Summary
Tumor inflammation promotes angiogenesis, immunosuppression and tumor growth, but the mechanisms controlling inflammatory cell recruitment to tumors are not well understood. We found that a range of chemoattractants activating G-protein coupled receptors (GPCRs), receptor tyrosine kinases (RTKs) and Toll-like/IL-1 receptors (TLR/IL1Rs) unexpectedly initiate tumor inflammation by activating the PI3-kinase isoform p110γ in Gr1+CD11b+ myeloid cells. Whereas GPCRs activate p110γ in a Ras/p101 dependent manner, RTKs and TLR/IL1Rs directly activate p110γ in a Ras/p87-dependent manner. Once activated, p110γ promotes inside-out activation of a single integrin, α4β1, causing myeloid cell invasion into tumors. Pharmacological or genetic blockade of p110γ suppressed inflammation, growth and metastasis of implanted and spontaneous tumors, revealing an important therapeutic target in oncology.
Introduction
Cancer and inflammation are intricately linked, as chronic inflammatory diseases such as Crohn s disease and Barrett s esophagus increase the risk of developing tumors (Grivennikov et al., 2010). Tumors induce host inflammatory responses that stimulate angiogenesis (De Palma et al., 2005; Du et al., 2008; Grunewald et al., 2006; Lin et al., 2006; Shojaei et al., 2007) immunosuppression (Bronte et al, 2000; Bunt et al., 2006; DeNardo et al., 2010; Gabrilovich and Nagaraj, 2009; Yang et al., 2006), and tumor metastasis (Kim et al., 2009). Neutrophils, monocytes and myeloid derived suppressor cells invade the tumor microenvironment in response to diverse tumor-derived chemoattractants, including chemokines, cytokines and growth factors. Myeloid cells may differentiate into tumor-associated macrophages (TAMs) or tumor-associated neutrophils (TANs), which express pro-angiogenic and immunosuppressive factors, thereby promoting tumor growth (Biswas and Mantovani, 2010; Fridlender et al., 2009; Lazennic and Richmond, 2010; Yang et al., 2010) and relapse after therapy (Ferrara, 2010). Thus, targeting tumor inflammation could provide substantial therapeutic benefit to cancer patients. However, effective suppression of tumor inflammation could require identification and targeting of mechanisms common to the many inflammatory pathways that are activated during tumor growth.
One family of signaling proteins implicated in inflammatory responses is the Class I PI3K family. This group of kinases is comprised of four catalytic subunit family members that phosphorylate PtdIns (4,5)P2 on the 3 hydroxyl position of the inositol ring to produce PtdIns (3,4,5)P3 (Vanhaesebroeck, et al., 2010). PI(3,4,5)P3 interacts with plextrin homology and other lipid-binding domains, promoting protein localization to membranes and protein activation. Current models hold that the Class IA PI3K isoforms p110α, β and δ are activated downstream of receptor tyrosine kinases (RTKs) through the engagement of the regulatory p85 subunit by receptor phosphotyrosines (Carpenter et al., 1993). In contrast, the Class IB isoform p110γ is activated by G-protein coupled receptors (GPCRs) via the β–γ subunits of heterotrimeric G proteins. Activated p110γ promotes chemotaxis and polarization of neutrophils in response to GPCR ligands, such as chemokines (Sasaki et al., 2000; Li et al., 2000, Hirsch et al., 2000).
The integrin family of adhesion proteins also plays key roles in inflammation (Lobb and Hemler, 1994; Rose et al., 2007; Jin et al., 2006). Activation of integrin α4β1 by inside-out signaling is required for lymphocyte extravasation (Feral et al., 2006; Rose et al., 2007). While extracellular stimuli induce conformational changes and activation in integrins (Arnaout et al., 2005; Luque et al., 1996), the signaling mechanisms by which integrins are activated are not well understood.
In the studies described here, we investigate the mechanisms that control tumor inflammation and growth by examining the roles of molecular signals that are commonly activated by diverse tumor-derived chemoattractants, including RTKs, Toll-like/IL1 receptors (TLR/IL1Rs) or GPCRs.
Results
To identify pathways that regulate immune cell trafficking during tumor inflammation, we characterized the extent and duration of myeloid cell recruitment to human and murine tumors. CD11b+ myeloid cells extensively populated spontaneous or orthotopic murine and human breast, pancreatic, and lung carcinomas but not corresponding normal tissues (Fig. 1A, S1A). These cells persistently invaded growing tumors over time until as much as 25% of a tumor s mass was comprised of myeloid cells (Fig. 1B-C). Furthermore, tumor inflammation was directly proportional to angiogenesis throughout the growth of the tumor (Fig.1B, S1B). Tumor-associated myeloid cells, which were isolated by proteolytic digestion of primary tumors and quantified by flow cytometry, primarily consisted of Gr1lo/negCD11b+ F4/80+ macrophages and a much smaller population of granulocytes (Fig.1C). In contrast, myeloid cells in peripheral blood (PB) and bone marrow (BM) of normal and tumor-bearing animals were comprised primarily of Gr1hiCD11b+ granulocytes (80%) and a smaller population of Gr1loCD11b+ monocytes (20%) (Fig.1D). However, the absolute number of myeloid cells in PB, BM and tumors increased progressively during tumor development, indicating that the host inflammatory response is an early and ongoing systemic process throughout tumor development (Fig.S1C-D). While both Gr1hi and Gr1lo subpopulations are able to enter tumors when adoptively transferred into tumor-bearing mice (Fig. S1E), only Gr1lo cells persist in tumors (Fig.1C-D). Together, these results indicate that macrophages are the major population of myeloid cells in tumors and that they are recruited throughout tumor growth.
Figure 1. Diverse tumor-derived chemoattractants promote myeloid cell trafficking to tumors.
(A) Left, CD11b+ pixels/field in normal human and murine breast and invasive ductal breast carcinoma, normal mouse pancreas and orthotopic Panc02 pancreatic carcinoma, and normal murine lung and orthotopic LLC (n=6–10), *p<0.001 vs normal tissue. Right, CD11b+ cells (red, arrowheads) and nuclei (blue) in normal murine and human breast and invasive ductal carcinoma; scale bars, 40 μm. (B) Graphs, quantification of myeloid (CD11b) and endothelial (CD31) cells over time in LLC tumors, *p<0.05 (n=10). Images, LLC tumor sections immunostained to detect myeloid (CD11b) and endothelial (CD31) cells; scale bars, 40 μm. (C) Flow cytometric quantification of Gr1+CD11b myeloid cells in tumors (n=3). Tumor-derived myeloid cells are comprised primarily of Gr1lo/negCD11b+F4/80+CD14+MHCII+ monocyte/macrophages. (E) Relative levels of chemoattractant gene expression in CD11b+ myeloid cells and CD11b- tumor cells from 14d orthotopic LLC tumors (n=4), *p<0.05 vs normal lung. (F) SDF-1α and IL-1β protein expression in tumor-derived CD11b+ myeloid and tumor cells from 14d LLC tumors (n=3), *p<0.05. See also Figure S1.
To investigate whether specific chemoattractants recruit myeloid cells to the tumor microenvironment, we determined which chemoattractants were commonly expressed in tumors. Orthotopic murine Lewis lung carcinomas (LLC) (Fig. S1F) and pancreatic carcinomas (not shown) expressed increasing levels of Sdf-1α, Vegf-A, Tnfα, Il-1β and Il-6 during tumor growth. Surprisingly, tumor-derived myeloid cells were the exclusive source of Il-1β and Il-6 in these tumors, while tumor cells were the exclusive source of Sdf-1α (Fig.1E-F). Importantly, a significant fraction of Vegf-A expressed in these tumors was derived from myeloid cells (Fig.1E). Indeed, previous studies have shown the important role of VEGF-A-expressing myeloid cells in tumor angiogenesis (Du et al., 2008). Our studies show that inflammatory factors in the tumor microenvironment derive from both tumor and inflammatory cells.
