Abstract
Objectives
Little is known about the effect of physical activity on diverticular complications. This study examined prospectively the association between physical activity and diverticular bleeding and diverticulitis.
Methods
We studied 47,230 US males in the Health Professionals Follow-up Study cohort who were aged 40–75 years and free of diverticular disease, gastrointestinal cancer and inflammatory bowel disease at baseline in 1986. Men reporting newly diagnosed diverticular disease on biennial follow-up questionnaires were sent supplemental questionnaires outlining details of diagnosis and treatment. Physical activity was assessed every 2 years. Men recorded the average time per week spent in 8 recreational activities, and flights of stairs climbed per day. Cox proportional hazards regression was used to calculate relative risks.
Results
During 18 years of follow-up, 800 cases of diverticulitis, and 383 cases of diverticular bleeding were identified. Total cumulative physical activity was associated with a decreased risk of diverticulitis and diverticular bleeding. After adjustment for potential confounders, the relative risk (RR) for men in the highest quintile of total activity (≥57.4 Metabolic Equivalent (MET h/wk) was 0.75 (95% CI 0.58–0.95) for diverticulitis, and 0.54 (95% CI, 0.38–0.77) for bleeding when compared to men in the lowest quintile (≤8.2 MET h/wk). Vigorous activity was inversely related to diverticulitis (multivariable RR 0.66, 95% CI, 0.51–0.86), and bleeding (multivariable RR 0.61, 95% CI, 0.41 – 0.90) in a high vs. low comparison, whereas non-vigorous activity was not. These results were similar for recent (simple updated) and baseline activity.
Conclusions
Data from this large prospective cohort suggest that physical activity lowers the risk of diverticulitis and diverticular bleeding. Vigorous activity appears to account for this association.
Keywords: Diverticular disease, diverticulitis, diverticular bleeding, physical activity
INTRODUCTION
Diverticulosis is one of the most common gastrointestinal disorders. An estimated two-thirds of adults will develop diverticulosis by the ninth decade.(1) Dietary fiber is considered the predominant risk factor for the development of diverticulosis.(2) Evidence also suggests that a low fiber diet contributes to symptomatic diverticular disease, although most studies include only patients with nonspecific gastrointestinal symptoms in the setting of diverticulosis, and not those with the objective and clinically relevant outcomes of diverticulitis and bleeding.(3, 4)
Other features of the Western or industrialized lifestyle parallel the adoption of a low-fiber diet, and a number of these have been identified as potential risk factors for complicated diverticular disease. These include red meat consumption, obesity and the use of non-steroidal anti-inflammatory drugs.(5–7) Physical activity may reduce the risk of colon cancer, and a number of other gastrointestinal disorders (8–10) by decreasing transit time, inflammation and colon pressures. These proposed mechanisms may also be beneficial in diverticular disease.
In a prior study of the first six years of the Health Professionals Follow-up Study, our group found that physical activity was inversely associated with symptomatic diverticular disease.(11) However, this study was not adequately powered to examine diverticulitis and diverticular bleeding separate from nonspecific gastrointestinal symptoms in the setting of diverticulosis, and did not adjust for body mass index or the use of non-steroidal anti-inflammatory drugs. We therefore aimed to use 18 years of prospective follow-up from the Health Professionals Follow-up study to examine the association between physical activity and diverticulitis and diverticular bleeding. We hypothesized that physical activity would be inversely related to both bleeding and diverticulitis but that the magnitude of the effect might differ.
METHODS
Participants were selected from the Health Professionals Follow-up Study, a study of 51,529 male dentists, veterinarians, pharmacists, optometrists, osteopathic physicians and podiatrists aged 40–75 years who have been followed since 1986 via self-administered medical and dietary questionnaires. We included in the analysis the 47,228 men who at baseline were free of diverticulosis or its complications, cancer (except non-melanoma skin cancer), and inflammatory bowel disease; and who answered the physical activity questions, and reported daily caloric intakes between 800–4,200 kcal/day.
Diverticulitis and Diverticular Bleeding Cases
Incident diverticulosis and diverticulitis were assessed on the biennial follow-up questionnaires beginning in 1990. Men reporting these diagnoses received a 5 question supplemental questionnaires outlining diagnosis and treatment. Trained abstractors performed double data entry. A study investigator (L.L.S) blinded to exposure status reviewed all questions pertaining to data entry. Diverticulitis and diverticular bleeding were the main study endpoints.
Diverticulitis was defined as the report of pain attributed to diverticular disease and one of the following: 1) Fistula, abscess, perforation or obstruction; 2) Treated with antibiotics, hospitalization, or surgery; 3) Categorized as severe or acute; presenting with fever, requiring medication, or evaluated with computed tomography. Diverticular bleeding was defined as rectal bleeding attributed to diverticular disease and one of the following: 1) Requiring hospitalization, intravenous fluids, blood transfusions, angiography, nuclear medicine scanning or surgery; 2) Described as profuse; or 3) Without other potential gastrointestinal, rectal or anal sources in men whose bleeding was not evaluated as part of a routine endoscopy or barium enema. We used the first 2 criteria for each endpoint definition in sensitivity analyses for the endpoint definitions. These definitions took into account the large number of patients with diverticulitis who are managed in the outpatient setting.(12) Men who reported asymptomatic diverticulosis or nonspecific symptoms such as pain or change in bowel habits but who did not meet these criteria were considered to have uncomplicated diverticulosis.
A total of 179 available medical charts from men reporting diverticular disease in 1990 and 1992 were reviewed to assess the validity of self-report. Chart review confirmed a diagnosis of diverticular disease in 97% of the cases. In 85% of cases, the diagnosis in the medical record matched our outcome definitions based on self-report. Fifty percent of the non-concordant cases had a chart diagnosis of diverticulitis or diverticular bleeding, but were classified as uncomplicated disease based on the response to the supplemental questionnaire.
