Abstract
The oral part of the pontine reticular formation (PnO) is a component of the ascending reticular activating system and plays a role in the regulation of sleep and wakefulness. The PnO receives glutamatergic and GABAergic projections from many brain regions that regulate behavioral state. Indirect, pharmacological evidence has suggested that glutamatergic and GABAergic signaling within the PnO alters traits that characterize wakefulness and sleep. No previous studies have simultaneously measured endogenous glutamate and GABA from rat PnO in relation to sleep and wakefulness. The present study utilized in vivo microdialysis coupled on-line to capillary electrophoresis with laser-induced fluorescence to test the hypothesis that concentrations of glutamate and GABA in the PnO vary across the sleep/wake cycle. Concentrations of glutamate and GABA were significantly higher during wakefulness than during NREM sleep and REM sleep. Regression analysis revealed that decreases in glutamate and GABA accounted for a significant portion of the variance in the duration of NREM sleep and REM sleep episodes. These data provide novel support for the hypothesis that endogenous glutamate and GABA in the PnO contribute to the regulation of sleep duration.
Keywords: NREM sleep, REM sleep, capillary electrophoresis, in vivo microdialysis
Glutamatergic signaling contributes to the regulation of sleep and wakefulness. In numerous brain regions, levels of glutamate are highest during wakefulness or rapid eye movement (REM) sleep (Azuma et al. 1996, Dash et al. 2009, John et al. 2008, Kodama & Honda 1999, Kodama et al. 1998, Léna et al. 2005, Lopez-Rodriguez et al. 2007). One exception is the thalamus, where levels of glutamate are highest during non-REM (NREM) sleep (Kekesi et al. 1997). The effects of glutamate receptor agonists on traits of sleep and wakefulness are dependent on glutamate receptor subtype (Datta et al. 2002, Lai & Siegel 1991), agonist concentration (Datta et al. 2001a, Datta et al. 2001b), and brain region (Boissard et al. 2002, Cape & Jones 2000, Pal & Mallick 2009, Wigren et al. 2007, Alam & Mallick 1994). The oral part of the pontine reticular formation (PnO) is a component of the ascending reticular activating system and plays a role in sleep cycle control (Brown et al. 2010). The PnO receives glutamatergic projections from the amygdala (Fung et al. 2011), the laterodorsal tegmental and pedunculopontine tegmental nuclei (Lai et al. 1993), and data from immunohistochemical studies suggest that the PnO contains glutamatergic neurons (Kaneko et al. 1989). Administration of glutamate receptor agonists into the PnO excites PnO neurons in cat (Greene & Carpenter 1985) and rat (Stevens et al. 1992), indicating that functional glutamatergic signaling occurs within the PnO.
GABAergic signaling also regulates sleep and wakefulness. Dependent upon brain region, levels of endogenous GABA are highest during REM sleep (Nitz & Siegel 1997a, Nitz & Siegel 1997b), NREM sleep (Kekesi et al. 1997, Nitz & Siegel 1996), or wakefulness (Kekesi et al. 1997, Vanini et al. 2011). GABAergic signaling also causes an increase in wakefulness (Mallick et al. 2001) or REM sleep (Mallick et al. 2001, Nitz & Siegel 1997a, Pal & Mallick 2009, Vanini et al. 2007) in a brain-region dependent manner. Within the PnO, GABA promotes wakefulness and inhibits REM sleep (Camacho-Arroyo et al. 1991, Flint et al. 2010, Xi et al. 1999, Watson et al. 2008, Marks et al. 2008, Sanford et al. 2003). GABAergic projection neurons from the thalamus, hypothalamus, basal forebrain, and ventral lateral periaqueductal gray terminate in the PnO (Rodrigo-Angulo et al. 2008, Boissard et al. 2003, Sapin et al. 2009). Each of these brain regions regulates sleep and wakefulness. The rostral brainstem also contains a column of GABAergic neurons that connects both sides of the PnO, and these neurons may modulate sleep and wakefulness (Liang & Marks 2009).
