Abstract
Concurrent cigarette smoking and cocaine use is well documented. However, the behavioral pharmacology of cocaine and nicotine combinations is poorly understood, and there is a need for animal models to examine this form of polydrug abuse. The purpose of this study was two-fold: first to assess the effects of nicotine on the discriminative stimulus effects of cocaine, and second, to study self-administration of nicotine/cocaine combinations in a novel polydrug abuse model. In drug discrimination experiments, nicotine increased the discriminative stimulus effects of low cocaine doses in two of three monkeys, but nicotine did not substitute for cocaine in any monkey. Self-administration of cocaine and nicotine alone, and cocaine + nicotine combinations was studied under a second-order fixed ratio 2, variable ratio 16 (FR2[VR16:S]) schedule of reinforcement. Cocaine and nicotine alone were self-administered in a dose-dependent manner. The combination of marginally reinforcing doses of cocaine and nicotine increased drug self-administration behavior above levels observed with the same dose of either cocaine or nicotine alone. These findings indicate that nicotine may increase cocaine’s discriminative stimulus and reinforcing effects in rhesus monkeys, and illustrate the feasibility of combining cocaine and nicotine in a preclinical model of polydrug abuse. Further studies of the behavioral effects of nicotine + cocaine combinations will contribute to our understanding the pharmacology of dual nicotine and cocaine dependence, and will be useful for evaluation of new treatment medications.
Keywords: Cocaine, Nicotine, Polydrug abuse, Self-administration, Drug discrimination, Rhesus monkey
INTRODUCTION
Research on drug addiction has often focused on a single drug, but there is considerable evidence that drug abusers typically use two or more substances concurrently. Nicotine is one of the most commonly used drugs, and often it is used in combination with other abused drugs (Budney, Higgins, Hughes, & Bickel, 1993; Mello, Mendelson, Sellers, & Kuehnle, 1980). Cocaine-dependent smokers often report increases in smoking during periods of cocaine use (Roll, Higgins, Budney, Bickel, & Badger, 1996), and these self-reported increases in cigarette smoking during cocaine use have been verified by biochemical measures (Roll, Higgins, & Tidey, 1997). Interestingly, cocaine-dependent smokers report using more cocaine than cocaine-dependent non-smokers (Budney et al., 1993). Increases in cigarette smoking following stimulant drug administration also have been reported in clinical laboratory studies. For example, under double-blind, placebo-controlled conditions, acute cocaine administration led to increases in cigarette smoking (Roll et al., 1996). Acute administration of other indirect dopamine agonists, such as amphetamine (Cousins, Stamat, & de Wit, 2001; Henningfield & Griffiths, 1981; Schuster, Lucchesi, & Emley, 1979; Tidey, O'Neill, & Higgins, 2000) and methylphenidate (Rush et al., 2005; Vansickel, Stoops, Glaser, & Rush, 2007) also increased cigarette smoking under controlled laboratory conditions.
The reinforcing and discriminative stimulus effects of some drug combinations (e.g., cocaine + heroin ‘speedball’) have been studied in nonhuman primates (Mello et al., 1995; Rowlett, Wilcox, & Woolverton, 1998), but little is known about the behavioral pharmacology of nicotine and cocaine combinations. Interactions between nicotine and cocaine have been studied in rodents using both conditioned and unconditioned behaviors, and the findings suggest that nicotine enhances cocaine’s behavioral effects. For example, nicotine enhanced the locomotor activating effects of cocaine and other indirect dopamine agonists (Collins & Izenwasser, 2004; Jutkiewicz, Nicolazzo, Kim, & Gnegy, 2008). There is also some overlap between the discriminative stimulus effects of nicotine and cocaine. Nicotine fully generalized to a cocaine discriminative stimulus in rats (Desai, Barber, & Terry, 1999, 2003) and partially generalized in rhesus monkeys (Ando & Yanagita, 1978; de la Garza & Johanson, 1983). However, cocaine did not fully generalize to a nicotine discriminative stimulus in rats (Desai et al., 1999, 2003; Mansbach, Rovetti, & Freedland, 1998). In drug self-administration studies, acute and repeated nicotine administration also has been shown to increase cocaine self-administration, reinstate cocaine-seeking behavior, and potentially lead to an escalation of cocaine self-administration over time (Bechtholt & Mark, 2002). Moreover, nicotine exposure has been reported to increase rates of acquisition of cocaine self-administration in rats (Horger, Giles, & Schenk, 1992).
The similarities between the pharmacological and behavioral effects of nicotine and cocaine include the enhancement of dopamine release. Microdialysis studies have shown that cocaine administration is followed by increases in extracellular dopamine levels in the nucleus accumbens in nonhuman primates (Iyer, Nobiletti, Jatlow, & Bradberry, 1995) and in rodents (Hemby, Co, Koves, Smith, & Dworkin, 1997; Hurd, Weiss, Koob, And, & Ungerstedt, 1989; H.O. Pettit & Justice, 1989; H .O. Pettit & Justice, 1991; Wise et al., 1995). Nicotine, like cocaine, activates the mesolimbic dopamine system and stimulates dopamine release. Moreover, administration of equipotent doses of nicotine and cocaine in combination produce additive effects on nucleus accumbens dopamine release (Gerasimov et al., 2000; Sziraki, Sershen, Benuck, Hashim, & Laitha, 1999; Zernig, O'Laughlin, & Fibiger, 1997). However, nicotine and cocaine increase extracellular dopamine levels by different mechanisms. Nicotine induces dopamine release by stimulating nicotinic acetylcholine receptors on the cell bodies of mesolimbic dopamine neurons (Corrigall, Coen, & Adamson, 1994; Nisell, Nomikos, & Svensson, 1994; I. P. Stolerman & Shoaib, 1991; Watkins, Koob, & Markou, 2000), whereas cocaine increases extracellular dopamine levels by blocking reuptake by the dopamine transporter (Kuhar, Ritz, & Boja, 1991; Ritz, Lamb, Goldberg, & Kuhar, 1987, 1988).
The purpose of this study was to examine the effects of nicotine on the reinforcing and discriminative stimulus effects of cocaine in nonhuman primates, in a new polydrug model involving self-administration of nicotine + cocaine combinations. The ability of nicotine to alter cocaine’s discriminative stimulus effects and to substitute for cocaine was studied. The reinforcing effects of nicotine and cocaine alone, and in combination, were evaluated in rhesus monkeys with extensive cocaine self-administration histories. On the basis of neurochemical, pharmacological, and clinical data, we hypothesized that nicotine would enhance the discriminative stimulus and reinforcing effects of cocaine.
METHODS
Subjects
Eight adult male rhesus monkeys (Macaca mulatta) weighing 7.8 to 10.2 kg were studied. Chow (Purina Jumbo Monkey Chow #503), fresh fruit and vitamins were given each day to maintain 95% free feeding weights. Water was freely available at all times. Animal maintenance and research were conducted in accordance with the guidelines provided by the Committee on Laboratory Animal Resources (ILAR-NRC, 1996) and the NIH Office of Laboratory Animal Welfare (OLAW).
Apparatus
Each monkey lived in a well ventilated, stainless steel chamber during studies of drug discrimination (56 × 71 × 69 cm) and drug self-administration (60 × 100 × 76 cm). An operant panel (28 × 28 cm) was mounted on the front wall of each chamber. Each panel contained three square translucent response keys arranged in a horizontal row, which could be transilluminated by red or green stimulus lights. A set of three vertical stimulus lights (red, green, yellow) was located beneath the center response key. A dispenser delivered 1-g banana-flavored food pellets (Formula 5TUR banana flavor grain-based Pellet, Purina Mills Test Diet, Richmond, IN) to a receptacle below the operant panel. Reinforcement schedules were controlled by IBM-compatible computers and Med-Associates interface systems (St. Albans, VT) located in an adjacent room.
Cocaine Discrimination
As in our previous studies (Mello, Negus, Knudson, Kelly, & Mendelson, 2008; Negus, Mello, Blough, Baumann, & Rothman, 2007), daily sessions consisted of 1 to 5 20-min cycles. A 15-min time-out period was followed by a 5-min response period. During the time-out, all stimulus lights remained off, and responding had no scheduled consequences. During the response period, the right and left response keys were illuminated with a red or green light, and monkeys could earn up to 10 food pellets by responding on a fixed-ratio 30 (FR 30) schedule. The colors of the response keys were counterbalanced, i.e., the left key was red and the right key was green for some monkeys, and the colors were reversed for the other monkeys. If all available food pellets were delivered before the end of the 5-min response period, the stimulus lights on the response keys were turned off, and responding had no scheduled consequences.
