Abstract
Catecholamines are present in saliva, but their influence on oral epithelium is not understood. Because psychological stress increases salivary catecholamines and impairs oral mucosal wound healing, we sought to determine if epithelial adrenergic signaling could link these two findings. We found that cultured human oral keratinocytes (HOK) express the α2B- and β2-adrenergic receptors (ARs). Exposure of HOK to either epinephrine or the β-AR agonist, isoproterenol, reduced migratory speed and decreased in vitro scratch wound healing. Incubation with the β-AR antagonist timolol reversed the catecholamine-induced effects, indicating that the observed response is mediated by β-AR. Epinephrine treatment decreased phosphorylation of the mitogen-activated protein kinases (MAPK) ERK1/2 and p38; these decreases were also reversed with timolol. Cultured HOK express enzymes of the epinephrine synthetic pathway, and generate epinephrine. These findings demonstrate that stress-induced elevations of salivary catecholamines signal through MAPK pathways, and result in impaired oral keratinocyte migration required for healing.
Keywords: catecholamines, stress, wound repair, adrenergic receptor, oral epithelium
Introduction
Adrenergic receptors (ARs) are G-protein-coupled transmembrane receptors for catecholamine ligands, including the classic “stress hormones” epinephrine and norepinephrine. These receptors are expressed widely, including within the epidermis and cornea (Walkenbach et al., 1984; Steinkraus et al., 1996; Schallreuter, 1997), where recent work has uncovered a role for AR signaling in cell migration and wound repair (Chen et al., 2002; Pullar et al., 2006a, 2007; Ghoghawala et al., 2008; Sivamani et al., 2009b). A role for ARs in the control of salivary gland function is also known (reviewed in Proctor and Carpenter, 2007); however, the function of ARs within the oral epithelium is not clear.
An understanding of the physiology of ARs in the oral epithelium is warranted, because this epithelium is bathed in catecholamine-containing saliva (Okumura et al., 1997). Levels of salivary catecholamines, both norepinephrine and epinephrine, increase after psychological stress (McClelland et al., 1985; Mitome et al., 1997; Okumura et al., 1997). Various stressors can induce differential increases in either epinephrine, norepinephrine, or both (Vogel and Jensh, 1988; Goldstein, 2003). Therefore, one might expect that adverse effects of stress, often mediated by elevations of the “stress hormones” epinephrine and norepinephrine, could manifest in the oral cavity. Indeed, stress-impaired healing of oral mucosal wounds has been reported (Marucha et al., 1998), and the impaired healing of oral aphthous ulcers (Natah et al., 2004) has also been associated with increased psychological stress (Albanidou-Farmaki et al., 2008). Yet, the relationship of stress, catecholamine mediators, and ARs in the oral epithelium is one that has not been explored, and that was the goal of the work we report here.
Activation of β2-AR is one mechanism by which stress decreases migration of human skin-derived keratinocytes or corneal epithelial in vitro, delays healing of a wound in a confluent sheet of cultured cells, and impairs healing in both explanted skin and cornea (Chen et al., 2002; Pullar et al., 2006b, 2007; Ghoghawala et al., 2008; Sivamani et al., 2009b). β2-AR blockade with receptor-specific antagonists reverses this effect and also, even in the absence of exogenously added agonists, accelerates wound healing in vitro (Pullar et al., 2006b) and in vivo in wound models (Ghoghawala et al., 2008; Sivamani et al., 2009b). AR antagonists, notably to the β2-AR, have therefore been proposed as therapeutic agents to improve healing, and are currently used in the care of burn wound patients (Pereira et al., 2007; Zhang et al., 2009).
We hypothesized that the “stress hormone” epinephrine would decrease oral epithelial cell migration by activation of the β2-AR, if this or other ARs were present in oral epithelial cells, and that this could lead to impaired oral wound healing.
Materials & Methods
Cell Isolation
HOK were isolated from oral gingiva attached to extracted third molars of healthy individuals and cultured as described (Krisanaprakornkit et al., 1998), with the modification of epinephrine being removed from the medium. At 70% confluence, cells were frozen or passaged, and 24 hrs after passaging, the medium was replaced with growth medium (KBM 0.15 mM calcium plus keratinocyte growth medium supplements; Cambrex, Walkersville, MD, USA) to facilitate differentiation.
Microarrays
For microarray studies, fourth-passage HOKs from three donors were tested in triplicate. Total RNA was isolated with TRIzol and purified with the RNeasy mini kit (Qiagen, Valencia, CA, USA) as previously described (Yin and Dale, 2007). RNA quality was assayed then processed for microarrays at the Center for Expression Array at the University of Washington for expression analysis on HG-U133A GeneChips (Affymetrix, Santa Clara, CA, USA). Array data were analyzed by GeneChip operating software (GCOS, V1.4) and further analyzed as previously described (Yin and Dale, 2007). Arrays were normalized with the median intensity over the entire array. Probe sets with mean expression value < 40 were similar to negative controls and considered to be under the limits of detection.