Measuring levels of glutamate and GABA across states of sleep and wakefulness presents an analytical challenge due to the short duration of rodent sleep episodes and analytical methods that require relatively large sample volumes (3 – 10 μL). To overcome these limitations, the present experiments took advantage of capillary electrophoresis with laser-induced fluorescence detection (CE-LIF), which has the capacity to rapidly measure analytes from small sample volumes. With the use of in vivo microdialysis coupled on-line to CE-LIF, the present study measured concentrations of glutamate, GABA, aspartate, taurine, serine, and glycine. These measures were used to test the hypothesis that concentrations of glutamate and GABA in rat PnO vary significantly across states of wakefulness, NREM sleep, and REM sleep. Concentrations of aspartate, taurine, serine, and glycine were not predicted to show state specific changes, as there is no existing evidence that any of these amino acids functions in the pontine reticular formation to modulate sleep and wakefulness.
Experimental Procedures
Chemicals, Solutions, and Materials
Unless otherwise indicated, chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sodium chloride, potassium chloride, and calcium chloride were purchased from Fisher Scientific (Pittsburgh, PA, USA). All solutions were made with 18.2 MΩ MilliQ water (Millipore Corporation, Billerica, MA, USA). Ringer’s solution consisted of 147 mM NaCl, 1.22 mM CaCl2, 2.68 mM KCl, and 1.01 mM MgSO4 at pH 6.0. Electrophoresis buffer contained 10 mM sodium tetraborate and 0.9 mM hydroxypropyl-β-cyclodextrin (0.8 molar substitution) at pH 9.5. Derivatization solution was comprised of 42.6 mM o-phthaldialdehyde, 148.6 mM β-mercaptoethanol, 9 mM sodium tetraborate, 0.81 mM hydroxypropyl-β-cyclodextrin and 10% (vol/vol) methanol at pH 9.5. Fused silica capillary was purchased from Polymicro Technologies (Phoenix, AZ, USA).
Animals, Surgery, and Conditioning
All experiments were approved by the University of Michigan Committee on Use and Care of Animals and adhered to guidelines established by the Guide for the Care and Use of Laboratory Animals, Eighth Edition (National Academies Press, Washington DC, 2011). Male, adult, Crl:CD*(SD) (Sprague Dawley) rats weighing 235 – 250 g were purchased from Charles River Laboratories (Wilmington, MA, USA) and housed in a temperature and humidity controlled environment. Rats were kept on a 12 h light/dark cycle (lights on at 0600), had ad libitum access to food and water, and were allowed a minimum of one week for adjustment to the housing facilities before undergoing any procedures.
Detailed descriptions of surgical methods for implanting a guide tube for microdialysis and electrodes for recording the cortical EEG and electromyogram (EMG) have been published (Watson et al. 2007, Watson et al. 2008, Brevig et al. 2010). Briefly, isoflurane (2.5% for induction; 1.5 – 2.0% for maintenance; delivered in 100% O2) was used to anesthetize rats during surgery. Three electrodes for recording the cortical EEG were positioned above the primary and secondary motor cortices. Two electrodes for recording the EMG were inserted into dorsal neck muscles, and one indifferent electrode was placed between the skin and muscle layers above the spine. Three anchor screws were implanted into the skull to hold the head cap in place. A guide tube (8IC312GP0XXC; Plastics One Inc., Roanoke, VA, USA) containing a stylet (8IC312DCXXXC, Plastics One, Inc.) was aimed (Paxinos & Watson 2007) 1 mm above the PnO (from bregma: 8.4 mm posterior, 1.2 mm lateral, 8.4 mm ventral). The EEG and EMG electrode leads, electrode pedestal, and guide tube were cemented in place with dental acrylic. Rats were given three days to recover from surgery and an additional two weeks for conditioning before being used for experiments. During the recovery period, rats were conditioned to handling, being housed in a Raturn experiment cage (Bioanalytical Systems Inc, West Lafayette, IN, USA), and being tethered to the six-channel cable (used for recording EEG and EMG signals) for at least 8 h per day. This conditioning paradigm has been successfully used previously (Brevig et al. 2010, Watson et al. 2007, Watson et al. 2008). For each experiment, rats were placed in the Raturn bowl the afternoon before the start of the experiment.
