Abstract
Organochlorine (OC) insecticides continue to occur in tissues of humans and wildlife throughout the world although they were banned in the United States a few decades ago. Low doses of the OC insecticide chlordecone (CD) alter hepatic disposition of lipophilic xenobiotics and perturb lipid homeostasis in rainbow trout, mice and rats. CD pretreatment altered tissue and hepatic subcellular distribution of exogenous [14C]cholesterol (CH) equivalents 4 and 16 h after a bolus intraperitoneal (ip) injection of 5 ml corn oil/kg that contained 10 mg CH/kg. CD pretreatment altered tissue distribution of exogenously administered [14C]CH by decreased hepatic and renal accumulation, and increased biliary excretion up to 300%. Biliary excretion of polar [14C]CH metabolites was not altered by CD. CD pretreatment decreased subcellular distribution of [14C]CH equivalents in hepatic cytosol and microsomes and lipoprotein-rich fraction-to-homogenate ratio. CD pretreatment increased the ratio of [14C]CH equivalents in high density lipoprotein (HDL) to that in plasma and reduced [14C]CH equivalents in the non-HDL fraction 4 h after a bolus lipid dose. CD pretreatment increased plasma non-HDL total CH by 80% 4 h after a bolus lipid dose. Scavenger receptor class B type I (SR-BI) and ATPbinding cassette transporter G8 (ABCG8) proteins were quantified by western blotting in hepatic membranes from control and CD treated mice. Liver membrane contents of SR-BI and ABCG8 proteins were unchanged by CD pretreatment. The data demonstrated that a single dose of CD altered CH homeostasis and lipoprotein metabolism.
Keywords: organochlorines, chlordecone, cholesterol disposition, liver, biliary excretion, lipoproteins
INTRODUCTION
Organochlorines (OC) are a major group of persistent organic pollutants including chlorinated dioxins and furans, polychlorinated biphenyls (PCBs), and the OC insecticides. Despite bans on most uses of OCs in the United States and Europe, they continue to occur in tissues of humans and wildlife throughout the world (Bocquene and Franco 2005; Luellen et al. 2006). Because they are highly lipophilic and generally very resistant to degradation, trophic transfer though food webs is the principle route of environmental exposure to these agents. Modes of action for OC insecticides are distinct from that prototypical for chlorinated dioxins and planar PCB congeners in that they exhibit negligible affinity for the aryl hydrocarbon receptor (Poland and Knutson, 1982). As is typical for OC insecticides, neurotoxicity is a prominent mode of action for CD: it is unusual in that there is extensive epidemiologic documentation of a major human poisoning following poor manufacturing and disposal practices (reviewed by Guzelian, 1982). Prominent weight loss despite normal appetite is a common sign of occupational CD poisoning in humans (10 of 23 cases).
The OC insecticides dieldrin and chlordecone (CD) alter hepatic disposition of lipophilic xenobiotics and perturb lipid homeostasis in rainbow trout, mice and rats (Barnhill et al., 2003; Carpenter and Curtis 1989, 1991; Carpenter et al. 1996; Donohoe et al. 1998; Gilroy et al. 1994). These effects of OC insecticides occur at liver concentrations in the 175 parts per million (ppm) range. Single, doses of CD and dieldrin decrease plasma triglycerides and total cholesterol (CH) in rats 21 and 60 days after administration, respectively (Ishikawa et al. 1978). A single dose of 5 mg CD/kg to C57BL/6 mice also significantly alters tissue distribution of exogenous [14C]CD or [14C]CH, decreasing hepatic disposition and increasing distribution to other tissues (Carpenter and Curtis 1991).
CH is an important constituent of cell membranes and a precursor of steroid hormones and bile acids (Chen and Raymond 2006; Tabas 2002). However accumulation of excess CH contributes to several diseases especially heart attacks and stroke by promoting atherosclerosis (Krieger 1999). CH metabolism is regulated by plasma lipoprotein-mediated extracellular CH transport, the sterol regulatory element binding protein (SREBP) system involved CH synthesis (Brown and Goldstein 1997), nuclear receptor-mediated CH catabolism to bile acids (Peet et al. 1998), and plasma membrane transporter-mediated excretion into bile (Yu et al. 2002).
Transport kinetic studies indicated that the rate of uptake of high density lipoprotein (HDL)-bound [14C]CD or [14C]CH was significantly lower in perfused livers from Sprague-Dawley rats pretreated with a single dose of 15 mg/kg CD (Gilroy et al. 1994). Gilory et al. (1994) reported a significantly increased rate of efflux of HDL-bound [14C]CD to perfusate and cumulative biliary excretion of HDL-bound 14C]CH in these same preparations. Plasma HDL transfers CH from peripheral tissue to the liver for biliary excretion by the reverse CH transport pathway (Fielding and Fielding 1995). Therefore HDL is an important source of CH for secretion into the bile in rodents (Fielding and Fielding 1995; Lee and Parks 2005). Plasma low-density lipoproteins (LDL) and very low-density lipoproteins (VLDL) deliver CH to peripheral tissues such as adipose. In contrast to other OC insecticides, such as dieldrin and DDT in which tissue distribution is proportional to tissue lipid contents (Lindstrom et al. 1974), CD distributes preferentially to the liver in both humans (Cohn et al. 1978) and rodents (Egle et al. 1978). Dieldrin and DDT are primarily associated with LDL and VLDL (Mick et al. 1971). CD binds preferentially with albumin and HDL in vitro, consistent with in vivo studies of human, rat, and pig plasma (Soine et al. 1984a; Soine et al. 1982) and this may account for its predominant distribution to the liver. These previous works indicate that a change in hepatic uptake of HDL-complexed CH is important in alteration of lipid homeostasis by CD. Low doses of CD pretreatment may modulate membrane proteins which transport CH thereby disturbing the distribution of the exogenous CH to liver and increasing delivery of CH into bile.
Scavenger receptor class B type I (SR-BI), best known as a physiological HDL receptor, mediates selective uptake of both cholesterol esters and other lipids from HDL (Hobbs and Rader 1999; Krieger 1999). SR-BI deficiency reduces the rate of the clearance of HDL-CH from plasma (Out et al. 2004). ATP-binding cassette (ABC) transporter G5 (ABCG5) and G8 (ABCG8) are members of the G family of ABC transporters. Co-expression of G5 and G8 which are obligate heterodimers promotes transport of CH from hepatocytes to bile and probably from enterocytes into the intestinal lumen (Yu et al. 2005; Yu et al. 2002).
