Abstract
Hypersensitivity to thermal and mechanical stimuli can occur in painful pulpitis. To explore the neuro-anatomical basis of heat and mechanical sensitivity, we evaluated expression of TRPV1 (heat) and TRPV2 (heat/mechanical) channels in the cell bodies and terminal arborizations of neurons that innervate the dental pulp (DP) and periodontal tissues (PDL). We report that ~50% of trigeminal ganglion (TG) neurons retrogradely labeled from the DP express TRPV2, and this was significantly greater than the general expression of this channel in the TG (15%) and slightly more than what is expressed in the PDL by retrograde labeling (40%). The TRPV1 receptor, however, was less prevalent in neurons innervating the DP than their general expression in the TG (17% vs. 26%) and was more extensively expressed in neurons innervating the PDL (26%). Co-labeling studies showed that 70% of neurons that innervate the DP are myelinated. Approximately 1/3 of the retrogradely labeled neurons from the DP were calcitonin-gene-related-peptide-positive (peptide-expressing), but very few expressed the IB4 marker of non-peptidergic unmyelinated afferents. These findings suggest that the DP has a unique neurochemical innervation with regard to TRP receptor expression, which has significant implications for the mechanisms contributing to odontogenic pain and management strategies.
Keywords: pain, TRPV2, TRPV1, dental pulp, heat
Introduction
Applying a heat stimulus to the tooth evokes pain by activating nociceptors that innervate dental pulp (DP), and these nociceptors in turn engage second-order neurons in the trigeminal nucleus (Chattipakorn et al., 2001). Inflammation of the dental pulp is associated with thermal and mechanical hypersensitivity, i.e., exaggerated sensitivity to cold or heat and enhanced mechanical activation of neurons in the dentinal tubules via hydrodynamic fluid movement (Närhi et al., 1982; Pau et al., 2005; Owatz et al., 2007). For example, heat hyperalgesia occurs in advanced painful cases of irreversible pulpitis and is associated with inflammatory and hyperemic histological changes in the pulp (Dachi, 1965; Cecic et al., 1983). The biological mechanisms of intra-oral thermal and mechanical allodynia and hyperalgesia, however, remain unclear. The hydrodynamic theory suggests that there is an important mechanism for indirect temperature-evoked activation of pulpal neurons, but it is likely that direct thermal activation of these neurons is also relevant (Brannström, 1986).
The discovery of transient receptor potential (TRP) ion channels has contributed greatly to our understanding of molecular mechanisms that mediate distinct sensory modalities (Patapoutian et al., 2009; Stucky et al., 2009). TRPV1 and TRPV2 are two TRP channels originally described as heat-sensitive (Caterina et al., 1997, 1999). Heat (> 43°C), pH, capsaicin, and inflammatory mediators activate or sensitize TRPV1, and the expression of TRPV1 is influenced by inflammatory mediators and nerve injury (Tominaga et al., 1998; Cesare et al., 1999; Caterina et al., 2000; Ji et al., 2002). Deletion of the TRPV1 receptor through both genetic and chemical means has confirmed a behavioral contribution of TRPV1 to heat sensitivity (Caterina et al., 2000; Cavanaugh et al., 2009). TRPV2, by contrast, has a much higher thermal activation threshold (> 52°C) (Caterina et al., 1999; Lewinter et al., 2004). Although TRPV2 was originally considered to process high-intensity heat, whether its in vitro properties are representative of its in vivo function has not yet been established. In fact, deletion of TRPV2 does not alter heat pain processing (Park et al., 2008). Other studies suggest that TRPV2 responds to membrane stretch (in aortic myocytes) and may serve a more general mechanosensitive function (Muraki et al., 2003; Lawson et al., 2008; Shibasaki et al., 2010).
To date, studies have demonstrated TRPV1 and TRPV2 receptors in neurons innervating dental pulp, but neither the relative expression of these channels nor their association with molecular markers of subpopulations of trigeminal ganglion neurons has been analyzed in detail (Chaudhary et al., 2001; Ichikawa and Sugimoto, 2001; Stenholm et al., 2002; Morgan et al., 2005). Here we use retrograde labeling methods combined with immunohistochemistry to compare the expression of TRPV1 and TRPV2 in two intra-oral heat and mechanosensitive tissues, namely, the dental pulp and periodontal tissues. This analysis established the relative contributions of neurochemically distinct TG neurons to pulpal and periodontal TRP channel innervation.
