Abstract
Individuals with periodontal disease have increased risk of tooth loss, particularly in cases with associated loss of alveolar bone and periodontal ligament (PDL). Current treatments do not predictably regenerate damaged PDL. Collagen I is the primary component of bone and PDL extracellular matrix. SPARC/Osteonectin (SP/ON) is implicated in the regulation of collagen content in healthy PDL. In this study, periodontal disease was induced by injections of lipopolysaccharide (LPS) from Aggregatibacter actinomycetemcomitans in wild-type (WT) and SP/ON-null C57/Bl6 mice. A 20-µg quantity of LPS was injected between the first and second molars 3 times a week for 4 weeks, whereas PBS control was injected into the contralateral maxilla. LPS injection resulted in a significant decrease in bone volume fraction in both genotypes; however, significantly greater bone loss was detected in SP/ON-null maxilla. SP/ON-null PDL exhibited more extensive degradation of connective tissue in the gingival tissues. Although total cell numbers in the PDL of SP/ON-null were not different from those in WT, the inflammatory infiltrate was reduced in SP/ON-null PDL. Histology of collagen fibers revealed marked reductions in collagen volume fraction and in thick collagen volume fraction in the PDL of SP/ON-null mice. SP/ON protects collagen content in PDL and in alveolar bone in experimental periodontal disease.
Keywords: SPARC, periodontal ligament, collagen, BM-40, osteonectin, extracellular matrix, matricellular, periodontal diseases, alveolar bone resorption
Introduction
Periodontal diseases are characterized by a bacterial biofilm-initiated inflammatory reaction which gives rise to gingival inflammation, bleeding, extracellular matrix (ECM) degradation, bone resorption, and tooth loss (Savage et al., 2009).
Collagen fibers are the structural elements of periodontal ligament (PDL) and span the width of the periodontal space, from alveolar bone to cementum. The collagen fibers that originate in PDL that weave into the bone and cementum are referred to as Sharpey’s fibers (SF). SF participate in the transmission of forces from tooth to bone. A reduction in SF is associated with increased bone resorption (Johnson, 2005). Therefore, SF are considered essential for the establishment of a functional PDL.
The collagen within PDL is primarily collagen type I. Collagen type I is produced by resident fibroblasts, and is secreted as a procollagen molecule with propeptides at the N and C termini that are cleaved prior to incorporation into the ECM. PDL has a high turnover of the collagenous ECM as compared with other collagen-rich matrices, such as skin (Sodek, 1977). Thus, proteins that participate in collagen extracellular processing, deposition, and assembly are also highly represented in the PDL. Specifically, expression of SP/ON, a matricellular collagen-binding protein, is found in PDL, cementum, and bone (Zeichner-David, 2006; Delany and Hankenson, 2009; Trombetta and Bradshaw, 2010). In addition, in patients with periodontal disease, an increase in saliva levels of SP/ON is associated with decreased alveolar bone loss (Ng et al., 2007; Scannapieco et al., 2007). SP/ON restricts proliferation and adhesion, promotes cell-cycle arrest, promotes angiogenesis, and alters collagen deposition within the ECM (Bradshaw and Sage, 2001). Based on previous results, a role of SP/ON in facilitating maturation of procollagen type I and thus promoting collagen deposition and fiber formation has been proposed (for review, see Bradshaw, 2009; McCurdy et al., 2010). We recently reported that SP/ON-null mice had collagen deficiencies in the PDL, most pronounced in young and aged mice. Because of the reduced collagen content within the PDL in SP/ON-null mice, we predicted that these mice would exhibit greater PDL loss with chronic exposure to LPS.
Materials & Methods
Animals
C57Bl6 SP/ON-null mice with global abrogation of SP/ON expression have been described previously (Norose et al., 1998). Mice were housed in the Medical University of South Carolina (MUSC) animal facility and fed a standard diet. The MUSC Institutional Animal Care and Use Committee approved all procedures in this study.