Physical Activity Assessment
Physical activity was assessed on biennial follow-up questionnaires. Beginning in 1986, participants were asked to note their average weekly time spent in 8 recreational activities during the previous year according to 13 response categories ranging from none to 40 or more hours per week. Activities included walking; jogging (> 10 minutes/mile); running (≤10 minutes/mile); bicycling (including stationary bike); lap swimming; tennis; squash or racquet ball; calisthenics, rowing, stair or ski machine. The number of flights of stairs climbed daily was also assessed. The reproducibility and validity of the physical activity questions was demonstrated previously in a random subset of 238 participants who returned a past-week recall and one-week activity diary during each of the 4 seasons.(13)
To calculate total physical activity, each activity was assigned a MET score based on energy expenditure.(14) (One MET is defined as the energy expended by a 70kg adult while at rest.) The MET score was multiplied by the duration of activity in hours and expressed in Metabolic Equivalents (MET)-hours per week. The MET-hours per week of all activities were summed to derive the total energy expended during physical activity. Activities with a MET score of less than 6 (walking, stair climbing) were classified as non-vigorous, and those with a MET score of 6 or more (all other specified activities) as vigorous. In addition, time spent in sedentary behaviors such as sitting, watching television and driving was collected beginning in 1988. Categories were created for missing values for the specific activities (e.g. running).
Other Potential Risk Factors
We also assessed a number of other factors that have been associated with diverticular bleeding or diverticulitis in our studies and in the literature. These included body mass index, use of non-steroidal anti-inflammatory drugs (NSAIDs) and acetaminophen, and consumption of fat, fiber, red meat, nuts and popcorn.(5, 7, 15–19). Dietary information was derived from a 131-item food frequency questionnaire mailed every 2 years. Dietary fat and fiber were adjusted for total caloric intake using regression analysis. Prior studies in this cohort have demonstrated the validity and reproducibility of the food frequency questionnaires and the self-reported anthropometric measurements.(20, 21)
Statistical Analysis
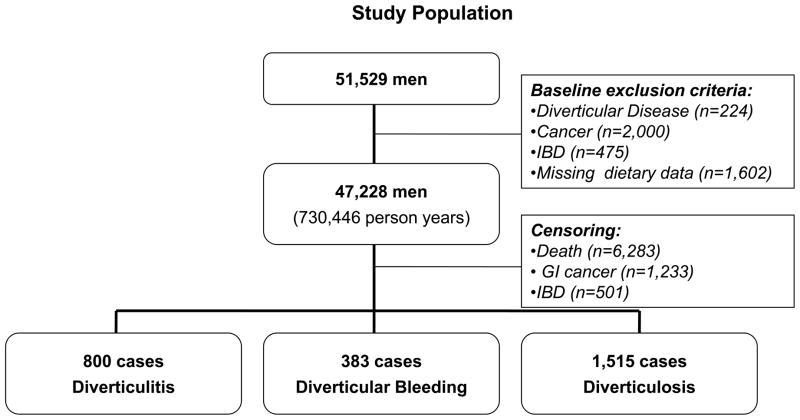
Participant follow-up time accrued from the date of return of the baseline questionnaire to the first of the following: date of diagnosis of diverticulosis or diverticular complications, date of death, or the end of follow-up (December 31, 2004). Men who reported a new diagnosis of gastrointestinal cancer, inflammatory bowel disease, diverticulosis or diverticular complications were censored at the date of diagnosis.(Figure 1) We censored men with a new diagnosis of uncomplicated diverticulosis because men with known asymptomatic diverticulosis likely represent a biased subset of men who have undergone lower endoscopy or abdominal imaging, and men with known diverticulosis are likely to alter their behaviors which could also bias the results. Because a large number of men likely have undetected asymptomatic disease, sufficient numbers remain to follow for the analysis.
Figure 1.
Men with diverticular disease, cancer (except non-melanoma skin cancer), inflammatory bowel disease (IBD), or missing or implausible dietary data were excluded at baseline. For the analysis, men were censored at the time of death, or diagnosis with GI cancer, IBD or diverticular disease (diverticulitis, diverticular bleeding, diverticulosis).
We used Cox proportional hazards models to calculate age-adjusted and multivariable relative risks (RR) and 95% Confidence Intervals (CI), comparing men in the highest versus lowest categories of physical activity.(22) Age-adjusted models included age in one-year intervals and study period in two-year intervals. Multivariable models adjusted for age and study period as well as sedentary behavior, BMI, use of NSAIDs, use of acetaminophen, dietary fat, fiber, red meat, combined nut, corn and popcorn consumption, and total calories. Vigorous and non-vigorous activity were simultaneously adjusted when examined.
Cumulative updated physical activity was used in the main analyses as the best measure of long-term exposure. To calculate cumulative activity, at the beginning of each follow-up period the activity data from all previous study periods was averaged. For example, the average of the 1986 and 1988 physical activity was used to calculate the risk for the period from 1988 to 1990. Simple updating (using data from the questionnaire immediately preceding the follow-up interval of interest) was used for the other covariates including BMI, medication use and dietary intake. We categorized total, vigorous and non-vigorous physical activity in quintiles. Body mass index was divided into 6 categories, and all dietary variables into quintiles. Use of NSAIDs and acetaminophen were categorized as binary variables.
We used the median value in each physical activity category as a continuous variable to test for linear trend. The proportional hazards assumption was checked for physical activity by testing for a significant (P <0.05) time variation in the hazard ratio. There were no significant departures. SAS software, version 9.1 (SAS Institute Inc., Cary, North Carolina) was used for the analyses. All reported values are two-sided, with a significance level of <0.05. The study was approved by the institutional review boards of the Harvard School of Public Health and Brigham and Women’s Hospital.
RESULTS
From 1986 to 2004 (729,080 person years), we identified 800 incident cases of diverticulitis, and 383 incident cases of diverticular bleeding. Baseline characteristics according to total and vigorous physical activity and standardized for age and study period are shown in Table 1. Men who exercised vigorously tended to be younger than those who did not, and physically active men were on average less likely to smoke and more likely to have a diet low in fat and red meat and high in fiber.
Table 1.