In Vivo Microdialysis and CE-LIF
Microdialysis probes were constructed according to a previously described method (Pettit & Justice 1989). Briefly, two lengths of fused silica capillary were offset by 1 mm, bound together with a small drop of cyanoacrylate, and fed through a guide tube connector (8IC313ICPNIT, Plastics One Inc.). The tips of the capillaries were then encased in a semi-permeable membrane (Spectrum Laboratories Inc., Rancho Dominguez, CA, USA) that was plugged on one end with polyimide resin (Alltech Associates, Deerfield, IL, USA). The regenerated cellulose membrane had a molecular weight cut-off of 18 kDaltons. The membrane was bonded to the capillaries and all non-active areas of the microdialysis probe (from tip to connector) were coated with polyimide resin. The final maximum dimensions of each dialysis probe were 1 mm active length with an outer diameter of 240 μm. Once screwed into position on the guide tube, the microdialysis membrane extended 1 mm below the bottom of the guide tube. The microdialysis probe was perfused at a flow rate of 0.4 μL/min for all experiments.
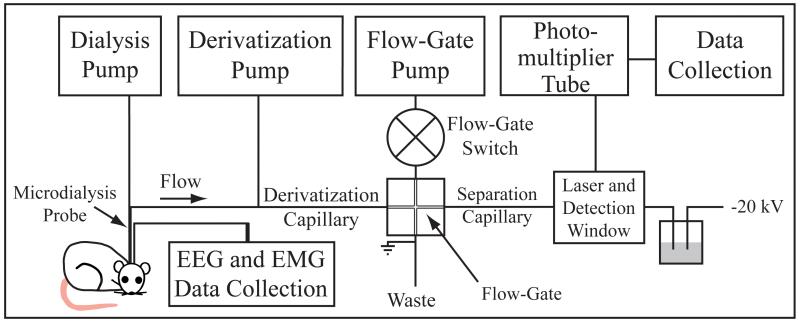
Coupling in vivo microdialysis on-line to CE-LIF has been described previously (Bowser & Kennedy 2001, Lada et al. 1997, Smith et al. 2004) and is schematized in Fig. 1. Briefly, the outlet of the microdialysis probe was connected on-line to a CE-LIF system. Derivatization solution and dialysate were mixed in a 1:2.5 ratio. Derivatized dialysate was then injected electrokinetically onto the separation capillary via the flow-gate interface. As the analytes migrated along the separation capillary, they passed through a transparent window of the capillary. A laser was focused through this window to induce fluorescence. Emitted fluorescence was collected by a photo-multiplier tube. The current from the photo-multiplier tube was amplified and then collected by software written using LabVIEW™ 8.0 (National Instruments, Austin, TX, USA). Concentrations of amino acids were quantified using the Cutter analysis program (Shackman et al. 2004).
Figure 1.
Schematic of the capillary electrophoresis (CE) instrument. Drawing on the lower left indicates placement of a microdialysis probe in the brain of an awake rat. Dialysate from the probe flows through the derivatization capillary into a flow-gate interface and then is injected onto the separation capillary. Analytes migrate along the separation capillary and through a detection window, where a laser beam induces fluorescence.
Recording and Analysis of Sleep and Wakefulness
EEG and EMG electrodes were connected to a Model 1700 differential AC amplifier (A-M Systems Inc., Sequim, WA, USA) via a six-channel cable. The amplified signal was then sent to a Micro 1401 mkII data acquisition unit (Cambridge Electronic Design Ltd. (CED), Cambridge, UK) for signal processing and storage (on a personal computer) by Spike2 version 5.14 software (CED). EEG and EMG signals were analyzed in 10-s bins for classification as wakefulness, NREM sleep, or REM sleep. Wakefulness displayed low amplitude, mixed frequencies in the EEG and moderate to high muscle tone in the EMG. NREM sleep was characterized by the presence of delta waves (0.5 – 4.0 Hz) or spindles (8.5 – 14.0 Hz) in the EEG and a decrease (compared to wakefulness) in the amplitude of the EMG. REM sleep was characterized by a predominant peak in the theta range (4.5 – 8.0 Hz) of the EEG and a further reduction of EMG amplitude. Two investigators, one of whom was blinded to the experimental conditions, scored every 10-s bin of each recording as wakefulness, NREM sleep, or REM sleep. A minimum of 98% agreement between the two investigators was achieved for each sleep record.