Materials & Methods
Experimental Animals
All studies were approved by the Institutional Animal Care and Use Committee and were conducted in compliance with the National Institutes of Health Guide and Public Health Service Policy on humane care and use of laboratory animals and the recommendations of the International Association for the Study of Pain. Two rats were housed per cage and maintained on a 12-hour light/dark schedule with ad libitum access to food and water. Twelve rats were used to complete these studies and none were dropped from the study.
Retrograde Labeling of Trigeminal Ganglion Neurons
The procedures used for retrograde (back) labeling of pulpal neurons, without inducing significant inflammation and necrosis, involve local application of the tracer Fluoro-Gold to dentin (Pan et al., 2003). Although it is unlikely that this tracer labels all trigeminal ganglion neurons that innervate the pulp, Fluoro-Gold likely reveals a more representative population than do other tracers (Zele et al., 2010). Dental Pulp (DP): Eight male adult Sprague-Dawley rats (200-250 g) were deeply anesthetized with a combination of ketamine HCl (60 mg/kg) and xylazine (7.5 mg/kg) by the intra-peritoneal route. After depth of anesthesia was confirmed, the jaw of each animal was propped open by means of a standard set of cotton forceps placed between the maxillary and mandibular molars. Using a ¼ round bur, we next made a shallow cavity into the dentin of the lingual surfaces of the left first and second maxillary molars and the mesial surface of the left maxillary first molar. Magnification and illumination via high-powered loupes and headlight allowed for adequate visualization. The cavity preparations were washed with sterile saline and dried with cotton pellets and air. The smear layer was removed from the cavities by etching with a gel containing 32% phosphoric acid (Young Dental, Earth City, MO, USA). The cavity was washed and dried again, and then a single 5% drop of the retrograde tracer Fluoro-Gold (Fluorochrome, Denver, CO, USA) was placed onto each cavity by means of a 10 µL Hamilton Syringe, and allowed to dry for 1-2 min. Periodontal Tissues (PDL): In a separate set of experiments with 4 adult male rats, we labeled periodontal tissues by injecting ~10 µL of 5% Fluoro-Gold into the periodontal ligament space located between the gingiva and the 1st and 2nd left maxillary molars, using an insulin syringe at multiple sites around the teeth.
Tissue Preparation and Immunocytochemistry
Animals were sacrificed by overdose with sodium pentobarbital (100 mg/kg) and perfused intracardially with 50 mL of 0.1 M phosphate-buffered saline (PBS), followed by 500 mL of 10% formalin in 0.1 M sodium phosphate buffer. The trigeminal ganglia and maxillary jaws were collected, post-fixed for 4 hrs, and then cryoprotected overnight in 30% sucrose in 0.1 M PB. Maxillae were decalcified in 10% EDTA (pH 7.6) for 2 to 3 wks. Tissues were sectioned in a cryostat (maxillae at 40 µm and TG at 18 µm) and thaw-mounted onto Superfrost Plus Microscope Slides (Sigma-Aldrich, St. Louis, MO, USA).
Tissue sections were incubated for 60 min at room temperature in a blocking solution of 5% normal goat serum in PBS with 0.3% Triton X-100. Slides were incubated overnight at 4°C with a rabbit anti-TRPV1 (1:5000) or rabbit anti-TRPV2 (1:1000) antibody, both kindly provided by Dr. David Julius (UCSF). Both antibodies were previously characterized for specificity (Caterina et al., 1999; Lewinter et al., 2004). Importantly, the TRPV1 antibody does not immunostain neurons in TRPV1-null mice. Sections were then incubated with either Alexa Fluorophore 488 or 546 goat anti-rabbit (Molecular Probes, Eugene, OR, USA; 1:700) for 2 hrs at room temperature. For double-label studies, antibodies used included mouse anti-CGRP (1:500; Sigma-Aldrich) and mouse anti-neurofilament 200 (1:10,000; Sigma-Aldrich). The lectin IB4 was visualized with biotinylated IB4 (1:500; Vector Laboratories, Burlingame, CA, USA) and Alexa Fluorophore 594 conjugated to streptavidin (1:1000; Invitrogen, Carlsbad, CA, USA). Sections were observed with either a fluorescence (Nikon Eclipse) or confocal microscope (Zeiss LSM510). Image J was used to count cells and measure cell diameters.