A single integrin, α4β1, promotes myeloid cell trafficking to tumors
As most tumors produce multiple chemoattractants, blockade of individual chemoattractants may not suppress tumor inflammation. Therefore, we sought to determine whether a common mechanism regulates myeloid cell recruitment to tumors. To exit the blood stream in response to signals released from diseased tissues, immune cells transiently adhere to and transmigrate through vascular endothelium. As immune cell extravasation depends on adhesion to endothelial cell (EC) receptors such as selectins, VCAM or ICAM (Lobb and Hemler 1994; Rose et al., 2007), we tested the ability of chemoattractants to promote myeloid cell adhesion to EC in vivo and in vitro. Primary Gr1hiCD11b+ and Gr1loCD11b+ myeloid cells isolated from BM of either normal or tumor-bearing (Fig. 2A,S2A) mice adhered strongly to ECs in vivo and in vitro after stimulation by diverse tumor-derived chemoattractants [SDF-1α, IL-1β, IL-6, VEGF-A, TNFα, and LLC conditioned medium (TCM), which primarily contains SDF-1α, VEGF-A and TGFβ].
Figure 2. Diverse tumor-derived chemoattractants promote integrin α4β1.
dependent myeloid cell trafficking (A) Adhesion of stimulated myeloid cells to EC in the presence of medium (untreated), control IgG (cIgG), anti-α4 or anti-αM integrin antibody, or small molecule inhibitor of integrin α4 (ELN476063) (n=3), *p<0.001 vs IgG. (B) Adhesion to EC of stimulated WT, α4Y991A, α4-/−, αM-/− and integrin α4 (Itga4) or αM (Itgam) siRNA transfected myeloid cells (n=3), *p<0.001 vs WT. (C) Left: HUTS21 antibody (activated integrin β1) binding to unstimulated, SDF-1α, IL1β, or Mn2+ stimulated human CD11b+ cells. Right: Mean fluorescence intensity per stimulus (MFI). (D) MFI of VCAM-1/Fc binding to stimulated WT or α4Y991A myeloid cells (n=3), *p<0.01 vs WT. (E) Trafficking to LLC tumors of WT, α4Y991A, integrin α4-/−, integrin αM-/− or α4 (itga4), αM (itgam) or non-silencing siRNA transfected myeloid cells (n=3–6), *p<0.001 vs WT cells. See also Figure S2.
Myeloid cells express two receptors (integrins α4β1 and αMβ2) that recognize the EC surface adhesion proteins VCAM-1 and ICAM-1 (Lobb and Hemler 1994; Rose et al., 2007). Antibody and peptide inhibitors of integrin α4β1 (Konradi et al., 2006, but not αMβ2, suppressed murine (Fig.2A, Fig.S2B) and human (Fig.S2C) myeloid cell adhesion to ECs and to recombinant VCAM-1, whereas antibody inhibitors of integrins α5β1, αvβ5 and αvβ3 had no effect on adhesion (not shown). Importantly, α4Y991A Gr1+CD11b+ cells isolated from mice with an inactivating point mutation in the α4 integrin cytoplasmic tail (Feral et al., 2006; Manevich et al., 2007) and α4−/− Gr1+CD11b+ cells isolated from the BM of Tie2Cre α4loxp/loxp mice (Scott et al., 2003), failed to adhere to ECs or VCAM-1, while αM−/− cells adhered normally to ECs and VCAM-1 but not to ICAM-1 (Fig.2B, S2D-G). Murine myeloid cells in which integrin α4 expression was ablated by siRNA also failed to adhere to EC and VCAM-1, while αM siRNA-transfected cells adhered normally (Fig. 2B, S2H-I). As chemoattractants had no effect on EC expression of α4 ligands during these assays (Fig.S2J), these results indicate that diverse chemoattractants stimulate myeloid cell adhesion by selectively increasing integrin α4β1 but not αMβ2 activity.
Extracellular stimuli induce integrin conformational changes that result in increased ligand-binding and cell adhesion, a process that is called “integrin activation” (Arnaout et al., 2005). We found that tumor-derived chemoattractants rapidly induced myeloid cell integrin β1 conformational changes, as measured by cell surface binding of HUTS21 (Luque et al., 1996), an antibody that recognizes an epitope expressed only on activated human β1 integrin (Fig.2C). These chemoattractants also rapidly stimulated the binding of VCAM-1 to myeloid cells from WT but not integrin α4Y991A mice (Fig.2D). Together, these results indicate that chemokines, cytokines and growth factors that activate RTKs, TLR/IL1Rs and GPCRs all activate integrin α4β1, thereby promoting murine and human myeloid cell adhesion to ECs.
To determine if integrin α4 activation is required for trafficking of myeloid cells to tumors in vivo, we adoptively transferred fluorescently labelled WT, α4Y991A, α4−/−, αM−/− and α4 and αM siRNA transfected myeloid cells into LLC-bearing WT mice. While WT, αM−/− and αM siRNA transfected cells arrested in tumors, cells with defective or ablated integrin α4β1 did not arrest (Fig.2E). These results provide evidence that integrin α4 activation is required for trafficking and infiltration of myeloid cells into tumors in vivo.
PI3-Kinase p110γ is necessary and sufficient for activation of myeloid cell integrin α4β1
Our results show that chemoattractants stimulating structurally diverse GPCRs, RTKs, TLR/IL1Rs and type I cytokine receptors, all activate myeloid cell integrin α4β1, indicating that a common downstream signalling pathway may link these receptors. To identify such a pathway, we evaluated inhibitors of various signaling pathways in myeloid cell adhesion assays. Selective inhibitors of PI3Kγ (p110γ) and Ras GTPases blocked myeloid cell adhesion to endothelium, while inhibitors of other PI3K isoforms and signaling proteins had no effect on adhesion (Fig. S3A). As we found that chemoattractant-induced adhesion to ECs is integrin α4β1-dependent, these results implied that p110γ activity is required for α4β1–mediated adhesion.
The class IB PI3K p110γ is well described as a GPCR-activated lipid kinase that promotes chemokine-stimulated chemotaxis and polarization of neutrophils, lymphocytes and thymocytes in vitro and in vivo (Sasaki et al., 2000; Li et al., 2000; Hirsch et al., 2000). Importantly, mice lacking p110γ (p110γ−/−) exhibit defects in granulocyte responses to chemokines, which signal through GPCRs (Sasaki et al., 2000; Li et al., 2000; Hirsch et al., 2000), as do p110γ kinase-dead (KD) mice, which express a K833R knockin mutation that inactivates p110γ catalytic activity without reducing its expression (Patrucco et al., 2004). Previous studies have found p110γ is essential for GPCR- but not RTK-mediated activation of PI3K activity (Sasaki et al., 2000; Chang et al, 2007). Our observation that p110γ inhibitors blocked adhesion in response to GPCR as well as RTK ligands was therefore unexpected. We investigated these results more extensively using p110γ specific knockout, knockin, and siRNA knockdown methods.
We found that growth factors, interleukins and chemokines, which are ligands for RTKs, TLR/IL-1Rs and GPCRs, promoted adhesion of WT but not of p110γ−/− or p110γKD myeloid cells to ECs or VCAM-1 (Fig.3A). As p110γ−/− and p110γKD myeloid cells express normal levels of α4 integrin (Fig. S3B), these results indicate that p110γ is necessary for integrin adhesive activity. siRNA knockdown of p110γ but not other PI3K catalytic subunits also suppressed adhesion to ECs or VCAM-1, regardless of the stimulus (Fig.3B, S3C). Selective inhibitors of p110γ, including TG100-115 (Fig. S3D-E) and AS605240 (Fig. S3E) (Camps et al., 2005; Doukas et. al., 2006; Palanki et al., 2007) suppressed p110γ activity (as measured by pAkt levels) (Fig. S3F) and blocked murine and human cell adhesion to ECs or VCAM, while inhibitors of p110β (TGX221) or p110α (PI3Kalpha) had minimal effects (Fig.3C-D, S3G). Furthermore, integrin α4 activation, as detected in human cells by HUTS21 antibody (Fig. S3H) and in murine cells by VCAM-1 binding (Fig. 3E), was also suppressed in p110γ inhibitor-treated and in p110γ-/− myeloid cells. Although prior studies have indicated that only GPCR ligands activate p110γ, our results indicate that ligands for RTKs and TLR/IL1Rs promote p110γ activity and p110γ–dependent integrin α4β1 activation. These results indicate p110γ is necessary for growth factor, cytokine and chemokine-induced integrin α4β1-mediated adhesion of myeloid cells.
Figure 3. p110γ PI3K activity is necessary and sufficient to promote myeloid cell trafficking to tumors.