Experiments
At the beginning of each experiment, the computer collecting data from the CE-LIF system and the computer collecting data from the EEG and EMG recording system were synchronized to a National Institute of Standards and Technology Internet time server to ensure correct time alignment between the data files. The time for the dialysate to travel from the microdialysis membrane to the detection window was calculated prior to each experiment. The mean ± SEM travel time was 8 min 40 s ± 56 s. For each experiment, a microdialysis probe was inserted into the implanted guide tube 2 h before the start of sample collection. This allowed adequate time for concentrations of amino acids to stabilize. This pre-experiment insertion time was determined by preliminary experiments (data not shown) and conformed to or exceeded pre-experiment insertion times previously published for measures of glutamate and GABA (Smith et al. 2004, Ferrario et al. 2008, Watson et al. 2007, Watson et al. 2008). Sample collection began between 1400 and 1600. At the end of each experiment, the rat was placed in its home cage and returned to its homeroom. Rats (n = 10) were allowed to sleep ad libitum prior to and during the experiment.
Histological Verification of Microdialysis Sites
Each rat was used for one experiment. At least three days after the experiment, rats were deeply anesthetized and decapitated. Brains were quickly removed, frozen, and placed in a Leica model CM3050S cryostat (Leica Microsystems, Nussloch, Germany). Serial, coronal sections (40 μm) were mounted on chrom-alum coated slides, fixed with hot paraformaldehyde vapor, and stained with cresyl violet. All sections containing the microdialysis site were digitized and compared to a stereotaxic atlas of the rat brain (Paxinos & Watson 2007) for determining the three-dimensional coordinates (in mm) of the dialysis site. Only experiments in which the microdialysis membrane was localized to the PnO were included in the data analysis.
Statistics
Statistical analyses were performed in consultation with the University of Michigan Center for Statistical Consultation and Research. Descriptive and inferential statistics were performed using GB-Stat™ PPC 6.5.6 (Dynamic Microsystems Inc., Silver Spring, MD, USA) and SAS version 9.2 (SAS Institute, Cary, NC, USA). Neurochemical data were analyzed using a linear mixed-model, repeated-measures ANOVA followed by Tukey-Kramer multiple comparisons tests. Data comparing episode duration for wakefulness, NREM sleep, and REM sleep to the duration of the decrease in amino acid concentration were analyzed using simple regression ANOVA.
Results
Concentrations of Glutamate and GABA Decreased Significantly during Sleep
A total of 4672 electropherograms were collected for this study. The conservative statistical approach constrained the degrees of freedom to the total number of rats used (n = 10). Electropherograms (Fig. 2) were obtained during polygraphically identified states of wakefulness, NREM sleep, and REM sleep (Fig. 3a). During every experiment, rats cycled spontaneously between these three behavioral states (Fig. 3b). The mean ± SEM percent of recording time spent in each state for all rats was 54.2 ± 3.7 for wakefulness, 38.1 ± 3.1 for NREM sleep, and 7.7 ± 1.8 for REM sleep. Histological analysis (Fig. 4) confirmed that samples of all analytes were collected from the PnO. The mean ± SEM stereotaxic coordinates for all 10 dialysis sites were 8.2 ± 0.1 mm posterior to bregma, 1.3 ± 0.1 mm lateral to the midline, and 8.9 ± 0.1 mm ventral to the skull surface.