Data Analysis
The results of cell counting were recorded in an Excel spreadsheet and then transferred to STATA (version 10) for analysis. Figures were generated using GraphPad Prism (ver. 5.0b). Standard descriptive statistics were used. For comparison between groups, we used an unpaired two-way t test. Significance was at the p < 0.05 level.
Results
Expression of TRPV1 and TRPV2 in Fluoro-Gold-labeled Pulpal and Periodontal Neurons of the Trigeminal Ganglia
We first evaluated the percentage of rat TG neurons that express the TRPV2 and TRPV1 receptors. Consistent with most other studies, we found that TRPV2 is expressed in about 15% of all neuronal cell bodies of the TG and TRPV1 in about 25% (Table). We then determined their tissue-specific distribution by retrograde labeling neurons that innervate the dental pulp (DP) and periodontal tissues (PDL) using Fluoro-Gold (FG). To ensure the feasibility of making injections that selectively target the DP and PDL, we examined the spread of FG in decalcified maxilla sections. Appendix Fig. 1 illustrates that it is indeed possible to localize injections, making our subsequent analysis of the innervation of the DP and PDL possible.
Table.
Comparison of TRPV1 and TRPV2 Expression in Neurons of the Dental Pulp (DP) with Expression of These Channels in the Periodontal Tissues (PDL) and Trigeminal Ganglion (TG)
| DP | PDL | TG | p-value | |
|---|---|---|---|---|
| TRPV2 | 50.2 ± 11.9 n = 8 (817) |
39.9 ± 10.3 n = 4 (563) |
14.5 ± 1.8 n = 4 (2082) |
DP vs. PDL: p = 0.17 DP vs. TG: p = 0.0002 PDL vs. TG: p = 0.003 |
| TRPV1 | 16.9 ± 6.8 n = 8 (682) |
25.8 ± 3.6 n = 4 (626) |
25.8 ± 2.6 n = 4 (2267) |
DP vs. PDL: p = 0.04 DP vs. TG: p = 0.03 PDL vs. TG: p = 0.99 |
Unpaired two-tailed t tests were used to determine the difference between groups. The average and SD represent the frequency of expression averaged by rat, n = number of rats used in analysis, and (x) = the number of Fluoro-Gold-positive profiles counted per analysis. A statistically significant difference between groups is considered at the p < 0.05 level.
To determine the tissue-specific neuronal distribution of TRPV1 and TRPV2 receptors, we counted the co-expression of TRPV1 and TRPV2 immunoreactivity in FG-labeled neurons that innervate the DP and PDL. We found that 50% (Table) of all DP cell bodies expressed TRPV2 (Figs. 1a, 1e, 1i, 1c, 1g, 1k), while only 17% expressed TRPV1 (Figs. 2a, 2e, 2i, 2c, 2g, 2k). There were slight differences in the innervation of the PDL relative to DP, with 40% of all retrogradely labeled cell bodies expressing TRPV2 and 26% expressing TRPV1 (Table). These results indicate that the DP and PDL innervation is dominated by afferents that express TRPV2, at least when compared with TRPV1. The relatively sparse TRPV1 innervation of the DP indicates that the DP is likely less responsive to heat via TRPV1-mediated mechanisms than other tissues of the trigeminal system, including periodontal tissues.
Figure 1.
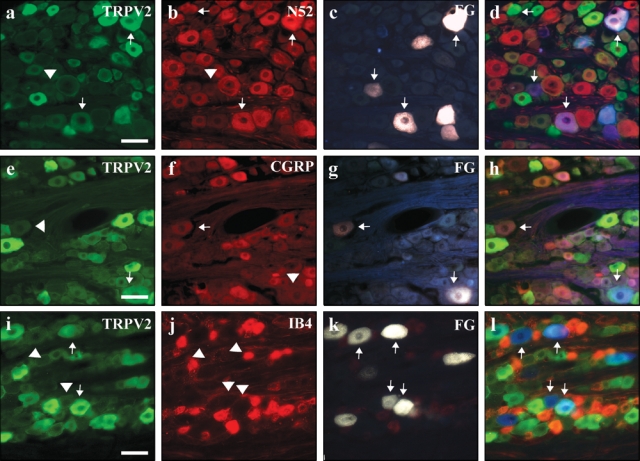
TRPV2 was highly expressed in the cell bodies of neurons innervating the dental pulp. Sections of trigeminal ganglia containing pulpal neurons that were retrogradely labeled with Fluoro-Gold were evaluated for TRPV2 expression. Tailed arrows point to cell bodies that expressed Fluoro-Gold and immunoreactivity for the indicated antigen. Arrowheads highlight cells that expressed Fluoro-Gold and were immunonegative for the indicated antigen. Fluoro-Gold-positive cells are shown in Panels c, g, and k. TRPV2 was expressed in pulpal neurons (Panels a, e, i) that almost always co-expressed NF200 (Panel b) and sometimes CGRP (Panel f). Overlay images are also included (Panels d, h, l). The non-peptidergic nociceptive marker IB4 was not found in pulpal neurons (Panel j). All scale bars = 200 µm.
