Abstract
Gene expression profiles of human ameloblastoma microdissected cells were characterized with the purpose of identifying genes and their protein products that could be targeted as diagnostic and prognostic markers as well as for potential therapeutic interventions. Five formalin-fixed, decalcified, paraffin-embedded samples of ameloblastoma were subjected to laser capture microdissection, linear mRNA amplification, and hybridization to oligonucleotide human 41,000 RNA arrays and compared with universal human reference RNA, to determine the gene expression signature. Assessment of the data by Significance Analysis of Microarrays (SAM) and cluster analysis showed that 38 genes were highly expressed (two-fold increase) in all samples, while 41 genes were underexpressed (two-fold reduction). Elements of the sonic hedgehog pathway and Wingless type MMTV integration site family were validated by immunohistochemistry. We have identified the expression of multiple genes and protein products that could serve as potential diagnostic, prognostic, and therapeutic targets.
Keywords: ameloblastoma, gene expression profile, human, microarray
Introduction
Ameloblastoma is an aggressive tumor of odontogenic epithelial origin, with devastating morbidity if left untreated, due to its unlimited growth potential. It is characterized by a high rate of recurrence (up to 70%, depending on the treatment modality) and potential to undergo malignant transformation and to metastasize (up to 2% of cases). Malignant ameloblastoma is defined as a histologically benign-appearing ameloblastoma with metastasis (Goldenberg et al., 2004; Cardoso et al., 2009). Surgical resection is the treatment of choice, which can cause further morbidity to the craniofacial complex, with loss of function and esthetics (Olasoji and Enwere, 2003).
Gene expression profiling of tumor cell populations has advanced our understanding of the pathogenesis of human tumors (Naderi et al., 2004). The identification of specific genes or groups of genes that are deregulated, and thus potentially playing a role in initiation, proliferation, and morphological determination of the tumor, could provide new diagnostic and therapeutic approaches. Laser capture microdissection (LCM) allows for the isolation of single cells, with no detrimental effect on PCR amplification, and can be coupled to high-density oligonucleotide arrays to obtain expression profiles from cell populations of complex tumors (Luzzi et al., 2003).
Knowledge of early events leading to and promoting tumorigenesis in ameloblastoma, and odontogenic tumors in general, remains limited, partly because studies directed at identifying molecular factors and events that initiate and drive tumorigenesis are inconclusive. There have been two microarray studies of ameloblastoma (Heikinheimo et al., 2002; Carinci et al., 2004), one of them using cDNA microarrays containing 19,000 human cDNAs, and, more recently, an oligonucleotide analysis of whole-tissue ameloblastoma in a 34,000 human RNA microarray (Lim et al., 2006). The objectives of our study were: (a) to characterize the gene expression profile of laser-captured microdissected ameloblastoma cells using oligonucleotide microarray technology; (b) to compare the gene expression profile with universal human reference RNA; and (c) to identify genes or gene products that may have diagnostic, prognostic, or therapeutic potential. This study is the first to successfully perform microgenomics on formaldehyde-fixed, decalcified, and paraffin-embedded odontogenic tumor tissue.
Materials & Methods
Case Selection
This study was approved by the Institutional Review Board of the University of North Carolina at Chapel Hill (UNC). Ameloblastoma samples were retrieved from the paraffin block archives of the departments of Surgical Pathology and Oral and Maxillofacial Pathology at UNC; the diagnosis was confirmed, based on the 2005 WHO Histologic Classification of Odontogenic Tumors (Barnes et al., 2005). Five ameloblastoma samples were selected (1 unicystic, 1 recurrent tumor, and 3 solid/multicystic), all of which were originally decalcified in Richard Allan Scientifics’ decalcifying solution containing water, hydrochloric acid, EDTA, tetrasodium tartrate, potassium sodium, and tartrate.