Training Procedure
Monkeys (N=3) were trained to discriminate 0.4 mg/kg, IM cocaine from saline. On training days, monkeys were given an IM injection of cocaine or an equal volume of saline 5-min after the beginning of each time-out period. Following saline administration, responding on only the green, saline-appropriate key produced food, whereas following cocaine administration, only responding on the red, drug-appropriate key produced food. Responses on the inappropriate key reset the FR requirement on the appropriate key. Training days consisted of 0 to 5 saline cycles followed by 0 to 1 drug cycles. Cocaine was administered only during the last cycle.
Acquisition of cocaine discrimination was defined by meeting three criteria for 7 of 8 consecutive training sessions: 1) the percent injection-appropriate responding prior to delivery of the first reinforcer was greater than or equal to 80% for all cycles; 2) the percent injection-appropriate responding for each cycle was greater than or equal to 90%; and 3) response rates during saline training cycles were greater than 0.5 responses per second.
Testing Procedure
Test sessions were identical to training sessions except that responding on either key produced food. Initially, a substitution procedure was used to determine the degree to which nicotine and different doses of cocaine generalized to the training dose of cocaine. Cocaine (0.013–1.3 mg/kg, IM) or nicotine (0.032–0.56 mg/kg, IM) was administered alone using a cumulative dosing procedure. Monkeys received an injection of nicotine or cocaine 5 min after the beginning of each cycle of a multiple-cyxtotal cumulative dose by ¼- or ½-log units. Dose-effect curves for cocaine and nicotine alone were determined once in each subject.
To evaluate the effects of nicotine on cocaine’s discriminative stimulus effects, a single dose of nicotine (0.32 mg/kg) was administered as a pretreatment 5 min after single doses of cocaine (0.04, 0.13, and 0.4 mg/kg), given 10 min before the sessions.
Data Analysis for Drug Discrimination Studies
The mean ± SEM percent cocaine-appropriate responding (for the entire response period) was plotted as a function of cocaine dose. Test drugs were considered to substitute for the training dose of cocaine if they produced ≥90% cocaine-appropriate responding. In addition, discrimination ED50 values were calculated by linear interpolation, and were defined as the dose of cocaine, nicotine, or cocaine + nicotine combinations that produced 50% cocaine-appropriate responding ED50 values for cocaine alone and cocaine + nicotine in combination were compared with a paired t-test (GraphPad Prism 5.00 for MacIntosh; GraphPad Software Inc.).
Cocaine, Nicotine and Cocaine+Nicotine Self-administration
Surgical Procedure
To permit intravenous drug delivery, a double-lumen silicone® catheter was surgically implanted into a jugular or femoral vein under aseptic conditions as described previously (Mello et al., 2008). The intravenous catheter was protected by a tether system consisting of a custom-fitted nylon vest connected to a flexible stainless steel cable and fluid swivel (Lomir Biomedical, Inc., Malone, NY). Two syringe pumps were mounted above each chamber. One syringe pump delivered self-administered drug or saline injections through one catheter lumen, and the second syringe pump was used for non-contingent delivery of saline through the second catheter lumen. Catheter patency was evaluated periodically by administration of a short-acting barbiturate, methohexital sodium (3 mg/kg) through the catheter lumen. Patency was inferred from a decrease in muscle tone within 10 sec.
Response Requirements
Procedures for evaluating the reinforcing effects of nicotine, cocaine, and cocaine + nicotine combinations were similar to those used in our previous studies of cocaine + heroin combinations (Mello & Negus, 1998, 1999, 2001, 2007; Mello et al., 1995). Schedules of reinforcement were programmed with custom-designed software and IBM compatible computers and interface systems (see\ (Mello et al., 1995). Each day consisted of four sessions and studies were conducted 7 d/week. Food was available for self-administration at 11am, 3pm, 7pm, and 6am the next morning, and the cocaine self-administration sessions began at 12pm, 4pm, 8pm, and 7am the next morning. Room lights were off during all experimental sessions.
Monkeys responded under an FR2 [VR16:S] second-order schedule of reinforcement for food pellets and drug or saline injections. At the beginning of each session, stimulus lights in the center response key were illuminated (red for food, green for drug). Completion of a variable ratio (VR) averaging 16 responses turned off that stimulus light and turned on the same colored stimulus light located below the response key for 1 s (VR16:S). Completion of a second VR16 turned off the stimulus light illuminating the response key, turned on the same colored stimulus light beneath it for 1 s and the circuitry dellivered a 1-g banana-flavored food pellet or a 0.1 ml injection of cocaine or saline over 1 second. Reinforcer delivery was followed by a 10-s time-out period during which stimulus lights remained off and responding had no scheduled consequences. If 25 food pellets or 20 injections were delivered before the end of the 1-hr session, then all stimulus lights were turned off, and responding had no scheduled consequences for the remainder of that session. Thus, a monkey could earn a maximum of 100 pellets/day and 80 injections/day. These procedures were used for all drug self-administration experiments.
Testing procedure
Training continued until monkeys met the following criteria for stable food and cocaine self-administration under the FR2 [VR16:S] schedule of reinforcement: 1) three consecutive days during which the number of drug injections/day varied by no more than 20% of the three-day mean with no upward or downward trend, and 2) the mean number of both food pellets and injections delivered per day was equal to or greater than 60. Once responding was stable, saline, and drug dose-effect curves for cocaine (0.001–0.1 mg/kg/inj), nicotine (0.001–0.1 mg/kg/inj), and cocaine (0.001–0.01 mg/kg/inj) + nicotine (0.001–0.01 mg/kg/inj) combinations were determined. Each dose was substituted for a minimum of 7 days and until responding was stable according to the above criteria, or for a maximum of 10 days. Following each substitution test, monkeys were returned to the maintenance dose of cocaine, 0.01 mg/kg/inj, for at least three days and until responding returned to baseline levels. In one exception, one monkey was maintained on 0.0032 mg/kg/inj cocaine prior to being tested with a combination of cocaine 0.0032 + nicotine 0.0032 mg/kg/inj and a combination of cocaine 0.0032 + nicotine 0.01 mg/kg/inj. The doses and drugs were presented in an irregular order that differed for each monkey.
Data Analysis for Drug Self-administration Studies
The primary dependent variables were the total number of drug or saline injections and food pellets self-administered per day. The number of injections self-administered during the last three days of each substitution condition were averaged. Changes in drug-maintained responding from baseline were evaluated using a one-way repeated measures analysis of variance (ANOVA). Differences between self-administration of cocaine alone and cocaine + nicotine in combination were evaluated using a two-way repeated measures ANOVA. A significant ANOVA (P < 0.05) was followed by a Bonferroni multiple comparison posthoc test when appropriate. (GraphPad Prism 5.0c for Macintosh; GraphPad Software Inc.).
Three monkeys completed all drug conditions of the study. Mean ED50 values, defined as the dose of cocaine that produced 50% of maximum possible numbers of injections per day (80 inj/day), were computed for cocaine and nicotine alone, and for the drug combinations.
Drugs
Cocaine HCl was provided by the National Institute on Drug Abuse (Rockville, MD, USA) and prepared in sterile saline (0.9%). (−)-Nicotine hydrogen tartrate was obtained commercially (Sigma-Aldrich, St. Louis, MO, USA) and solubilized in sterile water and buffered with NaOH to achieve a pH of 6–7. Self-administered drugs were sterile-filtered with a 22-micron syringe-driven filter. The cocaine + nicotine combinations were combined in the same syringe. Cocaine doses are expressed as the salt form, nicotine doses are expressed as the base.