Figure 2.
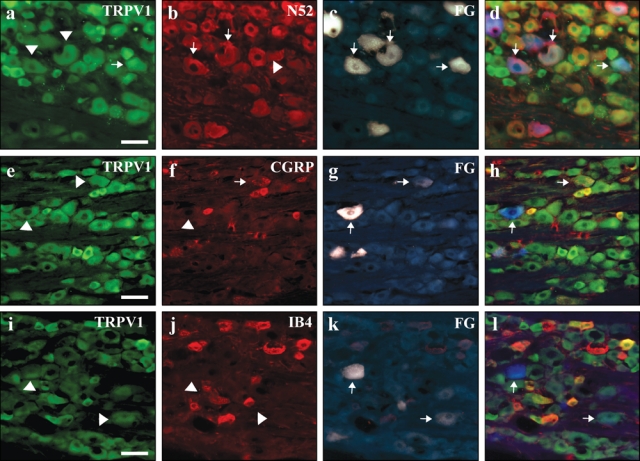
TRPV1 is infrequently expressed in the cell bodies of neurons innervating the dental pulp. Sections of trigeminal ganglia containing pulpal neurons that were retrogradely labeled with Fluoro-Gold were evaluated for TRPV1 and TRPV2 expression. Tailed arrows point to cell bodies that expressed Fluoro-Gold and/or immunoreactivity for the indicated antigen. Arrowheads highlight cells that expressed Fluoro-Gold and were immunonegative for the indicated antigen. TRPV1 was infrequently expressed in pulpal neurons (Panels a, e, i), but even these few were often NF200-positve (Panel b) and sometimes CGRP-positive (Panel f). The non-peptidergic nociceptive marker IB4 was not found in pulpal neurons (Panel j). All scale bars = 200 µm.
Cell diameter measurements showed that the TRPV2 innervation of the DP derives from neurons that ranged between 71.2 and 2577.8 µm2 (median = 868.6). The vast majority of these (87%) are of medium and large diameter (> 400 µm2) (Fig. 3a). By contrast, the TRPV1 innervation is derived from somewhat smaller-diameter neurons, 66.2-2256.2 µm2 (mean = 741.4). Again, as for the TRPV2 neurons, the great majority of these (82%) are medium and large diameter neurons (Fig. 3b).
Figure 3.
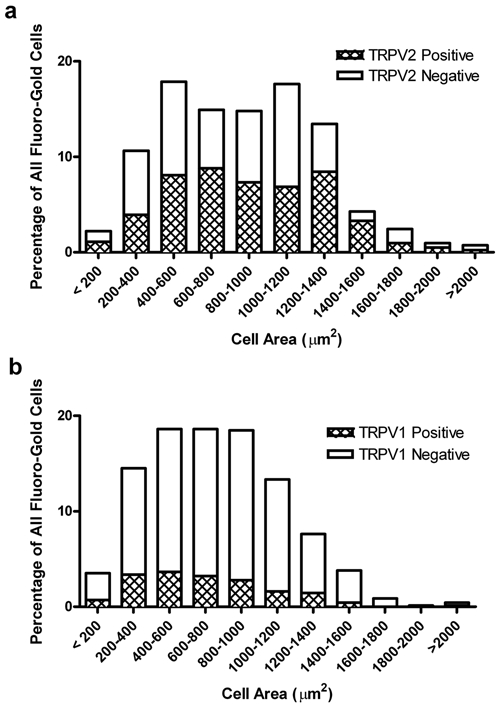
Cell size distribution analysis of TRPV1 and TRPV2 expressing pulpal neurons in the trigeminal ganglia.