The present studies examined the effect of CD pretreatment on the tissue distribution of exogenous [14C]CH 4 and 16 h after intraperitoneal (ip) injection of 5 ml corn oil/kg that contained 10 mg CH/kg. Hepatic subcellular distribution of [14C]CH was also determined. Blood plasma, hepatic and biliary total CH were examined in control and CD pretreated mice. This investigation determined whether the reduced disposition of [14C]CH to liver was associated with decreased SR-BI protein, SRBI was analyzed by immunoblotting with hepatic membranes from CD treated mice (15 mg/kg) or controls. Immunoblot analysis for ABCG8 was conducted with hepatic membranes from CD treated mice (15 mg/kg) or controls to determine whether the stimulation of biliary CH efflux by CD was coupled to the increased CH efflux pump (ABCG5/ABCG8).
Our results demonstrated that CD pretreatment altered plasma, tissue and hepatic subcellular distribution of exogenously administered [14C]CH. CD pretreatment also altered the amount of total CH in blood plasma and gallbladder. The reduced hepatic [14C]CH equivalents and increased biliary [14C]CH equivalents were not associated with altered hepatic membrane SR-BI or ABCG8 contents.
MATERIALS AND METHODS
Chemicals
CD (99 % purity) was purchased from Chem Service (West Chester, PA) and purity was confirmed by GC-EI/MS. [4-14C]CH (55 mCi/mmol) was obtained from American Radiolabeled Chemicals. Inc. (St. Louis, MO). Solvable tissue solubilizer and Hionicfluor scintillation cocktail were purchased from PerkinElmer (Boston, MA). All other chemicals and general reagents were purchased from Sigma (St. Louis, MO).
Treatment of mice
The CD and lipid bolus including [14C]CH doses and times of sampling were based on previous doseresponse and timecourse experiments (Carpenter and Curtis, 1989; 1991). Male C57BL/6 mice, 6 to 7-weeksold, were obtained from Simenson Laboratory (Gilroy, CA). The mice were randomly divided into three groups; control, 5 mg or 15 mg CD/kg body weight treatment. The animals were housed in a temperature controlled room (22 ± 1°C) with a daily cycle of 12 h of light and 12 h darkness and fed ad libitum with AIN93 diet (Dyets, Inc., Bethlehem, PA) with a free access to water. Treatments were initiated after seven days acclimatization.
[14C]Cholesterol tissue distribution experiments
CD was dissolved in corn oil. Mice received CD (5 or 15 mg/kg body weight) or corn oil alone by intraperitoneal injection (ip, 5 ml/kg body weight). After 3 days, a bolus lipid dose of corn oil (5 ml/kg) containing [14C]CH was administered (10 mg CH/kg body 14 weight, ip). [14 C]CH injected mice were housed individually and allowed free access to water. After 4 or 16 h animals were killed by CO2 anesthesia and exsanguination. Blood samples and tissues (liver, kidneys, gallbladder, adrenal gland and adipose tissue) were removed and prepared as required for each analysis. The procedures for animal use were approved by the Oregon State University Institutional Animal Care and Use Committee (IACUC).
Quantification of radioactivity in tissues, blood and hepatic subcellular fractions
Blood was collected by cardiac puncture in heparinized syringes. Plasma was isolated by centrifugation at 3,000g for 25 min at 4°C. The HDL fraction was separated after apolipoprotein-B containing lipoproteins were precipitated with dextran sulfate and magnesium (Rachem, San Diego, CA). Kidneys, fat, and adrenal glands were homogenized in homogenization buffer (0.01 M potassium phosphate, pH 7.5; 0.15 M KCl; 1.0 mM EDTA; 0.1 mM BHT (butylated hydroxytoluene); 0.1 mM PMSF (phenylmethylsulfonyl fluoride)) with a Dounce homogenizer. Liver was homogenized in homogenization buffer with a polytron PT 3000 (Brinkmann Instruments, Westbury, NY). Liver homogenate was centrifuged at 1200 g for 30 min. The pellet was cell debris. The supernatant was centrifuged at 9,000 g for 20 min to prepare mitochondria. The supernatant was again centrifuged at 100,000 g for 90 min at 4°C with Ti 70 rotor. The remaining supernatant and pellet were cytosolic and microsomal fractions, respectively. Subsamples of each tissue homogenate and each of hepatic subcellular fraction (cell debris, mitochondria, cytosol and microsomes) were solubilized with Solvable (PerkinElpmer, Boston, MA) at 50 to 60°C for 1.5 – 2 h and decolorized by adding 30% hydrogen peroxide. Plasma and HDL fraction were analyzed by liquid scintillation counting (LSC) without further treatment. Ten ml of HionicFluor (PerkinElmer, Boston, MA) liquid scintillation cocktail was added. After decay of chemiluminescnce liquid scintillation counting was conducted using a Beckman LS7500 liquid scintillation counter. Protein concentration was determined by BCA assay (Pierce Biotechnology, Inc. Rockford, IL).
Gallbladders plus bile from mice killed 4 hr after lipid bolus injections were digested, decolorized, and analyzed by LSC for total [14C]CH equivalents as described above. Lipid and polar fractions of gallbladders plus bile from mice killed 16 hr after [14C]CH injections were separated by liquid-liquid extraction. Neutral steroids were extracted with slight modifications of the method described by Miettinen et al (1965). A 2.9 ml volume of basic (1.5 N KOH) ethanol/water (2:1) was added to each gallbladder plus bile sample which was heated at 70°C for 30 min. One ml of water was added to cooled samples which were then extracted with 2 ml of hexame. The hexane layer was removed and dried under a nitrogen stream. The residue was resuspended in 1 ml 2-propanol that contained 10% Triton X-100. Duplicate 0.1 ml samples of the ethanol/water phase (polar metabolites) and the hexane residues containing neutral steroids were analyzed by LSC.
Plasma lipid analyses
Total plasma CH concentrations were measured enzymatically by using 2 ul of plasma and 200 ul of CH reagent from Sigma Diagnostic (St. Louis, MO). HDL-CH concentration was measured after apolipoprotein-B containing particles precipitation with dextran sulfate and magnesium (Rachem, San Diego, CA). NonHDL-CH concentrations were taken as the difference between the two.