Adhesion to VCAM-1 of chemoattractant-treated murine myeloid cells from (A) WT, p110γ−/− and p110γKD/KD mice (n=3), *p< 0.001 vs WT or (B) WT myeloid cells transfected with non-silencing, Pi3kα, β, γ, or δ siRNAs (n=3–6), *p< 0.001 vs non-silencing siRNA. (C) Adhesion to VCAM-1 of stimulated murine myeloid cells treated with TG100-115 and AS605240 (p110γ inhibitors), TGX221 (p110β inhibitor) or PI3Kα2 (p110α inhibitor). IC50TG100-115: IL-1β = 281 nM, SDF-1α = 158 nM; IC50AS605240: IL-β = 50 nM, SDF-1α = 50 nM. IC50 TGX221 and PI3Kα2 > 1 mM (n=3) *p<0.001 vs WT. (D) Adhesion to VCAM-1 of stimulated murine and human myeloid cells treated with TG100-115, AS605240, TGX221, or PI3Kα2. (E) VCAM-1/Fc binding to SDF-1α or IL-1β stimulated CD11b+ myeloid cells from WT or p110γ−/− mice or cells treated with 1 μM TG100-115, AS605240, PI3Kα2, TGX221 or control (n=3) *p<0.01 vs control. (F) Adhesion to VCAM-1 of unstimulated myeloid cells from WT and p110γCAAX mice (n=3), *p<0.01 vs WT. (G) Number/105 LLC tumor cells of adoptively transferred, fluorescently labeled myeloid cells transfected with non-silencing, Pi3k p110α, β, γ, or δ siRNAs, myeloid cells pretreated with TG100-115, PI3Kα2, or TGX221, and myeloid cells isolated from p110γ−/− mice (n=3), *p<0.001 vs non sil. siRNA. See also Figure S3.
Importantly, p110γ is also sufficient for integrin α4β1 activation, as cells from p110γCAAX mice, which express membrane-targeted, constitutively activated p110γ (Costa et al., 2007), adhered strongly to ECs even in the absence of stimulation (Fig.3F). Furthermore, siRNA-mediated knockdown and selective inhibition of p110α, β or δ isoforms had no effect on myeloid cell adhesion (Fig. 3B-D) or integrin activation (Fig. 3E, S3H). Taken together, these results indicate that p110γ is necessary and sufficient for integrin α4β1 activation.
To determine if p110γ is also required for trafficking of myeloid cells to tumors in vivo, we adoptively transferred fluorescently labelled WT, p110γ−/−, p110γ siRNA transfected and PI3Kγ inhibitor treated myeloid cells into tumor-bearing WT mice. While WT cells arrested in tumors, cells deficient in p110γ expression or activation failed to arrest in tumors (Fig.3G). In contrast, inhibition of other PI3K isoforms had no effect on cell trafficking to tumors (Fig.3G). Together, these data indicate that p110γ is necessary and sufficient to promote integrin α4β1-mediated myeloid cell trafficking to tumors in vivo.
Direct activation of p110γ by RTKs and TLR/IL-1Rs
To understand why p110γ but no other PI3K isoform is required for myeloid cell adhesion in vitro and in vivo, we examined the expression levels of various PI3K isoforms in primary murine myeloid cells, lymphocytes and Lewis lung carcinoma cells. Surprisingly, p110γ is the major PI3K catalytic isoform expressed in primary myeloid cells, as these cells expressed at least 73-fold more p110γ than p110α, 243-fold more p110γ than p110β and 25 fold more p110γ than p110δ (Fig.4A-B). Myeloid cells express the p110 regulatory subunits p85, p87 and p101 (Fig. S4A-B). In contrast, lymphocytes express large amounts of p110δ, while LLC cells express large amounts of p110α and β and little γ or δ (Fig.4A). Importantly, cytokines, growth factors and chemokines all rapidly activated p110γ in WT but not p110γ−/− primary myeloid cells, as shown by phosphoAkt immunoblotting analysis (Fig.4C). These results indicate that p110γ is the major catalytically active isoform in myeloid cells and that cytokines and growth factors can activate p110γ, refuting current dogma that p110γ can be activated only by GPCRs.
Figure 4. RTKs and TLR/IL-1Rs promote PI3-kinase p110γ catalytic activity.
(A) Western blotting of p110 isoforms in murine CD11b+ myeloid cells, lymphocytes and LLC tumor cells. (B) Quantification of protein and mRNA expression of p110 isoforms in murine CD11b+ myeloid cells. (C) Lysates of stimulated WT and p110γ−/− CD11b+ myeloid cells immunoblotted to detect pThr 308Akt and total Akt. (D) Upper graphs: time courses of p110γ activation in WT and p110γ−/− myeloid cells transiently expressing the PI(3,4,5)P3 reporter AKT-PH-EGFP. Primary myeloid cells were imaged live before and after treatment with 200ng/ml IL-1β, SDF-1α, or VEGF-A. Results are expressed as the mean ratio of AKT-PH-EGFP plasma membrane to cytosolic fluorescence +/− s.e.m, averaged over multiple experiments. t=0 corresponds to the time of growth factor addition. Lower graphs: time courses of IL-1β, SDF-1α, or VEGF-A stimulated myeloid cell fluorescence with and without addition of the p110γ selective inhibitor AS605240. t=0 corresponds to time of inhibitor addition. (E) Representative wild type or p110γ−/− primary myeloid cells transiently expressing the PI(3,4,5)P3 reporter AKT-PH-EGFP imaged live before and after treatment with AS605240, IL-1β, SDF-1α, or VEGF-A, scale bars, 5 μm. See also Figure S4.
To determine whether growth factors and cytokines/interleukins directly stimulate p110γ activity in myeloid cells, we measured p110γ activation in murine cells expressing the PI(3,4,5)P3 reporter Akt-PH-EGFP (Luo et al., 2005). IL-1β, SDF-1α, and VEGF-A each rapidly stimulated PIP3 production and reporter translocation to the membrane in WT but not in p110γ−/− primary myeloid cells (Fig.4D-E) within 5–10 seconds after stimulation. Continued production of PtdIns(3,4,5)P3 after stimulation by these factors was acutely dependent on p110γ activity, as treatment with the p110γ selective inhibitor AS605240 reversed Akt-PH-EGFP translocation to the membrane within 5-10s after its addition (Fig.4D-E). These results indicate that chemokines, growth factors and cytokines all rapidly and directly stimulate p110γ activation in primary myeloid cells.
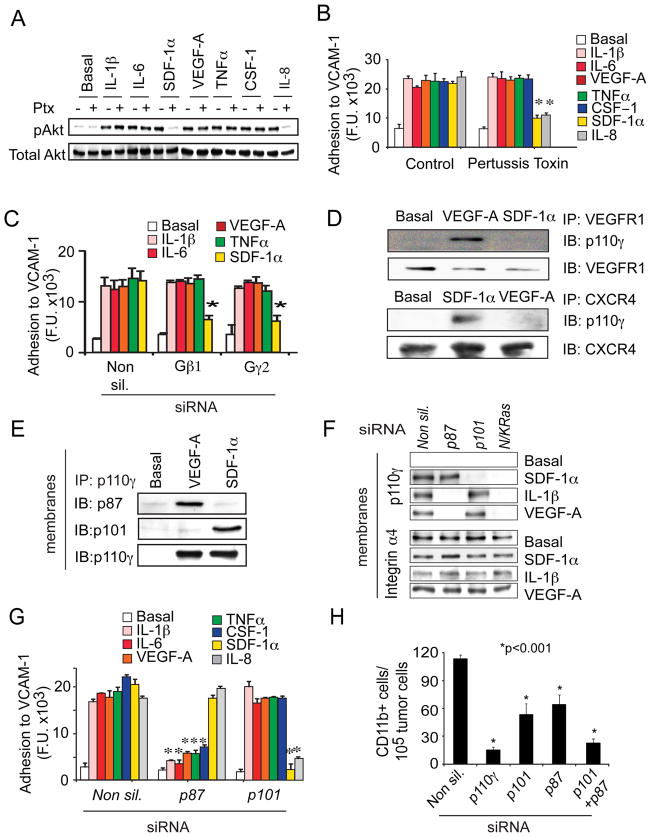
To examine further whether growth factors and cytokines directly activate p110γ, rather than indirectly through GPCRs, we tested the effect of the GPCR signaling inhibitor, pertussis toxin (Ptx) (Marrari et al., 2007; Suire et al., 2006), on p110γ activation and myeloid cell adhesion. Ptx had no effect on RTK- and TLR/IL1R-mediated Akt phosphorylation (Fig.5A) or cell adhesion (Fig.5B), although it blocked chemokine-mediated (SDF-1α and IL-8) effects. Additionally, siRNA-mediated inhibition of Gβ1 or Gγ2 expression blocked GPCR- but not RTK or TLR/IL1R-induced cell adhesion (Fig.5C, S5A). These results indicate that RTKs and TLR/IL1Rs directly activate p110γ in a GPCR-independent manner.
