Abstract
The use of intra-oral soft-tissue-engineered devices has demonstrated potential for oral mucosa regeneration. The aim of this study was to investigate the temporal expression of angiogenic biomarkers during wound healing of soft tissue reconstructive procedures comparing living cellular constructs (LCC) with autogenous free gingival grafts. Forty-four human participants bilaterally lacking sufficient zones of attached keratinized gingiva were randomly assigned to soft tissue surgery plus either LCC or autograft. Wound fluid samples were collected at baseline and weeks 1, 2, 3, and 4 post-operatively and analyzed for a panel of angiogenic biomarkers: angiogenin (ANG), angiostatin (ANT), PDGF-BB, VEGF, FGF-2, IL-8, TIMP-1, TIMP-2, GM-CSF, and IP-10. Results demonstrated a significant increase in expression of ANT, PDGF-BB, VEGF, FGF-2, and IL-8 for the LCC group over the autograft group at the early stages of wound repair. Although angiogenic biomarkers were modestly elevated for the LCC group, no clinical correlation with wound healing was found. This human investigation demonstrates that, during early wound-healing events, expression of angiogenic-related biomarkers is up-regulated in sites treated with LCC compared with autogenous free gingival grafts, which may provide a safe and effective alternative for regenerating intra-oral soft tissues (ClinicalTrials.gov number, NCT01134081).
Keywords: tissue engineering, gingival recession, regenerative medicine, wound repair, regeneration, angiogenesis, clinical trial
Introduction
Gingival augmentation procedures, such as free gingival grafts, are performed routinely in an attempt to maintain an adequate zone of keratinized gingiva and improve gingival health in patients. However, there is morbidity associated with the harvesting of autogenous gingival grafts. Furthermore, the palate possesses limited tissue, providing a limited treatment area in some individuals. Tissue-engineered solutions such as a living cellular construct (LCC; CelTx™, Organogenesis Inc., Canton, MA, USA) provide an unlimited source and can be a successful alternative to increasing the width of keratinized tissue without using autogenous palatal donor tissue. LCC is a living cellular and collagen construct, designed to regenerate intra-oral soft tissue comprised of type I bovine collagen and viable allogeneic human fibroblasts and keratinocytes isolated from human neonatal foreskin.
The living cellular construct evaluated in this study has been investigated for several different clinical indications, such as diabetic foot ulcers, venous leg ulcers, excision wounds, epidermolysis bullosa, and pressure ulcers (Falanga et al., 1998; Falabella et al., 1999; Veves et al., 2001; Fivenson et al., 2003). Recently, LCC was demonstrated to be safe and capable of generating keratinized tissue without the morbidity and potential clinical difficulties associated with donor site surgery (McGuire et al., 2008).
The mechanism of action of LCC is still unknown. However, it is speculated to modulate healing by secondary intention of the surrounding soft tissues. Living cellular constructs act as a temporary wound covering that is eventually replaced by host cells. DNA of allogeneic fibroblasts and keratinocytes placed over wounds was no longer present after 6 wks of healing (Griffiths et al., 2004). It is postulated that the population of live fibroblasts and keratinocytes improves the wound environment through growth factor interactions, matrix deposition and degradation, wound coverage, and a provision of responsive cells, leading to a clinically beneficial outcome (Sabolinski et al., 1996). The purpose of this study was to investigate the expression of angiogenic biomarkers expressed during oral wound repair using living cellular constructs to regenerate soft tissue.