RESULTS
Cocaine Discrimination
Cocaine discrimination performance
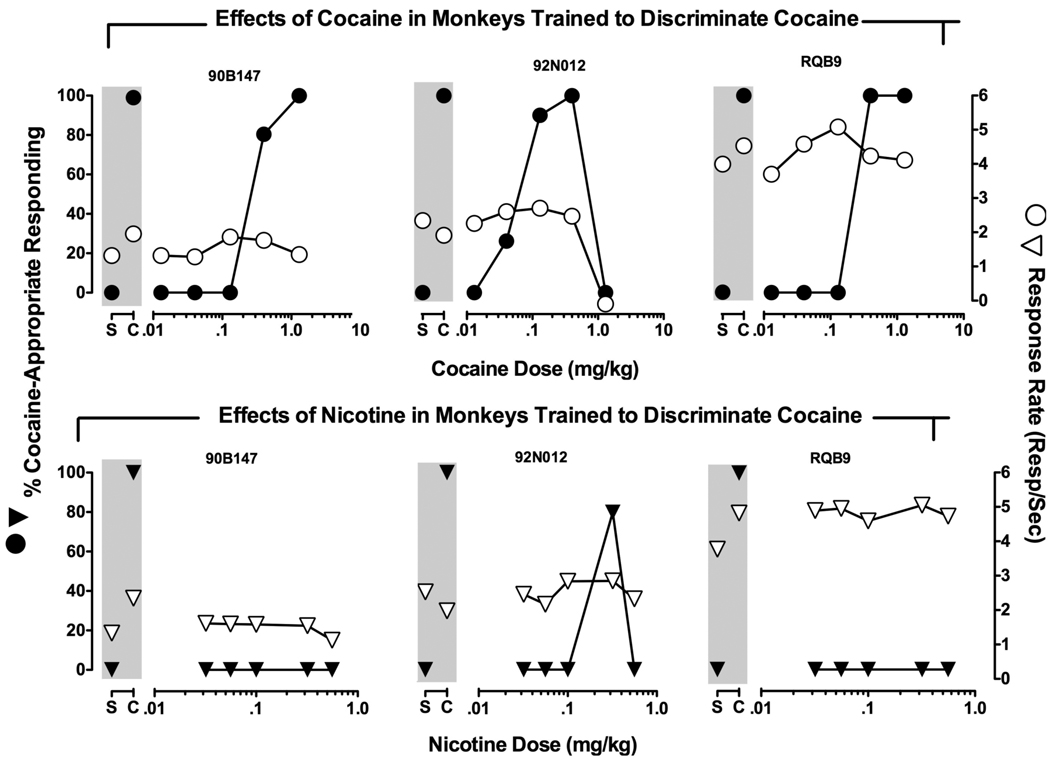
During the training days preceding test days, monkeys responded almost exclusively on the saline key during saline sessions and almost exclusively on the cocaine key during cocaine sessions (data not shown). Response rates were similar during saline (2.69 ±0.67 responses/second) and cocaine training sessions (3.16 ±0.83 responses/second). Figure 1 (upper panels) shows cocaine dose-effect curves following cumulative dosing in individual monkeys. Cocaine (0.04–1.3 mg/kg, IM) produced a dose-dependent increase in cocaine-appropriate responding in all monkeys. The ED50 values for producing 50% cocaine appropriate responding were 0.23 mg/kg for monkey 90B147, 0.06 mg/kg for monkey 92N012, and 0.26 mg/kg for monkey RQB9. Response rates were generally comparable at all doses tested and were within the range of control values; however, the highest dose of cocaine (1.3 mg/kg) suppressed responding in monkey 92N012.
Figure 1.
Cumulative dose-effect curves for cocaine (●, upper panels) and for nicotine (▼, lower panels) in three individual monkeys trained to discriminate 0.4 mg/kg cocaine from saline. Abscissae: Cocaine (Upper panels) and nicotine (Lower panels) dose in mg/kg (log scale). Left ordinates (Filled symbols): % Cocaine-appropriate responding. Right ordinates (Unfilled symbols): Response rate (responses/second). Data points above “S” and “C” show control data from saline and cocaine training cycles, respectively. Each point represents one determination at each dose. Each monkey’s identification number is located above the figures.
Effects of nicotine substitution
Figure 1 (lower panels) shows percent cocaine appropriate responding during cumulative dosing with nicotine in individual monkeys. Nicotine was substituted for cocaine to assess the extent to which the discriminative stimulus for nicotine was similar in cocaine-trained monkeys. Unlike cocaine, nicotine produced exclusively saline-appropriate responding in two monkeys. However, in one monkey, 92N012, 0.32 mg/kg nicotine produced 79% cocaine-appropriate responding. In this particular monkey, lower doses of cocaine also generalized to the cocaine training dose. Rates of food-maintained responding were unaffected by nicotine and were within the range of control values.
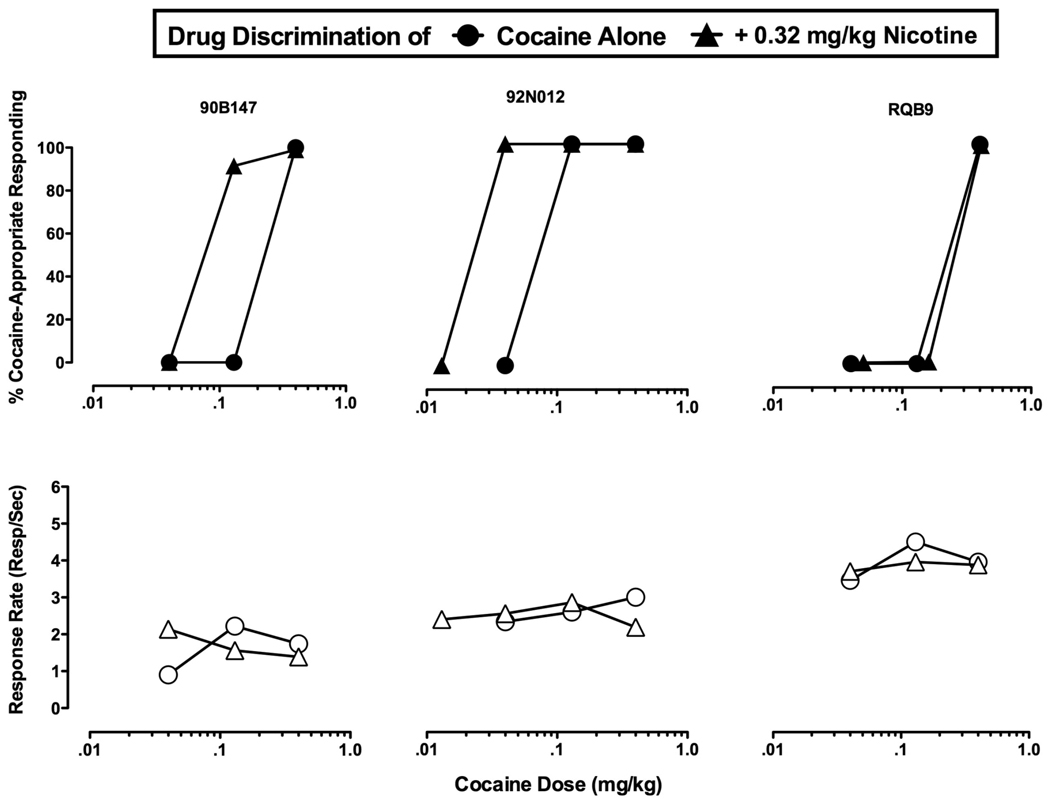
Effects of nicotine pretreatment on cocaine discrimination
Subsequently, single doses of cocaine alone and after treatment with 0.32 mg/kg nicotine were tested (Figure 2). As was observed with cumulative dosing, single doses of cocaine fully substituted for the cocaine training dose, 0.4 mg/kg, in a dose-dependent manner in all three monkeys. When nicotine (0.32 mg/kg, IM) was given as a pretreatment to cocaine, it enhanced the discriminative stimulus effects of low doses of cocaine that normally produce saline-like responding in two monkeys. Pretreatment with 0.32 mg/kg nicotine shifted the cocaine dose-effect curve leftward in two monkeys (90B147, 92N012), but produced no effect in monkey RQB9. ED50 values for cocaine alone and cocaine + 0.32 mg/kg nicotine, respectively, were 0.23 mg/kg and 0.08 mg/kg for monkey 90B147; 0.23 mg/kg and 0.023 mg/kg for monkey 92N012; and 0.23 mg/kg and 0.23 mg/kg for monkey RQB9. These ED50 values did not differ significantly as compared with a paired t-test [t (2)(=1.9]. Pretreatment with 0.32 mg/kg nicotine did not alter rates of food-maintained responding in any of the three monkeys.
Figure 2.
Effects of nicotine pretreatment on cocaine discrimination in individual monkeys. Abscissae: Cocaine dose in mg/kg (log scale). Ordinate (Upper panel): % Cocaine-appropriate responding. Ordinate (Lower panel): Response rates (responses/second). Upper figures show % cocaine-appropriate responding following administration of single doses of cocaine alone (●) and single doses of cocaine followed by pretreatment with 0.32 mg/kg nicotine (▼). Lower figures show response rates. Each monkey’s identification number is located above the figures.