Murine Model of LPS-induced Periodontitis
LPS-induced periodontitis was generated as previously described (Sartori et al., 2009). Briefly, 9 SP/ON-Null and 8 WT 4-month-old male mice were individually anesthetized with 1000 cc/min of O2 and 5% isofluorane. LPS was isolated from Aggregatibacter actinomycetemcomitans (Aa), a micro-organism associated with aggressive and chronic periodontal disease in humans (Kolenbrander and London, 1993). To induce periodontal inflammation and bone loss, we micro-injected 2 µL of LPS (10 µg/µL) into the palatal gingiva between the right maxillary first and second molars of each mouse. A 2-µL quantity of endotoxin-free PBS vehicle (Invitrogen, Carlsbad, CA, USA) was injected into the palatal gingiva between the left maxillary first and second molars as control (Rogers et al., 2007). Mice received injections thrice weekly over a 4-week time period.
Micro-computed Tomography and Bone Volume Fraction (BVF) Analysis
Upon completion of treatment, maxillae were removed, tumbled in 10% formalin at 4°C overnight, transferred to a 70% ethanol solution, and stored at 4°C. Specimens were scanned by micro-computed tomography (µCT), and results were analyzed with GE microview software for quantification of bone volume fraction (BVF). In each scan, a standardized tridimensional region of interest (ROI) was defined by the following landmarks: the apex of the palatal root of the 1st molar (inferior limit), mesial aspect of the palatal root of the 1st molar (posterior limit), distal aspect of the 2nd molar distal root (anterior limit), the most cervical portion of the furcation of the 1st molar (upper limit), palatal aspect of the 1st molar palatal root (medial limit), and buccal aspect of the 1st molar distal root (lateral limit). The analysis of BVF was calculated with a threshold of 1621 within the ROI.
Histology and Cell Counts
Following µCT analysis, maxillae were decalcified in 0.5M EDTA at 4°C for 2 wks. Samples were hemisected and embedded in paraffin, and 7-µm sections were generated. Sections were stained with hematoxylin and eosin (H&E), and total cell numbers were determined as described (Trombetta and Bradshaw, 2010). To determine the percentage of inflammatory cells, we established cells morphologically identified as inflammatory cells within PDL, characterized by a larger and granulated nucleus, per total nuclei in each section.
Analysis of Collagen Morphology and Collagen Volume Fraction
Sections were prepared and analyzed as described (Trombetta and Bradshaw, 2010). Values presented for “No injection” were taken from previously published results for 4-month WT and SP/ON-null PDL (Trombetta and Bradshaw, 2010).
Statistical Analysis
For comparisons within genotypes, we used a paired Student’s t test to calculate p values. Comparisons between genotypes with one condition were analyzed with a Student’s t test. For comparisons between genotypes with more than one condition, we used ANOVA followed by the Tukey test to calculate p values. For all analyses, p < 0.05 was considered statistically significant.
Results
Sequential microinjections of Aa LPS induced alveolar bone loss typical of periodontitis (Fig. 1). µCT scans of WT maxillae revealed significant bone loss in mice injected with LPS in comparison with PBS, as indicated by an increase in root structure visible outside of the bone socket (Fig. 1A, circles). Maxillae from SP/ON-null mice also demonstrated significant bone loss in LPS-injected sites vs. PBS control. Furthermore, the increase in LPS-mediated bone loss in SP/ON-null was more pronounced than that in WT mice (Fig. 1A, arrows).
Figure 1.