Laser Capture Microdissection
Serial 7-µm-thick sections were cut from each block in an RNAse-free lab environment for a total of 75 slides (15 from each block). In addition, tissue scrapes from each block of the 5 samples were processed for the assessment of total RNA fidelity, following the protocol of Optimum™ FFPE RNA Isolation Kit (Ambion, Inc., Austin, TX, USA) optimized for RT-PCR analysis. For the purposes of the present study, tissue blocks that were one year old or less were selected, since they provide better fidelity than older blocks. Once the quantity and quality of total RNA were established, the ameloblastoma sections were subjected to laser capture microdissection with the AutoPix™ with infrared diode laser (Arcturus Engineering, Santa Clara, CA, USA). The cells from the basal cell layer of the ameloblastoma samples, which have a uniform histologic architecture, were microdissected. For correct identification of the cells of interest, H&E sections were scanned in the Aperio ScanScope™ system and viewing software (Vista, CA, USA). Scanned images were viewed simultaneously with the LCM procedure. Microdissected cells were captured with a CapSure™ cap (Arcturus Engineering), and the cells were placed in RNA extraction buffer. Total RNA was isolated from the microdissected cells with the PicoPure RNA Isolation kit (Arcturus Bioscience, Santa Clara, CA, USA). The quality and yield of total RNA were assessed on an Agilent Bioanalyzer 2100 (Agilent Technologies, Palo Alto, CA, USA).
RNA Amplification
The TargetAmp™ 2-Round Aminoallyl-aRNA Amplification Kit (Epicentre Biotechnologies, Madison, WI, USA) was used. This protocol is based on an improved “Eberwine” (Van Gelder et al., 1990) linear amplification process. In parallel with the laser-captured and microdissected samples, 2 additional samples were included in the amplification, a positive control (human universal RNA) and a negative control (no RNA template).
Oligonucleotide Microarray Analysis
Whole Human Genome Oligo Microarrays (G4112A) (Agilent Technologies Inc.) including 60-mer oligonucleotide probe sequences of over 41,000 human genes and transcripts were used. Target labeling, hybridization, washing, scanning, and data extraction were performed following the oligonucleotide microarray hybridization protocol as previously described (Ohyama et al., 2002). Amplified aRNA from the laser-captured tissue was labeled with the Cy5 dye and hybridized to the arrays with a Cy3-labeled Stratagene Human Universal Reference (Santa Clara, CA, USA). The Universal Reference labeled cRNA from a single labeling reaction was used in all 5 hybridizations to minimize technical variance (Novoradovskaya et al., 2004). Precautions were taken to avoid small-size aRNA hybridization to gene oligos, by removing free nucleotides as previously described (Coudry et al., 2007).
Data Analysis
The hybridized arrays were scanned at 488 nm in a G2500 Scanner (Agilent). A normalization factor was estimated from ratios of the medians. The log2 ratios for all the targets on the array were calibrated by the normalization factor, and log2 ratios outside the 99.7% confidence interval were determined as significantly changed in the ameloblastoma cells. Cluster analysis of microarray data and statistical analysis were performed on the Agilent GeneSpring Analysis Platform™ as well as by Significance Analysis of Microarrays (SAM) (Larsson et al., 2005).
Validation of Protein Expression
Immunohistochemistry was performed on sections of the ameloblastomas used for LCM and microarray analysis as well as on additional samples from the Pathology laboratory archives. Standard protocols were used to evaluate expression and localization of sonic hedgehog pathway members (SHH, Gli, Ptch, and SMO) (R&D Systems Inc., Minneapolis, MN, USA) and WNT10 (ProSci Incorporated, Poway, CA, USA). Positive and negative controls were included for each antibody.
Results
Laser capture microdissection yielded an estimated 500 cells per sample (Table 1) and approximately 10-15 ng total RNA. The 260/280 ratio for the 5 samples was between 2.12 and 2.14. Anti-sense RNA generated from the ameloblastoma samples had an average size of 500 nucleotides. The microarray hybridization was performed with Agilent microarrays including more than 41,000 human genes and transcripts; the raw data have been submitted to the Gene Expression Omnibus (GEO) microarray database (accession number GPL1708). We considered genes based on Bonferroni’s inequality, which limits the chance of a false-positive result to be no more than by multiplying each nominal p-value by N (with a maximum of 1). Hierarchical cluster analysis of genes is depicted in Fig. 1. In total, 21 genes were highly expressed, with a two-fold increase over the reference RNA in all 5 samples (Table 2), while 41 genes were underexpressed (two-fold) in 4 out of 5 samples; among these was RAB31, a member of the RAS oncogene family (Entrez Gene ID 11031) T-cell acute lymphocytic leukemia 1 (Entrez Gene ID 6886). Variance was greater in genes expressed at low levels between these tumors, compared with the highly expressed genes between tumors.