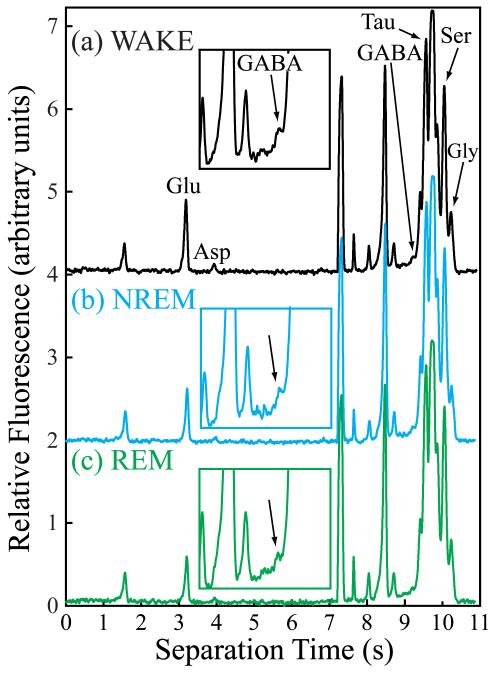
Figure 2.
Electropherograms collected from the same rat during episodes of (a) wakefulness (WAKE), (b) NREM sleep, and (c) REM sleep. Glutamate (Glu), aspartate (Asp), GABA, taurine (Tau), serine (Ser), and glycine (Gly) were separated and detected within 11 s of sample injection. The inset over each electropherogram magnifies the GABA peak (arrow).
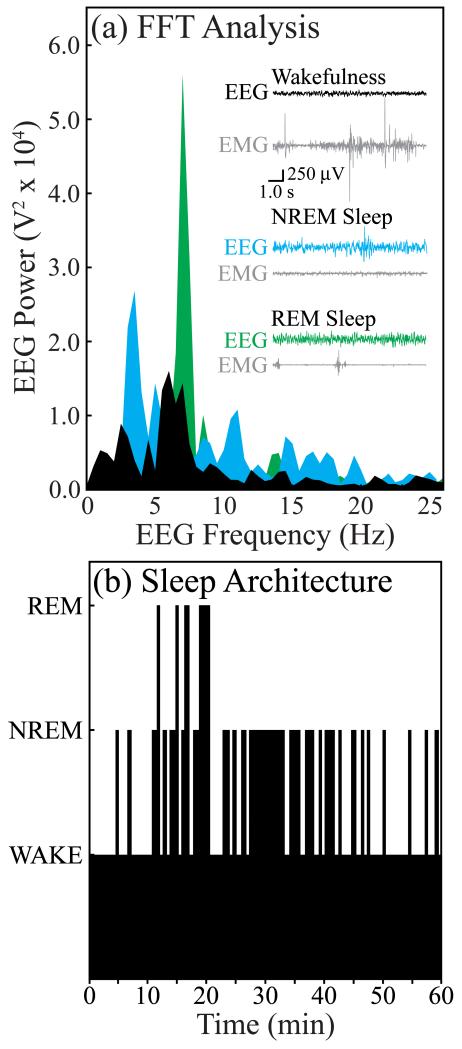
Figure 3.
Electrographic identification of sleep and wakefulness. (a) Representative 10-s recordings of cortical EEG and electromyogram (EMG) as well as a fast Fourier Transform of the respective EEG signals are shown for wakefulness (black), NREM sleep (blue), and REM sleep (green). (b) A time-course plot of sleep architecture during a typical experiment. The height of the black bars indicates the behavioral states of wakefulness (lowest bars), NREM sleep (intermediate height bars), or REM sleep (highest bars). Data shown in (a) and (b) were obtained from the same rat.