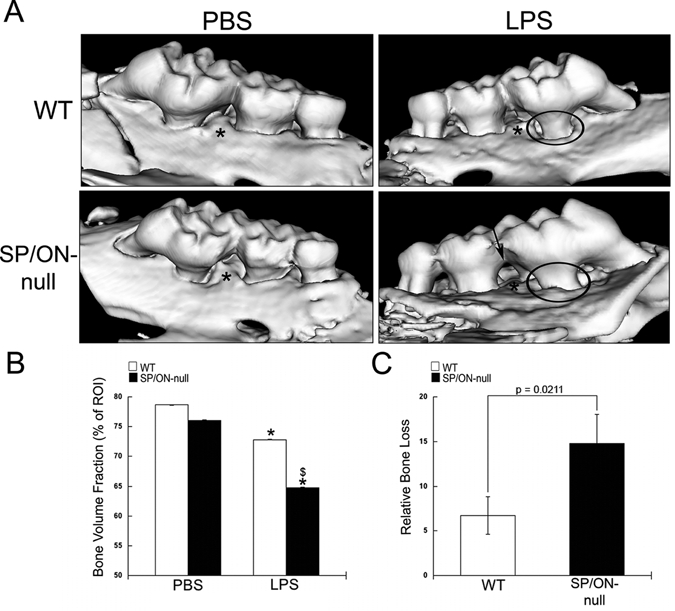
SP/ON-null maxillae injected with LPS demonstrated significantly decreased alveolar bone volume fraction (BVF) in comparison with WT maxillae. (A) Representative µCT reconstructions for WT and SP/ON-null maxillae injected with PBS and LPS. LPS injections caused significant bone resorption in both WT and SP/ON-null mice. In (A), * indicates the injection sites, circles indicate areas of bone loss with increased root structure visible above the alveolar bone crest, and arrow indicates extensive bone loss in the interproximal aspects of SP/ON-null LPS-injected mice. (B) Quantification of BVF reveals statistically significant decreases in bone volume associated with LPS injections in both WT (white bars) and SP/ON-null (black bars) mice in comparison with their respective PBS controls. * indicates p < 0.05 from PBS control. $ indicates p < 0.05 statistical significance of WT LPS vs. SP/ON-null LPS-injected mice. Error bars = standard deviation. (C) Comparison of relative bone loss indicates that bone loss caused by LPS injections in SP/ON-null mice was significantly greater than that in WT mice. P-value determined by Student’s t test. Error bars = SEM.
Quantification of images generated by µCT demonstrated similar levels of bone in PBS-injected sites of WT and SP/ON-null (Fig. 1B). However, significantly less BVF was detected in SP/ON-null LPS in comparison with WT (p = 0.015, Fig. 1B). To accommodate natural variation of BVF between animals, we calculated the relative bone loss. LPS-induced bone loss was significantly more severe in SP/ON-null than in WT (Fig. 1C).
Morphological differences in PDL between WT and SP/ON-null injected maxillae were assessed in H&E-stained tissue sections (Fig. 2). WT PBS PDL was grossly intact, with the majority of fibroblasts tightly surrounded by collagen fibers (Fig. 2, arrows). SP/ON-null PBS PDL was less organized than WT, with a greater number of fibroblasts inadequately surrounded by dense collagen fibers (Fig. 2A, arrows). Similar differences in PDL structure were observed previously in homeostatic PDL of SP/ON-null and WT mice at 4 mos of age (Trombetta and Bradshaw, 2010). In response to LPS, the PDL of SP/ON-null mice showed greater disorganization of collagen fibrils in comparison with the PDL of WT mice (Fig. 2A).
Figure 2.
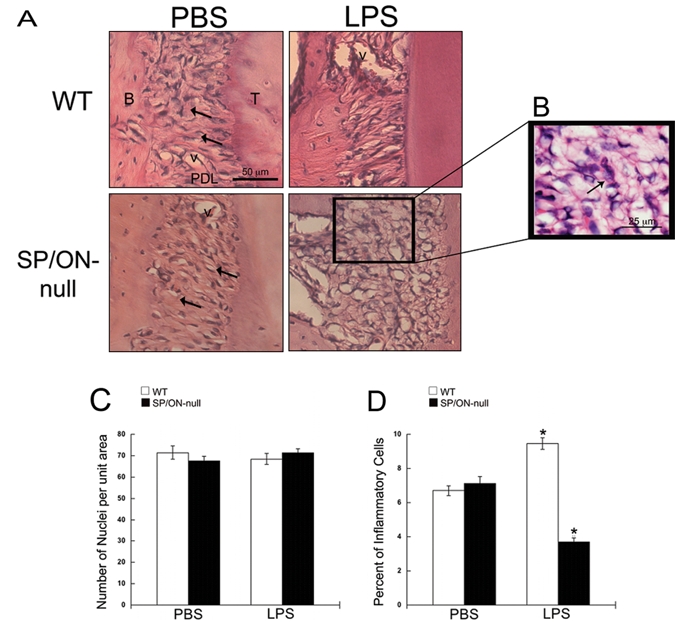
SP/ON-null exhibit disorganized collagen in the PDL and decreased inflammation. (A) Representative images of H&E-stained sections. The PDL in WT mice was highly organized, with fibroblasts tightly surrounded by interstitium (arrows), while the PDL in SP/ON-null mice exhibited less organized collagen ECM, with gaps (arrows). In LPS-injected sites, the PDL of SP/ON-null mice showed substantial disorganization compared with the PDL of WT mice. (B) Arrows indicate examples of cells that were morphologically identified as inflammatory cells in (D). (C) Quantification of nuclei revealed no statistically significant differences in the total number of cells in the PDL of WT and SP/ON-null mice, regardless of injection of PBS or LPS. Error bars = SEM. (D) Quantification of inflammatory cells revealed a significant decrease in inflammatory infiltrate in the LPS-injected PDL of SP/ON-null mice. * indicates p < 0.001 statistical significance of WT LPS vs. SP/ON-null LPS and PBS controls, and SP/ON-null LPS vs. WT LPS and PBS controls, as determined by one-way ANOVA analysis followed by the Tukey test. Error bars = SEM.