Western blot analysis
Liver crude membrane fractions were prepared as described (Voshol et al. 2001) from control or 15 mg CD/kg treated animals. Proteins were separated on Tris/glycine gels (Bio-Rad, Richmond, CA) under reducing conditions. Following gel electrophoresis at 100 V for 2 h, proteins were electrophoretically transferred onto PVDF membranes utilizing a Mini trans-blot transfer cell (Bio-Rad, Richmond, CA). Membranes were then blotted with rabbit anti-SR-BI (Abcam, Cambridge, MA) and probed with horseradish peroxidase conjugated goat anti-rabbit IgG. Membranes were also blotted with mice anti-ABCG8 (a generous gift from Professor Helen H. Hobbs, Univ. of Texas Southwestern Medical Center) and then probed with horseradish peroxidase conjugated rat anti-mouse IgG as the secondary antibody (Bio-Rad, Richmond, CA). The proteins were detected after development with enhanced chemiluminescence (ECL) detection (Amersham, Piscataway, NJ). Quantification of the intensity of the protein bands was performed with NIH-image software.
Biliary lipid analyses
Total CH was determined in the hexame extracts of bile treated with basic ethanol/water for 30 min at 70°C (detailed above). Duplicate subsamples in 2-propanol that contained 10% Triton X-100 were assayed with a Cholesterol Quantitation KitR BioVision, Mt. View, CA).
Statistical Methods
Statistical analyses were conducted with StatGraphics software (StatPoint, Herndon, VA). Normality of data sets were assessed with standardized kurtosis, standardized skewness, and homogeneity of variance. Data that failed one or more test for normality were log base 10 transformed. Transformed data were assessed for normality with the criteria specified above. Data were expressed as means ± SE. Comparisons among groups validated for normality were submitted to a one-way analysis of variance (ANOVA). For comparison between two groups, Student’s t-test with equal-variance was applied. In all analyses, a 95% confidential level was used as the criterion for significance. For outlier identification, Grubbs’ Test (assumes normality) was applied.
RESULTS
The mean body weights of the control, 5, and 15 mg/kg CD pretreated mice at 4 or 16 h after 10 mg [14C]CH/kg in 5 ml corn oil/kg (ip bolus lipid) were similar as were the liver weights (Table 1––1). No significant differences in gallbladder weights between control and CD pretreated mice were observed (Table 1). However, 16 h following ip bolus lipid dose, gallbladder weights were significantly smaller (~9 to 10 mg) than those at 4 h following ip bolus lipid dose (~15 to 17 mg) (Table 1).
Table 1.
Body and organ weight of vehicle control and CD pretreated mice that received a challenge dose of [14C]CH.
| Body mass (g) | Liver (g) | Gallbladder (mg) | ||||
|---|---|---|---|---|---|---|
| 4 hr | 16 hr | 4 hr | 16 hr | 4 hr | 16 hr | |
| Control | 22.2 ± 0.8 | 21.9 ± 0.4 | 1.21 ±0.03 | 1.19 ±0.05 | 15.0 ± 0.9 | 9.2 ± 0.5* |
| 5.0 mg CD/kg | 22.5 ± 0.7 | 21.8 ± 0.4 | 1.23 ±0.05 | 1.22 ±0.04 | 17.4 ± 0.7 | 10.7 ± 1.1* |
| 15.0 mg CD/kg | 22.9 ± 0.7 | 22.3 ± 0.3 | 1.32 ±0.05 | 1.22 ±0.02 | 16.2 ± 0.7 | 8.9 ± 0.5* |
The re were 6–7 mice in each group. Values are expressed mean ± SE.
Significantly different from the 4 hr (p < 0.05).
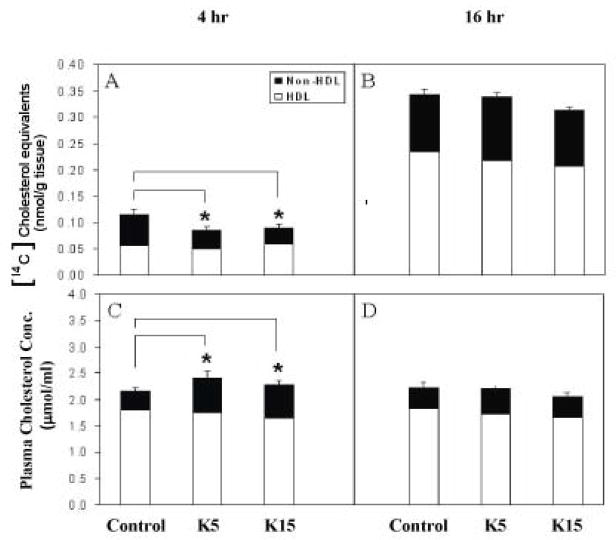
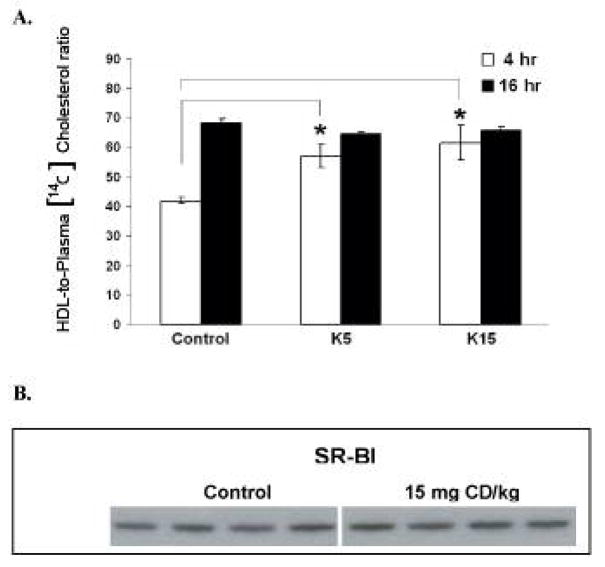
Plasma [14C]CH equivalents were approximately 3-fold higher at 16 h than at 4 h after the ip bolus lipid dose (p < 0.05, Fig. 1A, 1B), although plasma total CH concentration at 16 h was not different from that at 4 h after the ip bolus lipid (Fig. 1C, 1D). Plasma [14C]CH equivalents from CD (5 or 15 mg CD/kg) pretreated mice significantly decreased 4 h following the bolus lipid dose (31 %, p < 0.05)(Fig. 1A). CD pretreatment (5 or 15 mg CD/kg) significantly decreased [14C]CH equivalents in the non-HDL fraction compared with controls (45%, p < 0.05)(Fig. 1A). On the contrary, CD pretreatment increased total CH in non-HDL fraction 4 h following ip bolus lipid (about 1.8fold, p < 0.05)(Fig. 1C). At 16 h after the ip bolus lipid, neither [14C]CH equivalents nor total CH concentrations in plasma were altered by CD pretreatment (Fig. 1B, 1D). No significant differences in the [14C]CH equivalents and total CH in the either HDL or non-HDL fractions were observed 16 h following ip bolus lipid (Fig. 1B, 1D). Although the [14C]CH equivalents in the HDL fraction were unchanged, the ratio of [14C]CH equivalents in HDL fraction to that in plasma was mildly but statistically significantly increased by CD pretreatment 4 h after ip bolus lipid in a dose-dependent manner (Fig. 2A).