Figure 5. RTKs and TLR/IL-1Rs activate p110γ directly via p87.
(A) Immunoblots of pThr308Akt and total Akt in chemoattractant-stimulated myeloid cells treated with or without 100ng/ml Ptx. (B-C) Adhesion to VCAM-1 of chemoattractant-stimulated myeloid cells treated (B) with or without 100ng/ml Ptx or (C) transfected with non-silencing (Non sil.), Gβ1 or Gγ2 siRNA (n=3), *p<0.001 vs control (B) or vs Non silencing (C). (D) p110γ co-immunoprecipitation with VEGFR1 (upper) or CXCR4 (lower) in unstimulated (basal), VEGF-A or SDF-1α stimulated primary myeloid cells. (E) Co-immunoprecipitation of p87 or p101 with p110γ from membrane fractions of unstimulated (basal), VEGF-A or SDF-1α stimulated primary myeloid cells. (F) Immunoblots of p110γ and integrin α4 in membrane fractions from unstimulated (basal), SDF-1α, VEGF-A, and IL-1β stimulated myeloid cells transfected with non-silencing, p87, p101 and N/K Ras siRNAs. (G) Adhesion to VCAM-1 of non-silencing (Non sil.), p87, p101 and Ras siRNA transfected myeloid cells (n=3), *p<0.001 vs Non silencing siRNA. (H) Trafficking to tumors of myeloid cells transfected with non-silencing, p110γ, p101 or p87 siRNA (n=3). *p<0.001 vs Non-silencing siRNA. See also Figure S5.
Previous studies have shown that PI3K isoforms bind receptors or adaptors at the plasma membrane (Carpenter, 1993; Suire et al., 2006; Voigt et al., 2006; Kurig et al., 2009). We reasoned that if p110γ is directly activated by growth factors, it should closely associate with RTKs at the plasma membrane upon receptor activation. To test whether p110γ exhibits this behavior, we immunoprecipitated VEGFR1 (RTK) as well as CXCR4 (GPCR), the cell surface receptors for VEGF-A and SDF-1α, respectively, from unstimulated and VEGF-A and SDF-1α-stimulated myeloid cells. We found that p110γ, but not other p110 isoforms, co-immunoprecipitated with VEGFR1 upon stimulation with VEGF-A but not SDF-1α (Fig. 5D, S5B). In contrast, p110γ co-immunoprecipitated with the SDF-1α receptor CXCR4 upon stimulation with SDF-1α but not with VEGF-A (Fig.5D). Taken together, these results support the concept that RTKs and TLRs directly activate p110γ in a GPCR–independent manner, thereby promoting myeloid cell adhesion.
Myeloid cell RTKs activate p110γ in a p87-dependent manner
Upon stimulation by cell surface receptors, PI3K regulatory and catalytic subunits translocate to plasma membranes, where interactions with GPCR-activated Gβγ, tyrosine kinase receptors or Ras relieve regulatory subunit inhibition and stimulate catalytic activity. Chemoattractants activating GPCRs (SDF-1α), TLR/IL-1Rs (IL-1β) and RTKs (VEGF-A) all stimulated membrane translocation of myeloid cell p110γ but not p110δ, p110α or p110β (Fig. S5C). p110γ has been shown to associate with either of two regulatory subunits, p101 which is activated by binding to Gβγ, and p87, which is Gβγ-independent but Ras-dependent (Suire et al, 2006; Voigt et al, 2006; Kurig et al., 2009). We tested the roles of p101 and p87 in the activation of p110γ by RTKs and GPCRs and found that the RTK ligand VEGF-A stimulated p87 but not p101 membrane translocation and co-immunoprecipitation with p110γ, while the GPCR ligand SDF-1α induced p101 but not p87 membrane translocation and co-immunoprecipitation with p110γ in primary myeloid cells (Fig. 5E). Importantly, RTK and TLR-dependent p110γ translocation was blocked in p87 but not p101 siRNA transfected cells (Fig. 5F, S5D). GPCR-dependent p110γ membrane translocation was blocked in p101- but not in p87-siRNA transfected cells, while membrane localization induced by all stimuli was blocked by Ras siRNAs (Fig.5F, S5D). Similarly, growth factor and cytokine-dependent cell adhesion was blocked in p87 but not p101 siRNA transfected cells, while chemokine-dependent cell adhesion was blocked in p101 but not in p87 siRNA transfected cells (Fig.5G). Additionally, siRNA mediated suppression of p87 and p101 partially blocked myeloid cell invasion of tumors in vivo, and siRNA mediated suppression of both regulatory subunits completely blocked infiltration of myeloid cells, similarly to siRNA-mediated suppression of p110γ (Fig.5H). Taken together, these findings demonstrate that in primary myeloid cells, p110γ is activated by RTKs and TLR/IL1Rs in a p87-dependent manner and is activated by GPCRs in a p101-dependent manner in vitro and in vivo. Importantly, these results indicate that diverse chemoattractant signals converge on p110γ to promote tumor inflammation and suggest that blocking p110γ activity might be an effective strategy to suppress tumor inflammation.
Ras is necessary and sufficient to promote p110γ dependent cell adhesion
Ras is an important regulator of PI3K activity, as it enhances p110γ activation through direct interaction with the p110γ N-terminus (Suire et al., 2006; Kurig et al., 2009; Rubio et al., 1997; Pacold et al., 2000). In addition, the p87/p110γ complexes interact with Ras to promote p110γ membrane translocation and activation (Kurig et al., 2009). We found that ligands for GPCRs, RTKs and TLR/IL1Rs all stimulated Ras activity, although only GPCR ligands require heterotrimeric G proteins, as only Ras activation induced by SDF-1α or by IL-8 was blocked by Ptx (Fig.6A). Adhesion stimulated by RTK, TLR/IL1R, or GPCR ligands was blocked by a combination of N and K Ras siRNAs but not by Raf or MEK siRNAs or inhibitors (Fig.6B, S6A-C). These studies show that Ras is necessary for p110γ activation and myeloid cell adhesion in response to all chemoattractants.
Figure 6. Ras is necessary and sufficient to activate myeloid cell p110γ.
(A) Immunoblots of active (GTP-Ras) and total Ras in chemoattractant-stimulated myeloid cells with or without 100 ng/ml Ptx. (B) Adhesion to VCAM-1 of control (Non sil.), N/K Ras-, Raf-, and MEK-siRNA transfected myeloid cells (n=3), *p<0.01 vs control. (C) Adhesion to VCAM-1 of control-, RasV12-, RasV12C40-, and RasV12S35-transfected myeloid cells (n=3), *p<0.01 vs vector control. (D) Adhesion to VCAM-1 of RasV12 transfected cells treated with TG100-115; p110γ−/− myeloid cells transfected with RasV12, and WT myeloid cells transfected with RasV12 in combination with non-silencing, p110α, p110β, p110γ, p110δ, and itga4 siRNA. (n=3), *p<0.01 vs RasV12. (E) VCAM-1/Fc binding (MFI) to WT or p110γ−/− myeloid cells transfected with RasV12 in combination with non-silencing, p110α, β, γ, or δ siRNAs (n=3), *p<0.01 vs WT. (F) Trafficking to tumors of control or FTI treated myeloid cells and control transfected (Non-Sil.) or N+K ras siRNA transfected myeloid cells (n=3), *p<0.01 vs Ctrl. See also Figure S6.