Cell Migration
We determined the migratory speed of cultured HOK using image analysis of time-lapse images, as previously described (Sivamani et al., 2009b). Cells were exposed to either growth medium alone (control) or medium containing either the β-AR agonist isoproterenol (EMD Biosciences, La Jolla, CA, USA), the β-AR agonist epinephrine, the β-AR antagonist timolol (Sigma-Aldrich, St. Louis, MO, USA), or combinations of these agonists and the antagonist, and incubated at 37°C for another 30 min before imaging.
Immunocytochemistry
immunocytochemistry was performed as previously described (Pullar et al., 2006b) on HOK incubated with growth medium alone (control), or growth medium containing agonists and/or the antagonist for 1 hr prior to fixation in 10% buffered formalin. Cells were permeabilized for 5 min with 0.1% Triton-X-100 in PBS, and non-specific binding was blocked for 1 hr with 10% goat serum in PBS. Primary and secondary antibody dilutions were made in 1% goat serum in PBS. Imaging was done on a Nikon Eclipse TE2000 inverted microscope with a 60x objective (NA 1.4) with volume deconvolution and OpenLab image analysis software.
Scratch Wound Assay
The rate of healing scratch wounds made in confluent HOK cultures was examined by previously reported techniques (Pullar et al., 2006b). Cells were pre-treated for 1 hr with 10 µg/mL mitomycin C (EMD Biosciences) to inhibit cell proliferation that could confound the analysis of migration (Hayashi et al., 2007). Wounded cultures were incubated with growth medium alone (control) or growth medium containing agonists and/or the antagonist. We used ImageJ (NIH.gov) to measure the scratch wound area, and performed a two-sample, unequal variance, one-tailed Student’s t test to compare the average percentage healing.
Immunoblotting
HOK treated for 60 min with growth medium alone or growth medium containing agonists and/or the antagonist underwent lysis, and lysates were subjected to immunoblotting as previously described (Pullar et al., 2006b). Electrophoresis was performed with 5 µg protein per lane (P-ERK, ERK, P-p38 MAPK, and p38 MAPK) or 40 µg protein per lane (α2B-AR and β2-AR). Detection was as previously described (Pullar et al., 2006b).
Real-time RT-PCR
Total RNA was isolated from cells with the use of the RNeasy® Mini Kit (Qiagen), and reverse transcription was performed on 2 ng RNA (Applied Biosystems, Foster City, CA, USA). RT-PCR was performed in duplicate on 0.056 ng cDNA in a reaction volume of 10 µL with a 7500 Fast Real-Time PCR (Applied Biosystems). Expression of tyrosine hydroxylase (TH) and phenylethanolamine-N-methyl transferase (PNMT) was determined by TaqMan® Gene Expression Assays (Applied Biosystems) and normalized to 18S rRNA.
Epinephrine Immunoassay
We used an epinephrine enzyme immunoassay (Rocky Mountain Diagnostics, Colorado Springs, CO, USA) to measure the amount of epinephrine produced by HOK. Epinephrine levels were calculated as picogram/milligram of total protein. Epinephrine levels were also measured in HOK-conditioned medium.
Results
α2B-AR and β2-AR Were Expressed by HOK
Epinephrine can activate both α- and β-AR, so we first asked which AR subtypes are expressed by HOK. Both α2B-AR and β2-AR were detected by gene expression analysis (Fig. 1A), with the β2-AR being the most highly expressed AR gene in HOK. Protein expression of both α2B-AR and β2-AR was also confirmed in these cells (Fig. 1B).
Figure 1.