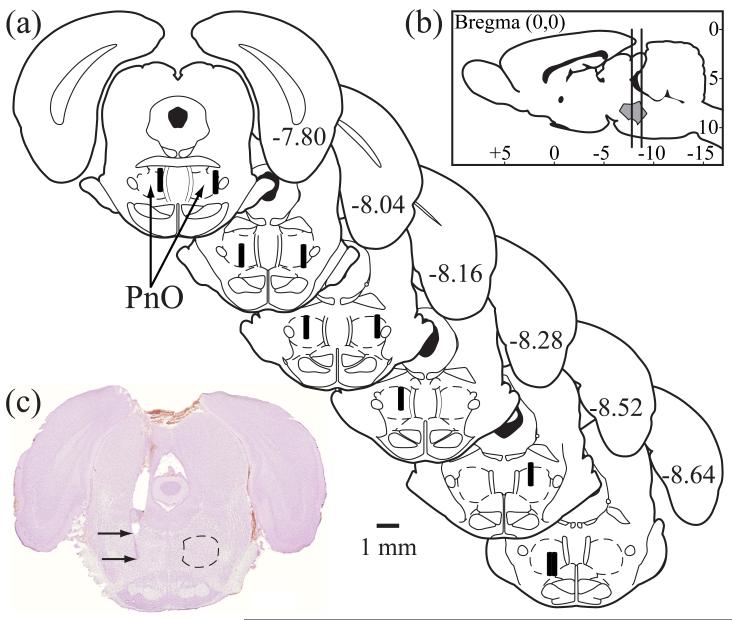
Figure 4.
Collection of analytes was localized to the oral part of the pontine reticular formation (PnO). (a) Schematic drawings of rat brainstem show the location of each microdialysis membrane (n = 10) as a black cylinder (drawn to scale). (b) A sagittal schematic of the rat brain has vertical lines to indicate the anterior-to-posterior span of the microdialysis sites shown in part (a). The shaded area between the vertical lines indicates the boundaries of the PnO. The schematics were modified from a rat brain atlas (Paxinos & Watson 2007). (c) A typical, cresyl violet-stained section with the upper arrow pointing to the location of the bottom of the guide tube and the lower arrow pointing to the deepest part of the microdialysis site in the PnO. Dotted line on the right side indicates the boundary of the PnO, according to Paxinos and Watson (2007).
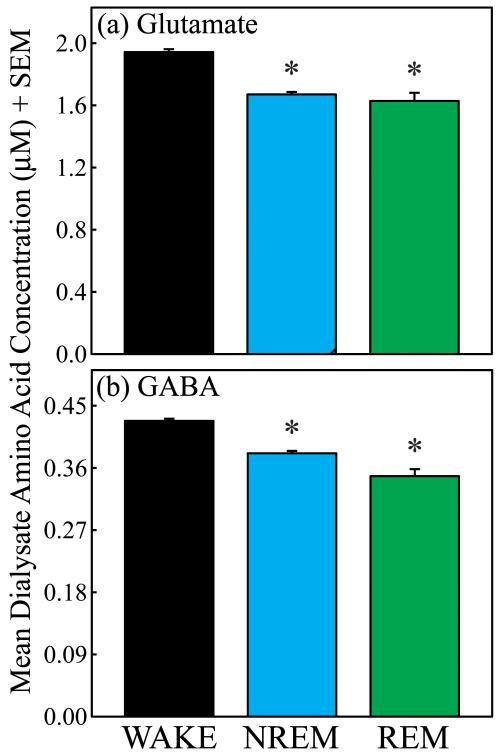
Fig. 5 summarizes the group data, based on 2447 amino acid measures obtained during wakefulness, 1963 measures collected during NREM sleep, and 262 measures taken during REM sleep. The mean ± SEM dialysate concentrations (μM) of each amino acid during wakefulness were: glutamate = 1.948 ± 0.018, aspartate = 0.522 ± 0.003, GABA = 0.428 ± 0.003, taurine = 7.442 ± 0.055, serine = 10.665 ± 0.083, and glycine = 4.942 ± 0.046. Repeated-measures ANOVA revealed significant changes in the concentration of glutamate (Fig. 5a; F = 31.2; df = 2, 10; p < 0.0001) and GABA (Fig. 5b; F = 15.27; df = 2, 10; p = 0.0009) across the sleep/wake cycle. As predicted, concentrations of aspartate, taurine, serine, and glycine did not vary significantly across states.
Figure 5.
Concentrations of glutamate and GABA decreased significantly below waking levels during sleep. Asterisks indicate a significant (p < 0.05) decrease compared to wakefulness. (a) The concentration of glutamate significantly decreased by 14.2% during NREM sleep and by 16.4% during REM sleep. (b) The concentration of GABA decreased significantly during NREM sleep (−11.0%) and REM sleep (−18.7%). There was no significant difference in concentrations of glutamate or GABA between NREM sleep and REM sleep.