Ras is also sufficient for promoting p110γ mediated integrin α4β1 activation in myeloid cells. WT but not p110γ−/− cells expressing activated RasG12V (V12Ras) exhibited constitutive adhesion (Fig.6C-D) and ligand binding (Fig.6E), indicating constitutive integrin activation. Expression of V12RasS35, which cannot bind p110γ, failed to stimulate adhesion, while V12RasC40, which binds p110γ but cannot bind to the Ras substrate Raf, stimulated constitutive adhesion (Fig.6C). Importantly, RasV12-mediated adhesion and ligand binding were blocked in cells expressing p110γ but not α, β or δ, siRNAs. Similarly, PI3Kγ inhibitor (TG100-115)-treated and p110γ−/− myeloid cells did not respond to RasV12 expression (Fig.6D). Additionally, expression of full-length p110γ in p110γ−/− cells restored adhesion to VCAM-1, but expression of p110γ with a Ras-binding domain deletion failed to restore adhesion in these cells (Fig.S6C). RasV12-mediated adhesion was also blocked in α4 integrin siRNA transfected cells (Fig.6D). Together, these data indicate that Ras is necessary and sufficient to activate p110γ-mediated integrin α4β1-dependent cell adhesion to VCAM-1. Finally, trafficking of myeloid cells to tumors was significantly suppressed in Ras siRNA transfected and farnesyltransferase inhibitor-treated cells (Fig.6F). Taken together with our data indicating the dependence of in vivo trafficking on p110γ and integrin α4β1, these results indicate that Ras is both necessary and sufficient for activation of p110γ and integrin α4 in primary myeloid cells during tumor inflammation. Our results thus indicate that GPCR ligands stimulate myeloid cell adhesion in a Gβγ-p101/p110γ-Ras-dependent manner, while RTK and TLR/IL1R ligands stimulate adhesion in a p87-p110γ-Ras-dependent but Gβγ-independent manner.
Myeloid cell p110γ is required for tumor inflammation, growth and metastasis
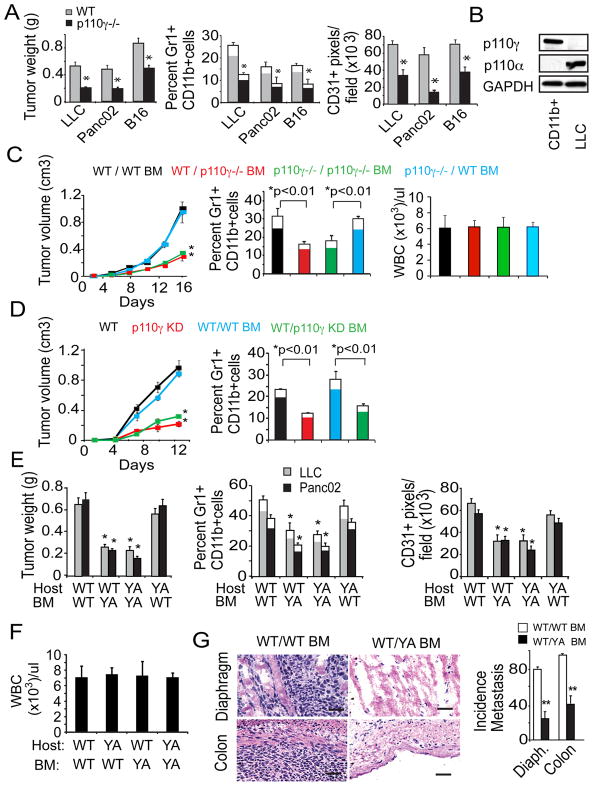
To explore the role of PI3Kγ in tumor inflammation and growth in vivo, we evaluated tumor growth in animals with deleted or catalytically inactive p110γ. Tumor growth, invasion of Gr1loCD11b+ and Gr1hiCD11b+ cells, angiogenesis and metastasis were substantially suppressed in p110γ−/− mice implanted with syngeneic subcutaneous LLCs, orthotopic pancreatic carcinoma and melanomas (Fig.7A, S7A-C). Importantly, myeloid cells but not tumor cells expressed p110γ, while tumor cells but not myeloid cells expressed p110α (Fig. 7B). Levels of circulating myeloid cells were identical in normal and tumor-bearing WT and p110γ−/− animals (Table S1). To determine whether tumor growth suppression resulted from decreased myeloid cell invasion, we characterized the growth of tumors in mice transplanted with BM from p110γ−/− and WT animals. BM from p110γ−/− and WT mice engrafted with equal efficacy (Fig. 7C, S7D); recruitment of Gr1loCD11b+ and Gr1hiCD11b+ cells and macrophages, angiogenesis and tumor growth were significantly suppressed in mice transplanted with p110γ−/− BM, but not in p110γ−/− or WT mice transplanted with WT BM (Fig7C, S7E).
Figure 7. p110γ and integrin α4β1 are required for tumor inflammation, growth and progression.
(A) Tumor weight, percent Gr1+CD11b+ cells (filled, Gr1lo; white, Gr1hi) in tumors, and CD31+ pixels/field in LLC, Panc02, and B16 tumors in WT and p110γ−/− mice (n=8–10), *p<0.01 vs WT. (B) Immunoblot analyses of p110α and p110γ in tumor-derived CD11b+ and LLC cells. (C) LLC tumor volume, percent Gr1+CD11b+ cells (filled, Gr1lo; white, Gr1hi) in tumors, and circulating WBCs/μl in WT mice with WT (black) or p110γ−/− bone marrow (BM, red), and in p110γ−/− mice with WT (blue) or p110γ−/− BM (green) bone marrow; *p<0.01 vs WT/WT. (D) Tumor volume and percent Gr1+CD11b+ cells in LLC tumors grown in WT (black line) and p110γKD (red line) animals and in WT animals with WT BM (blue) or p110γKD BM (green), (n=9–10 per group) *p<0.01 vs WT. (E) LLC or Panc02 tumor weight, percent Gr1+CD11b+ cells in tumor, and CD31+ pixels/field in WT or α4Y991A (YA) animals transplanted with WT or YA BM (n=8) *p<0.05 vs WT mice with WT BM. (F) Circulating WBCs/μl in WT mice with WT or α4Y991A BM. (G) Images, H&E-stained diaphragm and colon from BM transplanted, Panc02 implanted animals from E, scale bars, 40 μm. Graphs, incidence of colon and diaphragm metastases (n=8), *p<0.05 vs WT/WT. See also Figure S7 and Table S1.
As p110γ has been shown to exhibit kinase-independent adaptor protein functions (Patrucco et al., 2004), we determined if the catalytic activity of p110γ is required for tumor inflammation and growth by evaluating tumor growth in p110γKD mice and in WT mice transplanted with p110γKD BM. Although p110γKD and WT BM engrafted equally (Fig.S7F), we observed substantial impairment of myeloid cell recruitment and LLC tumor growth in p110γKD mice and in mice with p110γKD BM (Fig. 7D). As we have observed no intrinsic differences in the numbers of myeloid cells in peripheral blood or BM of normal or tumor-bearing mutant and WT animals and no defects in growth factor-induced angiogenesis in p110γ−/− mice (Serban et al., 2008), these studies indicate that p110γ-mediated integrin α4 activation in myeloid cells plays a key role in tumor inflammation and growth.
In support of our findings that p110γ plays a critical role in activating integrin α4β1 during tumor inflammation, we found that the growth of subcutaneous LLC and orthotopic Panc02 tumors were also suppressed in mice transplanted with BM from integrin α4Y991A mice. Recruitment of Gr1+CD11b+ myeloid cells, angiogenesis, tumor growth and spontaneous metastases were suppressed in mice transplanted with BM expressing α4Y991A, even though BM from α4Y991A animals and WT mice engrafted with equal efficacy (Fig. 7E-G; S7G-H), Myeloid cell infiltration, neovascularization and tumor growth were also suppressed in α4Y991A animals implanted with LLCs, orthotopic pancreatic carcinoma and orthotopic melanoma cells (Fig. S7I), even though α4Y991A animals exhibit normal myeloid cell levels (Table S1). These studies demonstrate that p110γ together with integrin α4 promote tumor inflammation, which leads to tumor angiogenesis, growth and metastasis.