Figure 1. [14C]CH equivalents and total CH concentration in plasma of vehicle control and CD pretreated mice that received a challenge dose of [14C]CH.
Male C57BL16 mice were pretreated with corn oil, 5, or 15 mg CD/kg. [14C]CH (10 mg/kg in 5 ml/kg corn oil) was administered 3 days following pretreatment. Determinations were performed 4 and 16 h after ip administration of [14C]CH. [14C]CH equivalents of plasma and HDL were determined as described under “Material and methods”. Plasma total CH and HDLCH was determined enzymatically as described under “Material and Methods”. Non-HDL-CH content was taken as the difference between the total plasma CH and HDL-CH. A. [14C]CH equivalents of plasma 4 h after ip lipid bolus. B. [14C]CH equivalents of plasma 16 h after ip lipid bolus. C. Plasma total CH 4 h after ip lipid bolus. D. Plasma total CH 16 h after ip lipid bolus. Values are expressed mean ± SE(n=6 or 7). *Significantly different from the 4 h (p < 0.05).
Figure 2. The ratio of [14C]CH equivalents in HDL to that in plasma and hepatic SRBI contents.
Treatments were as described in Figure 1. A. The ratio of [14C]CH equivalents in HDL to that in plasma. Values are expressed as mean ± SE. B. Hepatic SRBI contents. Corn oil or 15 mg CD/kg was treated to C57BL/6 mice. Hepatic plasma membrane fractions were prepared from animals (6 mice in each group) as described under “material and methods”. Hepatic SR-BI content was measured by western blotting with SRBI antibody. There were 6–7 mice in each group.* Indicates a statistically significant difference (p < 0.05) when compared with control.
SR-BI was analyzed by immunoblotting with hepatic membranes from CD treated mice (15 mg/kg) or controls. Liver membrane contents of SRBI protein in CD-treated mice were not different from control animals (Fig. 2B).
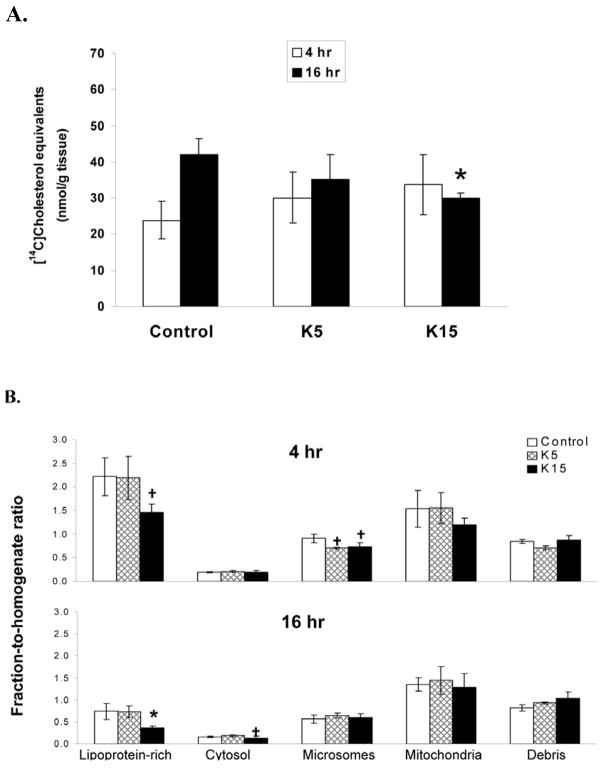
Consistent with previous results, CD decreased [14C]CH equivalents distribution to the liver at 16 h after the bolus lipid dose (43 %, p < 0.05)(Fig. 3A). Altered [14C]CH disposition in the liver of CD pretreated mice was further characterized in hepatic subcellular fractions (homogenate, lipoprotein-rich fraction, cytosol, microsomes, mitochondria and cell debris/nuclei). At 4 h after the bolus lipid dose, [14C]CH equivalents significantly decreased about 50 % in the microsomal fraction from CD (15 mg/kg) pretreated mice compared to control animals (10.1 ± 1.3 vs 5.9 ± 0.4 pmol/mg protein, p < 0.05). At 16 h after ip bolus lipid, [14C]CH equivalents significantly decreased about 30 % in hepatic cytosolic fraction from CD (15 mg/kg) pretreated mice compared to control animals ( 5.7 ± 0.4 vs 4.0 ± 0.3 pmol/mg protein, p < 0.05). To assess the hepatic compartmental re-distribution of 14C]CH, [14C]CH equivalents from each hepatic fraction were normalized with [14C]CH equivalents in homogenate. The ratios of [14C]CH lipoprotein-rich fraction-to-homogenate were lower at 16 h than at 4 h after the ip bolus lipid in both control and CD pretreated mice. Interestingly, 15 mg CD/kg pretreatment significantly reduced [14C]CH equivalents lipoprotein-rich fraction-to-homogenate ratio 16 h after ip bolus lipid, compared with control (about 50%, p < 0.05)(Fig. 3B). A trend for decreased [14C]CH in lipoprotein-rich and microsomal fraction-to-homogenate ratios at 4 and cytosolic fraction-to-homogenate ratios at 16 h after the ip bolus lipid in CD pretreated animals was observed (Fig. 3B).
Figure 3. Hepatic disposition of [14C]CH equivalents of vehicle control and CD pretreated mice that received a challenge dose of [14C]CH.
Treatments were as described in Figure 1. A. Hepatic [14C]CH equivalents 4 and 16 h after ip bolus lipid of [14C]CH. B. Hepatic subcellular fraction to homogenate ratios. Hepatic subcellular fractions were prepared and analyzed from animals (6 mice in each group) as described under “material and methods”. Data are normalized by the amount of [14C]CH equivalents per mg protein in the liver homogenate. Values are expressed as mean ± SE. There were 6–7 mice in each group.*Indicates a statistically significant difference (p < 0.05) when compared with control. Indicates a statistically difference (p< 0.1) when compared with control.