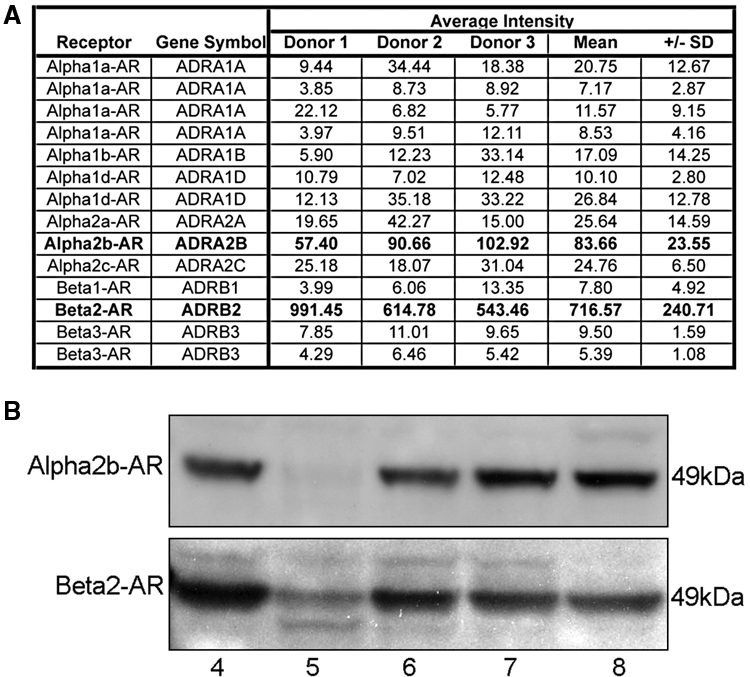
Expression of adrenergic receptors in HOK. (A) mRNA was isolated from HOK strains derived from three donors and hybridized to an Affymetrix HG-U133A microarray. The values in the table are signal intensities for hybridization with the microarray; values higher than 40 represent significant gene expression. Bold typeface indicates the 2 genes in the list that were significantly expressed. (B) Protein was isolated from HOK strains derived from four donors, and expression of the α2B-AR and β2-AR proteins was detected on a Western blot. Blots were stained with anti-α2B-AR (Genex Bioscience, Hayward, CA, USA) at a 1:5000 dilution (2.3 µg/mL), followed by HRP-linked anti-rabbit secondary antibody (Cell Signaling Technology, Danvers, MA, USA) at a 1:1000 dilution (0.2 µg/mL), or with anti-β2-AR (Abcam, Cambridge, MA, USA; clone Ab40834) at a 1:2500 dilution (0.2 µg/mL), followed by HRP-linked anti-goat secondary antibody (Abcam) at a 1:5000 dilution (0.4 µg/mL). α2B-AR was detected at 49 kDa as expected based on its amino acid composition. Immuno-detected β2-AR bands were evident at 49 kDa and 47 kDa, likely representing the palmitoylated and non-palmitoylated forms. Human epidermal keratinocytes were used as a positive control. 4, HOK from Donor 4; 5, HOK from Donor 5; 6, HOK from Donor 6; 7, HOK from Donor 7; 8, human epidermal keratinocytes from Donor 8.
β-AR Activation Reduced HOK Cell Migration and Wound Healing in vitro
HOK (control) migrated at 1.7 µm/min, and activation of the β-AR with 1 µM isoproterenol resulted in a 43% decrease in migratory speed (Fig. 2A, n = 7, p ≤ 0.01); 1 µM epinephrine treatment resulted in a similar 34% decrease (Fig. 2B, n = 3, p ≤ 0.01). Dose response testing demonstrated a plateau in the inhibition of migration at the 1-µM level (Appendix Fig. 1). Blocking the β-AR with 20 µM timolol completely reversed the agonist-induced effect (Fig. 2A, n = 7, p ≤ 0.01; Fig. 2B, n = 3, p ≤ 0.01). Treatment with 20 µM timolol alone resulted in a 14% increase in migratory speed (Figs. 2A, 2B, n = 7, p ≤ 0.01).
Figure 2.
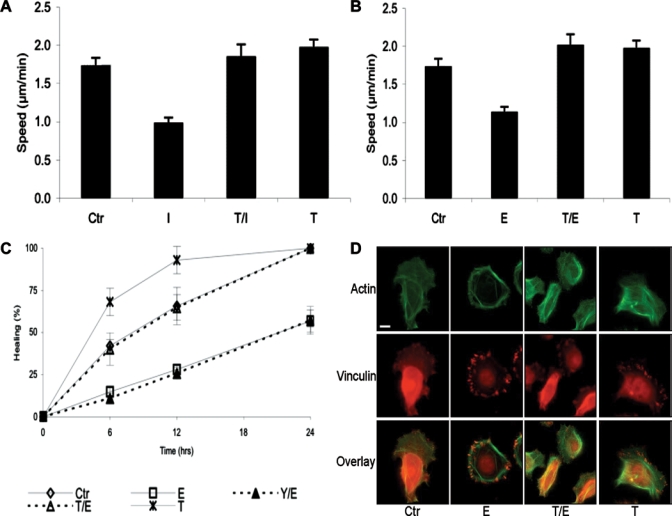
β-AR activation reduced HOK migration and in vitro wound closure. (A) Migratory speed of HOK that were plated on collagen-coated glass-bottomed culture dishes and treated with growth medium (Ctr), 1 µM isoproterenol (I), 20 µM timolol + 1 µM isoproterenol (T/I), or 20 µM timolol (T). Average speed in µm/min was calculated per treatment group, and we performed a one-way ANOVA followed by a two-sample, unequal variance, one-tailed Student’s t test to calculate significant differences between and among treatment groups. Panel A represents the mean values and standard errors of at least 7 experiments per treatment. The data represent 2 cell strains that were isolated from different donors. (B) Migratory speed of HOK that were treated with growth medium (Ctr), 1 µM epinephrine (E), 20 µM timolol + 1 µM epinephrine (T/E), or 20 µM timolol (T). Panel B represents the mean values and standard errors of at least 3 experiments per treatment. The data represent 6 cell strains that were isolated from different donors. *p ≤ 0.01 compared with Ctr; #p ≤ 0.05 compared with Ctr; **p ≤ 0.01 compared with I; and ***p ≤ 0.01 compared with E. (C) Scratch wounds were made in confluent cultures of HOK, previously treated with Mitomycin C, as described in MATERIALS & METHODS. Two scratches were made in each