Materials & Methods
This investigation enrolled 44 participants from 3 clinical centers (Fig. 1A). This sample size was selected for feasibility rather than to power specific hypotheses, given that this is a first-time-in-humans investigation. Patients involved in the study possessed bilateral mucogingival defects with ≤ 1 mm of attached gingiva located in contralateral quadrants requiring mucogingival surgery to augment the zone of keratinized and attached gingiva. The patients in this study were a cohort of 96 research subjects participating in a pivotal clinical trial designed to evaluate living cell constructs as an alternative to tissue from the palate to enhance oral soft-tissue regeneration and wound healing who agreed and consented to participate in this investigation (Clinical Trial Registration No. NCT00587834) (McGuire et al., 2010). The participants provided periodontal wound fluid (WF) after mucogingival surgeries with either LCC (Fig. 1C) or autogenous free gingival grafts (autograft). The 2 surgical sites of each patient were randomly selected to receive LCC as a donor material in one site (Fig. 1D) or a conventional autograft using keratinized tissue from the palate as the donor material at the contralateral site. A computer-generated randomization scheme provided allocations for both treatment assignments, and order of administration was contained in sealed envelopes. Informed consent was obtained at the initial visit prior to any research-related treatment or procedure. The study protocol conformed to the ethical guidelines as reflected in obtainment of approval by the University of Michigan’s Human Subjects Research Review Committee and the Western Institutional Review Board (WIRB). This human trial investigation was registered at the NIH Clinical Trials Registry (Clinical Trial Registration No. NCT01134081).
Figure 1.
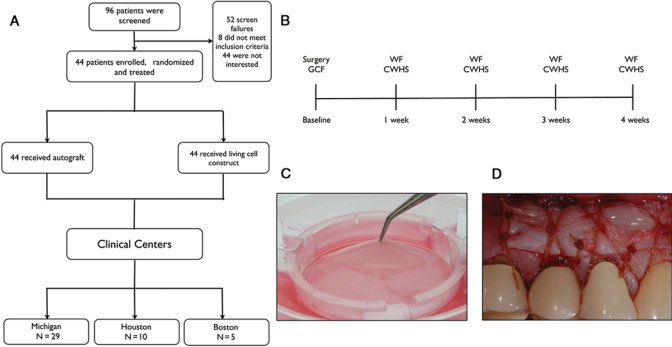
Patient distribution, time line, and living cellular construct transplantation. (A) Stratification of the study population was divided into 2 treatment groups at 3 clinical centers. (B) Study timeline. GCF, gingival crevicular fluid; WF, wound fluid; CWHS, clinical wound-healing score. (C) Sealed bioreactor with living cellular construct; (D) living cellular construct placed at the surgical site, 254 x 169 mm (300 x 300 DPI).
Inclusion/Exclusion Criteria
Individuals were enrolled in the study if they were between 18 and 70 yrs of age and possessed bilateral recession-type defects manifested by an insufficient zone of attached gingiva (≤ 1 mm), not requiring root tooth coverage. Patients were excluded: if they displayed a shallow vestibule, severe gingival recession, and tooth mobility (Grade ≥ 2); if they had any systemic conditions that could compromise wound healing and preclude periodontal surgery (i.e., diabetes mellitus, cancer, HIV, bone metabolic diseases); if they had received, within 2 mos prior to study entry, systemic corticosteroids, immunosuppressive agents, intravenous bisphosphonates, radiation therapy, and/or chemotherapy that could compromise wound healing and preclude periodontal surgery; if they had used any tobacco product within 3 mos; if they had only molar teeth suitable for soft-tissue grafting; if they had known hypersensitivity to bovine collagen; and if they had received an investigational drug or biological/bioactive treatment within 30 days prior to study enrollment.
Wound-healing Score
During the first four-week follow-up visits, the healing condition of the soft tissues at the surgical site was examined by visual inspection. A secondary endpoint, clinical wound-healing score (CWHS), was recorded to reflect the extent of healing at the surgical site and to further evaluate the significance of angiogenic biomarkers on clinical wound healing. A wound-healing scale was created uniquely to classify the possible post-surgical healing characteristics: 0 = Mature wound healing; 1 = Erythema; 2 = Bleeding; 3 = Graft mobility; 4 = Suppuration; and 5 = Necrosis.
Periodontal Wound Fluid Collection
Prior to the surgical procedure, gingival crevicular fluid (GCF) was collected from the 3 study teeth in each participant. One tooth received the autograft, and the other received the LCC. The GCF samples were taken from the mesio-buccal site of each tooth. The area around each sample site was air-dried, and the supra-gingival plaque biofilm was removed. A methylcellulose paper strip (Periopaper®, ProFlow Inc., Amityville, NY, USA) was gently inserted into the gingival sulcus until slight resistance was felt. The fluid sample was then collected for 30 sec, and the strips were placed in Eppendorf tubes and kept on ice.