Baseline Characteristics According to Total and Vigorous Physical Activity
| Total Physical Activitya | Vigorous Physical Activityb | |||||
|---|---|---|---|---|---|---|
| Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | |
| No. of individuals | 9,157 | 9,370 | 9,431 | 17,172 | 5,625 | 7,162 |
| Age (yr) | 54 | 54 | 53 | 56 | 53 | 50 |
| BMI (kg/m2) | 25.4 | 25.1 | 24.3 | 25.4 | 24.9 | 24.1 |
| MET (h/wk) | 1.1 | 11.9 | 61.5 | 8.5 | 14.9 | 64.3 |
| Current smoking (%) | 15.0 | 9.7 | 6.7 | 14.4 | 8.5 | 4.9 |
| Current NSAIDs (%) | 33 | 33 | 32 | 33 | 35 | 30 |
| Lower endoscopy (%)c | 62 | 68 | 69 | 63 | 69 | 71 |
| Physical exam (%)d | 58 | 63 | 62 | 59 | 64 | 63 |
| Total calories (kcal/day) | 1934 | 1981 | 2050 | 1980 | 1983 | 2017 |
| Alcohol (g/day) | 10.9 | 11.3 | 12.2 | 11.7 | 11.1 | 11.6 |
| Total fat (g/day) | 75 | 72 | 68 | 74 | 71 | 67 |
| Total fiber (g/day) | 19 | 21 | 23 | 20 | 22 | 23 |
| Red meat (servings/day) | 4.8 | 4.3 | 3.8 | 4.8 | 4.2 | 3.4 |
BMI, body mass index; MET, metabolic equivalent; NSAID, non-steroidal anti-inflammatory
Quintiles of total activity in MET-h/wk: Q1 ≤ 8.2; Q3 19.1–33.5; Q5 ≥ 57.4
Quintiles of vigorous activity in MET-h/wk: Q1 0; Q3 4.1–10; Q5 >=28.1
Proportion of participants reporting ever having a sigmoidoscopy or colonoscopy during the study period from 1986–2004.
Proportion of participants reporting a physical examination in 1988, the first year this information was ascertained
A statistically significant inverse association was seen between total physical activity and the risk of diverticulitis and diverticular bleeding (Tables 2 and 3). After adjustment for other known or potential confounders, men in the highest quintile of physical activity (≥57.4 MET-h/week) had a 25% and 46% reduction in the risk of diverticulitis and bleeding, respectively, compared to those in the lowest quintile (≤ 8.2 MET-h/week). Vigorous activity was also associated with a decreased risk of diverticulitis and diverticular bleeding in a highest to lowest quintile comparison (multivariable RR, 0.66; 95% CI, 0.51–0.86, and multivariable RR, 0.61; 95% CI, 0.41–0.90). However, non-vigorous activity was not significantly associated with either outcome. Time spent in sedentary behavior was also not significantly associated with diverticulitis or diverticular bleeding.
Table 2.
Physical activity and sedentary behavior and the risk of diverticulitis
| Total Physical Activity, MET-h/wk (quintiles) a | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | P value for Trend | |
| Casesb | 152 | 185 | 169 | 170 | 124 | |
| Person years | 129,642 | 143,765 | 149,069 | 151,888 | 153,792 | |
| Age-adjusted RRc | 1.00 | 1.08 | 0.95 | 0.94 | 0.68 | <0.001 |
| 95% CI | - | 0.87–1.34 | 0.77–1.18 | 0.75–1.17 | 0.54–0.86 | |
| Multivariable RRd | 1.00 | 1.08 | 0.97 | 0.98 | 0.75 | 0.005 |
| 95% CI | - | 0.87–1.34 | 0.77–1.21 | 0.78–1.23 | 0.58–0.95 | |
|
| ||||||
| Vigorous Physical Activity, MET-h/wk (quintiles)e | ||||||
| 1 | 2 | 3 | 4 | 5 | P value for Trend | |
|
| ||||||
| Casesb | 207 | 169 | 176 | 167 | 82 | |
| Person years | 174,557 | 141,175 | 138,942 | 158,234 | 115,901 | |
| Age-adjusted RRc | 1.00 | 0.93 | 0.98 | 0.83 | 0.57 | <0.001 |
| 95% CI | - | 0.75–1.14 | 0.80–1.20 | 0.68–1.03 | 0.44–0.74 | |
| Multivariable RRf | 1.00 | 0.91 | 0.99 | 0.89 | 0.66 | 0.002 |
| 95% CI | - | 0.74–1.12 | 0.81–1.22 | 0.72–1.10 | 0.51–0.86 | |
|
| ||||||
| Non-Vigorous Physical Activity, MET-h/wk (quintiles)g | ||||||
| 1 | 2 | 3 | 4 | 5 | P value for Trend | |
|
| ||||||
| Casesb | 149 | 158 | 163 | 160 | 171 | |
| Person years | 132,094 | 146,870 | 150,737 | 150,163 | 148,943 | |
| Age-adjusted RRc | 1.00 | 1.00 | 0.97 | 0.95 | 1.0 | 0.91 |
| 95% CI | - | 0.80–1.25 | 0.77–1.21 | 0.76–1.19 | 0.81–1.26 | |
| Multivariable RRf | 1.00 | 0.94 | 0.91 | 0.90 | 0.96 | 0.95 |
| 95% CI | - | 0.75–1.18 | 0.73–1.15 | 0.71–1.13 | 0.76–1.21 | |
|
| ||||||
| Sedentary Behavior, h/wk (quintiles)h | ||||||
| 1 | 2 | 3 | 4 | 5 | P value for Trend | |
|
| ||||||
| Casesa | 141 | 142 | 162 | 166 | 138 | |
| Person years | 136,483 | 99,647 | 146,156 | 147,544 | 120,844 | |
| Age-adjusted RRb | 1.00 | 1.19 | 1.04 | 1.07 | 1.02 | 0.83 |
| 95% CI | - | 0.94–1.5 | 0.83–1.31 | 0.85–1.34 | 0.80–1.29 | |
| Multivariable RRi | 1.00 | 1.14 | 1.02 | 1.03 | 0.97 | 0.56 |
| 95% CI | - | 0.90–1.45 | 0.81–1.28 | 0.82–1.29 | 0.76–1.23 | |
MET, Metabolic Equivalent; RR, Relative Risk; CI, Confidence Interval.