Co-localization of TRPV1 and TRPV2 with Neurochemical Markers of Nociceptors that Innervate the DP
We found that approximately 1/3 of FG-labeled pulpal neurons are peptidergic, i.e., co-label for CGRP (37 ± 11% of 486 FG cells counted; Figs. 1f-1h, Figs. 2f-2h). Of particular interest, many of these pulpal cell bodies give rise to myelinated axons, since they also express NF200, a neurofilament marker of myelinated axons (71 ± 13%, of 471 FG cells counted; Figs. 1b-1d, Figs. 2b-2d). Interestingly, we found that few FG-labeled cells bound the lectin IB4, which is a marker of non-peptidergic subpopulation of unmyelinated C-fibers (1 ± 1.7%, of 543 FG cells counted; Figs. 1j-1l, Figs. 2j-2l). In co-labeling studies, we found that about 1/3 of the FG-labeled TRPV2-ir cells from DP expressed CGRP (33%, 49/150; Figs. 1e-1h). This is considerably less than the pulpal TRPV1-ir cells, of which greater than half co-labeled with CGRP (60%, 23/38; Figs. 2e-2h). Finally, we found that the great majority of FG-labeled cells that express TRPV1 and TRPV2-ir are myelinated, as demonstrated by their high degree of co-localization with NF200 (TRPV1, 68%, 17/25; Figs. 2a-2d; TRPV2, 83%, 104/126; Figs. 1a-1d).
Direct Visualization of TRPV1 and TRPV2 Axonal Arbors in the Dental Pulp
We also examined the arborization of TRPV1 and TRPV2 axons in the dental pulp of rodent maxillary molars. We found that TRPV2 receptor immunoreactivity was abundantly expressed in NF200-positive axonal profiles in the dental pulp (Appendix Figs. 2a-2c). This supports the conclusions drawn from the Fluoro-Gold retrograde-labeling studies. We were not able to demonstrate convincingly any TRPV1 receptor expression in the dental pulp using immunohistochemistry (data not shown). This was expected, given the low expression of TRPV1 in cell bodies that innervate the DP (Table).
Discussion
In this study, we found that the TRPV2 receptor is frequently expressed in pulpal sensory neurons relative to their expression in trigeminal ganglia as a whole. By contrast, TRPV1 is underrepresented in pulpal sensory neurons relative to their expression in the trigeminal ganglia. These observations support the contention that the dental pulp is a uniquely innervated nociceptive tissue and may, in fact, have a limited capacity to detect heat via TRPV1, at least under uninjured conditions. This finding could be related to the clinical observation that heat is an unreliable stimulus to test pulp vitality (Petersson et al., 1999). Although the IB4-positive population of neurons is heat-sensitive (Albers et al., 2006), these afferents are quite limited in the dental pulp, and likely contribute minimally to heat sensitivity in vivo (Cavanaugh et al., 2009). Taken together with the fact that TRPV2 is no longer considered a major transducer of noxious heat stimuli, our results suggest either that very few TRPV1 afferents are required to mediate heat sensitivity of the DP, or that as-yet-unidentified heat transducers or mechanisms are involved.
Although the level of TRPV1 in the pulp was low relative to periodontal tissues and trigeminal ganglia, there is no question that the TRPV1 innervation is functional. Thus, capsaicin, which specifically targets TRPV1, can stimulate CGRP release from isolated dental pulp (Gibbs and Hargreaves, 2008; Fehrenbacher et al., 2009), and trans-dentinal application of capsaicin to incisor dental pulp can evoke nocifensive behavior in rats (Chidiac et al., 2002). Finally, electrophysiological studies have identified capsaicin-sensitive fibers in dental pulp (Ikeda et al., 1997; Chaudhary et al., 2001). However, other studies provide support for the hypothesis that TRPV1 is not a major contributor to pulpal nociception. Thus, depletion of capsaicin-sensitive fibers by neonatal administration of capsaicin has little effect on noxious electrical and mechanical tooth pulp stimulation, indicating a relatively minor role for capsaicin-sensitive fibers (Tal, 1984; He et al., 2000), at least for these modalities of pulpal nociception. Conceivably, TRPV1-expressing pulpal neurons are not major contributors to nociceptive processing in non-injured dental pulp, but take on a more critical role after injury, namely, in the setting of pulpal inflammation and neural degeneration.