well, and two fields of view were photographed per scratch by means of an inverted Nikon Diaphot microscope. Images of the same field were captured at 0, 6, 12, and 24 hrs after the scratch was made. After wounding, the medium was replaced with growth medium (Ctr), 1 µM epinephrine (E), 20 µM timolol + 1 µM epinephrine (T/E), 20 µM timolol (T), or 20 µM yohimbine + 1 µM epinephrine (Y/E). The graph represents 2 HOK strains and displays the mean percentage healing calculated from 4 image areas on 2 scratches per treatment. *p ≤ 0.01 compared with Ctr; **p ≤ 0.05 compared with Ctr. (D) Actin and vinculin immunostaining revealed that cells that were treated with E had a non-migratory morphology. Cells were incubated overnight at 4°C with 33 µg/mL anti-vinculin (Sigma-Aldrich), followed by a two-hour incubation with 10 µg/mL AF594-goat-anti-mouse IgG (Invitrogen, Carlsbad, CA, USA) and 33nM AF488-phalloidin (Invitrogen). Cells treated with Ctr, T/E, or T all showed a similar migratory morphology. Bar = 10 microns.
Similarly, in the scratch wound assay, HOK treated with 1 µM epinephrine demonstrated decreased rates of healing when compared to controls (Fig. 2C, n = 4, p ≤ 0.01 at 6 and 24 hrs after wounding, p ≤ 0.01 at 12 hrs; Appendix Fig. 2). Co-incubation with 20 µM timolol reversed the epinephrine-induced effect, and timolol alone resulted in more rapid healing compared with the control (n = 4, p ≤ 0.05 at 6 and 12 hrs after wounding). Since epinephrine (unlike isoproterenol, which solely activates β-AR) is also an agonist to the α2B-AR, that are expressed in HOK, the α2-AR-specific antagonist yohimbine was added in some experiments. Yohimbine did not reverse the epinephrine effect, suggesting that the epinephrine-induced impairment in healing was mediated solely by the β-AR.
The decrease in migration speed in epinephrine-treated cells was accompanied by morphologic changes characteristic of stationary cells: the presence of many large vinculin-containing focal adhesions symmetrically distributed at the cell periphery (Fig. 2D). Control cells, cells treated with timolol/epinephrine, or cells treated with timolol alone all displayed fewer focal adhesions, and these were concentrated in the lamellipodia at the leading edges of migrating cells. Fine branching networks of actin fibers extending into the lamellipodia were evident in migratory cells (control, epinephrine/timolol, or timolol alone), whereas broad actin fibers arranged symmetrically in the periphery characterized the stationary, epinephrine-treated cells.
Epinephrine Activation of β-AR Resulted in Decreased Phosphorylation of ERK1/2 and P38 MAPK
Activation of both ERK 1/ 2 and P38 MAP kinases are required for cell migration (reviewed in Huang et al., 2004; Fitsialos et al., 2007). Treatment of HOK with 1 µM epinephrine resulted in a 39-64% decrease in ERK1/2 phosphorylation (Fig. 3A, n = 3, p ≤ 0.01), reversed when the cells were pre-treated with 20 µM timolol, indicating that the decrease in ERK1/2 phosphorylation, like the decrease in migration, was mediated by epinephrine-induced β2-AR activation. Phosphorylation of the pro-migratory p38 MAP kinase was also decreased (38-42%) in HOK by epinephrine activation of the β-AR (Fig. 3B, n = 3, p ≤ 0.01), and this decrease was reversed by co-incubation with the β-AR antagonist timolol. Treatment with 20 µM timolol alone did not affect ERK phosphorylation compared with the control.
Figure 3.