Table 1.
Laser Capture Microdissection (LCM) and Analysis of Total RNA Obtained by LCM of Ameloblastoma Samples
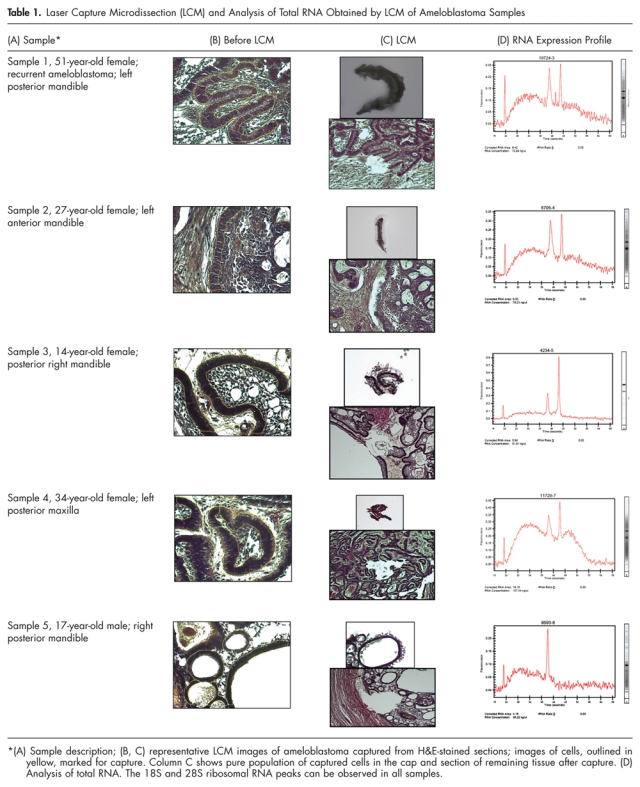
Figure 1.
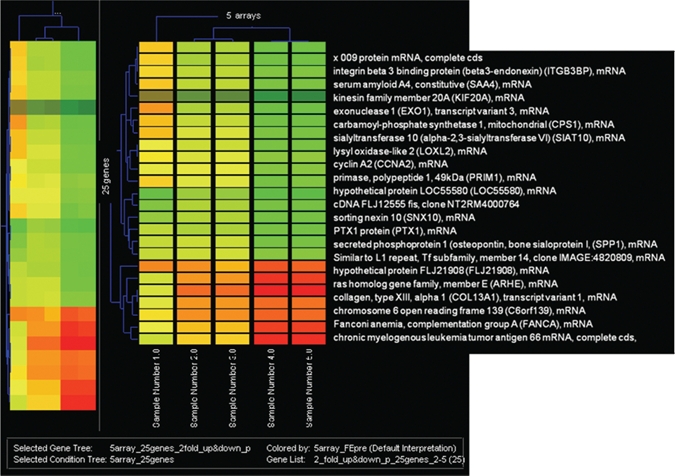
Hierarchical gene cluster analysis of ameloblastoma. Each column represents a tumor sample and each row a single gene. Gene expression levels are shown in descending order; red indicates two-fold up; green, two-fold down.
Table 2.