To determine whether increases in bone loss might be attributed to modulation of the inflammatory response, we quantified the total cell number and number of inflammatory cells within the WT and SP/ON-null PDL. No statistically significant differences in total nuclei between LPS and PBS or genotypes were detected (Fig. 2B). However, WT LPS PDL had significantly more inflammatory cells than WT PBS, as would be expected in this model. SP/ON-null LPS PDL had significantly fewer inflammatory cells than PBS-injected sites (Fig. 2B). Because there were no differences in the percentage of inflammatory cells in WT PBS PDL vs. SP/ON-null PBS mice at the end-point (Fig. 2B), these results suggest that the severity of LPS-induced inflammation was attenuated in SP/ON-null mice.
Effects of LPS injections on the organization of collagen fibers in PDL were further assessed in WT and SP/ON-null sections stained with PicroSirius Red (PSR) (Fig. 3). As predicted from initial characterizations of SP/ON-null PDL (Trombetta and Bradshaw, 2010), control sites injected with PBS contained fewer collagen fibers in SP/ON-null vs. WT PDL. In LPS-injected sites, there was a reduction in PDL collagen content. In WT LPS, fibers continued to span the width of PDL, with SF insertions into bone and cementum. In contrast, SP/ON-null LPS exhibited substantially fewer collagen fibers that spanned the width of the PDL, with some areas lacking portions of bone and containing no detectable collagen (Fig. 3A, box).
Figure 3.
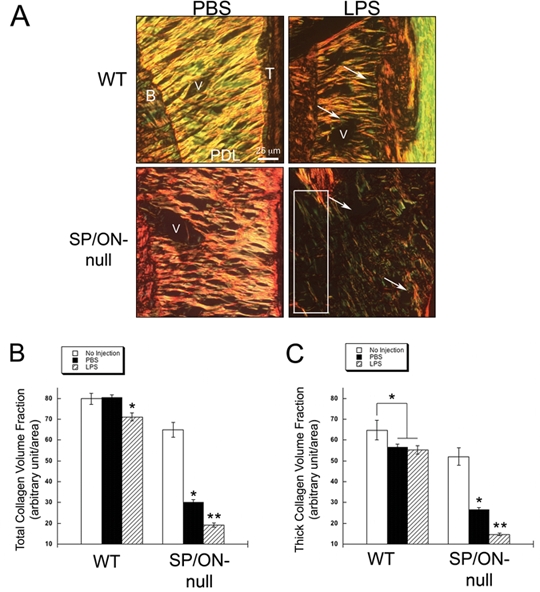
Collagen loss induced by LPS was more extensive in SP/ON-null mice. (A) Representative images of PicroSirius Red-stained sections demonstrate that fibrillar collagen morphology in LPS-injected maxilla caused loss of collagen in PDL of both WT and SP/ON-null mice. (B) Quantification of total collagen volume fraction. In WT mice injected with LPS, a statistically significant decrease in total collagen in PDL was apparent, whereas no differences in PBS injected PDL were detected. In contrast, PDL of SP/ON-null mice showed a statistically significant decrease in collagen content with PBS injections in comparison with the PDL of non-injected mice. LPS injections caused a further reduction in total collagen volume fraction in the PDL of SP/ON-null mice. * indicate p < 0.05, for LPS-vs. PBS-injected PDL in WT mice, as well as for PBS-injected vs. non-injected PDL in SP/ON-null mice. p values were determined by a Student’s t test. $ indicates p < 0.05 for LPS vs. PBS-injected and un-injected PDL sites in SP/ON-null mice as determined by ANOVA analysis. Error bars = SEM. (C) Quantification of thick collagen volume fraction. In WT mice, PDL of PBS- and LPS-injected sites presented similar levels of thick collagen; however, both sites had significantly less thick collagen than non-injected PDL sites. In SP/ON-null mice, PBS-injected sites had significantly less thick collagen than non-injected sites, and LPS injections caused a further decrease in thick collagen in comparison with PBS-injected sites. * indicates p < 0.05 for differences