Cocaine and nicotine self-administration
Self-administration of cocaine and nicotine alone
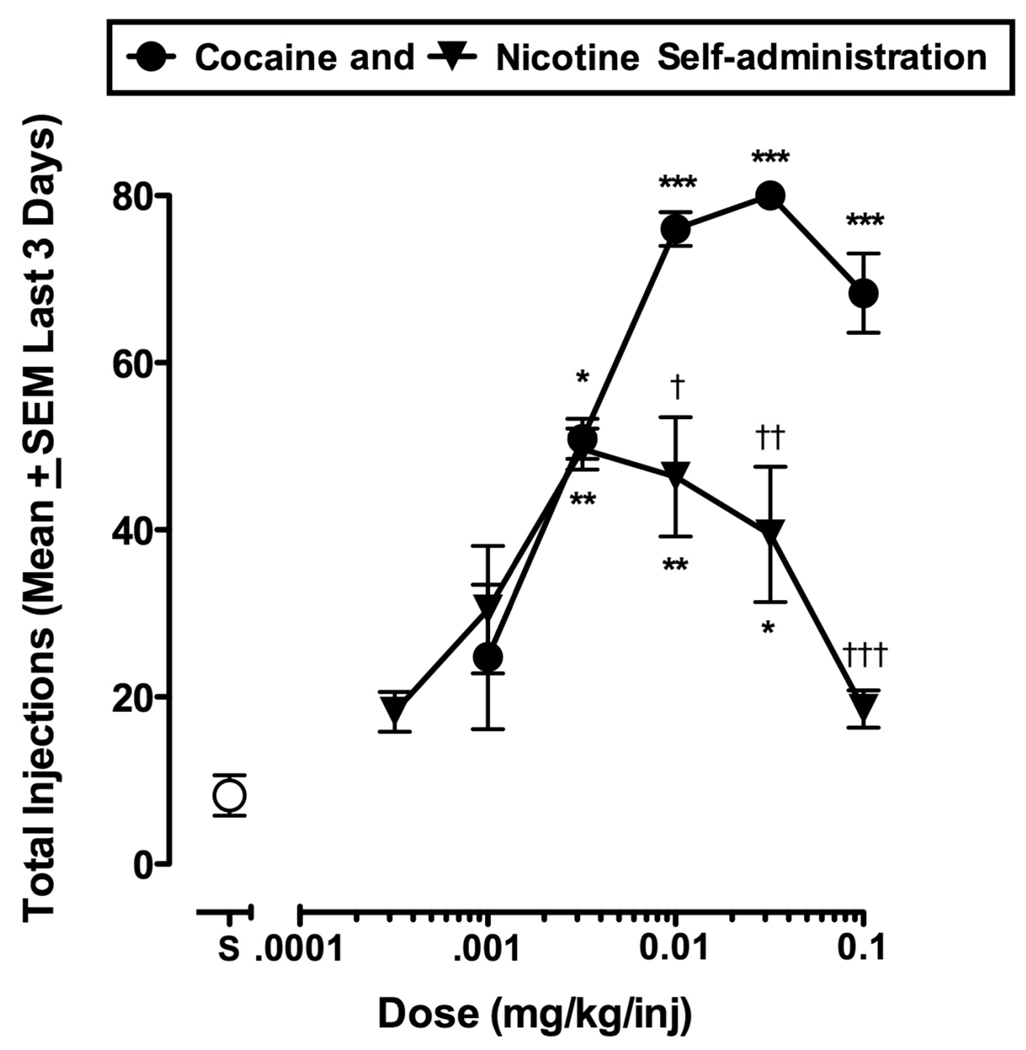
Baseline levels of cocaine self-administration were stable and 0.01 mg/kg/inj reliably maintained the near-maximum number of daily injections (76 ± 2.04 injections/day; Figure 3). Cocaine was self-administered in a dose-dependent manner and 0.032 mg/kg/inj maintained peak injection levels similar to 0.01 mg/kg/inj. When saline was substituted for cocaine, self-administration behavior decreased to low levels of 8.22 ± 2.42 injections/day. A one-way repeated measures ANOVA detected statistically significant differences between cocaine doses [F (10,32) = 15.27; P < 0.0001]. Significant differences in responding maintained by saline and cocaine 0.0032 mg/kg/inj (P < 0.05), as well as between saline and cocaine 0.01–0.1 mg/kg/inj (P < 0.0001) were detected by Bonferroni multiple comparison posthoc tests.
Figure 3.
Self-administration of cocaine alone (●) and nicotine alone (▼) in a group of three monkeys. Abscissa: Saline or drug unit dose (mg/kg/injection). Ordinate: Mean (±SEM) injections obtained on the last three days of each dose condition. The point above ‘S’ represents the number of injections when saline was available for self-administration. Asterisks indicate drug unit doses that differed significantly from saline self-administration (* P < 0.05; ** P < 0.01; *** P < 0.001). Daggers indicate unit doses of nicotine that maintained levels of responding that were significantly different from corresponding unit doses of cocaine († P < 0.05; †† P < 0.01; ††† P < 0.001).
Figure 3 also shows self-administration of nicotine alone after substitution for cocaine in the same group of monkeys. Nicotine maintained stable levels of responding and the relationship between number of injections and nicotine dose was characterized by an inverted U-shaped curve. Low doses of nicotine, (0.00032 and 0.001 mg/kg/inj), maintained levels of responding comparable to saline. The maximum number of nicotine injections was maintained by 0.0032 mg/kg/inj (46.44 ± 3.63 inj/day) and 0.01 mg/kg/inj (46.33 ± 7.18 inj/day). Higher nicotine doses (0.032 and 0.1 mg/kg/inj) were on the descending limb of the dose-effect curve. Bonferroni posthoc tests indicated statistically significant differences between saline and nicotine injections (0.0032 and 0.01 mg/kg/inj), (P < 0.01) and between saline and cocaine injections (0.032 mg/kg/inj) (P < 0.05)]. Intermediate to high doses of nicotine maintained significantly lower levels of responding than corresponding doses of cocaine. Statistically significant differences were detected between 0.01 mg/kg/inj cocaine and 0.01 mg/kg/inj nicotine (P < 0.05); between 0.032 mg/kg/inj cocaine and 0.032 mg/kg/inj nicotine (P < 0.01); and between 0.1 mg/kg/inj cocaine and 0.1 mg/kg/inj nicotine (P < 0.001).
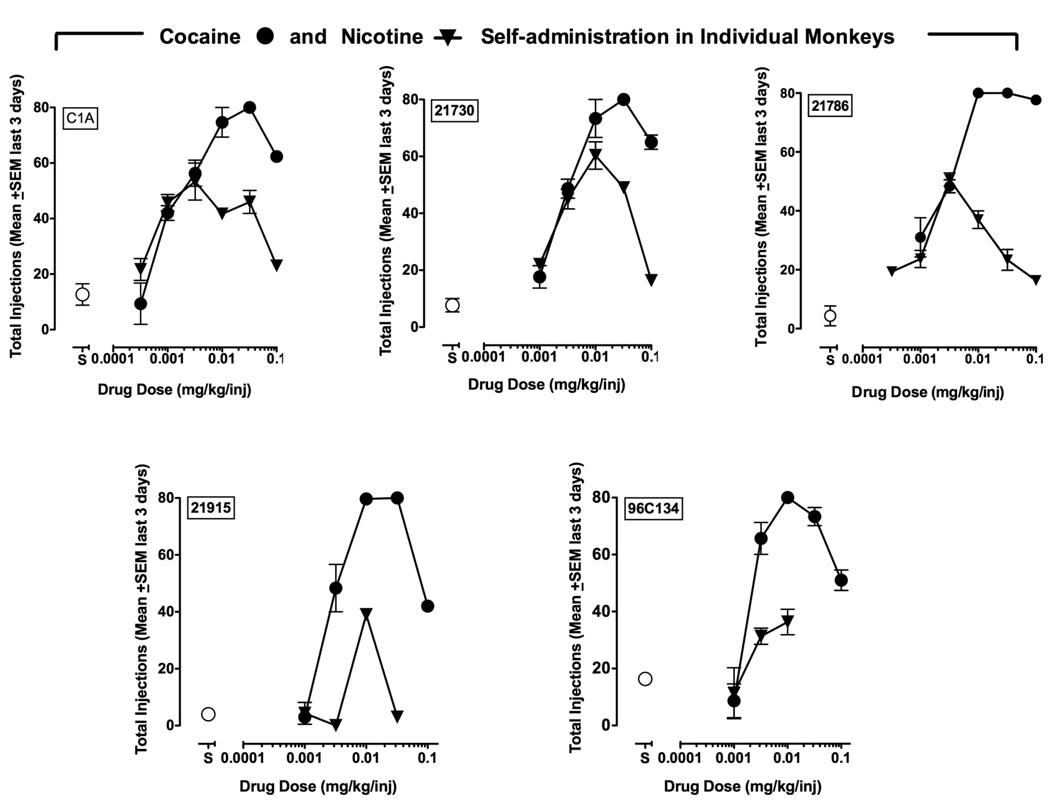
Figure 4 shows nicotine and cocaine self-administration dose-effect curves in five individual monkeys. Two animals that lost catheter patency prior to completing the nicotine dose-effect curve were not included in the statistical analysis or group figure. All monkeys self-administered both cocaine and nicotine dose-dependently, and these data are consistent with group data shown in Figure 3. Peak responding for nicotine occurred at unit doses of 0.0032 or 0.01 mg/kg/inj nicotine and peak responding for cocaine occurred at unit doses of 0.01 or 0.032 mg/kg/inj. The potency of nicotine and cocaine as reinforcers was similar. The highest doses of cocaine (0.1 mg/kg/inj) and nicotine (0.032 or 0.1 mg/kg/inj) maintained low levels of responding and were on the descending limb of the dose-effect curve.