Expression of WNT Family of Genes and Sonic Hedgehog Pathway Members in Ameloblastoma
| Expression of WNT Family of Genes in Ameloblastoma | ||
|---|---|---|
| Gene Name |
Annotation |
Average Fold Change |
| WISP2 | WNT1 inducible signaling pathway protein 2 | 1.15 |
| WISP1 | WNT1 inducible signaling pathway protein 1 | 1.26 |
| WISP3 | WNT1 inducible signaling pathway protein 3 | 1.13 |
| WISP1 | WNT1 inducible signaling pathway protein 1 | 1.18 |
| WNT11 | Wingless-type MMTV integration site family, member 11 | 0.62 |
| WNT1 | Wingless-type MMTV integration site family, member 1 | 1.36 |
| WNT10B | Wingless-type MMTV integration site family, member 10B | 1.00 |
| WNT10A | Wingless-type MMTV integration site family, member 10A | 5.10 |
| WNT11 |
Wingless-type MMTV integration site family, member 11 |
1.23 |
| Expression of the Sonic Hedgehog Pathway Members in Ameloblastoma | ||
| Gene Name |
Annotation |
Average Fold Change |
| SHH | Sonic hedgehog homolog (Drosophila) (SHH), mRNA | 0.88 |
| SMOX | Spermine oxidase | 1.13 |
| SMOC1 | SPARC-related modular calcium-binding protein 1 precursor | 1.00 |
| SMO | Smoothened homolog precursor (SMO) | 0.70 |
| SMOC2 | SPARC-related modular calcium-binding protein 2 precursor | 0.50 |
| PTCH | Protein patched homolog 1 (PTC1) (PTC). | 2.13 |
| PTCH2 | Patched homolog 2 (Drosophila) RP11-269F19.8 | 1.34 |
| GLi1 | Zinc finger protein GLI1 (Glioma-associated oncogene) | 1.28 |
| GLi2 | Zn-finger, C2H2 type | 0.98 |
| GLI4 | Zinc finger protein GLI4 (Kruppel-related zinc finger protein 4) | 0.56 |
| GLIPR1 | Glioma pathogenesis-related protein 1 precursor | 0.54 |
| GLI3 | GLI-Kruppel family member GLI3 | 0.43 |
The tumor cells consistently expressed enamel extracellular matrix genes, including AMELX, AMBN, ENAM, and KLK4, at levels similar to the reference RNA. Interestingly, MMP20 was not expressed at levels above background. Similarly, genes involved primarily in dentin formation, such as DSPP and DMP1, were not expressed above background.
Genes that were highly expressed compared with the control RNA included a variety of growth factors and transcription factors. Wingless-type MMTV integration site family member 10A (WNT10A) was strongly expressed in our ameloblastoma samples, with a fold change of 5.1. Members of the sonic hedgehog (SHH) pathway also showed variable expression compared with reference RNA GLI1, and all 5 ameloblastomas. Patched (PTCH) was highly expressed, while Smoothened (SMO) was expressed at levels less than the reference RNA (Fig. 2, Appendix Table).
Figure 2.
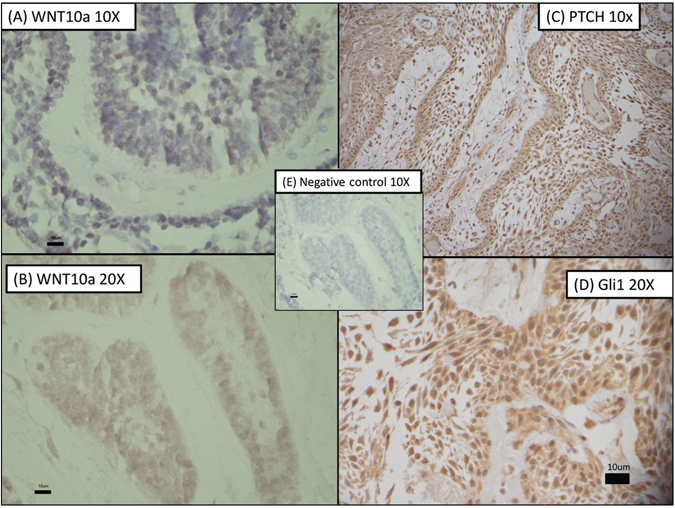
Confirmation of expression of WNT10a, PTCH, and Gli1 in ameloblastoma by immunohistochemistry. (A) Reactivity for WNT10a at 10x; (B) reactivity for WNT10a at 20x; (C) reactivity for PTCH at 10x; (D) reactivity for Gli1 at 20x; and (E) negative control at 10x.