in thick collagen in WT PDL of non-injected sites vs. sites injected with either PBS or LPS in WT mice. * also indicates a significant difference in thick collagen content between non-injected and either PBS- or LPS-injected PDL in SP/ON-null mice. p values determined by Student’s t test. ** indicates p < 0.05 for SP/ON-null PBS-injected thick collagen vs. LPS-injected PDL and un-injected PDL in SP/ON-null mice as determined by ANOVA analysis. Error bars = SEM.
To obtain further insight into the influence of SP/ON on collagen content in experimental periodontitis, we assessed total collagen volume fraction in injected PDL. Interestingly, a statistically significant decrease in total collagen in SP/ON-null PBS PDL was detected in comparison with previously reported values in aged-matched SP/ON-null mice with no injections. In contrast, there were no differences in the collagen content in WT PBS and non-injected WT PDL (Fig. 3B). SP/ON-null PBS PDL also demonstrated a significant reduction in collagen volume fraction compared with WT PBS PDL. These results suggest that the lack of SP/ON aggravates decreases in collagenous ECM, due to relatively minor stress/inflammatory changes such as those associated with PBS injections. LPS injections caused a significant decrease in collagen content in both WT and SP/ON-null mice, but this decrease was more severe in SP/ON-null (Fig. 3B). Values of thick collagen fiber volume fraction followed similar trends, with the exception that WT mice had reduced levels of thick fibers in PBS and LPS maxillae vs. non-injected WT mice (Fig. 3C).
SF, the collagen fibers that exit PDL and weave into bone and cementum, are identifiable in PSR sections viewed with high magnification at the junction of PDL and bone (Fig. 4A, arrows). Quantification of the number of SF in PBS control showed a greater number of SF in SP/ON-null mice in comparison with WT mice. However, SF in SP/ON were thinner than those of WT (Fig. 4C). Moreover, LPS injections decreased the number of SF in SP/ON-null mice, whereas, in WT, LPS injections were associated with a slightly higher, although not significant, number of SF vs. PBS control (Fig. 4B).
Figure 4.
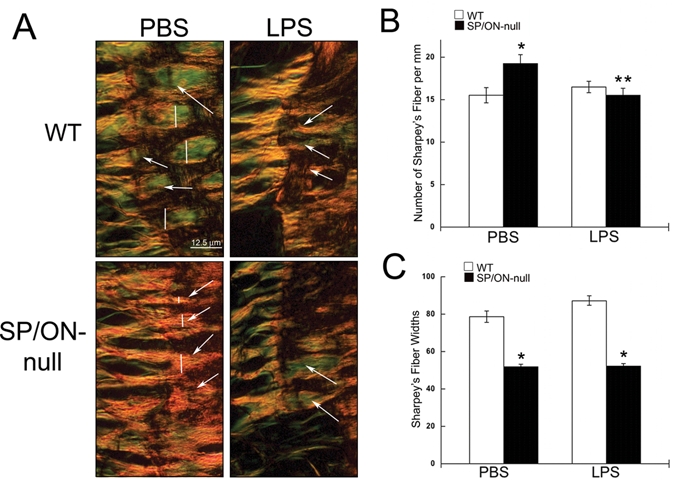
SP/ON-null PDL exhibited thinner Sharpey’s fibers (SF) in greater numbers. (A) High magnification of the PDL-bone interface allowed for visualization of SF. In PBS-injected sites, SF were thicker in WT than in SP/ON-null mice; however, greater numbers of SF were present in SP/ON-null mice. (B) Quantification showed that PBS-injected sites of SP/ON-null mice have the greatest number of SF. * indicates p < 0.05 SP/ON-null PBS vs. WT PBS; ** indicates a significant difference, p < 0.05, in LPS- vs. PBS-injected sites in SP/ON-null mice, as determined by Student’s t test. Error bars = SEM. (C) Quantification of SF widths showed that SP/ON-null mice had thinner SF in comparison with the SF in WT mice. * indicates a significant difference, p < 0.05, between SF thickness in SP/ON-null vs. SF in WT mice, as determined by Student’s t test. Error bars = SEM.