Quintiles of Total Physical Activity: ≤8.2, 8.3–19.0, 19.1–33.5, 33.6–57.3, ≥57.4
Cases numbers may vary because of missing values.
Age-adjusted models include age in 1-year intervals and study period in 2-year intervals
Multivariable models include age, study period, sedentary behavior, BMI, use of NSAIDs, use of acetaminophen, dietary fat, fiber, red meat, combined nut, corn and popcorn consumption, and total calories.
Quintiles of Vigorous Physical Activity: 0, 0.1–4, 4.1–10, 10.1–28, ≥28.1
Models of vigorous activity adjust for non-vigorous activity and vice versa in addition to other covariates.
Quintiles of Non-vigorous Activity: 0–2.9, 3–7.9, 8–15.9, 16–29.9, ≥30
Quintiles of Sedentary Behavior: 0–15.9, 16–26.9, 27–38, 38.1–51.7, ≥51.8
Models of sedentary behavior are adjusted for total physical activity in addition to other covariates.
Table 3.
Physical activity and sedentary behavior and the risk of diverticular bleeding
| Total Physical Activity, MET-h/wk (quintiles)a | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | P value for Trend | |
| Casesb | 91 | 93 | 75 | 69 | 55 | |
| Person years | 129,642 | 143,765 | 149,069 | 151,888 | 153,792 | |
| Age-adjusted RRc | 1.00 | 0.92 | 0.72 | 0.66 | 0.52 | <0.001 |
| 95% CI | - | 0.69–1.23 | 0.53–0.98 | 0.48–0.90 | 0.37–0.73 | |
| Multivariable RRd | 1.00 | 0.91 | 0.71 | 0.66 | 0.54 | <0.001 |
| 95% CI | - | 0.68–1.22 | 0.52–0.98 | 0.48–0.92 | 0.38–0.77 | |
|
| ||||||
| Vigorous Physical Activity, MET-h/wk (quintiles)e | ||||||
| 01 | 2 | 3 | 4 | 5 | P value for Trend | |
|
| ||||||
| Casesa | 110 | 98 | 63 | 75 | 37 | |
| Person years | 174,557 | 141,175 | 138,942 | 158,234 | 115,901 | |
| Age-adjusted RRb | 1.00 | 1.08 | 0.72 | 0.80 | 0.57 | 0.001 |
| 95% CI | - | 0.81–1.42 | 0.52–0.98 | 0.59–1.08 | 0.39–0.84 | |
| Multivariable RRf | 1.00 | 1.04 | 0.70 | 0.81 | 0.61 | 0.008 |
| 95% CI | - | 0.79–1.37 | 0.51–0.97 | 0.59–1.10 | 0.41–0.90 | |
| Non-Vigorous Physical Activity, MET-h/wk (quintiles)g | ||||||
| 1 | 2 | 3 | 4 | 5 | P value for Trend | |
|
| ||||||
| Casesa | 75 | 74 | 77 | 86 | 71 | |
| Person years | 132,094 | 146,870 | 150,737 | 150,163 | 148,943 | |
| Age-adjusted RRb | 1.00 | 0.93 | 0.92 | 0.99 | 0.78 | 0.14 |
| 95% CI | - | 0.68–1.29 | 0.67–1.26 | 0.72–1.36 | 0.56–1.08 | |
| Multivariable RRf | 1.00 | 0.90 | 0.89 | 0.96 | 0.77 | 0.17 |
| 95% CI | - | 0.65–1.25 | 0.64–1.23 | 0.70–1.33 | 0.55–1.07 | |
|
| ||||||
| Sedentary Behavior, h/wk (quintiles)h | ||||||
| 1 | 2 | 3 | 4 | 5 | P value for Trend | |
|
| ||||||
| Casesa | 83 | 69 | 70 | 73 | 63 | |
| Person years | 136,483 | 99,647 | 146,156 | 147,544 | 120,844 | |
| Age-adjusted RRb | 1.00 | 1.01 | 0.81 | 0.86 | 0.88 | 0.24 |
| 95% CI | - | 0.73–1.40 | 0.59–1.12 | 0.63–1.18 | 0.63–1.22 | |
| Multivariable RRi | 1.00 | 1.01 | 0.82 | 0.85 | 0.83 | 0.14 |
| 95% CI | - | 0.73–1.4 | 0.59–1.13 | 0.62–1.17 | 0.60–1.17 | |
MET, Metabolic Equivalent; RR, Relative Risk; CI, Confidence Interval.
Quintiles of Total Physical Activity: ≤8.2, 8.3–19.0, 19.1–33.5, 33.6–57.3, ≥57.4
Cases numbers may vary because of missing values.
Age-adjusted models include age in 1-year intervals and study period in 2-year intervals
Multivariable models include age, study period, sedentary behavior, BMI, use of NSAIDs, use of acetaminophen, dietary fat, fiber, red meat, combined nut, corn and popcorn consumption, and total calories.
Quintiles of Vigorous Physical Activity: 0, 0.1–4, 4.1–10, 10.1–28, ≥28.1
Models of vigorous activity adjust for non-vigorous activity and vice versa in addition to other covariates.
Quintiles of Non-vigorous Activity: 0–2.9, 3–7.9, 8–15.9, 16–29.9, ≥30
Quintiles of Sedentary Behavior: 0–15.9, 16–26.9, 27–38, 38.1–51.7, ≥51.8
Models of sedentary behavior are adjusted for total physical activity in addition to other covariates.