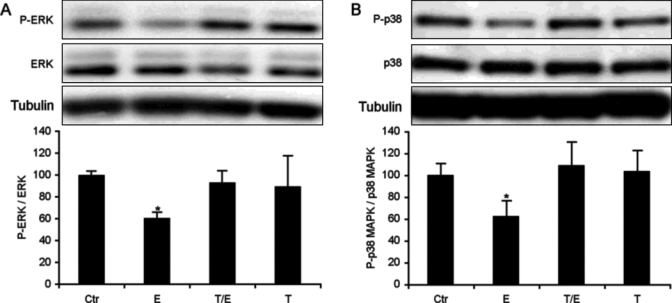
β-AR activation decreased ERK1/2 and p38 MAPK phosphorylation. Cells were grown in control medium until 60-80% confluent and treated for 60 min with growth medium (Ctr), 1 µM epinephrine (E), 20 µM timolol + 1 µM epinephrine (T/E), or 20 µM timolol (T). Immunoblotting with antibodies against P-ERK, ERK, P-p38 MAPK, and p38 MAPK (Cell Signaling Technology, Danvers, MA, USA) at a 1:1000 dilution (P-ERK, 0.1 µg/mL; ERK, 0.01 µg/mL; P-p38 MAPK, 35 ng/mL; p38 MAPK, 5 ng/mL) was followed by HRP-linked anti-rabbit secondary antibody (Cell Signaling Technology) at a 1:1000 dilution (0.2 µg/mL). Phosphorylated ERK and p38 MAPK were normalized to total ERK or p38 MAPK. The histogram represents the mean signal intensities and standard errors of at least 3 experiments per treatment. Panels A and B are data derived from 3 experiments. The data represent 2 cell strains that were isolated from different donors. *p ≤ 0.01 compared with Ctr.
HOK Produced and Secreted Epinephrine
Addition of the β-AR antagonist timolol to cultured HOK in the absence of exogenously added β-AR agonists increased their migratory speed and ability to heal a scratch wound (Fig. 2). This prompted the question of what endogenous β-AR agonist generated by the cultured HOK is blocked by the antagonist. Transcripts for 2 enzymes in the epinephrine synthetic pathway—tyrosine hydroxylase (TH, converts L-tyrosine to L-DOPA) and phenylethanolamine-N-methyltransferase (PNMT, converts norepinephrine into epinephrine)—were detected in HOK (Figs. 4A, 4B) by either microarray analysis or RT-PCR. Cultured HOK were also able to generate epinephrine detectable in cell lysates (Fig. 4C) and in the culture medium (Fig. 4D).
Figure 4.
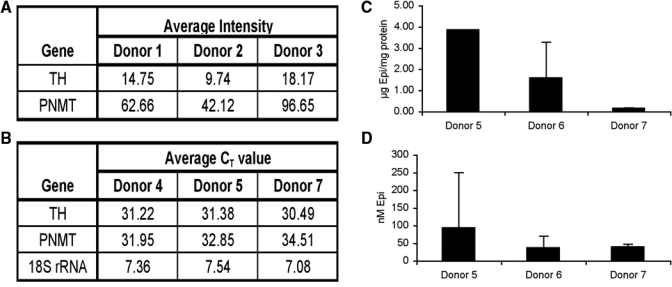
HOK can generate epinephrine. (A) Expression of enzymes tyrosine hydroxylase (TH) and phenylethanolamine-N-methyl transferase (PNMT), involved in epinephrine synthesis. mRNA was isolated from HOK strains derived from Donors 1, 2, and 3 and hybridized to an Affymetrix HG-U133A microarray. The values in the table are signal intensities for hybridization with the microarray; values higher than 40 represent significant gene expression. (B) mRNA was isolated from HOK strains derived from Donors 4, 5, and 7, and Ct values for TH and PNMT were determined by real-time RT-PCR; values lower than 40 represent significant gene expression. (C) Epinephrine measured in HOK cellular extracts. Confluent cultures were extracted in 100 µL 0.1 M hydrochloric acid/100-mm culture dish, and 4 dishes were combined, sonicated, and used for triplicate measurements. Results are expressed as pg epinephrine per µg total protein in the sample. (D) Epinephrine measured in the culture medium conditioned by HOK. Results are expressed as the final concentration in the sample.
Discussion
While the role of adrenergic signaling has been investigated in skin and corneal epithelia (Chen et al., 2002; Pullar et al., 2006b, 2007; Ghoghawala et al., 2008; Sivamani et al., 2009b), surprisingly, this receptor-mediated signaling pathway has not been explored in oral epithelium. Here, we demonstrate for the first time the existence of an adrenergic receptor signaling pathway in oral epithelial keratinocytes, report on the consequences of receptor activation and blockade, and suggest a role for endogenous catecholamine ligands in the physiology of healing wounds within the oral cavity. The β2-AR and α2b-AR were found to be expressed in cultured HOK, at both the message and protein levels, with the β2-AR being the predominantly expressed receptor. β-AR activation by epinephrine decreased HOK migration and their ability to heal a wound within a confluent sheet of cultured cells. That these effects could reversed by a β-AR antagonist, but not an α2-AR-antagonist, suggests that the migratory effects are mediated by the β-AR, and since the β2-AR is the only β-AR expressed, it is likely that the effects are mediated by the β2-AR. The catecholamine agonists also decrease the activity of the pro-migratory ERK and p38 MAPK, kinases required for epithelial cell migration (McCawley et al., 1999; Fitsialos et al., 2007; Storesund et al., 2008; Shi et al., 2009; Sivamani et al., 2009b) and convert HOK from a migratory to a stationary phenotype. Additionally, the HOK themselves can generate catecholamine ligands. Thus, this work establishes a regulatory autocrine pathway in oral epithelial cells wherein all the required components—the receptors, ligands, and downstream mediators—are expressed within the cell.