Inhibition of PI3-kinase p110γ blocks spontaneous breast tumor progression
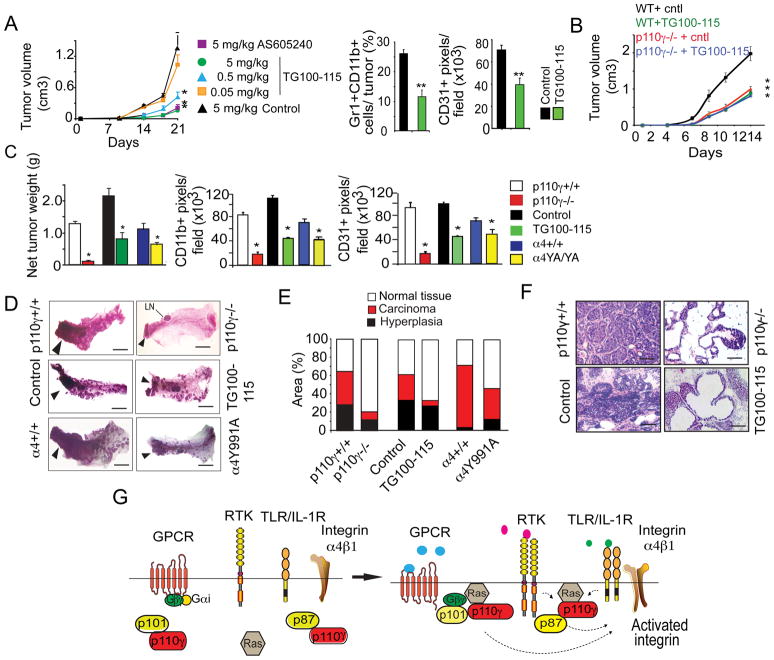
Pan-PI3kinase inhibitors are currently under clinical investigation to determine their usefulness in cancer therapy, but these agents broadly inhibit all PI3-kinase isoforms. Because we observed that all chemoattractant signals converge on p110γ, we evaluated the efficacy of two p110γ inhibitors, TG100-115 and AS605240 in tumor growth models. TG100-115 had no effect while pan-PI3 kinase inhibitors inhibited in vitro tumor cell proliferation (Fig. S8A). In contrast, a single 2.5mg/kg intravenous dose of TG100-115 rapidly and sustainably inhibited myeloid cell p110γ catalytic activity and adhesion to VCAM-1, with inhibition lasting approximately 12 hours (Fig.S8B). We therefore treated mice bearing LLC tumors with daily doses of 0.05, 0.5 and 5 mg/kg TG100-115, 5 mg/kg of AS605240 or 5 mg/kg of a chemically similar but inactive control compound (Control) for three weeks. TG100-115 and AS605240 suppressed lung carcinoma inflammation, angiogenesis and tumor growth, with an IC50 of 0.5 mg/kg for TG100-115 (Fig. 8A, S8C). TG100-115 had no direct effect on LLC tumor cell proliferation in vivo, as it did not inhibit LLC tumor growth in p110γ-/− mice (Fig. 8B, S8D). As p110γ is not expressed in LLC tumor cells but is mainly expressed in BM-derived cells, our studies indicate that PI3Kγ inhibitors block tumor growth by inhibiting tumor inflammation and angiogenesis without directly affecting tumor cells.
Figure 8. p110γ inhibition blocks spontaneous breast tumor growth and progression.
(A) Mice with LLC tumors were treated with control, AS605240, or TG100-115 (n=10). Left, tumor volume, *p<0.01 vs control. Middle, percent Gr1+CD11b+ cells, **p<0.001 vs control. Right, CD31+ pixels/field in control (black) and 0.5 mg/kg/day TG100-115 treated (green) tumors, **p<0.001 vs control. (B) Tumor volume in WT (black) and p110γ−/− (red) mice with LLC tumors treated with control, and WT mice (green) and p110γ−/− mice (blue) treated with TG100-115 at 5 mg/kg/day. (C) Tumor burden, CD11b+ myeloid cells and CD31+ blood vessels in spontaneous breast tumors from 9 week old control and TG100-115 treated, p110γ+/+ and p110γ−/−, and WT and α4Y991A FVB PyMT+ mice (n=10), *p<0.01 vs control. (D) Whole mounts of 4th mammary glands from B. Arrowheads, adenocarcinoma. LN, lymph node, scale bars, 400μm. (E) Percent area of normal, hyperplastic and carcinoma tissue from C (n=10), p<0.001 carcinoma vs WT, p<0.01 normal tissue vs WT and p= 0.45 hyperplasia vs WT. (F) H&E-stained mammary glands from C, scale bars, 40 μm. (G) Role of p110γ during tumor inflammation: cytokines and growth factors activate primary myeloid cell p110γ in a p87-Ras-dependent manner, and chemokines activate p110γ in a Gβγ-p101-Ras-dependent manner. Both pathways promote integrin α4β1 activation, with subsequent stimulation of myeloid cell trafficking, tumor growth and progression. See also Figure S8 and Table S2.
To determine whether myeloid cell p110γ and integrin α4β1 also regulate spontaneous tumor progression, we investigated the growth of PyMT spontaneous breast carcinomas in p110γ−/− and integrin α4Y991A mice and in mice treated with daily doses of 5 mg/kg TG100-115. Spontaneous breast carcinoma growth was strongly inhibited in p110γ−/− and integrin α4Y991A PyMT mice and in TG100-115 treated mice (Fig. 8C). Gr1+CD11b+ myeloid cell and macrophage recruitment was strongly reduced in mammary tumors from these mice (Fig. 8C, S8E). Importantly, the number of macrophages in TG100-115 treated or p110γ−/− tumors was reduced to or below the level of macrophages found in early hyperplastic lesions (Fig. S8E). Importantly, myeloid cells but not PyMT tumor cells express p110γ, while tumor cells but not myeloid cells expressed p110α (Fig. S8F-G). p110γ inhibitors had no effect on PyMT tumor cell growth in vitro (Fig. S8H). Together, these results indicate that p110γ inhibitors directly affect myeloid but not tumor cells.
Tumor angiogenesis was also suppressed in these animals (Fig. 8C). Tumor progression was strongly inhibited in p110γ−/−, α4991A and TG100-115 treated animals, as tumors in these animals exhibited substantially less carcinoma and more normal tissue than control-treated animals (Fig.8D-F; Table S2). Together, these studies indicate that p110γ-mediated integrin α4 activation in myeloid cells promotes spontaneous tumor inflammation and growth and demonstrate that p110γ inhibitors are useful in controlling spontaneous tumor growth and malignancy. Importantly, we found that expression of inflammatory and pro-angiogenic factors within tumors was substantially suppressed in both p110γ−/− and α4Y991A tumors, indicating that blockade of inflammatory cell infiltration of tumors has dramatic impacts on the pro-angiogenic, pro-inflammatory milieu of the tumor (Fig. S8I).
In conclusion, our studies demonstrate that tumor-derived chemokines, growth factors and cytokines promote tumor inflammation and progression by activating p110γ in inflammatory cells. Surprisingly, cytokines and growth factors activate p110γ in a p87-Ras-dependent manner, while chemokines activate p110γ in a Gβγ-p101-Ras-dependent manner in primary myeloid cells. Both pathways play key roles in tumor inflammation. p110γ then promotes integrin α4β1 activation, with subsequent stimulation of myeloid cell trafficking, tumor growth and progression (Fig. 8G).
Discussion
Beginning with the observation that tumor-derived chemoattractants ligands for RTKs, TLR/IL1Rs, and GPCRs promote tumor inflammation and progression, we found that RTK and TLR/IL1R signals can directly activate p110γ in inflammatory myeloid cells. We observed that RTKs and TLR/IL1Rs activate p110γ in a manner dependent on p87 and Ras but independent of heterotrimeric G proteins. In contrast, GPCRs activate p110γ in a Gβγ-p101-Ras-dependent manner. Both pathways promote α4β1 activation and myeloid cell adhesion to vascular endothelium in tumors, leading to tumor angiogenesis, growth and progression. Our studies refute current dogma that p110γ can be activated only by GPCRs, as we show that myeloid cell p110γ is directly and rapidly activated by RTKs and TLR/IL1Rs. Our studies demonstrate that tumor inflammation and growth are critically dependent on p110γ and that selective, small molecule inhibitors of p110γ strongly suppress spontaneous tumor growth by blocking inflammation. Importantly, as these observations were made in both murine and human myeloid cells, our results indicate that selective p110γ inhibitors have good potential as cancer therapeutic agents.
We also made the surprising observation that only integrin α4 and not αMβ2 or other integrins is required to promote adhesion of myeloid cells to tumor vascular endothelium in vivo. Although beta2 integrins have previously been shown to mediate lymphocyte adhesion to ECs, our studies indicate that this integrin is not required for myeloid cell invasion of tumors.