Given the high expression of TRPV2 in the dental pulp and the fact that this receptor is unlikely to contribute to heat detection, it is of great interest to determine its function in the DP. One intriguing possibility is that TRPV2 receptors are expressed on mechanosensitive nociceptors, as recently demonstrated in an electrophysiological study of somatic afferents (Lawson et al., 2008). Since the dental pulp is densely innervated with mechanosensitive nociceptive fibers, and TRPV2 is concentrated in myelinated afferents, it is conceivable that the two populations overlap, and that TRPV2 contributes to the mechanosensitivity of the pulpal afferent (Muraki et al., 2003). Although pharmacological antagonism of TRPV2 is not yet possible, TRPV2 knock-out mice are now available, so that functional studies in the pulp can be performed.
From this analysis, we conclude that afferents that innervate dental pulp and periodontal tissues have a neurochemical signature manifest in the differential expression of the heat-sensitive channel TRPV1 and the purported heat-/mechano-sensitive channel TRPV2. The expression pattern of these receptors in peripheral afferents of these tissues likely contributes to the high sensitivity of these tissues to detection of innocuous as well as noxious thermal and mechanical stimuli. Clearly, future studies should address the distribution of other TRP channels (e.g., the cold-sensitive TRPM8 channel) that likely defines a non-overlapping population of pulpal afferents.
Supplementary Material
Acknowledgments
This research was funded by a grant from the American Association of Endodontists Foundation as well as by NIH/NCRR/OD UCSF-CTSI Grant Number KL2RR024130 and NIH/NIDCR 1K23DE019461. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Albers KM, Woodbury CJ, Ritter AM, Davis BM, Koerber HR. (2006). Glial cell-line-derived neurotrophic factor expression in skin alters the mechanical sensitivity of cutaneous nociceptors. J Neurosci 26:2981-2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannström M. (1986). The hydrodynamic theory of dentinal pain: sensation in preparations, caries, and the dentinal crack syndrome. J Endod 12:453-457 [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. (1997). The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816-824 [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. (1999). A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 398:436-441 [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. (2000). Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288:306-313 [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, et al. (2009). Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci USA 106:9075-9080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecic PA, Hartwell GR, Bellizzi R. (1983). Cold as a diagnostic aid in cases of irreversible pulpitis. Report of two cases. Oral Surg Oral Med Oral Pathol 56:647-650 [DOI] [PubMed] [Google Scholar]
- Cesare P, Dekker LV, Sardini A, Parker PJ, McNaughton PA. (1999). Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron 23:617-624 [DOI] [PubMed] [Google Scholar]
- Chattipakorn SC, Light AR, Närhi M, Maixner W. (2001). The effects of noxious dental heating on the jaw-opening reflex and trigeminal Fos expression in the ferret. J Pain 2:345-353 [DOI] [PubMed] [Google Scholar]
- Chaudhary P, Martenson ME, Baumann TK. (2001). Vanilloid receptor expression and capsaicin excitation of rat dental primary afferent neurons. J Dent Res 80:1518-1523 [DOI] [PubMed] [Google Scholar]
- Chidiac JJ, Rifai K, Hawwa NN, Massaad CA, Jurjus AR, Jabbur SJ, et al. (2002). Nociceptive behaviour induced by dental application of irritants to rat incisors: a new model for tooth inflammatory pain. Eur J Pain 6:55-67 [DOI] [PubMed] [Google Scholar]
- Dachi SF. (1965). The relationship of pulpitis and hyperemia to thermal sensitivity. Oral Surg Oral Med Oral Pathol 19:776-785 [DOI] [PubMed] [Google Scholar]