It has been postulated that calretinin (CALB2) is a useful diagnostic marker to differentiate ameloblastoma from other less aggressive tumors that have a similar histological appearance (Altini et al., 2000). The microarray analysis we performed showed CALB2 to be overexpressed compared with the reference RNA in all 5 tumors. These results have been reported in detail elsewhere (DeVilliers et al., 2008).
Discussion
This is the first report of the gene expression profile of ameloblastoma using microgenomics. This study shows that LCM of ameloblastoma tissue provides an accurate way to select a specific tumor cell population, thereby reducing variance introduced by the inclusion of stromal tissue that confounds gene expression analysis. This is particularly valuable in the study of tumors that do not have a solid growth pattern, but rather are characterized by an infiltrative and cystic component that precludes the acquisition of uncontaminated tumor tissue by conventional bulk tissue analysis. In our study, the wingless-type MMTV integration site family, member 10A, MyoD family inhibitor (MDFI), and BTAF1 RNA polymerase II transcription factor were among the overexpressed genes (two-fold) in 5/5 samples of ameloblastoma (p < 0.05). In Heikinheimo’s study (Heikinheimo et al., 2002) of the gene expression profile of fresh-frozen ameloblastomas (using a cDNA microarray and comparing them with tooth germ), the oncogene FOS was the most overexpressed gene, followed by tumor-necrosis-factor-receptor 1 (TNFRSF1A). Genes from sonic hedgehog (SHH) pathway, TNF-receptor-associated-factor 3 (TRAF3), deleted in colorectal carcinoma (DCC), and transforming growth-factor-β1 (TGFβ1), among others, were underexpressed. Our data agreed with the overexpression of cFOS and underexpression of SHH; in contrast, we showed overexpression of PTCH (two-fold), which was not the case in the Heikinheimo study. In 2003, Carinci et al. studied the genetic expression profiling of 6 odontogenic tumors which included 3 ameloblastomas and 3 malignant odontogenic tumors, comparing them with gingival tissue (Carinci et al., 2003). This study focused on the description of the gene expression of malignant odontogenic tumors; we did not find any of those genes significantly expressed in our samples. Lim et al., in 2006, reported an oligonucleotide microarray analysis of 2 ameloblastomas compared with a dentigerous cyst (Lim et al., 2006). Overexpressed genes postulated to play a role in the tumorigenesis of ameloblastoma included VAV3, EGFR, TOM1L1, FUBP1, CDK2, TNKS, PTHRP, SMARCA2, KLK5, and CLU. Our study found significant overexpression of VAV3 and FUBP1, but not the others.
WNT Expression
Identification of the strong expression of WNT10A A in all tumors was noteworthy. The WNT gene family in humans consists of 19 structurally related genes that encode secreted signaling proteins. These proteins have been implicated in oncogenesis and in several developmental processes, including regulation of cell fate and patterning during embryogenesis. Interestingly, mutation of WNT10A is associated with odonto-onychodermal dysplasia (OMIM #257980), a type of ectodermal dysplasia characterized by developmental abnormalities in a variety of ectodermally derived tissues, including missing teeth. The WNT10A gene is located on chromosome 2q35 and codes for a 417-amino-acid protein that transduces signals by binding to a receptor on the cell surface. High expression levels of WNT10A could play a key role in carcinogenesis through activation of a canonical WNT-beta-catenin-TCF signaling pathway that is involved in cell fate determination (Molinolo et al., 2008). WNT10A is strongly expressed in the cell lines of promyelocytic leukemia and Burkitt’s lymphoma, as well as in colorectal cancer cell lines (Dorfman et al., 2003; Dhir et al., 2008). WNT10A expression in ameloblastomas could be mediated by tumor necrosis factor-α (TNF-α), which also had a high expression in our tumors. The potential induction of WNT10A by TNF-α and its potential role in the cytodifferentiation of ameloblastoma through the activation of the WNT-β-catenin-TCF signaling pathway deserves further study. Our results are supported by those of prior studies indicating an important role for TNF-α in ameloblastoma (Hendarmin et al., 2005; Kumamoto and Ooya, 2005; Sandra et al., 2005, 2006; Rizzardi et al., 2009).