Periodontal wound fluid was collected from the test and control graft sites at the 4 corners of the surgical site (Fig. 2). The surgical site was gently dried with gauze to remove excess saliva. Methylcellulose strips were gently inserted at the corners of the grafts. WF was collected as with GCF. Both GCF and WF samples were subsequently kept on dry ice and stored at -80°C until needed for analysis. The collection of WF occurred at wks 1, 2, 3, and 4 after surgery (Fig. 1B).
Figure 2.
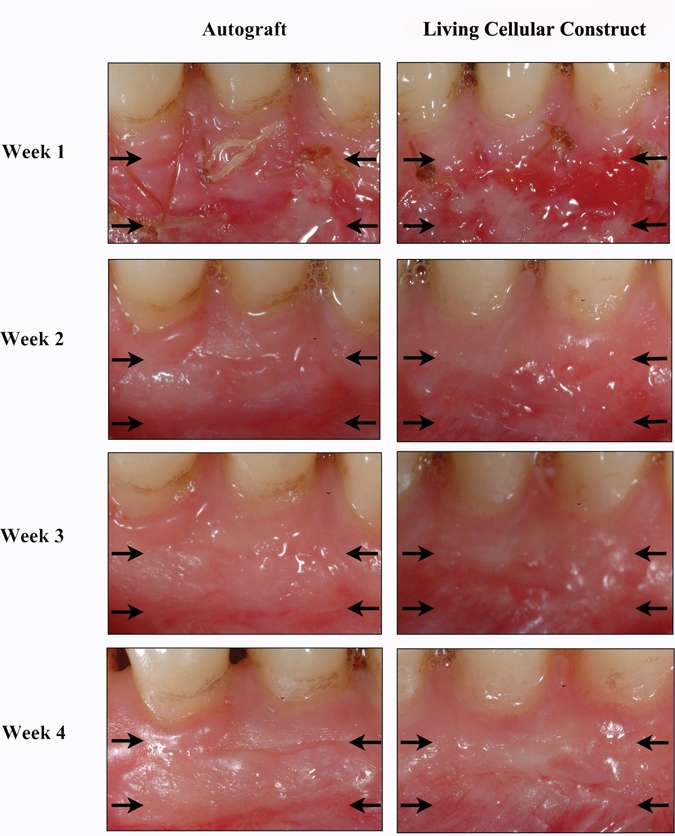
Wound fluid collection at the borders of the wound during the 4 wks post-operatively for both autograft and living cellular construct. 156 x 179 mm (300 x 300 DPI).
Angiogenic Biomarker Analysis
Prior to biomarker analysis, GCF and WF were thawed at RT. In addition, the 4 WF samples from each site were pooled, and proteins were eluted as previously described (Giannobile et al., 1995). Angiogenic biomarker expression was quantified with the use of a human angiogenesis custom array kit for the presence of 10 different biomarkers simultaneously according to the manufacturer’s protocol (Quantibody®, Ray-Biotech, Inc., Norcross, GA, USA), which included recombinant protein standards for standard curve generation. The biomarkers selected for the array were: TIMP-1, TIMP-2, VEGF, IP-10, PDGF-BB, GM-CSF, IL-8, FGF-2, angiogenin (ANG), and angiostatin (ANT). Slides were scanned and measured for fluorescent signal intensity, and data were imported to RayBio® Antibody Array Analysis software for assessment (including data sorting, averaging signal intensities, background subtraction, normalization, and obtaining protein levels that were then exported for statistical analysis; see below, “Statistical Analysis”).