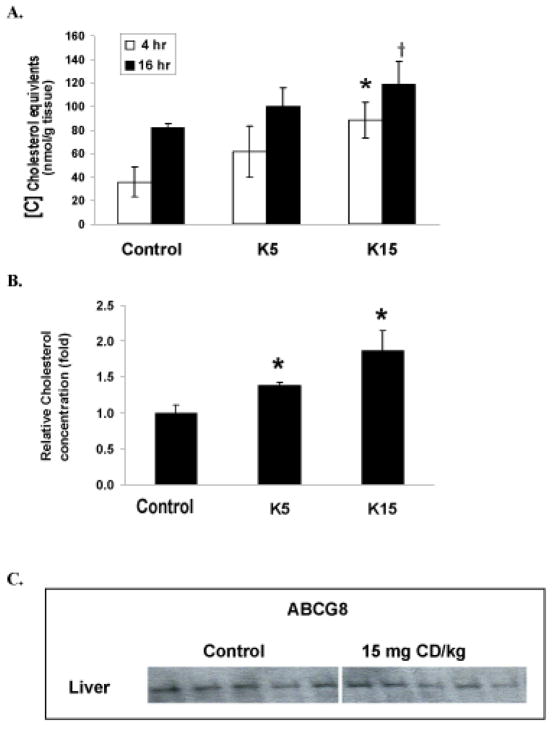
CD pretreatment increased [14C]CH equivalents of gallbladder 4 and 16 h after the ip bolus lipid dose, in a dosedependent manner (Fig. 4A). A statistically significant 300% increase total [14C]CH equivalents was observed 4 h after the tracer in 15 mg CD/kg pretreated animals (p < 0.05). A trend for increased [14C]CH equivalents in the neutral steroid fraction from gallbladders at 16 h after the ip bolus lipid in 15 mg CD/kg pretreated animals was observed (p < 0.056, Fig. 4A). Polar metabolites constituted 5.6-10.7% of total biliary [14C]CH equivalents at 16 hr after lipid bolus and there were no significant differences between treatment groups. The absolute polar biliary [14C]CH equivalents for control, 5 and 15 mg CD/kg pretreatments were 1.6, 1.7 and 0.9 nmol/g. CD pretreatment increased total CH concentration in gallbladder 16 h after lipid bolus compared with control (Fig. 4B). Immunoblot analysis for ABCG8 was conducted with hepatic membrane from CD treated mice (15 mg/kg) or controls. Liver membrane content of ABCG8 protein was unchanged by CD treatment (Fig. 4C). Statistically significant reductions of [14C]CH distribution to the kidney (48 %, p < 0.05) was observed in 15 mg CD/kg pretreated animals (data not shown). No significant change was observed in other tissues (data not shown).
Figure 4. [14C]CH equivalents and total cholesterol concentration in gallbladder and hepatic ABCG8 content.
Treatments were as described in Figure 1. A. Biliary [14C]CH equivalents 4 and 16 h after ip administration of [14C]CH. B. Biliary total CH concentrations were determined 16 h after ip administration of [14C]CH using an enzymatic method. C. Hepatic ABCG8 contents. Hepatic plasma membrane fractions were prepared from animals (6 mice in each group) as described under “material and methods”. Hepatic ABCG8 level was measured by western blotting with ABCG8 antibody. Values are expressed as mean ± SE. There were 6–7 mice in each group.* The asteric indicates a statistically significant difference (p < 0.05) when compared with control. +Indicates a statistically difference (p< 0.1) when compared with control.
DISCUSSION
The present results demonstrate that pretreatment with a single, dose of CD alters plasma (Figs. 1, 2), tissue and hepatic subcellular distribution of exogenously administered [14C]CH (Figs. 3, 4). There were a number of limitations in interpretation of plasma [14C]CH equivalents for explanation of CD-altered disposition of the exogenous lipid bolus. Total plasma [14C]CH equivalents included material: (1) newly absorbed from the peritoneal cavity associated with chylomicrons; (2) subjected to intraplasmic exchange between lipoprotein classes; and (3) secreted from the liver as nascent HDL or as VLDL. Nonetheless comparisons of data for plasma [14C]CH equivalents and plasma total CH in fractions treated with dextran sulfate and magnesium demonstrated CD-dependent differences (Fig. 1A, 1C). CD pretreatment decreased plasma non-HDL [14C]CH and increased plasma total non-HDL-CH 4 h after the ip lipid bolus dose (Fig. 1A, 1C). There were at least two mutually nonexclusive explanations. (1) CD delayed [14C]CH incorporation into non-HDL plasma lipoproteins, perhaps into hepatic VLDL synthesized for secretion. Exogenous CH occurred in a hepatic pool distinct from that synthesized in the liver (Oram and Vaughan 2006). Consistent with this, in control mice 4 h after the ip bolus lipid dose about 50 % of plasma [14C]CH equivalents were in the non-HDL fraction, while about 17 % of plasma total CH appeared in this fraction. (2) CD perhaps increased clearance of non-HDL-[14C]CH. Stimulated plasma clearance of non-HDL-[14C]CH seemed unlikely since total non-HDL-CH was elevated in CD pretreated mice 4 h after the bolus lipid (Fig. 1C). Plasma non-HDL-[14C]CH equivalents in CD pretreated mice were lower than controls 4 but not 16 h after the bolus lipid dose (Figs. 3A, 3B). This indicated that CD altered a CH exchange pathway or pool that delayed [14C]CH incorporation into nonHDL lipoproteins. At 16 h after ip lipid bolus dose [14C]CH appeared integrated into the total plasma CH pool (Fig. 1B). In fact, it appeared preferentially retained or secreted in the non-HDL pool since 32 to 35% of [14C]CH equivalents were in that fraction compared 18 to 20% of total CH at 16 h. Elevated total plasma non-HDL CH in CD pretreated mice 4 h after the ip lipid bolus (fig. 1C) was of specific interest. This reflected approximately doubled “bad CH” associated with promotion of atherosclerosis (Krieger, 1999). Plasma HDL contained 80–90% of total plasma CH in control mice (fig. 1C, 1D), typical for this species. Since non-HDL lipoproteins predominate in humans, the shift in plasma CH distribution after lipid bolus in CD pretreated mice was not directly applicable to human risk assessment. However, the results suggested work in animals with lipoprotein profiles similar to humans was warranted.