β-catenin is involved in the Wnt signaling pathway in tumorigenesis by T-cell factor transcription factor (TCF); this is a mechanism that has been well-studied in gastric cancer (Kirikoshi et al., 2001). We found strong expression of TCF supporting activation of the canonical WNT pathway or, alternatively, a loss of TCF regulation through the NKL cascade of the non-canonical pathway. WNT10A expression has been observed in the enamel knot, a critical signaling center for SHH and WNT pathways that are involved in tooth morphogenesis (Thesleff and Jernvall, 1997). The role of WNT10A in tooth development is not fully understood; however, its expression in the epithelial-derived enamel knot and the loss of tooth development when it is mutated clearly demonstrate that it is critical for normal odontogenesis. WNT10A could play an important role in regulating the growth and morphological patterning of ameloblastoma tumors. Further investigation of the role of WNT10A in ameloblastoma tumorigenesis is warranted.
Patched (PTCH)
The SHH pathway induces carcinogenesis or promotes cell survival in cancers in multiple organs (Berman et al., 2002; Yanai et al., 2007), plays a critical role in tooth development (Gritli-Linde et al., 2002), and appears to be involved in odontogenic tumorigenesis (Koyama et al., 2001). We identified the overexpression of GLI1 and PTCH in all 5 ameloblastomas and confirmed it by immunohistochemistry (Fig. 2). Other studies (Heikinheimo et al., 2002; Kumamoto et al., 2004) observed the underexpression of sonic hedgehog (SHH) in ameloblastoma; our data agree with this finding. The high expression of some of the SHH pathway members is important, given that several therapeutic compounds have proven effective as antagonists of the SHH pathway. Cyclopamine, a plant-derived SHH pathway antagonist, acts at the level of SHH signaling and is effective in reducing the viability of cancer cells by blocking activation of the SHH response pathway and abnormal cell growth. Several cyclopamine studies have shown successful responses in breast, pancreatic, and gastric cancer cells, medulloblastoma, and oral squamous cell carcinoma cells (Nishimaki et al., 2004; Mukherjee et al., 2006). We suggest that the SHH pathway members could play an important role in the tumorigenesis of ameloblastomas and provide a potential therapeutic opportunity. Studies are under way to investigate the effect of cyclopamine on ameloblastoma cells.
Genes that were underexpressed (two-fold down) in our study included RAB31, a member of the RAS oncogen family and a GTPase-mediated signal transduction; T-cell acute lymphocytic leukemia 1, important in cell differentiation, cell proliferation, and regulation of transcription and DNA binding; and ST13 (suppression of tumorigenicity 13, colon carcinoma), which functions in protein binding and bridging.
In this paper, we described how amplified aRNA generated from ameloblastoma cells harvested by laser capture microdissection and microarray analysis provided a highly efficient approach for establishing gene expression profiles for specific tumor cell populations. Several of the genes and their proteins that were highly expressed in ameloblastoma cells have good potential as therapeutic targets, including the SHH pathway member PTCH. The protein WNT10A was highly expressed in our ameloblastoma samples, and it may be mediated by tumor necrosis factor-α (TNF-α), which was also highly expressed. Presently, radical surgery remains the therapy of choice for ameloblastomas. It is critical to identify potential molecular pathways that provide an opportunity for novel therapies directed at reducing the size of these tumors prior to surgical intervention and potentially achieving a lower incidence of recurrence and metastasis.