Duration of Sleep Episodes Varied as a Function of the Decrease in the Concentrations of Glutamate and GABA
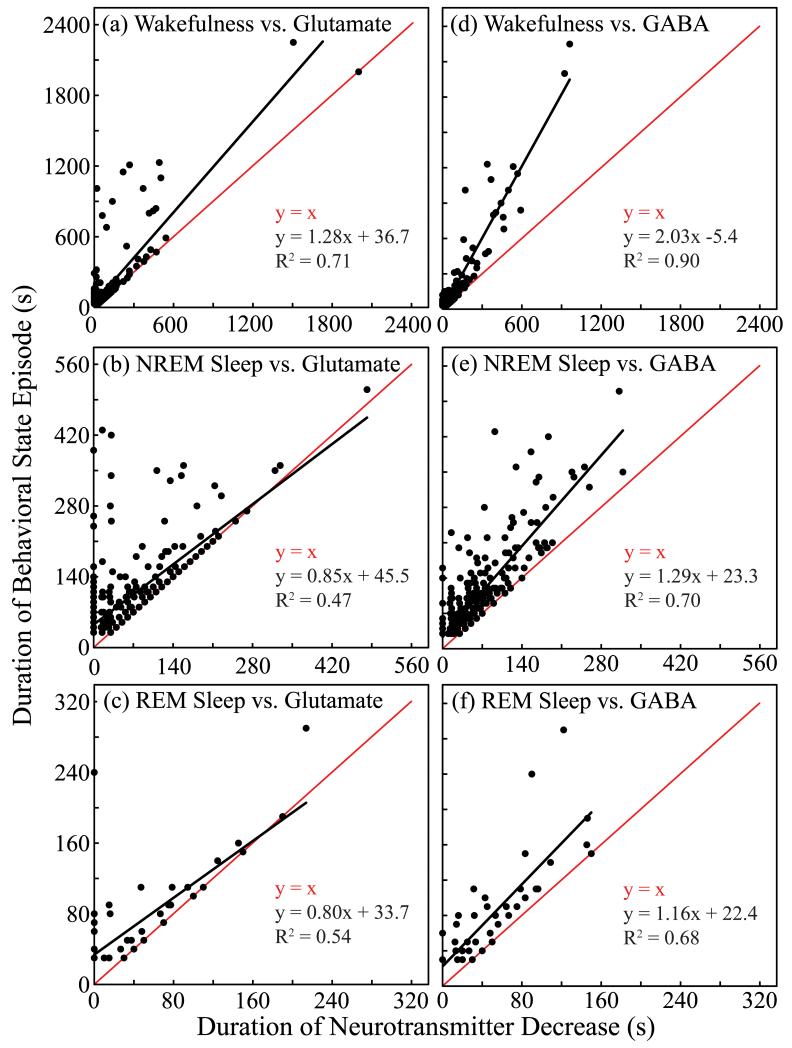
To determine if decreases in concentrations of glutamate and GABA accounted for the duration of behavioral state episodes, the duration of wakefulness, NREM sleep, and REM sleep episodes was plotted versus the duration of the decrease in glutamate or GABA concentrations (Fig. 6). Points on the line of identity (y = x) indicate episodes in which glutamate or GABA concentrations were decreased during the entire episode. Points on the y-axis (x = 0) indicate that the concentrations of glutamate or GABA did not decrease at any time during the state episode. Points falling between the y-axis and the line of identity indicate episodes during which glutamate or GABA decreased for part of the episode, but not for the entire duration of the episode. For each plot shown in Fig. 6, the duration of the decrease in glutamate or GABA accounted for a significant (p < 0.0001) amount of variance in the duration of the behavioral state.
Figure 6.
Duration of behavioral state episode varied as a function of duration of the decrease in concentrations of glutamate and GABA. Each plot indicates that for both glutamate (a-c) and GABA (d-f), the lower-level phenotype of duration of amino acid decrease (abscissa) accounted for a significant amount of the variance in the higher-level phenotype of behavioral state duration (ordinate). Black line plots y as a function of x; red line plots the line of identity. (b, c, f) Slopes of the black line near or less than the slope of the line of identity (red line) indicate that the longer the decrease in amino acid concentration, the longer the duration of NREM sleep (b) or REM sleep (c and f).