Statistical Analysis
For each angiogenic biomarker, the primary outcome was the within-subject difference between the concentrations at the autograft and LCC surgical areas. These differences were measured in each participant prior to surgery, as well as 1, 2, 3, and 4 wks after surgery, leading to a series of 5 longitudinal, within-mouth differences for each individual. For each marker, the differences at all 5 time-points were analyzed simultaneously by a linear mixed model (LMM), in which the actual marker levels were modeled as a function of time (categorical), treatment group, and the interaction of time and treatment, while accounting for the repeated measures on each individual with a random subject effect. Given that both time and treatment are categorical, our approach was a generalized version of repeated-measures ANOVA, since our LMM allowed for differing variability in marker levels at each time-point. Within each LMM, we also tested for a difference between the 2 treatment groups with respect to the overall time pattern in marker levels. A Bonferroni adjustment for multiple comparisons was applied to the p-values presented in Fig. 3. Statistical significance was defined as a p-value < 0.05.
Figure 3.
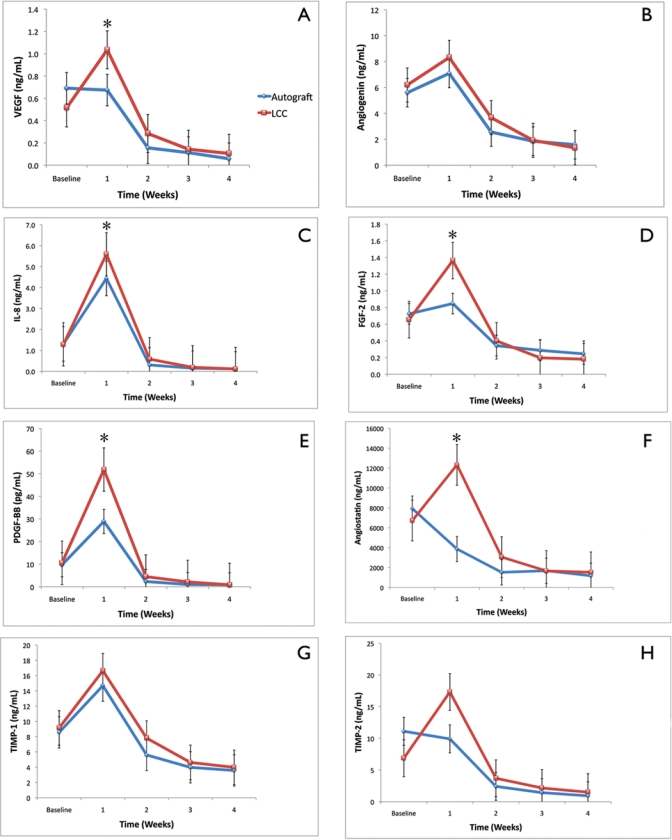
Expression of angiogenic biomarkers of living cellular construct (LCC) and autograft from baseline to week 4. (A) Mean expression of VEGF. Inter-group analysis (*) demonstrated a statistically significant expression of VEGF for living cellular constructs at week 1 (p < 0.05). (B) Mean expression of ANG. (C) Mean expression of IL-8. Inter-group analysis (*) demonstrated a statistically significant expression for living cellular constructs at week 1 (p < 0.05). (D) Mean expression of FGF-2. Inter-group analysis (*) demonstrated a statistically significant expression for living cellular constructs at week 1 (p < 0.05). (E) Mean expression of PDGF-BB. Inter-group analysis (*) demonstrated a statistically significant expression for living cellular constructs at week 1 (p < 0.05). (F) Mean expression of ANT. Inter-group analysis (*) demonstrated a statistically significant difference in the expression for living cellular constructs at week 1 (p < 0.05). (G) Mean expression of TIMP-1. (H) Mean expression of TIMP-2. Bars indicate standard error measurements (SEM). 319 x 374 mm (300 x 300 DPI).
Results
In total, 44 individuals were evaluated in the investigation (Table). Wound-healing scores measured after surgical procedure decreased during the 4 time-points (Appendix Table). No differences were found between groups regarding wound-healing scores (Appendix Fig. 1). Hence, the expression of the angiogenic biomarkers tested did not significantly correlate with the wound-healing score (Appendix Fig. 2).
Table.