Hepatic [14C]CH equivalents were not different between control and CD pretreated mice at 4 h after ip lipid bolus dose. CD exhibited specific binding affinity with albumin and HDL and was preferentially accumulated in the liver (Soine et al. 1982). Gilroy et al. (1994) suggested a competitive interaction between CH and CD by the observation of decreased uptake of HDL-bound [14C]CH in the CD treated perfused rat liver. CD was an agonist for the human pregnane × receptor (PXR) in a reporter gene assay and this was supported by CYP3A protein induction in mouse liver by CD (Lee et al., unpublished data). PXR agonists but not a selective constitutive androstane receptor agonist increased expression of the apoA-I gene in mice (Bachmann et al. 2004), the principal component of HDL. Sporstol et al. (2005) reported the down regulation of SR-BI by a PXR agonist in vitro. The HDL receptor SR-BI plays critical roles in the uptake of plasma CH by the liver. Therefore, hepatic SR-BI protein content was determined, this assessed whether hepatic uptake of HDL-CH was important in alteration of plasma CH by CD. SR-BI content in hepatic plasma membrane was not changed by CD treatment (Fig. 2B). Even though SR-BI was a physiological HDL receptor, it exhibited binding affinity for HDL, LDL, and VLDL (Acton et al. 1996; Calvo et al. 1998). Over expression of SRBI in the liver increased the clearance of LDL and VLDL (Wang et al. 1998). More non-spherical and heterogeneous HDL particles were observed in plasma from CD treated animals compared with controls (Lee et al., unpublished data). Therefore it was possible that CD binding to HDL particles perturbed the SR-BI mediated selective cholesterol ester uptake from HDL while clearance of LDL and VLDL was not affected. However it seemed unlikely since total non-HDL-CH was elevated in CD pretreated mice 4 h after the bolus lipid (Fig. 1C). Lower lipoprotein-rich fraction-to-liver homogenate [14C]CH equivalents ratios in 15 mg CD/kg pretreated mice were consistent with suppression of incorporation of exogenous CH into lipoprotein complexes (Fig. 2–3B). Markedly reduced [14C]CH lipoprotein-rich fraction-to-homogenate ratio from 4 to 16 h after the bolus lipid dose in both control and CD pretreated mice suggested the secretory phase peaked before 16 h (Fig. 3B). Elevation in plasma non-HDL-total CH at 4 h compared to 16 h after the lipid dose (Figs. 3C, 3D) in CD pretreated mice supported this interpretation.
Gallbladder bile of mice pretreated with 15 mg CD/kg contained 3-fold more [14C]CH equivalents than controls 4 h after the ip lipid bolus. A trend for this persisted after 16 h but was not statistically significant. Since gallbladder weight was lower at 16 than 4 h after the lipid bolus (Table 1) leakage or ejection of bile probably occurred more extensively before the later than the earlier sampling time. This likely reduced accuracy of gallbladder bile [14C]CH equivalents for estimation of biliary secretion at 16 compared to 4 h after the ip lipid bolus. Polar [14C]CH equivalents in gallbladder bile 16 hr after ip lipid bolus were unchanged (Results). This indicated CD stimulated biliary CH excretion itself, not CH metabolism to bile salts or other polar metabolites. Biliary CH secretion is one of the major pathways to eliminate excess CH from body. Heterodimers of ABCG5 and ABCG8 regulate and the whole-body retention of plant sterols and promote hepatobiliary secretion of CH (Kosters et al. 2006; Yu et al. 2002). CH feeding upregulates expression of ABCG5/ABCG8 coordinately through activation of LXR (liver X receptor) although the binding sites of LXR to these genes have not been mapped (Repa et al. 2002). The mRNA level of ABCG5/ABCG8 was higher in the liver of mice lacking LXRα/LXRβ (Repa et al. 2002). CD moderately inhibited LXRα activation and strongly suppressed LXRβ activation in a reporter gene assay (Lee et al., unpublished data). We hypothesized that increased basal activity of ABCG5/ABCG8 by CD stimulated biliary CH excretion. Hepatic membrane ABCG8 protein contents were not different between controls and 15 mg CD/kg treatment (Fig. 2–4C). CD pretreatment increased not only [14C]CH equivalents but also total CH in gallbladder 4 and 16 h after the ip lipid bolus dose, respectively. This indicated that the lipid bolus dose affected biliary CH secretion in CD pretreated mice compared with controls. Gallbladder bile total CH was not increased in CD pretreated mice compared to controls in animals that received no ip lipid bolus dose (Lee et al., unpublished data). Therefore it was possible that CD pretreatment and ip bolus lipid dose somehow synergistically stimulated an ABCG5/8-dependent or ABCG5/8-independent pathway of biliary CH secretion. Another possible explanation was SRBII mediated stimulation of biliary CH excretion. SR-BII is an alternatively spliced form of SR-BI. SR-BII is expressed intracellulary and suggested to mediate the rapid internalization of HDL for biliary excretion (Eckhardt et al. 2004). Although HDL was an important source of CH for biliary excretion in rodents, HDL-CH was the preferred substrate for bile acid synthesis. CH is secreted into bile after arrival at the canalicular membrane. An endocytic/retroendocytic pathway of HDL has been suggested to play a important role for the delivery of CH to the apical membrane for release into the bile (Wustner et al. 2004). In addition to membrane transporter, cytoplasmic proteins such as liver fatty acid-binding protein and sterol carrier protein 2 were suggested to play a role in the intracellular transport and biliary CH secretion (Kosters et al. 2005). Essentially all CD existed in pig liver cytosol in a protein-bound form (Soine et al. 1982). [14C]CH equivalents significantly decreased in the hepatic microsomal (~ 50 %) and cytosolic (~30%) fractions from CD pretreated mice compared to control animals. Sonie et al. (1982) suggested that CD and CH shared a common transport pathway in liver cytosol and CD interacted with CH transport and metabolism since isolated-CD binding proteins bind both CD and CH (Soine et al. 1984b). Decreased hepatic disposition of [14C]CH equivalents in the microsomal fraction suggested that binding of CD to cytosolic proteins perhaps not only inhibited CH transport to microsomes but also modulated biliary CH secretion. However, a definitive explanation for increased biliary CH excretion in CD pretreated mice remains elusive.