Supplementary Material
Acknowledgments
This investigation was supported by USPHS Research Grant DE016079 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD 20892, USA.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Altini M, Coleman H, Doglioni C, Favia G, Maiorano E. (2000). Calretinin expression in ameloblastomas. Histopathology 37:27-32 [DOI] [PubMed] [Google Scholar]
- Barnes L, Eveson JW, Reichart P, Sidransky D, editors (2005). Pathology and genetics of head and neck tumors. Switzerland/France: WHO Press/IARC Press; [Google Scholar]
- Berman DM, Karhadkar SS, Hallahan AR, Pritchard JI, Eberhart CG, Watkins DN, et al. (2002). Medulloblastoma growth inhibition by hedgehog pathway blockade. Science 297:1559-1561 [DOI] [PubMed] [Google Scholar]
- Cardoso A, Lazow SK, Solomon MP, Berger JR, Rock A. (2009). Metastatic ameloblastoma to the cervical lymph nodes: a case report and review of literature. J Oral Maxillofac Surg 67:1163-1166 [DOI] [PubMed] [Google Scholar]
- Carinci F, Francioso F, Piattelli A, Rubini C, Fioroni M, Evangelisti R, et al. (2003). Genetic expression profiling of six odontogenic tumors. J Dent Res 82:551-557 [DOI] [PubMed] [Google Scholar]
- Carinci F, Palmieri A, Delaiti G, Rubini C, Fioroni M, Martinelli M, et al. (2004). Expression profiling of ameloblastic carcinoma. J Craniofac Surg 15:264-269 [DOI] [PubMed] [Google Scholar]
- Coudry RA, Meireles SI, Stoyanova R, Cooper HS, Carpino A, Wang X, et al. (2007). Successful application of microarray technology to microdissected formalin-fixed, paraffin-embedded tissue. J Mol Diagn 9:70-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVilliers P, Liu H, Suggs C, Simmons D, Daly B, Zhang S, et al. (2008). Calretinin expression in the differential diagnosis of human ameloblastoma and keratocystic odontogenic tumor. Am J Surg Pathol 32:256-260 [DOI] [PubMed] [Google Scholar]
- Dhir M, Montgomery EA, Glockner SC, Schuebel KE, Hooker CM, Herman JG, et al. (2008). Epigenetic regulation of WNT signaling pathway genes in inflammatory bowel disease (IBD) associated neoplasia. J Gastrointest Surg 12:1745-1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman DM, Greisman HA, Shahsafaei A. (2003). Loss of expression of the WNT/beta-catenin-signaling pathway transcription factors lymphoid enhancer factor-1 (LEF-1) and T cell factor-1 (TCF-1) in a subset of peripheral T cell lymphomas. Am J Pathol 162:1539-1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg D, Sciubba J, Koch W, Tufano RP. (2004). Malignant odontogenic tumors: a 22-year experience. Laryngoscope 114:1770-1774 [DOI] [PubMed] [Google Scholar]
- Gritli-Linde A, Bei M, Maas R, Zhang XM, Linde A, McMahon AP. (2002). Shh signaling within the dental epithelium is necessary for cell proliferation, growth and polarization. Development 129:5323-5337 [DOI] [PubMed] [Google Scholar]
- Heikinheimo K, Jee KJ, Niini T, Aalto Y, Happonen RP, Leivo I, et al. (2002). Gene expression profiling of ameloblastoma and human tooth germ by means of a cDNA microarray. J Dent Res 81:525-530 [DOI] [PubMed] [Google Scholar]
- Hendarmin L, Sandra F, Nakao Y, Ohishi M, Nakamura N. (2005). TNFalpha played a role in induction of Akt and MAPK signals in ameloblastoma. Oral Oncol 41:375-382 [DOI] [PubMed] [Google Scholar]
- Kirikoshi H, Sekihara H, Katoh M. (2001). Up-regulation of WNT10A by tumor necrosis factor alpha and Helicobacter pylori in gastric cancer. Int J Oncol 19:533-536 [PubMed] [Google Scholar]
- Koyama E, Wu C, Shimo T, Iwamoto M, Ohmori T, Kurisu K, et al. (2001). Development of stratum intermedium and its role as a Sonic hedgehog-signaling structure during odontogenesis. Dev Dyn 222:178-191 [DOI] [PubMed] [Google Scholar]
- Kumamoto H, Ohki K, Ooya K. (2004). Expression of Sonic hedgehog (SHH) signaling molecules in ameloblastomas. J Oral Pathol Med 33:185-190 [DOI] [PubMed] [Google Scholar]