Patient Baseline Demographics
| Characteristics | Autograft, N = 44 | Living Cellular Construct, N = 44 | P-value |
|---|---|---|---|
| Age (yrs) | |||
| Mean (SD) | 47.1 (13.1) | ND | |
| Gender | |||
| Male | 25 (57%) | ND | |
| Female | 19 (43%) | ND | |
| Race | |||
| Caucasian | 40 | ND | |
| African-American | 1 | ND | |
| Hispanic | 2 | ND | |
| Smoking status | |||
| Non-smokers | 27 (60%) | ND | |
| Former smokers | 17 (40% | ND | |
| Mean clinical measurements (mm) | |||
| Keratinized tissue width | 1.43 ± 0.69 | 1.41 ± 0.72 | NS |
| Attached gingiva width | 0.08 ± 0.79 | 0.02 ± 0.76 | NS |
ND, not determined; NS, not significant.
VEGF concentrations over time for the LCC and autograft groups are shown in Fig. 3A. Both groups demonstrated an increased expression of VEGF at week 1 compared with baseline, with a progressive reduction from weeks 2-4. By week 1, LCC showed a significant increase in VEGF levels compared with autograft (p < 0.05). In addition, over time, LCC demonstrated significantly higher VEGF levels compared with autograft (p < 0.05). Endogenous ANG released over time for both LCC and autograft groups is shown in Fig. 3B. ANG expression levels reached their peak expression at week 1 and progressively decreased for both the LCC and autograft groups. No statistically significant difference was noted between groups.
IL-8 expression over time for all groups is demonstrated in Fig. 3C. Both groups showed an increase in IL-8 expression by 1 wk. Further, LCC demonstrated significantly higher IL-8 expression levels compared with autograft (p < 0.05), which was also confirmed in the overall time analysis. FGF-2 WF levels are depicted in Fig. 3D. LCC demonstrated a higher expression of FGF-2 compared with autograft only at week 1 (p < 0.05). PDGF-BB released over time for all groups is demonstrated in Fig. 3E. At week 1, LCC demonstrated a significantly higher expression compared with autograft (p < 0.05), which was also noted at the overall time analysis (p < 0.05).
ANT levels over time for both groups are shown in Fig. 3F. At week 1, LCC showed an increased expression in ANT expression compared with autograft (p < 0.05). The overall time analysis also demonstrated a significantly higher expression in favor of the LCC group (p < 0.05). TIMP-1 expression over time for all groups is shown in Fig. 3G. LCC demonstrated a modestly increased concentration of TIMP-1 at weeks 1 and 2 compared with autograft, but did not show significant difference between groups. The expression of TIMP-2 over time for both groups is illustrated in Fig. 3H. At week 1, the LCC group showed an increase in TIMP-2 levels compared with autograft, but, again, did not show significant difference between groups.
Due to minimal-no expression of GM-CSF and IP-10, no statistical analysis was performed involving these proteins (data not shown).
Discussion
The oral wound-healing field has reported few studies investigating soft-tissue-engineered biomaterials for regeneration of mucosal tissue (Izumi et al., 2003; McGuire and Nunn, 2005; McGuire et al., 2008). However, to our knowledge, this study was the first investigation to evaluate the angiogenic biomarker profiles of autologous gingival grafts and soft-tissue-engineered constructs. The rationale for analyzing angiogenic biomarkers is that angiogenesis is an essential feature of normal wound repair, which is partly responsible for the formation of fibrovascular tissue containing fibroblasts, collagen, and blood vessels that are the hallmark of an established healing response.
During the study, VEGF was highly expressed during the first week after the surgery, and its levels progressively decreased to the fourth week of healing. Many studies have described the presence of VEGF collected directly from the surgical wound fluid.
The presence of VEGF was analyzed in surgical WF, and it was demonstrated that VEGF production and VEGF-mediated angiogenic activity would rise in the early hypoxic wound and then fall when neovascularization was complete and wound perfusion was restored (Nissen et al., 1998). Additionally, VEGF together with other angiogenic factors have also been found in the WF of oral wounds (Cooke et al., 2006; Sakai et al., 2006).