Chronic exposures of humans and wildlife via tophic transfer through food webs is the most widestpread pathway of exposure to persistent organic pollutants, including OC insecticides. Residues occur as complex mixtures (Lordo et al, 1996) and direct extrapolation of results after ip administration to a single compound is tenuous. There is value in illucidation of potential modes of action, however. The ip route of exposure in mice yielded a hepatic CD concentration quite similar to that in rats after dietary exposure. Ten mg CD/kg ip in mice yielded 75 ppm in liver 3 days after dosing (Carpenter and Curtis, 1989). Livers of rats fed a diet that contained 10 pm CD for 15 days accumulated 52 ppm CD (Curtis and Mehendale, 1981). To the extent that liver OC concentration mediates pathophysiological changes, ip administration provides a convenient alternative to longterm feeding studies.
In summary, CD pretreatment altered the plasma, tissue and hepatic subcellular disposition of subsequently administered [14C]CH. Plasma non-HDL CH almost doubled 4 h after a bolus lipid dose without a significant change in HDL total CH. Our data demonstrated that SR-BI and ABCG8 protein contents in hepatic plasma membranes were unchanged by CD treatment indicating that mechanisms other than SR-BI or ABCG8 may be involved in modulating CD induced CH homeostasis and lipoprotein metabolism.
Acknowledgments
This work was supported by the Oregon Agricultural Experiment Station (ORE00871) and grant number T32 ES007060 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SRBI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- Bachmann K, Patel H, Batayneh Z, Slama J, White D, Posey J, Ekins S, Gold D, Sambucetti L. PXR and the regulation of apoA1 and HDL-cholesterol in rodents. Pharmacol Res. 2004;50:237–246. doi: 10.1016/j.phrs.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Barnhill ML, Rosemond MVM, Curtis LR. Dieldrin stimulates biliary excretion of 14C-benzo [a] pyrene polar metabolites but does not change the biliary metabolite profile in rainbow trout (Oncorhynicus mykiss) Toxicol Sci. 2003;75:249–259. doi: 10.1093/toxsci/kfg192. [DOI] [PubMed] [Google Scholar]
- Bocquene G, Franco A. Pesticide contamination of the coastline of Martinique. Mar Pollut Bull. 2005;51:612–619. doi: 10.1016/j.marpolbul.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- Calvo D, Gomez-Coronado D, Suarez Y, Lasuncion MA, Vega MA. Human CD36 is a high affinity receptor for the native lipoproteins HDL, LDL, and VLDL. J Lipid Res. 1998;39:777–788. [PubMed] [Google Scholar]
- Carpenter HM, Curtis LR. A characterization of chlordecone pretreatment-altered pharmacokinetics in mice. Drug Metab Dispos. 1989;17:131–138. [PubMed] [Google Scholar]
- Carpenter HM, Curtis LR. Low dose chlordecone pretreatment altered cholesterol disposition without induction of cytochrome P-450. Drug Metab Dispos. 1991;19:673–678. [PubMed] [Google Scholar]
- Chen J, Raymond K. Nuclear receptors, bile-acid detoxification, and cholestasis. Lancet. 2006;367:454–456. doi: 10.1016/S0140-6736(06)68156-7. [DOI] [PubMed] [Google Scholar]
- Cohn WJ, Boylan JJ, Blanke RV, Fariss MW, Howell JR, Guzelian PS. Treatment of chlordecone (Kepone) toxicity with cholestyramine. Results of a controlled clinical trial. N Engl J Med. 1978;298:243–248. doi: 10.1056/NEJM197802022980504. [DOI] [PubMed] [Google Scholar]
- Curtis LR, Mehendale HM. Hepatobiliary dysfunction and inhibition of adenosine triphosphatase activity of bile canaliculi enriched fraction following in vivo mirex, photomirex and chlordecone exposures. Toxivol Appl Pharmacol. 1981;61:429–440. doi: 10.1016/0041-008x(81)90366-5. [DOI] [PubMed] [Google Scholar]
- Donohoe RM, Zhang Q, Siddens LK, Carpenter HM, Hendricks JD, Curtis LR. Modulation of 7,12-dimethylbenz[a]anthracene disposition and hepatocarcinogenesis by dieldrin and chlordecone in rainbow trout. J Toxicol Environ Health A. 1998;54:227–242. doi: 10.1080/009841098158926. [DOI] [PubMed] [Google Scholar]
- Eckhardt ER, Cai L, Sun B, Webb NR, van der Westhuyzen DR. High density lipoprotein uptake by scavenger receptor SR-BII. J Biol Chem. 2004;279:14372–14381. doi: 10.1074/jbc.M313793200. [DOI] [PubMed] [Google Scholar]
- Egle JL, Fernandez JB, Guzelian PS, Borzelleca JF. Distribution and excretion of chlordecone (Kepone) in the rat. Drug Metab Dispos. 1978;6:91–95. [PubMed] [Google Scholar]
- Fielding CJ, Fielding PE. Role of an N-ethylmaleimide-sensitive factor in the selective cellular uptake of lowdensity lipoprotein free cholesterol. Biochemistry. 1995;34:14237–14244. doi: 10.1021/bi00043a031. [DOI] [PubMed] [Google Scholar]
- Gilroy DJ, Carpenter HM, Curtis LR. Chlordecone pretreatment alters [14C]chlordecone and [14C]cholesterol transport kinetics in the perfused rat liver. Fundam Appl Toxicol. 1994;22:286–292. doi: 10.1006/faat.1994.1032. [DOI] [PubMed] [Google Scholar]
- Guzelian PS. Comparative toxicology of chlordecone (kepone) in humans and experimental animals. Ann Rev Pharmacol Toxicol. 1982;22:89–113. doi: 10.1146/annurev.pa.22.040182.000513. [DOI] [PubMed] [Google Scholar]
- Hobbs HH, Rader DJ. ABC1: connecting yellow tonsils, neuropathy, and very low HDL. J Clin Invest. 1999;104:1015–1017. doi: 10.1172/JCI8509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, McNeeley S, Steiner PM, Glueck CJ, Mellies M, Gartside PS, McMillin C. Effects of chlorinated hydrocarbon on plasma alpha-lipoprotein cholesterol in rats. Metabolism. 1978;27:89–96. doi: 10.1016/0026-0495(78)90127-0. [DOI] [PubMed] [Google Scholar]