While we have shown that RTK, TLR/ILR and GPCR-mediated activation of Ras and p110γ promote integrin α4 activation, the pathway by which p110γ promotes integrin α4 activation is not yet clear. The requirement for p110γ kinase activity indicates that PtdIns(3,4,5)P3 production is crucial. However, the target of PtdIns(3,4,5)P3 that mediates integrin activation remains unknown.The small GTPase Rap1 promotes integrin activation by inducing talin binding to integrin β chains (de Bruyn et al, 2002; Lee et al., 2009). This event disrupts electrostatic interactions between integrin α and β chains and leads to a shift in the conformation of the extracellular domain of the integrin and increases ligand binding (Costa et. al., 2007). While our preliminary studies indicate that p110γ activates Rap1 to promote integrin activation (not shown), it is currently unclear how PI3Kγ activates Rap1. An attractive model would be the binding of PH-domain-containing Rap1 exchange factors to PtdIns(3,4,5)P3, effectively activating and localizing Rap1 at the plasma membrane where it might act on integrin α4β1 (Bergmeier et al., 2007).
PI3Ks have become attractive targets for cancer therapy. Activating mutations in p110α occur frequently in human cancers, and more generally, the RTK-Class 1A PI3K pathway (utilizing p110α and p110β) is one of the most common targets of oncogenic mutations. A number of clinical trials are underway testing pan-PI3K inhibitors in cancer patients (Cleary and Shapiro, 2010). However, our studies indicate that p110γ is an excellent target for a relatively non-toxic cancer therapeutic, as this isoform is primarily expressed by myeloid cells and is a convergence point of diverse chemoattractant signaling pathways that are required for tumor inflammation and tumor progression. PI3K p110γ inhibitors strongly suppressed tumor growth and progression in mice without apparent side effects. In conclusion, our data demonstrate alternate pathways whereby tumor derived factors that activate GPCRs, RTKs and TLRs promote PI3K p110γ to activate integrin α4β1-dependent tumor inflammation, growth and progression.
Experimental Procedures
Institutional approvals
All studies involving human tissues were approved by the University of California, San Diego IRB and were considered exempt according to federal guidelines. All experiments on live animals were performed in accordance with institutional and national guidelines and regulations, under approval by the UCSD IACUC.
Adhesion assays
1 X 105 calcein-AM labelled human or murine CD11b+ cells were incubated on human or murine EC monolayers or plastic plates coated with 5 μg/ml recombinant soluble human or murine VCAM-1 or ICAM-1 (R&D Systems) for 30 minutes at 37ºC in the presence of TCM or DMEM containing 200ng/ml SDF1α, IL-1β, IL-6, IL-8, TNFα, C5a, CSF-1 or VEGF-A (R&D Systems). After washing with warmed medium, adherent cells were quantified using a plate fluorimeter (GeniosPro, TECAN).
Gene expression
Total RNA was isolated from cells and tissues using ISOGEN (Nippon Gene). cDNA was prepared from 1μg RNA/sample, and qPCR was performed using gene specific QuantiTect Primer Assay primers from Qiagen. Relative expression levels were normalized to gapdh expression according to the formula <2^− (Ct gene of interest – Ct gapdh)>. Fold increase in expression levels were calculated by comparative Ct method <2^− (ddCt)>.
Animal studies
Integrin α4Y991A and p110γ−/− mice in C57BL6 or FVB lineages were used for tumor studies. p110γ−/− mice were obtained from Dr. Joseph Penninger, Institute of Molecular Biotechnology, Vienna, Austria. p110γKD mice and p110γCAAX mice were developed and maintained in the Hirsch lab. αMβ2−/− mice were from Jackson Laboratories. 5-10X105 Panc02, LLC or B16 cells were injected subcutaneously or orthotopically into syngeneic 6–8wk old mice; tumors dimensions and weights were recorded regularly. Tumors were cryopreserved in OCT, solubilized for RNA purification, or collagenase-digested for flow cytometric analysis of CD11b and Gr1 expression. Angiogenesis was measured by CD31 immunostaining. All tumor experiments were performed 3–4 times with n=8–14, except those in p110γKD mice and p110γKD BM transplanted mice were performed once (n=10).
Quantification of AKT-PH-EGFP translocation
Freshly isolated BM-derived murine CD11b+ cells were transfected with AKT-PH-EGFP expression plasmid (Luo et al, 2005) by electroporation using Amaxa macrophage transfection protocols. Cells were imaged live using an Olympus IX81-ZDC spinning disc confocal microscope controlled by Slidebook software. Cells in suspension were seeded onto a glass bottom dish contained within a 37°C, 5% CO2 chamber above a heated 37°C 60× 1.4 NA objective. Confocal GFP images were collected every 2 seconds before and after addition of 200 ng/ml IL-1β, SDF-1α, VEGF-A, or basal media. The ratio of plasma membrane to cytosolic intensity was measured using Image J software.
Statistical Methods
In vitro assay data (n=3–5/group) were analyzed for significance using Student s two-tailed t-test. In vivo data (n=6–14/group) were analyzed by one-way ANOVA, coupled with posthoc Tukey s test for pairwise comparison. All errors reported are s.e.m. All animal studies were performed 2–4 times.
Additional procedures are described in Supplemental Information.
Supplementary Material
Acknowledgments
We thank Dr. Hanjoong Jo for immortalized murine ECs. These studies were supported by fellowships from Novartis, SNSF and the California TRDRP to MCS, NIH grants R01CA83133 and R01CA126820 to JAV, DP2OD004265 and R01CA138676 to SJF, AR27214 and HL31950 to MHG, R01CA50286 and R01CA45726 to DAC, R01CA118182 to LGE, and a grant from Cariplo to EH. The authors declare no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Significance
As inflammation stimulates tumor angiogenesis, immunosuppression and growth, understanding how inflammatory cells are recruited to the tumor microenvironment could lead to additional avenues for tumor therapy. We discovered that tumor-derived chemoattractants stimulating myeloid cell RTKs, TLR/IL1Rs, and GPCRs activate a single PI3-kinase isoform, p110γ, and a single integrin, α4β1, to promote myeloid cell recruitment to tumors and tumor progression. Myeloid cell p110γ is unexpectedly activated by RTKs and TLR/IL1Rs via Ras and p87, refuting current dogma that p110γ is activated only by GPCRs. Our studies reveal that PI3Kγ is a single convergent point controlling tumor inflammation and progression. Selective inhibitors of p110γ could thus serve as therapeutics to suppress tumor malignancy by blocking diverse pathways promoting tumor inflammation.
