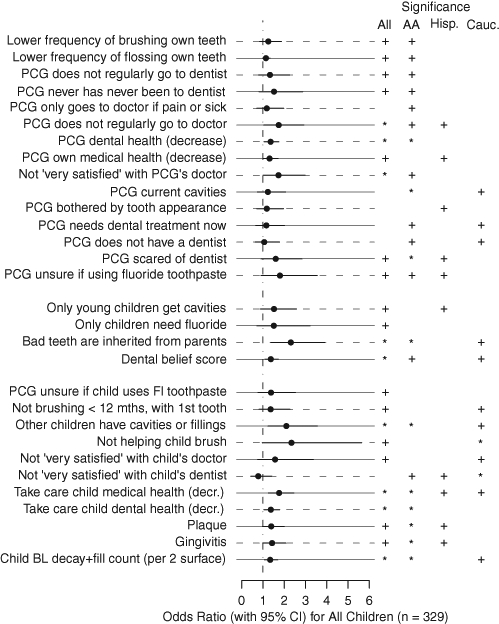
Odds ratios with 95% confidence intervals for dental/medical history, dental habits, and dental beliefs for prediction of at least 1 new ICDAS score of 3 or higher, at least 1 new filling, and/or at least 1 previous lesion progressing from a 3 or 4 to a 5 or 6 for all children. AA = Non-Hispanic African-American. Hisp = Hispanic. Cauc = Non-Hispanic Caucasian. PCG = Primary Caregiver. “+” indicates a p-value less than 0.30 (the cut-off for inclusion in the multiple-variable models). “*” indicates a p-value less than 0.05.
Because significance of the predictors varied across racial/ethnic groups, outcomes were also examined by group (Appendix Figs.). The multiple-variable model for African-American-Non-Hispanic children (n = 166, Table; AUC = 0.82) included: PCG has other children with fillings, child does not snack on fresh fruit, child snacks on popcorn, PCG not very satisfied with child’s dentist, did not respond “false” to ‘bad teeth are inherited from parents’, PCG is afraid of going to the dentist, and child born by vaginal birth. The multiple-variable model for the Caucasian-Non-Hispanic group (n = 96, Table; AUC = 0.75) included: PCG snacks on unhealthy snacks, and fewer adults with jobs in the child’s household. The multiple-variable model for Hispanic children (n = 67, Table; AUC = 0.63) included “child drinks regular soda between meals”.
In summary, family caries experience, transmission-related behaviors, dietary factors, health beliefs, and lower income were identified as risk factors for caries progression toward cavitation in very young children. Some differences were found in identified risk factors based on race/ethnicity.
Discussion
Risk assessment is essential in decision-making for targeted caries prevention and management. Our goal to identify the “highest of the high” caries-risk children influenced the predictive outcome selection. Using the AAPD (2008) definition of ECC would have identified 42% of the sample at the end of the study. In many of these children, lesions did not progress and/or worsen over time (i.e., at baseline 30% already had an ICDAS score ≥ 1). Thus, we restricted “high risk” to those children who would develop and/or have lesions progress over one year toward a break in the surface and/or extensive dentin involvement (23% at study end, and 14% at baseline) (Pienihäkkinen et al., 2004).
Although many factors are associated with caries development in children (Stamm et al., 1988; Demers et al., 1990, 1992; Disney et al., 1992; Zero et al., 2001; AAPD, 2008), there are few quality longitudinal caries risk studies in young toddlers, and existing studies involve primarily selective populations in Northern Europe (SBU, 2007), with two recent US studies (Ismail et al., 2009; Warren et al., 2009), so results are difficult to generalize. These studies conclude that correctly identifying preschool children at risk is possible, especially when a combination of risk factors is used (Twetman and Fontana, 2009; Gao et al., 2010).
Many of the risk factors we identified have also been identified in other longitudinal studies in toddlers. A combination of socio-demographic factors, dietary habits, and mutans streptococci counts was predictive of caries in one-year-olds at 3.5 yrs of age (Grindefjord et al., 1995). As these children’s age increased, presence of lesions became the best predictor at 2.5 yrs of age (Grindefjord et al., 1996). In another Nordic study (Pienihäkkinen et al., 2004), the greatest precision in prediction was achieved by a combination of caries experience, dietary habits, and mutans streptococci (AUC = 0.75). In the US, presence of mutans streptococci and dietary habits were significant risk factors identified in a low-SES rural community (Warren et al., 2009), while higher consumption of soda drinks, older age of child, greater weight-for-age, fewer dental treatment visits, higher baseline caries of children and caregivers, dental fatalism, and neighborhood disadvantage status were important in a low-SES African-American community (Ismail et al., 2009).
In contrast to other studies (Alaluusua and Malmivirta, 1994; Grindefjord et al., 1995, 1996; Pienihäkkinen and Jokela, 2002; Pienihäkkinen et al., 2004; AAPD, 2006), we did not find mutans streptococci counts, proportion of mutans streptococci/total streptococci, baseline caries experience, dental plaque, and gingivitis to be significant predictors of caries progression. Whether this is related to the caries outcome definition or to the population under study is unclear. However, this is promising, since it suggests that the identification of young “high risk” toddlers may be possible in primary-healthcare settings without requiring a dental examination (Gao et al., 2010).