IL-8 is a chemokine produced by macrophages, epithelial cells, and endothelial cells. Koch and co-workers have demonstrated that IL-8 is involved in angiogenesis-dependent disorders such as rheumatoid arthritis, tumor growth, and wound repair (Koch et al., 1992). IL-8 has also been reported to enhance endothelial cell proliferation, survival, and regulation of angiogenesis (Li et al., 2003). In the current study, both living cellular constructs and autografts displayed higher expression of IL-8 during week 1, with higher expression for living cellular constructs over time.
Tissue inhibitors of metalloproteinases (TIMPs) have been found to have potential roles as growth factors, survival factors, growth inhibitors, and inhibitors of angiogenesis (Stetler-Stevenson and Seo, 2005; Chirco et al., 2006; Stetler-Stevenson, 2008). The current investigation was able to detect the expression of both TIMP-1 and TIMP-2 during all evaluation periods, which peaked at week 1. Living cellular constructs showed an overall higher expression of both TIMP-1 and TIMP-2 compared with autograft. Osborne and Schmid evaluated the production of TIMPs by living cellular constructs and demonstrated that the induced expression of TIMP-1 and TIMP-2 may have the potential to counteract the imbalance between matrix production and degradation and thus may support wound re-epithelialization (Osborne and Schmid, 2002).
It is important to note that while several angiogenic biomarkers appeared to be elevated at early time-points, this study was not able to translate this directly to angiogenesis actually occurring in vivo. A proxy measure of this was the erythema component of the CWHS, which was notably present during weeks 1 and 2. However, studies evaluating the early wound healing of mucosal and skin wounds demonstrated that the presence of angiogenic biomarkers was positively related to increased blood vessel formation (Szpaderska et al., 2005; Kumar et al., 2009).
Although the present study compared the differences in angiogenic biomarker expression between living cellular constructs and autografts, it is important to understand that both living cellular constructs and autografts heal by two different healing processes. Autografts heal by primary intention repair. Conversely, a living cellular construct is not integrated into the surrounding tissues, but rather, it modulates the healing activity of the underlying and adjacent tissue. It is postulated that the supply of live fibroblasts and keratinocytes improves the wound environment through growth factor interactions, matrix deposition and degradation, wound coverage, and a provision of responsive cells, leading to therapeutic functions (Sabolinski et al., 1996).
In conclusion, the results of this study demonstrated that, during early wound-healing events, expression of angiogenic-related biomarkers is up-regulated in sites treated with LCC compared with autogenous free gingival grafts. The use of LCC may affect the regeneration of intra-oral soft tissues or the treatment of chronic soft-tissue lesions.
Supplementary Material
Acknowledgments
The authors thank James Sugai for technical support. This investigation was funded by Organogenesis, Inc. and NIH/NCRR UL 1RR024986. Dr. Bates is an employee of Organogenesis, Inc. Dr. McGuire has received honoraria and is a consultant to Organogenesis, Inc. Dr. Giannobile previously served on the Scientific Advisory Board for Organogenesis, Inc.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Chirco R, Liu XW, Jung KK, Kim HR. (2006). Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev 25:99-113 [DOI] [PubMed] [Google Scholar]
- Cooke JW, Sarment DP, Whitesman LA, Miller SE, Jin Q, Lynch SE, et al. (2006). Effect of rhPDGF-BB delivery on mediators of periodontal wound repair. Tissue Eng 12:1441-1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falabella AF, Schachner LA, Valencia IC, Eaglstein WH. (1999). The use of tissue-engineered skin (Apligraf) to treat a newborn with epidermolysis bullosa. Arch Dermatol 135:1219-1222 [DOI] [PubMed] [Google Scholar]