References
- Arnaout MA, Mahalingam B, Xiong JP. Integrin structure, allostery, and bidirectional signaling. Annu Rev Cell Dev Biol. 2005;21:381–410. doi: 10.1146/annurev.cellbio.21.090704.151217. [DOI] [PubMed] [Google Scholar]
- Bergmeier W, Goerge T, Wang HW, Crittenden JR, Baldwin AC, Cifuni SM, Housman DE, Graybiel AM, Wagner DD. Mice lacking the signaling molecule CalDAG-GEFI represent a model for leukocyte adhesion deficiency type III. J Clin Invest. 2007;117:1699–1707. doi: 10.1172/JCI30575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- Bronte V, Apolloni E, Cabrelle A, Ronca R, Serafini P, Zamboni P, Restifo NP, Zanovello P. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96:3838–3846. [PMC free article] [PubMed] [Google Scholar]
- Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- Camps M, Ruckle T, Ji H, Ardissone V, Rintelen F, Shaw J, Ferrandi C, Chabert C, Gillieron C, Françon B, et al. Blockade of PI3K γ suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nature Medicine. 2005;11:936–943. doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
- Carpenter CL, Auger KR, Chanudhuri M, Yoakim M, Schaffhausen B, Shoelson S, Cantley LC. Phosphoinositide 3-kinase is activated by phosphopeptides that bind to the SH2 domains of the 85-kDa subunit. J Biol Chem. 1993;268:9478–9483. [PubMed] [Google Scholar]
- Cleary JM, Shapiro GI. Development of phosphoinositide-3 kinase pathway inhibitors for advanced cancer. Curr Oncol Rep. 2010;12:87–94. doi: 10.1007/s11912-010-0091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa C, Barberis L, Ambrogio C, Manazza AD, Patrucco E, Azzolino O, Neilsen PO, Ciraolo E, Altruda F, Prestwich GD, et al. Negative feedback regulation of Rac in leukocytes from mice expressing a constitutively active phosphatidylinositol 3-kinase gamma. Proc Natl Acad Sci USA. 2007;10:14354–14359. doi: 10.1073/pnas.0703175104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruyn KM, Rangarajan S, Reedquist KA, Figdor CG, Bos JL. The small GTPase Rap1 is required for Mn(2+)- and antibody-induced LFA-1- and VLA-4-mediated cell adhesion. J Biol Chem. 2002;277:29468–29476. doi: 10.1074/jbc.M204990200. [DOI] [PubMed] [Google Scholar]
- DeNardo DG, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. 2010;29:309–316. doi: 10.1007/s10555-010-9223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doukas J, Wrasidlo W, Noronha G, Dneprovskaia E, Fine R, Weis S, Hood J, Demaria A, Soll R, Cheresh D. Phosphoinositide 3-kinase gamma/delta inhibition limits infarct size after myocardial ischemia/reperfusion injury. Proc Natl Acad Sci U S A. 2006;103:19866–19871. doi: 10.1073/pnas.0606956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du R, Petritsch C, Liu P, Ganss R, Passegué E, Song H, Vandenberg S, Johnson RS, Werb Z, Bergers G. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feral CC, Rose DM, Han J, Fox N, Silverman GJ, Kaushansky K, Ginsberg MH. Blocking the alpha 4 integrin-paxillin interaction selectively impairs mononuclear leukocyte recruitment to an inflammatory site. J Clin Invest. 2006;116:715–723. doi: 10.1172/JCI26091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N. Role of myeloid cells in vascular endothelial growth factor-independent tumor angiogenesis. Curr Opin Hematol. 2010;17:219–224. doi: 10.1097/MOH.0b013e3283386660. [DOI] [PubMed] [Google Scholar]
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of Tumor-Associated Neutrophil Phenotype by TGF-b: N1 versus N2 TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature Reviews Immunology. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov GI, Greten FR, Karin M. Immunity, Inflammation and Cancer. Cell. 2010;140:883–840. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Central role for G protein coupled phosphoinositide 3-kinase γ in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- Jin H, Su J, Garmy-Susini B, Kleeman J, Varner J. Integrin alpha4beta1 promotes monocyte trafficking and angiogenesis in tumors. Cancer Res. 2006;66:2146–2152. doi: 10.1158/0008-5472.CAN-05-2704. [DOI] [PubMed] [Google Scholar]
- Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi AW, Pleiss MA, Semko CM, Yednock T, Smith JL. Multimeric VLA-4 antagonists comprising polymer moieties. 20060013799 A1 US. 2006
- Kurig B, Shymanets A, Bohnacker T, Prajwal, Brock C, Ahmadian MR, Schaefer M, Gohla A, Harteneck C, Wymann MP, et al. Ras is an indispensable coregulator of the class IB phosphoinositide 3-kinase p87/p110gamma. Proc Natl Acad Sci U S A. 2009;106:20312–20317. doi: 10.1073/pnas.0905506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazennec G, Richmond A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol Med. 2010;16:133–144. doi: 10.1016/j.molmed.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Lim CJ, Puzon-McLaughlin W, Shattil SJ, Ginsberg MH. RIAM activates integrins by linking talin to ras GTPase membrane-targeting sequences. J Biol Chem. 2009;284:5119–5127. doi: 10.1074/jbc.M807117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D. Roles of PLC-β2 and –β3 and PI3kγ in chemoattractant mediated signal transduction. Science. 2000;287:1046–1049. doi: 10.1126/science.287.5455.1046. [DOI] [PubMed] [Google Scholar]
- Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- Lobb RR, Hemler ME. The pathophysiologic role of alpha 4 integrins in vivo. J Clin Invest. 1994;94:1722–1728. doi: 10.1172/JCI117519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque A, Gómez M, Puzon W, Takada Y, Sánchez-Madrid F, Cabañas C. Activated conformations of very late activation integrins detected by a group of antibodies (HUTS) specific for a novel regulatory region (355–425) of the common beta 1 chain. J Biol Chem. 1996;271:11067–11075. doi: 10.1074/jbc.271.19.11067. [DOI] [PubMed] [Google Scholar]
- Luo J, Field SJ, Lee JY, Engelman JA, Cantley LC. The p85 regulatory subunit of phosphoinositide 3-kinase down-regulates IRS-1 signaling via the formation of a sequestration complex. J Cell Biol. 2005;170:455–464. doi: 10.1083/jcb.200503088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manevich E, Grabovsky V, Feigelson SW, Alon R. Talin 1 and paxillin facilitate distinct steps in rapid VLA-4-mediated adhesion strengthening to vascular cell adhesion molecule 1. J Biol Chem. 2007;282:25338–25348. doi: 10.1074/jbc.M700089200. [DOI] [PubMed] [Google Scholar]
- Marrari Y, Crouthamel M, Irannejad R, Wedegaertner PB. Assembly and trafficking of heterotrimeric G proteins. Biochemistry. 2007;46:7665–7677. doi: 10.1021/bi700338m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacold ME, Suire S, Perisic O, Lara-Gonzalez S, Davis CT, Walker EH, Hawkins PT, Stephens L, Eccleston JF, Williams RL. Crystal structure and functional analysis of Ras binding to its effector phosphoinositide 3-kinase gamma. Cell. 2000;103:931–943. doi: 10.1016/s0092-8674(00)00196-3. [DOI] [PubMed] [Google Scholar]
- Palanki MS, Dneprovskaia E, Doukas J, Fine RM, Hood J, Kang X, Lohse D, Martin M, Noronha G, Soll RM, et al. Discovery of 3,3′-(2,4-diaminopteridine-6,7-diyl)diphenol as an isozyme-selective inhibitor of PI3K for the treatment of ischemia reperfusion injury associated with myocardial infarction. Journal of Medicinal Chemistry. 2007;50:4279–4294. doi: 10.1021/jm051056c. [DOI] [PubMed] [Google Scholar]
- Patrucco E, Notte A, Barberis L, Selvetella G, Maffei A, Brancaccio M, Marengo S, Russo G, Azzolino O, Rybalkin SD, et al. PI3Kγ modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and–independent events. Cell. 2004;118:375–387. doi: 10.1016/j.cell.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Rose DM, Alon R, Ginsberg MH. Integrin modulation and signaling in leukocyte adhesion and migration. Immunol Rev. 2007;218:126–134. doi: 10.1111/j.1600-065X.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- Rubio I, Rodriguez-Viciana P, Downward J, Wetzker R. Interaction of Ras with phosphoinositide 3-kinase gamma. Biochem J. 1997;326:891–895. doi: 10.1042/bj3260891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, et al. Function of PMK7 in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- Scott LM, Priestley GV, Papayannopoulou T. Deletion of alpha4 integrins from adult hematopoietic cells reveals roles in homeostasis, regeneration, and homing. Mol Cell Biol. 2003;23:9349–9360. doi: 10.1128/MCB.23.24.9349-9360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serban D, Leng J, Cheresh D. H-ras regulates angiogenesis and vascular permeability by activation of distinct downstream effectors. Circ Res. 2008;102:1350–1358. doi: 10.1161/CIRCRESAHA.107.169664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaei F, Singh M, Thompson JD, Ferrara N. Bv8 regulates myeloid-cell-dependent tumor angiogenesis. Nature. 2007;450:825–831. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
- Suire S, Condliffe AM, Ferguson GJ, Ellson CD, Guillou H, Davidson K, Welch H, Coadwell J, Turner M, Chilvers ER, et al. Gbetagammas and the Ras binding domain of p110gamma are both important regulators of PI(3)Kgamma signalling in neutrophils. Nat Cell Biol. 2006;8:1303–1309. doi: 10.1038/ncb1494. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nature Reviews Molecular Cell Biology. 2010;11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- Voigt P, Dorner MB, Schaefer M. Characterization of p87PIKAP, a novel regulatory subunit of phosphoinositide 3-kinaseγ that is highly expressed in heart and interacts with PDE3B. J Biol Chem. 2006;281:9977–9986. doi: 10.1074/jbc.M512502200. [DOI] [PubMed] [Google Scholar]
- Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31:220–227. doi: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Shephard F, Kim HB, Palmer IR, McHarg S, Fowler GJ, O’Neill LA, Kiss-Toth E, Qwarnstrom EETIRR. A novel IL1R1 co-receptor potentiates MyD88 recruitment to control Ras-dependent amplification of NF-kB. J Biol Chem. 2010;285:7222–7232. doi: 10.1074/jbc.M109.073429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.