Figure 4.
Self-administration of cocaine alone (●) and nicotine alone (▼) in individual monkeys. Abscissae: Saline or drug unit dose (mg/kg/injection). Ordinates: Mean (±SEM) injections obtained on the last three days of each dose condition. Points above ‘S’ represent numbers of injections when saline was available for self-administration. Each monkey’s identification number is shown in the upper left of each panel. Where no error bars are visible, the error bar is contained within the symbol.
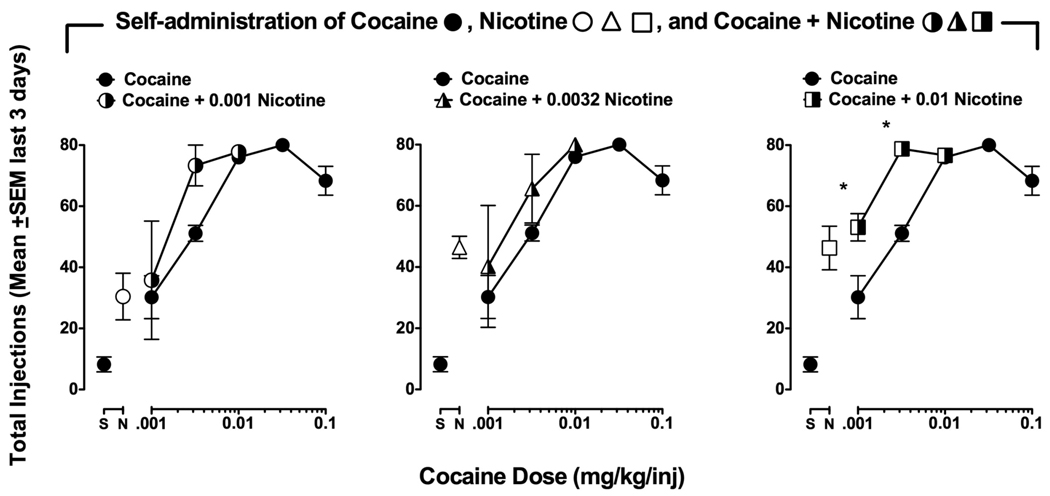
Self-administration of nicotine + cocaine combinations
Figure 5 compares self-administration of cocaine + nicotine combinations to cocaine alone. We examined the reinforcing effects of combinations of unit doses of nicotine and cocaine that fell on the ascending limbs of the dose effect curves when tested individually. Combinations of cocaine + nicotine were self-administered in a dose-dependent manner. A combination of 0.001 mg/kg nicotine with cocaine (0.001–0.1 mg/kg/inj) produced a slight leftward shift in the cocaine dose-effect curve (Figure 5, left panel). Increasing the nicotine dose to 0.0032 mg/kg/inj did not further increase the shift in the cocaine dose-effect curve (Figure 5, middle panel). However, increasing the nicotine dose by an additional ½-log unit to 0.01 mg/kg/inj produced a significant upward and leftward shift in the cocaine dose-effect curve (Figure 5, right panel).
Figure 5.
Self-administration of cocaine alone (●) and in combination with 0.001 mg/kg/inj nicotine (◑), 0.0032 mg/kg/inj nicotine (◮), and 0.01 mg/kg/inj nicotine (◨) in a group of three monkeys. Abscissae: Saline or drug unit dose (mg/kg/injection). Ordinates: Total injections obtained on the last three days of each dose condition. Points above ‘S’ represent number of injections when saline was available for self-administration and points above ‘N’ represent number of injections when only nicotine (0.001, 0.0032, and 0.01 mg/kg/inj) was available for self-administration. The cocaine alone dose-effect curve is repeated on each graph for comparison with the cocaine + nicotine combinations. Asterisks indicate unit doses of cocaine + nicotine that were significantly different from corresponding doses of cocaine alone (* P < 0.05).
A two-way repeated measures ANOVA comparing cocaine alone to cocaine + nicotine revealed a significant main effect of drug (i.e., cocaine vs. cocaine + nicotine) [F(3,18) = 4.05, P < 0.05], and of dose [F(2,18) = 6.49, P < 0.05]. Bonferroni posthoc tests revealed significant differences between cocaine alone and cocaine (0.001 and 0.0032 mg/kg/inj) combined with 0.01 mg/kg/inj nicotine (P < 0.05). Responding maintained by 0.01 mg/kg/inj cocaine was not affected by the addition of any nicotine dose. However, this unit dose of cocaine maintains maximum responding under baseline conditions, so a ceiling effect may have precluded further increases. A combination of 0.01 mg/kg/inj nicotine with cocaine produced a 3-fold shift in ED50 values for cocaine self-administration. The ED50 values for cocaine alone and when combined with 0.01 mg/kg/inj nicotine were 0.0023 mg/kg/inj (95% CL: 0.0018–0.0028) and 0.0007 mg/kg/inj (95% CL: 0.0007–0.0008) respectively. No dose of nicotine up to 0.01 mg/kg/inj reduced cocaine self-administration. Responding for food was maintained at maximum levels (100 pellets/day) throughout all drug conditions and was not affected by any drug self-administration condition (data not shown).
DISCUSSION
The goal of this study was to examine the interactions between cocaine and nicotine in a novel polydrug model of cocaine and nicotine self-administration. Our major findings were that nicotine enhanced the discriminative stimulus and reinforcing effects of cocaine. Nicotine dose-dependently shifted the cocaine self-administration dose-effect curve to the left. Importantly, self-administration of a combination of low doses of nicotine + cocaine was greater than self-administration of the same dose of either nicotine or cocaine alone. These findings are consistent with our previous reports that self-administration of another frequently abused drug combination, cocaine + heroin (‘speedball’), is enhanced under similar conditions in rhesus monkeys (Mello & Negus, 1998, 1999, 2001, 2007; Mello et al., 1995). The relation of these data to the literature and some implications of these findings are discussed below.
Drug Discrimination: Cocaine-Nicotine Interactions
Although nicotine alone did not produce cocaine-like discriminative stimulus effects in the present study, pretreatment with nicotine produced a slight leftward shift in the cocaine dose-effect curve. Similarly in rats, pretreatment with nicotine produced an increase in cocaine’s discriminative stimulus effects (Desai et al., 1999). Taken together, these findings suggest that nicotine may enhance the discriminative stimulus effects of cocaine. Previous studies in rhesus monkeys have suggested that nicotine may substitute for the cocaine stimulus in monkeys trained to discriminate a low dose of cocaine (0.25 mg/kg) (de la Garza & Johanson, 1983). It is often argued that use of a low training dose may reduce the pharmacological selectivity of the training cue, and increase the maximal effects of individual drugs and/or the overall range of drugs that produce substitution (Kantak, Riberdy, & Spealman, 1999; J. W. Smith & Stolerman, 2009; I.P. Stolerman, 1993; Terry, Witkin, & Katz, 1994).
Drug Self-administration: Cocaine-Nicotine Interactions
Cocaine self-administration was dose-related in a typical inverted U-shaped pattern. When nicotine was substituted for cocaine, nicotine maintained less responding than cocaine, but more than saline. However, when nicotine was combined with marginally reinforcing doses of cocaine, nicotine dose-dependently and significantly increased cocaine-maintained responding. These data are consistent with a recent study of the reinforcing effects of cocaine + nicotine combinations in rhesus monkeys using a progressive ratio schedule (Freeman & Woolverton, 2009). The addition of nicotine to IV cocaine solutions also shifted the cocaine dose-effect curve leftward but did not increase the progressive ratio break point (Freeman & Woolverton, 2009). The “breakpoint” is highest ratio of responses that is emitted for a single drug injection, and is the primary measure of reinforcing efficacy. These data were interpreted as reflecting an increase in cocaine’s potency, rather than its reinforcing strength (Freeman & Woolverton, 2009).