We also examined the specific vigorous activities (running/jogging, racquet ball, biking, swimming, tennis, rowing/calisthenics), as well as walking. Running was the only activity significantly associated with a decreased risk of diverticulitis. After adjustment for total physical activity, the other vigorous activities, walking, sedentary hours, and other potential confounders, the RR for diverticulitis was 0.53 (95% CI, 0.32–0.88) in men who ran at least 24 MET-hours per week compared to men who did not run. The magnitude of risk reduction was similar for diverticular bleeding, although the association did not reach statistical significance (multivariable RR 0.52; 95% CI, 0.23–1.18). Racquet ball was also associated with a decreased risk of both diverticulitis and diverticular bleeding, but the power to detect a statistically significant result may have been limited. The multivariable RR was 0.68 (94% CI, 0.37–1.27) for diverticulitis, and 0.50 (95% CI, 0.15–1.60) for bleeding when comparing frequent to infrequent players.. Walking was not associated with either outcome. The multivariable RR for diverticulitis was 1.06 (95% CI, 0.78–1.44), and for bleeding was 1.22 (95% CI, 0.77–1.92). We further explored the effect of walking in older men (> 70 years), and in men in the lowest 2 quintiles of vigorous activity, and again found no significant associations.
In addition to cumulative updated activity, we analyzed baseline and recent activity and found similar results. For recent activity using simple updating, the multivariable RR for the most physically active men was 0.79 (95% CI, 0.63–0.99) for diverticulitis, and 0.59 (95% CI, 0.42–0.82) for bleeding when compared to the least active men. The multivariable RRs for baseline activity in 1986 were 0.78 (95% CI, 0.61–0.98) for diverticulitis, and 0.61 (95% CI, 0.44–0.86) for bleeding.
We explored the possibility that the effect of physical activity may differ in obese versus non-obese individuals, by creating interaction terms between BMI (<25, 25–29, ≥30) and physical activity (in tertiles). In adjusted analyses, the P for interaction was 0.34 for diverticulitis and 0.98 for bleeding, suggesting the absence of effect modification by body size. Nevertheless, the risk associated with physical inactivity was greatest in obese men. The multivariable RR was 1.62 (95% CI, 1.16–2.26) for diverticulitis and 2.81 (95% CI, 1.76–4.46) for bleeding in men with a BMI ≥30 and in the lowest tertile of activity compared to men with a BMI of < 25 and in the highest tertile of activity.
We restricted the analysis to men who had undergone a lower endoscopy to investigate the possibility of detection bias. The results were unchanged for diverticular bleeding (multivariable RR 0.49; 95% CI 0.31–0.79), but lost significance for diverticulitis (multivariable RR 0.91; 95% CI, 0.65–1.26) in high to low activity comparisons. We also performed a sensitivity analysis for our study endpoints by including only men meeting either of the first 2 outcome criteria. The magnitudes of the risk reductions were similar, but lost significance. The multivariable RR was 0.79 (95% CI, 0.51–1.22) for diverticulitis, and 0.64 (95% CI, 0.29–1.41) for bleeding.
Lastly, we studied the association between physical activity and uncomplicated diverticulosis (diverticulosis without diverticulitis or diverticular bleeding) in the subset of men who had undergone a lower endoscopy (to limit the possibility of detection bias). When comparing men in the highest quintile of activity to those in the lowest, the multivariable RR was 0.93 (95% CI, 0.78–1.12). However, men who spent more than 52 hours per week in sedentary behaviors were at an increased risk of uncomplicated diverticulosis when compared to men who spent less than 16 hours per week (multivariable RR, 1.29; 95% CI, 1.08–1.54). (Table 4)
Table 4.
Physical activity and sedentary behavior and the risk of uncomplicated diverticulosisa
| Total Physical Activity, MET-h/wk (quintiles)b | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | P value for Trend | |
| Casesc | 224 | 306 | 340 | 345 | 300 | |
| Person years | 68,701 | 75,740 | 79,510 | 81,197 | 80,962 | |
| Age-adjusted RRd | 1.00 | 1.21 | 1.28 | 1.26 | 1.10 | 0.97 |
| 95% CI | - | 1.02–1.44 | 1.08–1.51 | 1.06–1.49 | 0.93–1.31 | |
| Multivariable RRe | 1.00 | 1.05 | 1.07 | 1.04 | 0.93 | 0.36 |
| 95% CI | - | 0.88–1.24 | 0.90–1.27 | 0.88–1.24 | 0.78–1.12 | |
|
| ||||||
| Vigorous Physical Activity, MET-h/wk (quintiles)f | ||||||
| 1 | 2 | 3 | 4 | 5 | P value for Trend | |
|
| ||||||
| Casesc | 294 | 307 | 330 | 366 | 218 | |
| Person years | 85,711 | 75,528 | 76,854 | 87,118 | 61,201 | |
| Age-adjusted RRd | 1.00 | 1.11 | 1.18 | 1.19 | 1.05 | 1.00 |
| 95% CI | - | 0.94–1.30 | 1.01–1.38 | 1.02–1.39 | 0.88–1.25 | |
| Multivariable RRg | 1.00 | 0.97 | 1.02 | 1.06 | 0.96 | 0.96 |
| 95% CI | - | 0.82–1.14 | 0.87–1.20 | 0.90–1.24 | 0.80–1.16 | |
|
| ||||||
| Non-Vigorous Physical Activity, MET-h/wk (quintiles)h | ||||||
| 1 | 2 | 3 | 4 | 5 | P value for Trend | |
|
| ||||||
| Casesc | 223 | 298 | 350 | 317 | 327 | |
| Person years | 71,710 | 76,741 | 79,322 | 80,020 | 78,623 | |
| Age-adjusted RRd | 1.00 | 1.25 | 1.37 | 1.19 | 1.23 | 0.45 |
| 95% CI | - | 1.05–1.49 | 1.16–1.62 | 1.00–1.42 | 1.03–1.46 | |
| Multivariable RRg | 1.00 | 1.03 | 1.09 | 0.93 | 0.96 | 0.50 |
| 95% CI | - | 0.87–1.23 | 0.92–1.29 | 0.78–1.11 | 0.80–1.14 | |
|
| ||||||
| Sedentary Behavior, h/wk (quintiles)i | ||||||
| 1 | 2 | 3 | 4 | 5 | P value for Trend | |
|
| ||||||
| Casesc | 227 | 265 | 354 | 330 | 311 | |
| Person years | 70,276 | 57,720 | 75,759 | 76,560 | 66,688 | |
| Age-adjusted RRd | 1.00 | 1.27 | 1.43 | 1.34 | 1.40 | 0.0001 |
| 95% CI | - | 1.06–1.52 | 1.21–1.69 | 1.13–1.59 | 1.17–1.66 | |
| Multivariable RRj | 1.00 | 1.18 | 1.34 | 1.25 | 1.29 | 0.008 |
| 95% CI | - | 0.99–1.42 | 1.13–1.59 | 1.05–1.48 | 1.08–1.54 | |
MET, Metabolic Equivalent; RR, Relative Risk; CI, Confidence Interval
The analyses of uncomplicated diverticulosis were limited to men having undergone a lower endoscopy to reduce the risk of detection bias.