- Fehrenbacher JC, Sun XX, Locke EE, Henry MA, Hargreaves KM. (2009). Capsaicin-evoked iCGRP release from human dental pulp: a model system for the study of peripheral neuropeptide secretion in normal healthy tissue. Pain 144:253-261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs JL, Hargreaves KM. (2008). Neuropeptide Y Y1 receptor effects on pulpal nociceptors. J Dent Res 87:948-952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He YF, Ichikawa H, Sugimoto T. (2000). The effect of neonatal capsaicin on the c-Fos-like immunoreactivity induced in subnucleus oralis neurons by noxious intraoral stimulation. Brain Res 860:203-207 [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Sugimoto T. (2001). VR1-immunoreactive primary sensory neurons in the rat trigeminal ganglion. Brain Res 890:184-188 [DOI] [PubMed] [Google Scholar]
- Ikeda H, Tokita Y, Suda H. (1997). Capsaicin-sensitive A delta fibers in cat tooth pulp. J Dent Res 76:1341-1349 [DOI] [PubMed] [Google Scholar]
- Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. (2002). p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 36:57-68 [DOI] [PubMed] [Google Scholar]
- Lawson JJ, McIlwrath SL, Woodbury CJ, Davis BM, Koerber HR. (2008). TRPV1 unlike TRPV2 is restricted to a subset of mechanically insensitive cutaneous nociceptors responding to heat. J Pain 9:298-308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinter RD, Skinner K, Julius D, Basbaum AI. (2004). Immunoreactive TRPV-2 (VRL-1), a capsaicin receptor homolog, in the spinal cord of the rat. J Comp Neurol 470:400-408 [DOI] [PubMed] [Google Scholar]
- Morgan CR, Rodd HD, Clayton N, Davis JB, Boissonade FM. (2005). Vanilloid receptor 1 expression in human tooth pulp in relation to caries and pain. J Orofac Pain 19:248-260 [PubMed] [Google Scholar]
- Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, et al. (2003). TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res 93:829-838 [DOI] [PubMed] [Google Scholar]
- Närhi MV, Hirvonen TJ, Hakumaki MO. (1982). Responses of intradental nerve fibres to stimulation of dentine and pulp. Acta Physiol Scand 115:173-178 [DOI] [PubMed] [Google Scholar]
- Owatz CB, Khan AA, Schindler WG, Schwartz SA, Keiser K, Hargreaves KM. (2007). The incidence of mechanical allodynia in patients with irreversible pulpitis. J Endod 33:552-556 [DOI] [PubMed] [Google Scholar]
- Pan Y, Wheeler EF, Bernanke JM, Yang H, Naftel JP. (2003). A model experimental system for monitoring changes in sensory neuron phenotype evoked by tooth injury. J Neurosci Methods 126:99-109 [DOI] [PubMed] [Google Scholar]
- Park UV, Guan Y, Raja SN, Koltzenburg M, Caterina MJ. (2008). Evaluation of TRPV2 function in mouse thermal nociception. 12th World Congress on Pain/Nociceptors - New Biology. Edinburgh, Scotland: International Association for the Study of Pain [Google Scholar]
- Patapoutian A, Tate S, Woolf CJ. (2009). Transient receptor potential channels: targeting pain at the source. Nat Rev Drug Discov 8:55-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pau A, Croucher R, Marcenes W, Leung T. (2005). Development and validation of a dental pain-screening questionnaire. Pain 119:75-81 [DOI] [PubMed] [Google Scholar]
- Petersson K, Söderström C, Kiani-Anaraki M, Levy G. (1999). Evaluation of the ability of thermal and electrical tests to register pulp vitality. Endod Dent Traumatol 15:127-131 [DOI] [PubMed] [Google Scholar]
- Shibasaki K, Murayama N, Ono K, Ishizaki Y, Tominaga M. (2010). TRPV2 enhances axon outgrowth through its activation by membrane stretch in developing sensory and motor neurons. J Neurosci 30:4601-4612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenholm E, Bongenhielm U, Ahlquist M, Fried K. (2002). VRl- and VRL-l-like immunoreactivity in normal and injured trigeminal dental primary sensory neurons of the rat. Acta Odontol Scand 60:72-79 [DOI] [PubMed] [Google Scholar]
- Stucky CL, Dubin AE, Jeske NA, Malin SA, McKemy DD, Story GM. (2009). Roles of transient receptor potential channels in pain. Brain Res Rev 60:2-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal M. (1984). The threshold for eliciting the jaw opening reflex in rats is not increased by neonatal capsaicin. Behav Brain Res 13:197-200 [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, et al. (1998). The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21:531-543 [DOI] [PubMed] [Google Scholar]
- Zele T, Sketelj J, Bajrovic FF. (2010). Efficacy of fluorescent tracers in retrograde labeling of cutaneous afferent neurons in the rat. J Neurosci Methods 191:208-214 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