- Kumamoto H, Ooya K. (2005). Expression of tumor necrosis factor alpha, TNF-related apoptosis-inducing ligand, and their associated molecules in ameloblastomas. J Oral Pathol Med 34:287-294 [DOI] [PubMed] [Google Scholar]
- Larsson O, Wahlestedt C, Timmons JA. (2005). Considerations when using the significance analysis of microarrays (SAM) algorithm. BMC Bioinformatics 6:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Ahn H, Min S, Hong SD, Lee JI, Hong SP. (2006). Oligonucleotide microarray analysis of ameloblastoma compared with dentigerous cyst. J Oral Pathol Med 35:278-285 [DOI] [PubMed] [Google Scholar]
- Luzzi V, Mahadevappa M, Raja R, Warrington JA, Watson MA. (2003). Accurate and reproducible gene expression profiles from laser capture microdissection, transcript amplification, and high density oligonucleotide microarray analysis. J Mol Diagn 5:9-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinolo AA, Amornphimoltham P, Squarize CH, Castilho RM, Patel V, Gutkind JS. (2008). Dysregulated molecular networks in head and neck carcinogenesis. Oral Oncol 45:324-334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Frolova N, Sadlonova A, Novak Z, Steg A, Page GP, et al. (2006). Hedgehog signaling and response to cyclopamine differ in epithelial and stromal cells in benign breast and breast cancer. Cancer Biol Ther 5:674-683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderi A, Ahmed AA, Barbosa-Morais NL, Aparicio S, Brenton JD, Caldas C. (2004). Expression microarray reproducibility is improved by optimising purification steps in RNA amplification and labelling. BMC Genomics 5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimaki H, Kasai K, Kozaki K, Takeo T, Ikeda H, Saga S, et al. (2004). A role of activated Sonic hedgehog signaling for the cellular proliferation of oral squamous cell carcinoma cell line. Biochem Biophys Res Commun 314:313-320 [DOI] [PubMed] [Google Scholar]
- Novoradovskaya N, Whitfield ML, Basehore LS, Novoradovsky A, Pesich R, Usary J, et al. (2004). Universal Reference RNA as a standard for microarray experiments. BMC Genomics 5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama H, Mahadevappa M, Luukkaa H, Todd R, Warrington JA, Wong DT. (2002). Use of laser capture microdissection-generated targets for hybridization of high-density oligonucleotide arrays. Methods Enzymol 356:323-333 [DOI] [PubMed] [Google Scholar]
- Olasoji HO, Enwere ON. (2003). Treatment of ameloblastoma—a review. Niger J Med 12:7-11 [PubMed] [Google Scholar]
- Rizzardi C, Leocata P, Ventura L, Zweyer M, Brollo A, Schneider M, et al. (2009). Apoptosis-related factors (TRAIL, DR4, DR5, DcR1, DcR2, apoptotic cells) and proliferative activity in ameloblastomas. Anticancer Res 29:1137-1142 [PubMed] [Google Scholar]
- Sandra F, Hendarmin L, Nakao Y, Nakamura N, Nakamura S. (2005). TRAIL cleaves caspase-8, -9 and -3 of AM-1 cells: a possible pathway for TRAIL to induce apoptosis in ameloblastoma. Tumour Biol 26:258-264 [DOI] [PubMed] [Google Scholar]
- Sandra F, Hendarmin L, Nakao Y, Nakamura N, Nakamura S. (2006). Inhibition of Akt and MAPK pathways elevated potential of TNFalpha in inducing apoptosis in ameloblastoma. Oral Oncol 42:39-45 [DOI] [PubMed] [Google Scholar]
- Thesleff I, Jernvall J. (1997). The enamel knot: a putative signaling center regulating tooth development. Cold Spring Harb Symp Quant Biol 62:257-267 [PubMed] [Google Scholar]
- Van Gelder RN, von Zastrow ME, Yool A, Dement WC, Barchas JD, Eberwine JH. (1990). Amplified RNA synthesized from limited quantities of heterogeneous cDNA. Proc Natl Acad Sci USA 87:1663-1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai K, Nagai S, Wada J, Yamanaka N, Nakamura M, Torata N, et al. (2007). Hedgehog signaling pathway is a possible therapeutic target for gastric cancer. J Surg Oncol 95:55-62 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