The presence of the β2-AR was previously detected by immunohistology in intact human oral mucosal epithelium, and its expression is increased in intra-oral squamous cell carcinomas (SCC) (Shang et al., 2009). Our work focused on non-transformed HOK, grown as primary cell cultures as physiologic representatives of the oral epithelium. We identified both β2-AR and α2b-AR protein in HOK, providing the rationale to characterize their function in these cells.
Epinephrine in the saliva of non-stressed humans is reported to range from 0.08 to 1.0 nM (Okumura et al., 1997), derived by transfer of epinephrine from the plasma and by synthesis and secretion by salivary sympathetic nerves (Kennedy et al., 2001). Increased levels of salivary catecholamines have been reported in psychologically stressed individuals, such as those undergoing dental procedures, public speaking, or taking academic examinations (McClelland et al., 1985; Mitome et al., 1997; Okumura et al., 1997). These earlier methods record levels only in the nM range; however, plasma levels of epinephrine can increase to the µM range with severe stress (Pacak et al., 1998), and salivary levels may likewise rise to this range. Both acute and chronic stress have been associated with delayed wound healing (Kiecolt-Glaser et al., 1995; Marucha et al., 1998; Christian et al., 2006; Robles et al., 2009), and both acute and chronic stress are accompanied by increases in circulating epinephrine levels (Schmidt and Kraft, 1996; Pike et al., 1997). Additionally, we show here that HOK themselves are a source of epinephrine and that nanomolar levels of epinephrine are secreted into the culture medium. Thus, the oral epithelium has the capacity to respond to an autocrine or paracrine signaling network that may be activated in either acute or chronic stress situations. Such a network has been suggested in the skin, where epidermal keratinocytes can generate catecholamines that then up-regulate the expression of, and/or activate, α1-, β1-, and β2-AR on keratinocytes or other cell types in the skin (Schallreuter, 1997; Sivamani et al., 2009a). It is reasonable to propose that stress increases the levels of circulating catecholamine agonists and, via a paracrine signaling mechanism, impairs healing. Additionally, autocrine signaling, via epithelial-cell-generated catecholamine ligands, may also impair healing in some circumstances. Autocrine signaling may play other, more homeostatic, roles in the epithelium, and further study will be required to discover this potential role.
Because catecholamine β2-AR agonists can impair HOK migration and wound healing in vitro, it is logical to propose that the stress-related elevations in salivary catecholamines contribute to the well-documented impairment of wound healing in both acutely and chronically stressed individuals (Kiecolt-Glaser et al., 1995; Marucha et al., 1998). Likewise, aphthous stomatitis, characterized by slowly healing ulcers in the oral epithelium, has also been associated with psychological stress (Albanidou-Farmaki et al., 2008), and here too, increased salivary catecholamines could play a role in impairing healing. Additionally, since infection of the oral epithelium with bacterial pathogens increases expression of the β2-AR in HOK (Handfield et al., 2005), bacterial infection may possibly impair healing in associated oral wounds, partly by β2-AR-mediated impairment of HOK motility and diminution of their ability to re-epithelialize a wound. Other studies have demonstrated that β2-AR agonists impair both endothelial angiogenesis and fibroblast-mediated gel contraction, and that β2-AR antagonists reverse these impairments (Pullar and Isseroff, 2005; Pullar and O’Leary, 2009). In each of these proposed clinical scenarios, the application of topical β2-AR antagonists might provide a novel therapeutic approach to improve healing, by acting as a classic antagonist to prevent binding of the increased levels of epinephrine, either present in saliva or HOK-generated. Continued investigations, including correlation of salivary catecholamines and oral β2-AR expression with chronic vs. acute stress, as well as clinical trials to determine the efficacy of adrenergic receptor-mediated treatment in improving healing in oral ulceration, will be needed to translate these findings into the clinical arena.
Supplementary Material
Acknowledgments
We thank Dr. Alex Tomaich, DDS, MD for providing tissue. This work was supported by VA Merit Award 104679, NIH AR044518, and Shriners Hospitals for Children® grant 8550 (RRI), NIH/NIDCR R01 DE 16961 and DE13573 (BAD), and NCRR UL1 RR024146 (NF).