Quintiles of Total Physical Activity: ≤8.2, 8.3–19.0, 19.1–33.5, 33.6–57.3, ≥57.4
Cases numbers and person years may vary because of missing values.
Age-adjusted models include age in 1-year intervals and study period in 2-year intervals
Multivariable models include age, study period, sedentary behavior, BMI, use of NSAIDs, use of acetaminophen, dietary fat, fiber, red meat, combined nut, corn and popcorn consumption, and total calories.
Quintiles of Vigorous Physical Activity: 0, 0.1–4, 4.1–10, 10.1–28, ≥28.1
Models of vigorous activity adjust for non-vigorous activity and vice versa in addition to other covariates.
Quintiles of Non-vigorous Activity: 0–2.9, 3–7.9, 8–15.9, 16–29.9, ≥30
Quintiles of Sedentary Behavior: 0–15.9, 16–26.9, 27–38, 38.1–51.7, ≥51.8
Models of sedentary behavior are adjusted for total physical activity in addition to other covariates.
DISCUSSION
In this large prospective study of men, we found that physical activity significantly decreased the risk of diverticulitis and diverticular bleeding. This association was due to vigorous rather than non-vigorous exercise. In fact, running was the only specific activity associated with a significantly decreased risk. Men who were obese and inactive were at a particularly high risk of diverticular complications. Sedentary behaviors, but not recreational physical activity, significantly influenced the risk of uncomplicated diverticulosis.
Our results verify and expand a previous study from our group on physical activity and symptomatic diverticular disease.(11) In this prior study, diverticulitis and diverticular bleeding were studied as a combined endpoint using a less specific definition that included nonspecific abdominal pain, change in bowel movements and low-grade or occult bleeding in the setting of diverticulosis. We utilized 12 additional years of follow-up to study diverticulitis and diverticular bleeding, separate from uncomplicated diverticulosis. This is important because bleeding and diverticulitis are thought to arise through different biologic mechanisms. Based on histological studies, bleeding appears to occur as a result of chronic vascular injury to the vasa recta in the absence of inflammation or diverticulitis, whereas obstruction, trauma or bacterial stasis may trigger an inflammatory process in diverticulitis.(23, 24) Moreover, it is difficult to ascribe chronic blood loss and nonspecific or functional gastrointestinal complaints to diverticular disease and physical activity may ameliorate the latter. The increased sample size also improved our ability to analyze the influence of specific recreational activities. In addition, we made adjustments for obesity and the use of non-steroidal anti-inflammatory drugs, risk factors for diverticulitis and diverticular bleeding that are potentially linked to physical activity; and studied the effect of timing of exercise, and the interaction between physical activity and BMI.
Long-term physical activity is believed to have a number of positive effects on the digestive system, and may reduce the risk of other gastrointestinal disorders including certain cancers, cholelithiasis, and gastrointestinal bleeding through a variety of proposed but poorly understood mechanisms.(8–10, 25, 26) Of particular relevance to our findings, the up and down motion of jogging and running may impart distinct benefits to the colon perhaps by stimulating defecation.(27) Physical activity may also decrease intra-colonic pressure, which is felt to play a key role in the development of diverticular complications, perhaps through alterations in intestinal autonomic activity(28)and/or transit time.(29–31) Decreases in colon transit time may also prevent stasis of bacteria and other toxins.(32) Exercise is also known to affect a variety of hormones which can influence gastrointestinal motility and secretion such as prostaglandins, catecholamines and motilin.(27) Long-term physical activity could protect against the chronic vascular changes found at sites of diverticular bleeding.(33) Lastly, physical activity may modulate intestinal immune function and inflammation.(30, 34)
Our results suggest that vigorous activities, and perhaps running specifically, underlie the beneficial effects of activity on diverticulitis and diverticular bleeding. This is in contrast to many other medical disorders for which walking and other moderate activities have a protective effect.(35–38) These findings are unlikely to have been the result of inadequate assessment of less vigorous activities. Non-vigorous activities have been associated with a number of other outcomes in the Health Professionals cohort including colon cancer and cardiovascular disease.(39, 40) It is also unlikely that vigorous activity obscured any true effect of non-vigorous activity because our results were similar in a sub-analysis of men in the lowest 2 quintiles of vigorous activity.
In our study, we found no relationship between uncomplicated diverticulosis (diverticulosis without diverticulitis or diverticular bleeding) and physical activity. However, men who spent the most hours in sedentary behaviors (sitting) were at a 30% increased risk of uncomplicated diverticulosis, after adjustment for physical activity, dietary fiber and other potential confounders. This finding parallels a study in Greece which found that the prevalence of diverticulosis was higher (although not statistically significant) in individuals with sedentary occupations compared to manual workers.(41) A sedentary lifestyle or prolonged sitting may predispose to the development of diverticulosis through alterations in colon transit.(42)
The consistent relationship between diverticulitis and bleeding and physical activity across time periods reinforces our findings. It also suggests that both baseline and cumulative activity are important. Physical activity may also be correlated over time.