We found that nicotine was reliably self-administered when it was substituted for cocaine, whereas Freeman and Woolverton did not (Freeman & Woolverton, 2009). However, nicotine does not appear to be as readily self-administered by laboratory animals as other drugs of abuse studied under similar conditions (for a review, see Le Foll, Wertheim, & Goldberg, 2007). Under choice conditions, rats reliably self-administer cocaine over nicotine (Manzardo, Stein, & Belluzzi, 2002). Taken together, these findings suggest that nicotine may be a weaker reinforcer than cocaine. However, the duration of nicotine exposure and the schedule of reinforcement may be important determinants of differences in nicotine and cocaine self-administration. In the present study for example, nicotine self-administration may have been facilitated by continuous exposure over days. We evaluated each dose of nicotine for 7–10 consecutive days and re-established cocaine self-administration baselines between periods of nicotine substitution. In contrast, Freeman and Woolverton studied each dose alone and each dose combination for only 2 consecutive sessions (Freeman & Woolverton, 2009).
Another important difference between our study and the report by Freeman and Woolverton (Freeman & Woolverton, 2009) is the schedule of reinforcement used to maintain drug responding. Freeman and Woolverton used a progressive ratio schedule that increases the response requirement following delivery of each successive reinforcer. The beginning ratios in the Freeman and Woolverton study ranged from 50 to 200, which may have been too high to initiate nicotine self-administration. Successful establishment of nicotine self-administration typically begins with training under low ratio values or with interval schedules (Le Foll et al., 2007; Slifer & Balster, 1985; Spealman & Goldberg, 1982). Additionally, second-order schedules with interval and ratio response components have been shown to facilitate nicotine self-administration in previous studies (Goldberg, Spealman, & Goldberg, 1981; Spealman & Goldberg, 1982). The second-order schedule of reinforcement (FR2[VR16:S]) used in the present study was similarly effective in maintaining nicotine self-administration. Finally, the monkeys used in our study had extensive histories of cocaine self-administration (≥ 3 years), but it is unlikely that this was a critical factor. Previous studies have shown that cocaine self-administration history alone may not be sufficient to establish reliable nicotine self-administration (Ator & Griffiths, 1983; de la Garza & Johanson, 1987). It appears that the schedule of reinforcement and duration of nicotine exposure, may be the most important antecedent conditions for establishing nicotine as a reinforcer.
The nicotine + cocaine dose combinations examined in the present study maintained self-administration near the maximum number of injections available (80 injections/day). This limit on the total number of injections may have precluded further increases in the peak of the dose-effect curve. Higher doses of nicotine may have increased or decreased cocaine self-administration. Interestingly, response-contingent nicotine can be a punisher at the same doses that maintain responding (Goldberg & Spealman, 1983; Goldberg, Spealman, Risner, & Henningfield, 1983). However, the highest dose of nicotine (0.1 mg/kg/inj) that we tested in combination with cocaine, did not decrease drug-maintained responding. Similarly, combining cocaine with a high dose of nicotine (0.05 mg/kg/inj) did not decrease drug self-administration in previous studies (Freeman & Woolverton, 2009).
Concordance between Clinical and Preclinical Data
The present findings are in agreement with some findings from clinical laboratory and self-report studies. As noted in the Introduction, concurrent use of cocaine is often associated with increased nicotine intake. Cocaine users report smoking more cigarettes during periods of cocaine use (Roll et al., 1996; Roll et al., 1997). Also, amphetamine dose-dependently increased number of cigarettes smoked and ratings of cigarette satisfaction in a clinical laboratory study (Henningfield & Griffiths, 1981). Nicotine may also set the occasion for cocaine-seeking behavior. Nicotine enhanced reports of craving induced by cocaine-related cues in individuals with cocaine use histories (Reid, Mickalian, Delucchi, & Berger, 1999; Reid, Mickalian, Delucchi, Hall, & Berger, 1998).
Although cocaine maintained higher levels of IV self-administration than nicotine in our rhesus monkeys, a direct comparison of subjective reactions to cocaine and nicotine in man yields a complex picture. Nicotine produced higher ratings on Visual Analog Scale (VAS) measures of “High”, “Stimulated”, “Rush”, “Good Effect”, as well as “Bad Effect” and “Jittery” (Jones, Garrett, & Griffiths, 1999). Only “Drug Liking” was slightly higher for cocaine, and subjects were willing to pay more money for cocaine than for nicotine (Jones et al., 1999). When cocaine and nicotine doses were equated for VAS reports of “Drug Effect”, reports of “Good Effects” were greater for cocaine and “Bad Effects” were greater for nicotine (Jones et al., 1999). The extent to which the aversive effects of IV nicotine may have contributed to the observed differences between nicotine and cocaine self-administration in the present study cannot be determined with certainty from these data.
Another study evaluated the effects of transdermal nicotine on the subjective and reinforcing effects of cocaine (Sobel, Sigmon, & Griffiths, 2004). VAS ratings and choice for cocaine vs money were measured during chronic treatment with either a transdermal nicotine or placebo patch. Although the differences in positive subject-rated effects of cocaine under active nicotine vs placebo treatment conditions were not statistically significant, most VAS ratings were higher under the active nicotine condition. Subjects’ choice for cocaine vs money also did not differ between the nicotine and placebo patch conditions (Sobel et al., 2004). In contrast, another clinical study reported that transdermal nicotine attenuated the positive subjective ratings of cocaine after 12 hours of nicotine exposure (Kouri, Stull, & Lukas, 2001).
Possible Mechanisms Underlying Cocaine-Nicotine Interactions
Both cocaine and nicotine produce their reinforcing effects through increases in extracellular dopamine (DiChiara & Imperato, 1988). The combination of marginally reinforcing doses of each drug may produce an additive increase in dopamine that could explain our finding that cocaine + nicotine combinations were more reinforcing than either drug alone. Indeed, nicotine augments cocaine-induced increases of extracellular dopamine within the nucleus accumbens in a pharmacologically additive manner (Gerasimov et al., 2000; Sziraki et al., 1999; Zernig et al., 1997). Additive or supra-additive effects on dopamine release have also been observed for combinations of cocaine and heroin (Hemby, Co, Dworkin, & Smith, 1999; J. E. Smith, Co, Coller, Hemby, & Martin, 2006). Moreover, pharmacological studies have shown that some effects of cocaine and nicotine can be mimicked by dopamine agonists or blocked by dopamine antagonists (Corrigall & Coen, 1991; Desai et al., 2003). Interestingly, mecamylamine, a noncompetitive nicotine receptor antagonist, can prevent the escalation of cocaine self-administration in rats (Hansen & Mark, 2007). In clinical studies, mecamylamine may reduce reports of cue-induced cocaine craving in individuals who are co-dependent on nicotine and cocaine (Reid et al., 1999).
Enhancement of cocaine’s reinforcing effects may also reflect a nicotine-related increase in the saliency of stimuli associated with cocaine. We used a second-order schedule, in which response-contingent drug-associated stimuli are intermittently presented between drug injections. An early study in squirrel monkeys found that behavior maintained by nicotine-associated stimuli and nicotine injections was greater than behavior maintained by nicotine alone (Goldberg & Gardner, 1981; Goldberg et al., 1981). In this laboratory, we have found that second-order schedules reliably maintain self-administration of opioids, cocaine, and nicotine as well as combinations of cocaine and heroin and cocaine and nicotine (Mello & Negus, 1998, 1999, 2001, 2007; Mello et al., 1995). Moreover, this procedure has been useful for evaluations of candidate treatment medications and results have shown good concordance with clinical trials (Mello, 2005). Taken together, findings from preclinical and clinical studies provide compelling evidence for pharmacological and behavioral interactions between nicotine and cocaine, which could influence treatment of dependence on one or both drugs.
Because nicotine and cocaine are frequently used together, a preclinical model incorporating the self-administration of both drugs simultaneously will be useful for evaluating potential medications or medication combinations for treating this form of polydrug abuse. A number of previous studies have demonstrated that medication combinations are more effective in reducing dependence upon a drug combination, such as cocaine and heroin (Mello, 2005; Mello & Negus, 1998, 1999, 2001, 2007). Moreover, this model will be important for understanding the reinforcing effects of nicotine and cocaine combinations.