Discussion
Previous characterization of SP/ON-null PDL revealed deficiencies in collagen volume fraction and collagen fiber thickness that varied with age (Trombetta and Bradshaw, 2010). Since SP/ON was shown to be relevant for collagen homeostasis in periodontal tissues, we assessed the role of SP/ON on collagen catabolism associated with periodontitis. Alveolar bone loss associated with LPS-induced periodontitis increased 2.2-fold in SP/ON-null mice in comparison with WT mice. The increased loss of bone in SP/ON-null mice was not surprising, since these mice are reported to have osteopenia and decreased bone mineral density in long bones. Defects in bone are predicted to predispose these mice to accelerated bone loss in this model (Delany et al., 2000).
Interestingly, PDL of SP/ON-null LPS had significantly fewer inflammatory cells than the PDL of LPS-injected WT mice. The reduction in inflammatory cell number in SP/ON-null LPS was somewhat unexpected, given the increases in bone loss in these animals. However, previous reports have indicated a dampened immune response to LPS in SP/ON-null mice. For example, SP/ON-null animals were unable to mount an immune response to an LPS footpad challenge (Rempel et al., 2007). In addition, altered inflammatory cell recruitment in tumors grown in SP/ON-null mice has been reported (Arnold et al., 2010). SP/ON-dependent mechanisms for controlling bone and PDL collagen turnover are more likely to arise from deficiencies in cellular deposition of fibrillar collagen. In skin fibroblasts, the absence of SPARC resulted in aberrant procollagen processing and decreased collagen deposition to insoluble ECM (Rentz et al., 2007). In vitro studies of human dermal fibroblasts have also demonstrated that collagen production is diminished in cells with reduced SP/ON expression (Zhou et al., 2005).
Hence, the decrease in collagen volume in SP/ON-null PDL might result from deficient collagen deposition in response to the ECM remodeling that occurs in response to injury. Effects on collagenous ECM deposition are not necessarily dependent upon inflammatory mediators. For example, increased deposition of collagen has been found to occur in the presence of increased mechanical tension (Levental et al., 2009). These results suggest that cellular factors and parameters such as tissue integrity/tension contribute to ECM deposition independent of inflammatory response.
Alternatively, PDL fibroblasts lacking SP/ON might produce collagen with altered fibrillar structure, rendering it more sensitive to degradation in response to mechanical or inflammatory insult, e.g., a higher amount of thin collagen fibers (Bradshaw et al., 2003b). PDL fibroblasts expressing SP/ON would then be predicted to produce collagen that is more resistant to collagenase digestion, hence maintaining PDL integrity. Increased sensitivity to collagenase digestion associated with inefficient collagen incorporation into insoluble ECM might account for the greater loss of tissue (alveolar bone and PDL collagen) in the absence of a robust inflammatory response in SP/ON-null mice.
In health, PDL and SF contribute to homeostasis of bone by regulating occlusal forces transmitted to the bone (McCulloch et al., 2000). An increase in SF is indicative of bone remodeling (Cochran et al., 2003; Diedrich et al., 2003; Johnson, 2005). SP/ON-null mice have decreased trabecular bone formation (Boskey et al., 2003) and diminished deposition of collagen (Bradshaw et al., 2003a,b, 2009; Trombetta and Bradshaw, 2010). The increase in the number of SF in the PDL of SP/ON-null vs. that of WT might reflect a compensation for the inherently weaker ECM associated with SP/ON-null PDL and alveolar bone. Despite substantially reduced PDL collagen content, SP/ON-null mice injected with PBS maintained alveolar BVF, possibly due to higher numbers of SF vs. WT. However, LPS injections caused significant decreases in bone, collagenous PDL, and SF in SP/ON-null mice.