Questions concerning pre-term birth and/or vaginal/cesarean-section birth of the child were added at the one-year visit, because it had been suggested that cesarean-section-delivered children acquired S. mutans earlier (Li et al., 2005), and early acquisition of mutans streptococci has been identified as a risk factor for caries in primary teeth (Kohler et al., 1988). Interestingly, we found vaginal birth to be a significant risk predictor, especially in Non-Hispanic African-Americans. It is also possible that pre-term children have delayed development, reducing caries-risk early on, and pre-term and cesarean-section children may receive more attention/care, resulting in decreased caries risk.
This study had several limitations associated with the large number of risk variables that needed to be individually screened in the selected sample size: (1) Problems inherent in the large number of individual tests were addressed by the development of multivariable prediction models. Multifactorial modeling allows for the simultaneous analysis and weighing of risk indicators, with the resulting odds-ratios providing the strength of the association with caries increment. (2) Even though some questions may be unreliable if looked at independently, we chose not to combine responses during this screening process. (3) Since the study was powered to look at the overall group, we recognize the reduced power within the ethnic/racial subgroups in our analyses. These exploratory analyses were done to evaluate whether the ethnicity/racial differences might be further explored in a larger follow-up study. (4) The sample was localized (Indiana). However, it provided enough variability in the responses to allow for the findings of the tested risk models to be generalizable and used in future validation studies.
In conclusion, family caries experience, transmission-related behaviors, dietary factors, health beliefs, and lower income were identified as risk factors for caries progression toward cavitation in very young children. Models differed in predictors/predictive power by race/ethnicity, with addition of clinical variables not improving the prediction. A viable next step, based on these results, would be to further validate a questionnaire based on the predictors identified here for use in primary-healthcare settings, and to determine predictors’ weight and how they may change with age.
Supplementary Material
Acknowledgments
This study was supported by grant NIH-R21-DE16451-01 from the National Institute of Dental and Craniofacial Research. We thank Hafsteinn Eggertsson for help in ICDAS calibration, and the following for their assistance in questionnaire development: James Bader, Brian Burt, Gustavo Cruz, Raul Garcia, Catherine Hayes, Amid Ismail, Steve Levy, Daniel Shugars, Carlos Ugarte, John Warren, James Wefel, Julie Meek, and Phil Weinstein.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Alaluusua S, Malmivirta R. (1994). Early plaque accumulation: a sign of caries in young children. Community Dent Oral Epidemiol 22(5 Pt 1):273-276 [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatric Dentistry (AAPD) (2006). Policy on Use of a Caries-risk Assessment Tool (CAT) for Infants, Children, and Adolescents, adopted 2002, revised 2006. Available at: http://www.aapd.org/media/Policies_Guidelines/P_CariesRiskAssess.pdf (URL accessed 06/23/2010).
- American Academy of Pediatric Dentistry (AAPD) (2008). Policy on Early Childhood Caries (ECC): Classification, Consequences, and Preventive Strategies, Council on Clinical Affairs. adopted 1978, last revised 2008; found at: http://www.aapd.org/media/Policies_Guidelines/P_ECCClassifications.pdf (URL accessed 6/23/2010).
- Anderson M. (2002). Risk assessment and epidemiology of dental caries: review of the literature. Pediatric Dent 24:377-385 [PubMed] [Google Scholar]
- Beltrán-Aguilar ED, Barker LK, Canto MT, Dye BA, Gooch BF, Griffin SO, et al. (2005). Surveillance for dental caries, dental sealants, tooth retention, edentulism, and enamel fluorosis—United States, 1988-1994 and 1999-2002. MMWR Surveill Summ 54:1-43 [PubMed] [Google Scholar]
- Demers M, Brodeur JM, Simard PL, Mouton C, Veilleux G, Frechette S. (1990). Caries predictors suitable for mass-screening in children. A literature review. Community Dent Health 7:11-21 [PubMed] [Google Scholar]
- Demers M, Brodeur JM, Mouton C, Simard PL, Trahan L, Veilleux G. (1992). A multivariate model to predict caries increment in Montreal children aged 5 years. Community Dent Health 9:273-281 [PubMed] [Google Scholar]
- Disney JA, Graves RC, Stamm JW, Bohannan HM, Abernathy JR, Zack DD. (1992). The University of North Carolina Caries Risk Assessment study: further developments in caries risk prediction. Community Dent Oral Epidemiol 20:64-75 [DOI] [PubMed] [Google Scholar]
- Dye BA, Tan S, Smith V, Lewis BG, Barker LK, Thornton-Evans G, et al. (2007). Trends in oral health status: United States, 1988-1994 and 1999-2004. Vital Health Stat 11:1-92 [PubMed] [Google Scholar]
- Gao X-L, Hsu CY, Xu Y, Hwarng HB, Loh T, Koh D. (2010). Building caries risk assessment models for children. J Dent Res 89:637-643 [DOI] [PubMed] [Google Scholar]
- Gold OG, Jordan H, van Houte J. (1973). A selective medium for Streptococcus mutans. Arch Oral Biol 18:1357-1364 [DOI] [PubMed] [Google Scholar]
- Grindefjord M, Dahllöf G, Nilsson B, Modéer T. (1995). Prediction of dental caries development in 1-year-old children. Caries Res 29:343-348 [DOI] [PubMed] [Google Scholar]
- Grindefjord M, Dahllöf G, Nilsson B, Modéer T. (1996). Stepwise prediction of dental caries in children up to 3.5 years of age. Caries Res 30:256-266 [DOI] [PubMed] [Google Scholar]
- Hale KJ, American Academy of Pediatrics (AAP), Section on Pediatric Dentistry (2003). Oral health risk assessment timing and establishment of the dental home. Pediatrics 111:1113-1116 [DOI] [PubMed] [Google Scholar]
- International Caries Detection & Assessment System Coordinating Committee. The International Caries Detection and Assessment System. ICDAS II Criteria (2005). Available at: http://www.dundee.ac.uk/dhsru/docs/Final%20ICDAS%20II%20criteria%20document.doc (URL accessed 6/23/2010).