- Kosters A, Frijters RJ, Kunne C, Vink E, Schneiders MS, Schaap FG, Nibbering CP, Patel SB, Groen AK. Diosgenin-induced biliary cholesterol secretion in mice requires Abcg8. Hepatology. 2005;41:141–150. doi: 10.1002/hep.20540. [DOI] [PubMed] [Google Scholar]
- Kosters A, Kunne C, Looije N, Patel SB, Oude Elferink RP, Groen AK. The mechanism of ABCG5/ABCG8 in biliary cholesterol secretion in mice. J Lipid Res. 2006;47:1959–1966. doi: 10.1194/jlr.M500511-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger M. Charting the fate of the "good cholesterol": identification and characterization of the highdensity lipoprotein receptor SR-BI. Annu Rev Biochem. 1999;68:523–558. doi: 10.1146/annurev.biochem.68.1.523. [DOI] [PubMed] [Google Scholar]
- Lee JY, Parks JS. ATP-binding cassette transporter AI and its role in HDL formation. Curr Opin Lipidol. 2005;16:19–25. doi: 10.1097/00041433-200502000-00005. [DOI] [PubMed] [Google Scholar]
- Lindstrom FT, Gillett JW, Rodecap SE. Distribution of HEOD (dieldrin) in mammals. I. Preliminary model. Arch Environ Contam Toxicol. 1974;2:9–42. doi: 10.1007/BF01985798. [DOI] [PubMed] [Google Scholar]
- Lordo RA, Dinh KT, Schwemberger JG. Semivolatile organic compounds in adipose tissue: estimated averages for the US population and selected subpopulations. Amer J Pb Hlth. 1996;86:1253–1259. doi: 10.2105/ajph.86.9.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luellen DR, Vadas GG, Unger MA. Kepone in James River fish: 1976–2002. Sci Total Environ. 2006;358:286–297. doi: 10.1016/j.scitotenv.2005.08.046. [DOI] [PubMed] [Google Scholar]
- Mick DL, Long KR, Dretchen JS, Bonderman DP. Aldrin and Dieldrin in human blood components. Arch Environ Health. 1971;23:177–180. doi: 10.1080/00039896.1971.10665982. [DOI] [PubMed] [Google Scholar]
- Miettinen TA, Ahrens EH, Grundy SM. Quantitative isolation and gas-liquid chromatographic analysis of total dietary and fecal neutral steroids. J Lipid Res. 1965;6:411–424. [PubMed] [Google Scholar]
- Oram JF, Vaughan AM. ATP-Binding cassette cholesterol transporters and cardiovascular disease. Circ Res. 2006;99:1031–1043. doi: 10.1161/01.RES.0000250171.54048.5c. [DOI] [PubMed] [Google Scholar]
- Out R, Hoekstra M, Spijkers JA, Kruijt JK, van Eck M, Bos IS, Twisk J, Van Berkel TJ. Scavenger receptor class B type I is solely responsible for the selective uptake of cholesteryl esters from HDL by the liver and the adrenals in mice. J Lipid Res. 2004;45:2088–2095. doi: 10.1194/jlr.M400191-JLR200. [DOI] [PubMed] [Google Scholar]
- Poland A, Knutson JC. 2, 3, 7, 8- Tetrachlordibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Ann Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Repa JJ, Berge KE, Pomajzl C, Richardson JA, Hobbs H, Mangelsdorf DJ. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J Biol Chem. 2002;277:18793–18800. doi: 10.1074/jbc.M109927200. [DOI] [PubMed] [Google Scholar]
- Soine PJ, Blanke RV, Chinchilli VM, Schwartz CC. Highdensity lipoproteins decrease the biliary concentration of chlordecone in isolated perfused pig liver. J Toxicol Environ Health. 1984a;14:319–335. doi: 10.1080/15287398409530583. [DOI] [PubMed] [Google Scholar]
- Soine PJ, Blanke RV, Guzelian PS, Schwartz CC. Preferential binding of chlordecone to the protein and high density lipoprotein fractions of plasma from humans and other species. J Toxicol Environ Health. 1982;9:107–118. doi: 10.1080/15287398209530146. [DOI] [PubMed] [Google Scholar]
- Soine PJ, Blanke RV, Schwartz CC. Isolation of chlordecone binding proteins from pig liver cytosol. J Toxicol Environ Health. 1984b;14:305–317. doi: 10.1080/15287398409530582. [DOI] [PubMed] [Google Scholar]
- Sporstol M, Tapia G, Malerod L, Mousavi SA, Berg T. Pregnane X receptor-agonists downregulate hepatic ATP-binding cassette transporter A1 and scavenger receptor class B type I. Biochem Biophys Res Commun. 2005;331:1533–1541. doi: 10.1016/j.bbrc.2005.04.071. [DOI] [PubMed] [Google Scholar]
- Tabas I. Cholesterol in health and disease. J Clin Invest. 2002;110:583–590. doi: 10.1172/JCI16381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voshol PJ, Schwarz M, Rigotti A, Krieger M, Groen AK, Kuipers F. Down-regulation of intestinal scavenger receptor class B, type I (SRBI) expression in rodents under conditions of deficient bile delivery to the intestine. Biochem J. 2001;356:317–325. doi: 10.1042/0264-6021:3560317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Arai T, Ji Y, Rinninger F, Tall AR. Liver-specific overexpression of scavenger receptor BI decreases levels of very low density lipoprotein ApoB, low density lipoprotein ApoB, and high density lipoprotein in transgenic mice. J Biol Chem. 1998;273:32920–32926. doi: 10.1074/jbc.273.49.32920. [DOI] [PubMed] [Google Scholar]
- Wustner D, Mondal M, Huang A, Maxfield FR. Different transport routes for high density lipoprotein and its associated free sterol in polarized hepatic cells. J Lipid Res. 2004;45:427–437. doi: 10.1194/jlr.M300440-JLR200. [DOI] [PubMed] [Google Scholar]
- Yu L, Gupta S, Xu F, Liverman AD, Moschetta A, Mangelsdorf DJ, Repa JJ, Hobbs HH, Cohen JC. Expression of ABCG5 and ABCG8 is required for regulation of biliary cholesterol secretion. J Biol Chem. 2005;280:8742–8747. doi: 10.1074/jbc.M411080200. [DOI] [PubMed] [Google Scholar]
- Yu L, Hammer RE, Li-Hawkins J, Von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc Natl Acad Sci U S A. 2002;99:16237–16242. doi: 10.1073/pnas.252582399. [DOI] [PMC free article] [PubMed] [Google Scholar]