In humans, bone loss associated with periodontal disease is thought to occur from the persistence of inflammatory cells in gingival tissues that subject alveolar bone and PDL to injury and degradation (Jain et al., 2008). The host inflammatory response is thus one essential determinant in the progression and severity of periodontal disease (Seymour, 1991; Hart et al., 2004). Our results suggest that differences in ECM assembly and composition are also critical factors in determining the severity of periodontal disease. Of future interest is investigation into the function of SP/ON in mediating periodontal repair, since SP/ON might be closely associated with variations in clinical response to nonsurgical and regenerative periodontal treatments.
Acknowledgments
We thank Kylie Martin, Yuhua Zhang, Lauren Card, and Robert Zinna for technical support. This work was supported by the National Institutes of Health [T32DE017551, R25 HL092611, 2P20RR017696, HL094517, R01DE018290] and by a Veteran’s Administration Merit Award.
References
- Arnold SA, Rivera LB, Miller AF, Carbon JG, Dineen SP, Xie Y, et al. (2010). Lack of host SPARC enhances vascular function and tumor spread in an orthotopic murine model of pancreatic carcinoma. Dis Model Mech 3:57-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskey AL, Moore DJ, Amling M, Canalis E, Delany AM. (2003). Infrared analysis of the mineral and matrix in bones of osteonectin-null mice and their wildtype controls. J Bone Miner Res 18:1005-1011 [DOI] [PubMed] [Google Scholar]
- Bradshaw AD. (2009). The role of SPARC in extracellular matrix assembly. J Cell Commun Signal 3:239-246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD, Sage EH. (2001). SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest 107:1049-1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD, Graves DC, Motamed K, Sage EH. (2003a). SPARC-null mice exhibit increased adiposity without significant differences in overall body weight. Proc Natl Acad Sci USA 100:6045-6050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD, Puolakkainen P, Dasgupta J, Davidson JM, Wight TN, Sage EH. (2003b). SPARC-null mice display abnormalities in the dermis characterized by decreased collagen fibril diameter and reduced tensile strength. J Invest Dermatol 120:949-955 [DOI] [PubMed] [Google Scholar]
- Bradshaw AD, Baicu CF, Rentz TJ, Van Laer AO, Boggs J, Lacy JM, et al. (2009). Pressure overload-induced alterations in fibrillar collagen content and myocardial diastolic function: role of secreted protein acidic and rich in cysteine (SPARC) in post-synthetic procollagen processing. Circulation 119:269-280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran DL, Jones A, Heijl L, Mellonig JT, Schoolfield J, King GN. (2003). Periodontal regeneration with a combination of enamel matrix proteins and autogenous bone grafting. J Periodontol 74:1269-1281 [DOI] [PubMed] [Google Scholar]
- Delany AM, Hankenson KD. (2009). Thrombospondin-2 and SPARC/osteonectin are critical regulators of bone remodeling. J Cell Commun Signal 3:227-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delany AM, Amling M, Priemel M, Howe C, Baron R, Canalis E. (2000). Osteopenia and decreased bone formation in osteonectin-deficient mice. J Clin Invest 105:915-923; erratum in J Clin Invest 105:1325, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrich P, Fritz U, Kinzinger G, Angelakis J. (2003). Movement of periodontally affected teeth after guided tissue regeneration (GTR)—an experimental pilot study in animals. J Orofac Orthop 64:214-227 [DOI] [PubMed] [Google Scholar]
- Hart GT, Shaffer DJ, Akilesh S, Brown AC, Moran L, Roopenian DC, et al. (2004). Quantitative gene expression profiling implicates genes for susceptibility and resistance to alveolar bone loss. Infect Immun 72:4471-4479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N, Jain GK, Javed S, Iqbal Z, Talegaonkar S, Ahmad FJ, et al. (2008). Recent approaches for the treatment of periodontitis. Drug Discov Today 13:932-943 [DOI] [PubMed] [Google Scholar]