- Falanga V, Margolis D, Alvarez O, Auletta M, Maggiacomo F, Altman M, et al. (1998). Rapid healing of venous ulcers and lack of clinical rejection with an allogeneic cultured human skin equivalent. Human Skin Equivalent Investigators Group Arch Dermatol 134:293-300 [DOI] [PubMed] [Google Scholar]
- Fivenson DP, Scherschun L, Choucair M, Kukuruga D, Young J, Shwayder T. (2003). Graftskin therapy in epidermolysis bullosa. J Am Acad Dermatol 48:886-892 [DOI] [PubMed] [Google Scholar]
- Giannobile WV, Lynch SE, Denmark RG, Paquette DW, Fiorellini JP, Williams RC. (1995). Crevicular fluid osteocalcin and pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP) as markers of rapid bone turnover in periodontitis. A pilot study in beagle dogs. J Clin Periodontol 22:903-910 [DOI] [PubMed] [Google Scholar]
- Griffiths M, Ojeh N, Livingstone R, Price R, Navsaria H. (2004). Survival of Apligraf in acute human wounds. Tissue Eng 10:1180-1195 [DOI] [PubMed] [Google Scholar]
- Izumi K, Feinberg SE, Iida A, Yoshizawa M. (2003). Intraoral grafting of an ex vivo produced oral mucosa equivalent: a preliminary report. Int J Oral Maxillofac Surg 32:188-197 [DOI] [PubMed] [Google Scholar]
- Koch AE, Polverini PJ, Kunkel SL, Harlow LA, et al. (1992). Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 258(5089):1798-1801 [DOI] [PubMed] [Google Scholar]
- Kumar I, Staton CA, Cross SS, Reed MW, Brown NJ. (2009). Angiogenesis, vascular endothelial growth factor and its receptors in human surgical wounds. Br J Surg 96:1484-1491 [DOI] [PubMed] [Google Scholar]
- Li A, Dubey S, Varney ML, Dave BJ, Singh RK. (2003). IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol 170:3369-3376 [DOI] [PubMed] [Google Scholar]
- McGuire MK, Nunn ME. (2005). Evaluation of the safety and efficacy of periodontal applications of a living tissue-engineered human fibroblast-derived dermal substitute. I. Comparison to the gingival autograft: a randomized controlled pilot study. J Periodontol 76:867-880 [DOI] [PubMed] [Google Scholar]
- McGuire MK, Scheyer ET, Nunn ME, Lavin PT. (2008). A pilot study to evaluate a tissue-engineered bilayered cell therapy as an alternative to tissue from the palate. J Periodontol 79:1847-1856 [DOI] [PubMed] [Google Scholar]
- McGuire MK, Scheyer ET, Nevins M, Neiva R, Cochran DL, Mellonig JT, et al. (2011). Living cellular construct for the treatment of mucogingival defects: results from a randomized, within-patient, controlled trial. J Periodontol (manuscript in review). [DOI] [PubMed] [Google Scholar]
- Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA. (1998). Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol 152: 1445-1452 [PMC free article] [PubMed] [Google Scholar]
- Osborne CS, Schmid P. (2002). Epidermal-dermal interactions regulate gelatinase activity in Apligraf, a tissue-engineered human skin equivalent. Br J Dermatol 146:26-31 [DOI] [PubMed] [Google Scholar]
- Sabolinski ML, Alvarez O, Auletta M, Mulder G, Parenteau NL. (1996). Cultured skin as a ‘smart material’ for healing wounds: experience in venous ulcers. Biomaterials 17:311-320 [DOI] [PubMed] [Google Scholar]
- Sakai A, Ohshima M, Sugano N, Otsuka K, Ito K. (2006). Profiling the cytokines in gingival crevicular fluid using a cytokine antibody array. J Periodontol 77:856-864 [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson WG. (2008). Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal 1(27):re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler-Stevenson WG, Seo DW. (2005). TIMP-2: an endogenous inhibitor of angiogenesis. Trends Mol Med 11:97-103 [DOI] [PubMed] [Google Scholar]
- Szpaderska AM, Walsh CG, Steinberg MJ, DiPietro LA. (2005). Distinct patterns of angiogenesis in oral and skin wounds. J Dent Res 84: 309-314 [DOI] [PubMed] [Google Scholar]
- Veves A, Falanga V, Armstrong DG, Sabolinski ML. (2001). Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care 24:290-295 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