ACKNOWLEDGEMENTS
This research was supported by R01-DA026892 (NKM) and R01-DA024642 (NKM) from the National Institute on Drug Abuse, NIH. We thank Peter Fivel, Meredith Mahnke, Olga Smirnova, and Nicholas Taft for their expert technical assistance. A special thanks to Dr. Jack Bergman for his helpful comments on an earlier version of the manuscript. Preliminary data were presented at the annual meeting of the College on Problems of Drug Dependence in 2010.
Footnotes
The authors do not have any conflict of interest to disclose.
REFERENCES
- Ando K, Yanagita T. The discriminative stimulus properties of intravenously administered cocaine in rhesus monkeys. In: Colpaert FC, Rosecrans JS, editors. Stimulus Properties of Drugs: Ten Years of Progress. Amsterdam: Elsevier; 1978. pp. 125–136. [Google Scholar]
- Ator NA, Griffiths RR. Nicotine self-administration in baboons. Pharmacol Biochem Behav. 1983;19(6):993–1003. doi: 10.1016/0091-3057(83)90406-9. [DOI] [PubMed] [Google Scholar]
- Bechtholt AJ, Mark GP. Enhancement of cocaine-seeking behavior by repeated nicotine exposure in rats. Psychopharmacology (Berl) 2002;162(2):178–185. doi: 10.1007/s00213-002-1079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Hughes JR, Bickel WK. Nicotine and caffeine use in cocaine-dependent individuals. J Subst Abuse. 1993;5(2):117–130. doi: 10.1016/0899-3289(93)90056-h. [DOI] [PubMed] [Google Scholar]
- Collins SL, Izenwasser S. Chronic nicotine differentially alters cocaine-induced locomotor activity in adolescent vs. adult male and female rats. Neuropharmacology. 2004;46(3):349–362. doi: 10.1016/j.neuropharm.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Selective dopamine antagonists reduce nicotine self-administration. Psychopharmacology (Berl) 1991;104(2):171–176. doi: 10.1007/BF02244174. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653(1–2):278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Stamat HM, de Wit H. Acute doses of d-amphetamine and bupropion increase cigarette smoking. Psychopharmacology (Berl) 2001;157(3):243–253. doi: 10.1007/s002130100802. [DOI] [PubMed] [Google Scholar]
- de la Garza R, Johanson CE. The discriminative stimulus properties of cocaine in the rhesus monkey. Pharmacol Biochem Behav. 1983;19(1):145–148. doi: 10.1016/0091-3057(83)90323-4. [DOI] [PubMed] [Google Scholar]
- de la Garza R, Johanson CE. The effects of food deprivation on the self-administration of psychoactive drugs. Drug Alcohol Depend. 1987;19(1):17–27. doi: 10.1016/0376-8716(87)90083-4. [DOI] [PubMed] [Google Scholar]
- Desai RI, Barber DJ, Terry P. Asymmetric generalization between the discriminative stimulus effects of nicotine and cocaine. Behav Pharmacol. 1999;10(6–7):647–656. doi: 10.1097/00008877-199911000-00011. [DOI] [PubMed] [Google Scholar]
- Desai RI, Barber DJ, Terry P. Dopaminergic and cholinergic involvement in the discriminative stimulus effects of nicotine and cocaine in rats. Psychopharmacology (Berl) 2003;167(4):335–343. doi: 10.1007/s00213-003-1426-x. [DOI] [PubMed] [Google Scholar]
- DiChiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman KB, Woolverton WL. Self-administration of cocaine and nicotine mixtures by rhesus monkeys. Psychopharmacology (Berl) 2009;207(1):99–106. doi: 10.1007/s00213-009-1637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimov MR, Franceschi M, Volkow ND, Rice O, Schiffer WK, Dewey SL. Synergistic interactions between nicotine and cocaine or methylphenidate depend on the dose of dopamine transporter inhibitor. Synapse. 2000;38(4):432–437. doi: 10.1002/1098-2396(20001215)38:4<432::AID-SYN8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Gardner ML. Second-order schedules: extended sequences of behavior controlled by brief environmental stimuli associated with drug self-administration. NIDA Res Monogr. 1981;37:241–270. [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD. Suppression of behavior by intravenous injections of nicotine or by electric shocks in squirrel monkeys: effects of chlordiazepoxide and mecamylamine. J Pharmacol Exp Ther. 1983;224(2):334–340. [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD, Goldberg DM. Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science. 1981;214(4520):573–575. doi: 10.1126/science.7291998. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD, Risner ME, Henningfield JE. Control of behavior by intravenous nicotine injections in laboratory animals. Pharmacol Biochem Behav. 1983;19(6):1011–1020. doi: 10.1016/0091-3057(83)90408-2. [DOI] [PubMed] [Google Scholar]
- Hansen ST, Mark GP. The nicotinic acetylcholine receptor antagonist mecamylamine prevents escalation of cocaine self-administration in rats with extended daily access. Psychopharmacology (Berl) 2007;194(1):53–61. doi: 10.1007/s00213-007-0822-z. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Co C, Dworkin SI, Smith JE. Synergistic elevations in nucleus accumbens extracellular dopamine concentrations during self-administration of cocaine/heroin combinations (Speedball) in rats. J Pharmacol Exp Ther. 1999;288(1):274–280. [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology (Berl) 1997;133(1):7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Griffiths RR. Cigarette smoking and subjective response: effects of d-amphetamine. Clin Pharmacol Ther. 1981;30(4):497–505. doi: 10.1038/clpt.1981.194. [DOI] [PubMed] [Google Scholar]
- Horger BA, Giles MK, Schenk S. Preexposure to amphetamine and nicotine predisposes rats to self-administer a low dose of cocaine. Psychopharmacology (Berl) 1992;107(2–3):271–276. doi: 10.1007/BF02245147. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Weiss F, Koob GF, And NE, Ungerstedt U. Cocaine reinforcement and extracellular dopamine overflow in rat nucleus accumbens: An in vivo microdialysis study. Brain Res. 1989;498:199–203. doi: 10.1016/0006-8993(89)90422-8. [DOI] [PubMed] [Google Scholar]
- ILAR-NRC. Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council) 1996:125. [Google Scholar]
- Iyer RN, Nobiletti JB, Jatlow PI, Bradberry CW. Cocaine and cocaethylene: effects on extracellular dopamine in the primate. Psychopharmacology. 1995;120:150–155. doi: 10.1007/BF02246187. [DOI] [PubMed] [Google Scholar]
- Jones HE, Garrett BE, Griffiths RR. Subjective and physiological effects of intravenous nicotine and cocaine in cigarette smoking cocaine abusers. J Pharmacol Exp Ther. 1999;288(1):188–197. [PubMed] [Google Scholar]
- Jutkiewicz EM, Nicolazzo DM, Kim MN, Gnegy ME. Nicotine and amphetamine acutely cross-potentiate their behavioral and neurochemical responses in female Holtzman rats. Psychopharmacology (Berl) 2008;200(1):93–103. doi: 10.1007/s00213-008-1159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Riberdy A, Spealman RD. Cocaine-opioid interactions in groups of rats trained to discriminate different doses of cocaine. Psychopharmacology (Berl) 1999;147(3):257–265. doi: 10.1007/s002130051165. [DOI] [PubMed] [Google Scholar]
- Kouri EM, Stull M, Lukas SE. Nicotine alters some of cocaine's subjective effects in the absence of physiological or pharmacokinetic changes. Pharmacol Biochem Behav. 2001;69(1–2):209–217. doi: 10.1016/s0091-3057(01)00529-9. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. TINS. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Wertheim C, Goldberg SR. High reinforcing efficacy of nicotine in non-human primates. PLoS One. 2007;2(2):e230. doi: 10.1371/journal.pone.0000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbach RS, Rovetti CC, Freedland CS. The role of monoamine neurotransmitter systems in the nicotine discriminative stimulus. Drug Alcohol Depend. 1998;52(2):125–134. doi: 10.1016/s0376-8716(98)00085-4. [DOI] [PubMed] [Google Scholar]
- Manzardo AM, Stein L, Belluzzi JD. Rats prefer cocaine over nicotine in a two-lever self-administration choice test. Brain Res. 2002;924(1):10–19. doi: 10.1016/s0006-8993(01)03215-2. [DOI] [PubMed] [Google Scholar]