- Johnson RB. (2005). Synthesis of alveolar bone Sharpey’s fibers during experimental tooth movement in the rat. Anat Rec A Discov Mol Cell Evol Biol 284:485-490 [DOI] [PubMed] [Google Scholar]
- Kolenbrander PE, London J. (1993). Adhere today, here tomorrow: oral bacterial adherence. J Bacteriol 175:3247-3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. (2009). Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139:891-906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch CA, Lekic P, McKee MD. (2000). Role of physical forces in regulating the form and function of the periodontal ligament. Periodontol 2000 24:56-72 [DOI] [PubMed] [Google Scholar]
- McCurdy S, Baicu CF, Heymans S, Bradshaw AD. (2010). Cardiac extracellular matrix remodeling: fibrillar collagens and Secreted Protein Acidic and Rich in Cysteine (SPARC). J Mol Cell Cardiol 48:544-549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng PY, Donley M, Hausmann E, Hutson AD, Rossomando EF, Scannapieco FA. (2007). Candidate salivary biomarkers associated with alveolar bone loss: cross-sectional and in vitro studies. FEMS Immunol Med Microbiol 49:252-260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norose K, Clark JI, Syed NA, Basu A, Heber-Katz E, Sage EH, et al. (1998). SPARC deficiency leads to early-onset cataractogenesis. Invest Ophthalmol Vis Sci 39:2674-2680 [PubMed] [Google Scholar]
- Rempel SA, Hawley RC, Gutierrez JA, Mouzon E, Bobbitt KR, Lemke N, et al. (2007). Splenic and immune alterations of the Sparc-null mouse accompany a lack of immune response. Genes Immun 8:262-274 [DOI] [PubMed] [Google Scholar]
- Rentz TJ, Poobalarahi F, Bornstein P, Sage EH, Bradshaw AD. (2007). SPARC regulates processing of procollagen I and collagen fibrillogenesis in dermal fibroblasts. J Biol Chem 282:22062-22071 [DOI] [PubMed] [Google Scholar]
- Rogers JE, Li F, Coatney DD, Rossa C, Bronson P, Krieder JM, et al. (2007). Actinobacillus actinomycetemcomitans lipopolysaccharide-mediated experimental bone loss model for aggressive periodontitis. J Periodontol 78:550-558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartori R, Li F, Kirkwood KL. (2009). MAP kinase phosphatase-1 protects against inflammatory bone loss. J Dent Res 88:1125-1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage A, Eaton KA, Moles DR, Needleman I. (2009). A systematic review of definitions of periodontitis and methods that have been used to identify this disease. J Clin Periodontol 36:458-467 [DOI] [PubMed] [Google Scholar]
- Scannapieco FA, Ng P, Hovey K, Hausmann E, Hutson A, Wactawski-Wende J. (2007). Salivary biomarkers associated with alveolar bone loss. Ann NY Acad Sci 1098:496-497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour GJ. (1991). Importance of the host response in the periodontium. J Clin Periodontol 18:421-426 [DOI] [PubMed] [Google Scholar]
- Sodek J. (1977). A comparison of the rates of synthesis and turnover of collagen and non-collagen proteins in adult rat periodontal tissues and skin using a microassay. Arch Oral Biol 22:655-665 [DOI] [PubMed] [Google Scholar]
- Trombetta JM, Bradshaw AD. (2010). SPARC/osteonectin functions to maintain homeostasis of the collagenous extracellular matrix in the periodontal ligament. J Histochem Cytochem 58:871-879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeichner-David M. (2006). Regeneration of periodontal tissues: cementogenesis revisited. Periodontol 2000 41:196-217 [DOI] [PubMed] [Google Scholar]
- Zhou X, Tan FK, Guo X, Wallis D, Milewicz DM, Xue S, et al. (2005). Small interfering RNA inhibition of SPARC attenuates the profibrotic effect of transforming growth factor beta1 in cultured normal human fibroblasts. Arthritis Rheum 52:257-261 [DOI] [PubMed] [Google Scholar]