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Albanidou-Farmaki E, Poulopoulos AK, Epivatianos A, Farmakis K, Karamouzis M, Antoniades D. (2008). Increased anxiety level and high salivary and serum cortisol concentrations in patients with recurrent aphthous stomatitis. Tohoku J Exp Med 214:291-296 [DOI] [PubMed] [Google Scholar]
- Chen J, Hoffman BB, Isseroff RR. (2002). Beta-adrenergic receptor activation inhibits keratinocyte migration via a cyclic adenosine monophosphate-independent mechanism. J Invest Dermatol 119:1261-1268 [DOI] [PubMed] [Google Scholar]
- Christian LM, Graham JE, Padgett DA, Glaser R, Kiecolt-Glaser JK. (2006). Stress and wound healing. Neuroimmunomodulation 13:337-346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitsialos G, Chassot AA, Turchi L, Dayem MA, LeBrigand K, Moreilhon C, et al. (2007). Transcriptional signature of epidermal keratinocytes subjected to in vitro scratch wounding reveals selective roles for ERK1/2, p38, and phosphatidylinositol 3-kinase signaling pathways. J Biol Chem 282:15090-15102 [DOI] [PubMed] [Google Scholar]
- Ghoghawala SY, Mannis MJ, Pullar CE, Rosenblatt MI, Isseroff RR. (2008). Beta2-adrenergic receptor signaling mediates corneal epithelial wound repair. Invest Ophthalmol Vis Sci 49:1857-1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS. (2003). Catecholamines and stress. Endocr Regul 37:69-80 [PubMed] [Google Scholar]
- Handfield M, Mans JJ, Zheng G, Lopez MC, Mao S, Progulske-Fox A, et al. (2005). Distinct transcriptional profiles characterize oral epithelium-microbiota interactions. Cell Microbiol 7:811-823 [DOI] [PubMed] [Google Scholar]
- Hayashi K, Storesund T, Schreurs O, Khuu C, Husvik C, Karatsaidis A, et al. (2007). Nerve growth factor beta/pro-nerve growth factor and their receptors in normal human oral mucosa. Eur J Oral Sci 115:344-354 [DOI] [PubMed] [Google Scholar]
- Huang C, Jacobson K, Schaller MD. (2004). MAP kinases and cell migration. J Cell Sci 117(Pt 20):4619-4628 [DOI] [PubMed] [Google Scholar]
- Kennedy B, Dillon E, Mills PJ, Ziegler MG. (2001). Catecholamines in human saliva. Life Sci 69:87-99 [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Marucha PT, Malarkey WB, Mercado AM, Glaser R. (1995). Slowing of wound healing by psychological stress. Lancet 346(8984):1194-1196 [DOI] [PubMed] [Google Scholar]
- Krisanaprakornkit S, Weinberg A, Perez CN, Dale BA. (1998). Expression of the peptide antibiotic human beta-defensin 1 in cultured gingival epithelial cells and gingival tissue. Infect Immun 66:4222-4228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marucha PT, Kiecolt-Glaser JK, Favagehi M. (1998). Mucosal wound healing is impaired by examination stress. Psychosom Med 60:362-365 [DOI] [PubMed] [Google Scholar]
- McCawley LJ, Li S, Wattenberg EV, Hudson LG. (1999). Sustained activation of the mitogen-activated protein kinase pathway.A mechanism underlying receptor tyrosine kinase specificity for matrix metalloproteinase-9 induction and cell migration. J Biol Chem 274:4347-4353 [DOI] [PubMed] [Google Scholar]
- McClelland DC, Ross G, Patel V. (1985). The effect of an academic examination on salivary norepinephrine and immunoglobulin levels. J Human Stress 11:52-59 [DOI] [PubMed] [Google Scholar]
- Mitome M, Shirakawa T, Kikuiri T, Oguchi H. (1997). Salivary catecholamine assay for assessing anxiety in pediatric dental patients. J Clin Pediatr Dent 21:255-259 [PubMed] [Google Scholar]
- Natah SS, Konttinen YT, Enattah NS, Ashammakhi N, Sharkey KA, Hayrinen-Immonen R. (2004). Recurrent aphthous ulcers today: a review of the growing knowledge. Int J Oral Maxillofac Surg 33:221-234 [DOI] [PubMed] [Google Scholar]
- Okumura T, Nakajima Y, Matsuoka M, Takamatsu T. (1997). Study of salivary catecholamines using fully automated column-switching high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 694:305-316 [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M, Yadid G, Kvetnansky R, Kopin IJ, Goldstein DS. (1998). Heterogeneous neurochemical responses to different stressors: a test of Selye’s doctrine of nonspecificity. Am J Physiol 275(4 Pt 2):R1247-R1255 [DOI] [PubMed] [Google Scholar]
- Pereira CT, Jeschke MG, Herndon DN. (2007). Beta-blockade in burns. Novartis Found Symp 280:238-248 [DOI] [PubMed] [Google Scholar]