- Mello NK. Marian W. Fischman Memorial Lecture (2004). Evaluation of drug abuse treatment medications: Concordance between clinical and preclinical studies. In: Dewey WL, editor. Problems of Drug Dependence 2004: Proceedings of othe 66th Annual Scientific Meeting, The College on Problems of Drug Dependence, Inc. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health; 2005. pp. 82–104. [Google Scholar]
- Mello NK, Mendelson JH, Sellers ML, Kuehnle JC. Effects of heroin self-administration on cigarette smoking. Psychopharmacology. 1980;67:45–52. doi: 10.1007/BF00427594. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. The effects of buprenorphine on self-administration of cocaine and heroin "speedball" combinations and heroin alone by rhesus monkeys. J Pharmacol Exp Ther. 1998;285(2):444–456. [PubMed] [Google Scholar]
- Mello NK, Negus SS. Effects of flupenthixol and quadazocine on self-administration of speedball combinations of cocaine and heroin by rhesus monkeys. Neuropsychopharmacology. 1999;21(4):575–588. doi: 10.1016/S0893-133X(99)00056-1. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Effects of indatraline and buprenorphine on self-administration of speedball combinations of cocaine and heroin by rhesus monkeys. Neuropsychopharmacology. 2001;25(1):104–117. doi: 10.1016/S0893-133X(00)00247-5. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Effects of d-amphetamine and buprenorphine combinations on speedball (cocaine+heroin) self-administration by rhesus monkeys. Neuropsychopharmacology. 2007;32(9):1985–1994. doi: 10.1038/sj.npp.1301319. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS, Knudson IM, Kelly M, Mendelson JH. Effects of estradiol on cocaine self-administration and cocaine discrimination by female rhesus monkeys. Neuropsychopharmacology. 2008;33(4):783–795. doi: 10.1038/sj.npp.1301451. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS, Lukas SE, Mendelson JH, Sholar JW, Drieze J. A primate model of polydrug abuse: cocaine and heroin combinations. J Pharmacol Exp Ther. 1995;274(3):1325–1337. [PubMed] [Google Scholar]
- Negus SS, Mello NK, Blough BE, Baumann MH, Rothman RB. Monoamine releasers with varying selectivity for dopamine/norepinephrine versus serotonin release as candidate "agonist" medications for cocaine dependence: Studies in assays of cocaine discrimination and cocaine self-administration in rhesus monkeys. J. Pharmacol. Exp. Ther. 2007;320:627–636. doi: 10.1124/jpet.106.107383. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse. 1994;16(1):36–44. doi: 10.1002/syn.890160105. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Justice JB. Dopamine in the nucleus accumbens during cocaine self-administration as studied by in vivo microdialysis. Pharmacol. Biochem. Behav. 1989;34:899–904. doi: 10.1016/0091-3057(89)90291-8. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Justice JB. Effect of dose on cocaine self-administration behavior and dopamine levels in the nucleus accumbens. Brain Res. 1991;539:94–102. doi: 10.1016/0006-8993(91)90690-w. [DOI] [PubMed] [Google Scholar]
- Reid MS, Mickalian JD, Delucchi KL, Berger SP. A nicotine antagonist, mecamylamine, reduces cue-induced cocaine craving in cocaine-dependent subjects. Neuropsychopharmacology. 1999;20(3):297–307. doi: 10.1016/S0893-133X(98)00076-1. [DOI] [PubMed] [Google Scholar]
- Reid MS, Mickalian JD, Delucchi KL, Hall SM, Berger SP. An acute dose of nicotine enhances cue-induced cocaine craving. Drug Alcohol Depend. 1998;49(2):95–104. doi: 10.1016/s0376-8716(97)00144-0. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine self-administration appears to be mediated by dopamine uptake inhibition. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1988;12:233–239. doi: 10.1016/0278-5846(88)90040-1. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Budney AJ, Bickel WK, Badger GJ. A comparison of cocaine-dependent cigarette smokers and non-smokers on demographic, drug use and other characteristics. Drug Alcohol Depend. 1996;40(3):195–201. doi: 10.1016/0376-8716(96)01219-7. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Tidey J. Cocaine use can increase cigarette smoking: evidence from laboratory and naturalistic settings. Exp Clin Psychopharmacol. 1997;5(3):263–268. doi: 10.1037//1064-1297.5.3.263. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Wilcox KM, Woolverton WL. Self-administration of cocaine-heroin combinations by rhesus monkeys: antagonism by naltrexone. J Pharmacol Exp Ther. 1998;286(1):61–69. [PubMed] [Google Scholar]
- Rush CR, Higgins ST, Vansickel AR, Stoops WW, Lile JA, Glaser PE. Methylphenidate increases cigarette smoking. Psychopharmacology (Berl) 2005;181(4):781–789. doi: 10.1007/s00213-005-0021-8. [DOI] [PubMed] [Google Scholar]
- Schuster C, Lucchesi B, Emley G. The effects of d-amphetamine, meprobomate, and lobeline on the cigarette smoking behavior of normal human subjects. NIDA Res Monogr. 1979;23:91–99. [PubMed] [Google Scholar]
- Slifer BL, Balster RL. Intravenous self-administration of nicotine: with and without schedule-induction. Pharmacol Biochem Behav. 1985;22(1):61–69. doi: 10.1016/0091-3057(85)90487-3. [DOI] [PubMed] [Google Scholar]
- Smith JE, Co C, Coller MD, Hemby SE, Martin TJ. Self-administered heroin and cocaine combinations in the rat: additive reinforcing effects-supra-additive effects on nucleus accumbens extracellular dopamine. Neuropsychopharmacology. 2006;31:139–150. doi: 10.1038/sj.npp.1300786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JW, Stolerman IP. Recognizing nicotine: The neurobiological basis of nicotine discrimination. In: Henningfield JE, London ED, Pogun S, editors. Nicotine Psychhopharmacology. Heidelberg: Springer-Verlag; 2009. pp. 295–333. [DOI] [PubMed] [Google Scholar]
- Sobel BF, Sigmon SC, Griffiths RR. Transdermal nicotine maintenance attenuates the subjective and reinforcing effects of intravenous nicotine, but not cocaine or caffeine, in cigarette-smoking stimulant abusers. Neuropsychopharmacology. 2004;29(5):991–1003. doi: 10.1038/sj.npp.1300415. [DOI] [PubMed] [Google Scholar]
- Spealman RD, Goldberg SR. Maintenance of schedule-controlled behavior by intravenous injections of nicotine in squirrel monkeys. J Pharmacol Exp Ther. 1982;223(2):402–408. [PubMed] [Google Scholar]
- Stolerman IP. Drug discrimination. In: Van Haaren F, editor. Techniques in the behavioral and neural sciences: Methods in behavioral pharmacology. Amsterdam: Elsevier; 1993. pp. 217–243. [Google Scholar]
- Stolerman IP, Shoaib M. The neurobiology of tobacco addiction. Trends Pharmacol Sci. 1991;12(12):467–473. doi: 10.1016/0165-6147(91)90638-9. [DOI] [PubMed] [Google Scholar]
- Sziraki I, Sershen H, Benuck M, Hashim A, Laitha A. Differences in receptor system participation between nicotine- and cocaine-induced dopamine overflow in nucleus accumbens. N.Y. Acad. Sci. 1999;877:800–802. doi: 10.1111/j.1749-6632.1999.tb09326.x. [DOI] [PubMed] [Google Scholar]
- Terry P, Witkin JM, Katz JL. Pharmacological characterization of the novel discriminative stimulus effects of a low dose of cocaine. J Pharmacol Exp Ther. 1994;270(3):1041–1048. [PubMed] [Google Scholar]
- Tidey JW, O'Neill SC, Higgins ST. d-amphetamine increases choice of cigarette smoking over monetary reinforcement. Psychopharmacology (Berl) 2000;153(1):85–92. doi: 10.1007/s002130000600. [DOI] [PubMed] [Google Scholar]
- Vansickel AR, Stoops WW, Glaser PE, Rush CR. A pharmacological analysis of stimulant-induced increases in smoking. Psychopharmacology (Berl) 2007;193(3):305–313. doi: 10.1007/s00213-007-0786-z. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob. Res. 2000;2:19–37. doi: 10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]
- Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice J., Jr Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1995;120:10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]
- Zernig G, O'Laughlin IA, Fibiger HC. Nicotine and heroin augment cocaine-induced dopamine overflow in nucleus accumbens. Eur J Pharmacol. 1997;337(1):1–10. doi: 10.1016/s0014-2999(97)01184-9. [DOI] [PubMed] [Google Scholar]