The prospective design, large sample size, and detailed, updated information on health-related exposures are important strengths of this study. The univariate and multivariable analyses produced similar results suggesting that physical activity has an independent effect on diverticular bleeding and diverticulitis apart from other aspects of a healthy lifestyle such as diet, and body mass index. Nonetheless, the observational design does not enable us to exclude residual confounding or to make causal inferences. The self-reported nature of physical activity and diverticular outcomes is a potential limitation of our study. However, exposure and outcome data have been validated,(13, 20, 21) self-reports from health care professionals are more likely to be accurate, and our results were similar in a number of secondary analyses. Furthermore, exposure data was reported prior to outcomes so any misclassification is likely to have been random, and to have diminished any true association. Our study did not evaluate occupational physical activity, but this is presumably low among healthcare professionals. Whether our results can be generalized to women, younger age groups, or non-health professionals is unknown, although gender differences in diverticular disease or exercise and the gastrointestinal tract have not been clearly demonstrated.
In conclusion, we found inverse associations between physical activity and diverticulitis and diverticular bleeding, which were largely due to the influence of vigorous activity. Men who engaged in at least 28 MET-hours/week of vigorous physical activity (approximately 3 hours of running) had a 34% reduction in the risk of diverticulitis, and a 39% reduction in the risk of diverticular bleeding when compared to men who did not exercise vigorously. These findings suggest a role for exercise in the prevention of diverticular complications, although the level of activity needed may be higher than that recommended for prevention of other chronic diseases.(38) Indeed, running was the only specific activity to show a statistically significant benefit. The prevalence of diverticular disease, the substantial risk for recurrent events, and the need for preventative strategies short of prophylactic colectomy highlight the implications of these findings for clinical care and public health.
Study Highlights.
-
What is current knowledge
Diverticular disease is very common
Little is known about the effect of physical activity on diverticular complications
-
What is new here
Physical activity is inversely associated with diverticulitis and diverticular bleeding
Vigorous activity appears to account for this association.
Running was the only specific activity to show a statistically significant benefit
Acknowledgments
Funding /Support: The project described was funded by grants from the Agency for Healthcare Research and Quality (K08 HS14062, Strate), the National Cancer Institute (PO1CA055075, Giovannucci) and the National Heart, Lung and Blood Institute (R01 HL035464, Giovannucci). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute, the National Institutes of Health or the Agency for Healthcare Research and Quality.
Role of the Sponsors: The sponsors had no role in the design and conduct of the study; collection, management, analyses, and interpretation of the data; or preparation, review, or approval of the manuscript. The content is solely the responsibility of the authors and should not be constituted to represent the official views of the National Cancer Institute or the National Institutes of Health.
We thank the investigators, staff, and participants of the Health Professionals Follow-up Study, especially the following for their contributions Siobhan Saint-Surin, Mira Kaufman, Luba Bondarenko, Cheryl Jones and Shiyu Wei for help with data collection, entry and management, and Elizabeth Frost-Hawes for administrative support.
Footnotes
Author Contributions: Dr. Giovannucci and Ms. Liu had full access to all of the data in the study and take responsibility for the integrity of the data and accuracy of the data analyses.
Study concept and design; obtained funding: Strate, Giovannucci
Acquisition of data: Strate, Liu, Aldoori, Giovannucci
Statistical Analysis: Strate, Liu, Giovannucci
Analysis and interpretation of data; critical revision of the manuscript for important intellectual content: Strate, Liu, Aldoori, Giovannucci
Drafting of the manuscript: Strate
Study supervision: Strate, Aldoori, Giovannucci
References
- 1.Welch CE, Allen AW, Donaldson GA. An appraisal of resection of the colon for diverticulitis of the sigmoid. Ann Surg. 1953;138:332–43. doi: 10.1097/00000658-195313830-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burkitt DP, Walker AR, Painter NS. Effect of dietary fibre on stools and the transit-times, and its role in the causation of disease. Lancet. 1972;2:1408–12. doi: 10.1016/s0140-6736(72)92974-1. [DOI] [PubMed] [Google Scholar]
- 3.Aldoori WH, Giovannucci EL, Rockett HR, et al. A prospective study of dietary fiber types and symptomatic diverticular disease in men. J Nutr. 1998;128:714–9. doi: 10.1093/jn/128.4.714. [DOI] [PubMed] [Google Scholar]
- 4.Hyland JM, Taylor I. Does a high fibre diet prevent the complications of diverticular disease? Br J Surg. 1980;67:77–9. doi: 10.1002/bjs.1800670202. [DOI] [PubMed] [Google Scholar]
- 5.Manousos O, Day NE, Tzonou A, et al. Diet and other factors in the aetiology of diverticulosis: an epidemiological study in Greece. Gut. 1985;26:544–9. doi: 10.1136/gut.26.6.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strate LL, Liu YL, Aldoori WH, et al. Obesity Increases the Risks of Diverticulitis and Diverticular Bleeding. Gastroenterology. 2008 doi: 10.1053/j.gastro.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laine L, Connors LG, Reicin A, et al. Serious lower gastrointestinal clinical events with nonselective NSAID or coxib use. Gastroenterology. 2003;124:288–92. doi: 10.1053/gast.2003.50054. [DOI] [PubMed] [Google Scholar]
- 8.Colditz GA, Cannuscio CC, Frazier AL. Physical activity and reduced risk of colon cancer: implications for prevention. Cancer Causes Control. 1997;8:649–67. doi: 10.1023/a:1018458700185. [DOI] [PubMed] [Google Scholar]