- Ismail AI, Sohn W, Tellez M, Amaya A, Sen A, Hasson H, et al. (2007). The International Caries Detection and Assessment System (ICDAS): an integrated system for measuring dental caries. Community Dent Oral Epidemiol 35:170-178 [DOI] [PubMed] [Google Scholar]
- Ismail AI, Sohn W, Lim S, Willem JM. (2009). Predictors of dental caries progression in primary teeth. J Dent Res 88:270-275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keels MA, Hale KJ, Thomas HF, Davis MJ, Czerepak CS, Weiss PA, collaborators. Section of Pediatric Dentistry and Oral Health (2008). Policy Statement: Preventive oral health intervention for pediatricians. Pediatrics 122:1387-1394 [DOI] [PubMed] [Google Scholar]
- Köhler B, Andréen I, Jonsson B. (1988). The earlier the colonization by mutans streptococci, the higher the caries prevalence at 4 years of age. Oral Microbiol Immunol 3:14-17 [DOI] [PubMed] [Google Scholar]
- Li Y, Caufield PW, Dasanayake AP, Wiener HW, Vermund SH. (2005). Mode of delivery and other maternal factors influence the acquisition of Streptococcus mutans in infants. J Dent Res 84:806-811 [DOI] [PubMed] [Google Scholar]
- Mouradian WE, Wehr E, Crall JJ. (2000). Disparities in children’s oral health and access to dental care. JAMA 284:2625-2631 [DOI] [PubMed] [Google Scholar]
- Pienihäkkinen K, Jokela J. (2002). Clinical outcomes of risk-based caries prevention in preschool-aged children. Community Dent Oral Epidemiol 30:143-150 [DOI] [PubMed] [Google Scholar]
- Pienihäkkinen K, Jokela J, Alanen P. (2004). Assessment of caries risk in preschool children. Caries Res 38:156-162 [DOI] [PubMed] [Google Scholar]
- Pitts N. (2004). “ICDAS”—an international system for caries detection and assessment being developed to facilitate caries epidemiology, research and appropriate clinical management. Community Dent Health 21:193-198 [PubMed] [Google Scholar]
- SBU: The Swedish Council on Technology Assessment in Health Care (2007). Report: Caries – diagnosis, risk assessment and non-invasive treatment. A systematic review. Summary and conclusions. Report No 188. ISBN:978-91-85413-21-8; ISSN:1440-1403. Available at: www.sbu.se/en/ (URL accessed 06/23/2010).
- Stamm JW, Disney JA, Graves RC, Bohannan HM, Abernathy JR. (1988). The University of North Carolina Caries Risk Assessment Study I: Rationale and content. J Public Health Dent 48:225-232 [DOI] [PubMed] [Google Scholar]
- Twetman S, Fontana M. (2009). Patient caries risk assessment. Monogr Oral Sci 21:91-101 [DOI] [PubMed] [Google Scholar]
- Trahan L, Söderling E, Drean MF, Chevrier MC, Isokangas P. (1992). Effect of xylitol consumption on the plaque-saliva distribution of mutans streptococci and the occurrence and long-term survival of xylitol-resistant strains. J Dent Res 71:1785-1791 [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. Oral Health in America (2000). A Report of the Surgeon General - Executive Summary. Rockville, MD: US Department of Health and Human Services, National Institute of Dental and Craniofacial Research, National Institutes of Health; Accessed at: http://www.surgeongeneral.gov/library/oralhealth/ (URL accessed 06/23/2010). [Google Scholar]
- Warren JJ, Weber-Gasparoni K, Marshall TA, Drake DR, Dehkordi-Vakil F, Dawson DV, et al. (2009). A longitudinal study of dental caries risk among very young low SES children. Community Dent Oral Epidemiol 37:116-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zero D, Fontana M, Lennon AM. (2001). Clinical applications and outcomes of using indicators of risk in caries management. J Dent Educ 65:1126-1132 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