- Pike JL, Smith TL, Hauger RL, Nicassio PM, Patterson TL, McClintick J, et al. (1997). Chronic life stress alters sympathetic, neuroendocrine, and immune responsivity to an acute psychological stressor in humans. Psychosom Med 59:447-457 [DOI] [PubMed] [Google Scholar]
- Proctor GB, Carpenter GH. (2007). Regulation of salivary gland function by autonomic nerves. Auton Neurosci 133:3-18 [DOI] [PubMed] [Google Scholar]
- Pullar CE, Isseroff RR. (2005). Beta 2-adrenergic receptor activation delays dermal fibroblast-mediated contraction of collagen gels via a cAMP-dependent mechanism. Wound Repair Regen 13:405-411 [DOI] [PubMed] [Google Scholar]
- Pullar CE, O’Leary A. (2009). The role of beta-adrenoceptors in wound angiogenesis [abstract]. J Invest Dermatol 129:80 [Google Scholar]
- Pullar CE, Grahn JC, Liu W, Isseroff RR. (2006a). Beta2-adrenergic receptor activation delays wound healing. FASEB J 20:76-86 [DOI] [PubMed] [Google Scholar]
- Pullar CE, Rizzo A, Isseroff RR. (2006b). Beta-adrenergic receptor antagonists accelerate skin wound healing: evidence for a catecholamine synthesis network in the epidermis. J Biol Chem 281:21225-21235 [DOI] [PubMed] [Google Scholar]
- Pullar CE, Zhao M, Song B, Pu J, Reid B, Ghoghawala S, et al. (2007). Beta-adrenergic receptor agonists delay while antagonists accelerate epithelial wound healing: evidence of an endogenous adrenergic network within the corneal epithelium. J Cell Physiol 211:261-272 [DOI] [PubMed] [Google Scholar]
- Robles TF, Brooks KP, Pressman SD. (2009). Trait positive affect buffers the effects of acute stress on skin barrier recovery. Health Psychol 28:373-378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallreuter KU. (1997). Epidermal adrenergic signal transduction as part of the neuronal network in the human epidermis. J Investig Dermatol Symp Proc 2:37-40 [DOI] [PubMed] [Google Scholar]
- Schmidt C, Kraft K. (1996). Beta-endorphin and catecholamine concentrations during chronic and acute stress in intensive care patients. Eur J Med Res 1:528-532 [PubMed] [Google Scholar]
- Shang ZJ, Liu K, Liang DF. (2009). Expression of beta2-adrenergic receptor in oral squamous cell carcinoma. J Oral Pathol Med 38:371-376 [DOI] [PubMed] [Google Scholar]
- Shi J, Zeng X, Zhou M, Chen Q. (2009). Activation of ERK-FAK signaling pathway and enhancement of cell migration involved in the early interaction between oral keratinocytes and Candida albicans. Mycopathologia 167:1-7 [DOI] [PubMed] [Google Scholar]
- Sivamani RK, Porter SM, Isseroff RR. (2009a). An epinephrine-dependent mechanism for the control of UV-induced pigmentation. J Invest Dermatol 129:784-787 [DOI] [PubMed] [Google Scholar]
- Sivamani RK, Pullar CE, Manabat-Hidalgo CG, Rocke DM, Carlsen RC, Greenhalgh DG, et al. (2009b). Stress-mediated increases in systemic and local epinephrine impair skin wound healing: potential new indication for beta blockers. PLoS Med 6:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkraus V, Mak JC, Pichlmeier U, Mensing H, Ring J, Barnes PJ. (1996). Autoradiographic mapping of beta-adrenoceptors in human skin. Arch Dermatol Res 288:549-553 [DOI] [PubMed] [Google Scholar]
- Storesund T, Hayashi K, Kolltveit KM, Bryne M, Schenck K. (2008). Salivary trefoil factor 3 enhances migration of oral keratinocytes. Eur J Oral Sci 116:135-140 [DOI] [PubMed] [Google Scholar]
- Vogel WH, Jensh R. (1988). Chronic stress and plasma catecholamine and corticosterone levels in male rats. Neurosci Lett 87:183-188 [DOI] [PubMed] [Google Scholar]
- Walkenbach RJ, Gibbs SR, Bylund DB, Chao WT. (1984). Characteristics of beta-adrenergic receptors in bovine corneal epithelium: comparison of fresh tissue and cultured cells. Biochem Biophys Res Commun 121:664-672 [DOI] [PubMed] [Google Scholar]
- Yin L, Dale BA. (2007). Activation of protective responses in oral epithelial cells by Fusobacterium nucleatum and human beta-defensin-2. J Med Microbiol 56(Pt 7):976-987 [DOI] [PubMed] [Google Scholar]
- Zhang XJ, Meng C, Chinkes DL, Finnerty CC, Aarsland A, Jeschke MG, et al. (2009). Acute propranolol infusion stimulates protein synthesis in rabbit skin wound. Surgery 145:558-567 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