- 9.Leitzmann MF, Rimm EB, Willett WC, et al. Recreational physical activity and the risk of cholecystectomy in women. N Engl J Med. 1999;341:777–84. doi: 10.1056/NEJM199909093411101. [DOI] [PubMed] [Google Scholar]
- 10.Oliveria SA, Christos PJ. The epidemiology of physical activity and cancer. Ann N Y Acad Sci. 1997;833:79–90. doi: 10.1111/j.1749-6632.1997.tb48595.x. [DOI] [PubMed] [Google Scholar]
- 11.Aldoori WH, Giovannucci EL, Rimm EB, et al. Prospective study of physical activity and the risk of symptomatic diverticular disease in men. Gut. 1995;36:276–82. doi: 10.1136/gut.36.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schechter S, Mulvey J, Eisenstat TE. Management of uncomplicated acute diverticulitis: results of a survey. Dis Colon Rectum. 1999;42:470–5. doi: 10.1007/BF02234169. discussion 475–6. [DOI] [PubMed] [Google Scholar]
- 13.Chasan-Taber S, Rimm EB, Stampfer MJ, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7:81–6. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Aldoori WH, Giovannucci EL, Rimm EB, et al. A prospective study of diet and the risk of symptomatic diverticular disease in men. Am J Clin Nutr. 1994;60:757–64. doi: 10.1093/ajcn/60.5.757. [DOI] [PubMed] [Google Scholar]
- 16.Aldoori WH, Giovannucci EL, Rimm EB, et al. Use of acetaminophen and nonsteroidal anti-inflammatory drugs: a prospective study and the risk of symptomatic diverticular disease in men. Arch Fam Med. 1998;7:255–60. doi: 10.1001/archfami.7.3.255. [DOI] [PubMed] [Google Scholar]
- 17.Dobbins C, Defontgalland D, Duthie G, et al. The relationship of obesity to the complications of diverticular disease. Colorectal Dis. 2006;8:37–40. doi: 10.1111/j.1463-1318.2005.00847.x. [DOI] [PubMed] [Google Scholar]
- 18.Morris CR, Harvey IM, Stebbings WS, et al. Anti-inflammatory drugs, analgesics and the risk of perforated colonic diverticular disease. Br J Surg. 2003;90:1267–72. doi: 10.1002/bjs.4221. [DOI] [PubMed] [Google Scholar]
- 19.Rosemar A, Angeras U, Rosengren A. Body Mass Index and Diverticular Disease: A 28- Year Follow-Up Study in Men. Dis Colon Rectum. 2007 doi: 10.1007/s10350-007-9172-5. [DOI] [PubMed] [Google Scholar]
- 20.Rimm EB, Giovannucci EL, Stampfer MJ, et al. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–26. doi: 10.1093/oxfordjournals.aje.a116211. discussion 1127–36. [DOI] [PubMed] [Google Scholar]
- 21.Rimm EB, Stampfer MJ, Colditz GA, et al. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–73. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Cox DR. Regression models and life tables. J R Stat Soc (B) 1972;34:187–220. [Google Scholar]
- 23.Jacobs DO. Clinical practice. Diverticulitis. N Engl J Med. 2007;357:2057–66. doi: 10.1056/NEJMcp073228. [DOI] [PubMed] [Google Scholar]
- 24.Meyers MA, Alonso DR, Gray GF, et al. Pathogenesis of bleeding colonic diverticulosis. Gastroenterology. 1976;71:577–83. [PubMed] [Google Scholar]
- 25.Leitzmann MF, Giovannucci EL, Rimm EB, et al. The relation of physical activity to risk for symptomatic gallstone disease in men. Ann Intern Med. 1998;128:417–25. doi: 10.7326/0003-4819-128-6-199803150-00001. [DOI] [PubMed] [Google Scholar]
- 26.Pahor M, Guralnik JM, Salive ME, et al. Physical activity and risk of severe gastrointestinal hemorrhage in older persons. JAMA. 1994;272:595–9. [PubMed] [Google Scholar]
- 27.Sullivan SN. The effect of running on the gastrointestinal tract. J Clin Gastroenterol. 1984;6:461–5. doi: 10.1097/00004836-198410000-00013. [DOI] [PubMed] [Google Scholar]
- 28.McMurray RG, Forsythe WA, Mar MH, et al. Exercise intensity-related responses of beta-endorphin and catecholamines. Med Sci Sports Exerc. 1987;19:570–4. [PubMed] [Google Scholar]
- 29.Morris CR, Harvey IM, Stebbings WS, et al. Epidemiology of perforated colonic diverticular disease. Postgrad Med J. 2002;78:654–8. doi: 10.1136/pmj.78.925.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters HP, De Vries WR, Vanberge-Henegouwen GP, et al. Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract. Gut. 2001;48:435–9. doi: 10.1136/gut.48.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cordain L, Latin RW, Behnke JJ. The effects of an aerobic running program on bowel transit time. J Sports Med Phys Fitness. 1986;26:101–4. [PubMed] [Google Scholar]
- 32.Reddy BS, Wynder EL. Metabolic epidemiology of colon cancer. Fecal bile acids and neutral sterols in colon cancer patients and patients with adenomatous polyps. Cancer. 1977;39:2533–9. doi: 10.1002/1097-0142(197706)39:6<2533::aid-cncr2820390634>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 33.Steiner S, Niessner A, Ziegler S, et al. Endurance training increases the number of endothelial progenitor cells in patients with cardiovascular risk and coronary artery disease. Atherosclerosis. 2005;181:305–10. doi: 10.1016/j.atherosclerosis.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol. 2005;45:1563–9. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 35.Hamer M, Chida Y. Walking and Primary Prevention: A Meta-analysis of Prospective Cohort Studies. Br J Sports Med. 2007 doi: 10.1136/bjsm.2007.039974. [DOI] [PubMed] [Google Scholar]
- 36.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–34. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 37.Jeon CY, Lokken RP, Hu FB, et al. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care. 2007;30:744–52. doi: 10.2337/dc06-1842. [DOI] [PubMed] [Google Scholar]
- 38.Pate RR, Pratt M, Blair SN, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–7. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 39.Giovannucci E, Ascherio A, Rimm EB, et al. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122:327–34. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 40.Tanasescu M, Leitzmann MF, Rimm EB, et al. Exercise type and intensity in relation to coronary heart disease in men. JAMA. 2002;288:1994–2000. doi: 10.1001/jama.288.16.1994. [DOI] [PubMed] [Google Scholar]
- 41.Manousos ON, Vrachliotis G, Papaevangelou G, et al. Relation of diverticulosis of the colon to environmental factors in Greece. Am J Dig Dis. 1973;18:174–6. doi: 10.1007/BF01071969. [DOI] [PubMed] [Google Scholar]
- 42.Liu F, Kondo T, Toda Y. Brief physical inactivity prolongs colonic transit time in elderly active men. Int J Sports Med. 1993;14:465–7. doi: 10.1055/s-2007-1021212. [DOI] [PubMed] [Google Scholar]