Episode Ratios for Glutamate and GABA Decreased below Waking Levels during NREM Sleep and REM Sleep
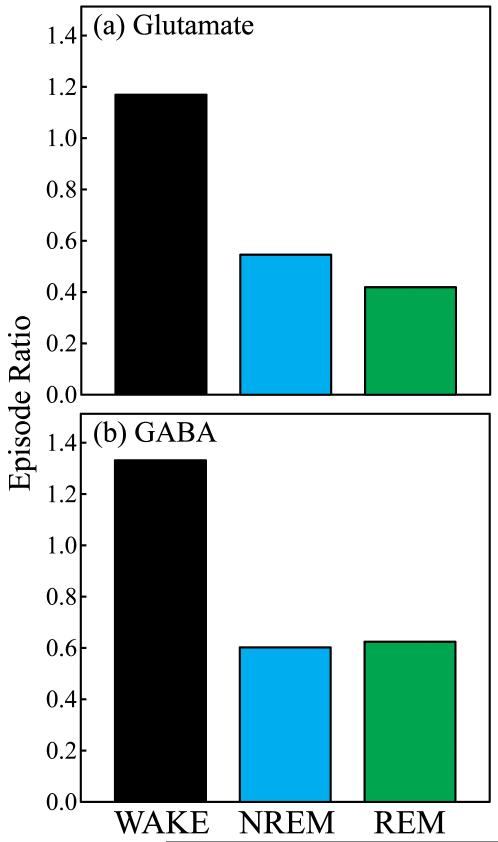
Episode ratio was defined as the number of episodes with concentrations of amino acid greater than the average concentration during wakefulness divided by the number of episodes with concentrations of amino acid less than the average concentration during wakefulness. Fig. 7 plots the episode ratio of glutamate and GABA for wakefulness, NREM sleep, and REM sleep. Ratio values greater than 1.0 indicate that more than half of the episodes were associated with concentrations of glutamate and GABA above 100% of control (average concentrations during wakefulness). NREM sleep and REM sleep showed a predominance of episodes characterized by concentrations of glutamate and GABA below 100%, as evidenced by ratio values at or below 0.6. For glutamate, the episode ratio decreased by 58% during NREM sleep and 67% during REM sleep compared to wakefulness. The episode ratios for GABA during NREM sleep and REM sleep both decreased by 50% compared to wakefulness.
Figure 7.
Episode ratios of glutamate and GABA decreased during sleep. Each plot indicates the state-specific episode ratio of amino acid concentrations during wakefulness (WAKE), NREM sleep, and REM sleep for glutamate (a) and GABA (b).
Discussion
In vivo microdialysis coupled on-line to CE-LIF was used to demonstrate that concentrations of glutamate and GABA in the PnO were greater during wakefulness than during NREM sleep and REM sleep (Fig. 5). A significant amount of the variance in the duration of episodes for each state was accounted for by the duration of the decrease in glutamate and GABA (Fig. 6). Episode ratios for NREM sleep and REM sleep (Fig. 7) revealed that most sleep episodes were associated with decreases in concentrations of glutamate and GABA. The significance of these findings is discussed below.
State-Specific Concentrations of GABA and Glutamate
Studies using cat have shown that state-specific changes in GABA concentrations are brain-region specific. In locus coeruleus (Nitz & Siegel 1997b) and dorsal raphé nucleus (Nitz & Siegel 1997a), concentrations of GABA are significantly higher during REM sleep than during NREM sleep or wakefulness. In the posterior hypothalamus (Nitz & Siegel 1996) and thalamus (Kekesi et al. 1997), however, concentrations of GABA are highest in NREM sleep. In the thalamus (Kekesi et al. 1997) concentrations of GABA are also significantly lower during REM sleep than during wakefulness.
The present study revealed for the first time in rat that the concentration of GABA in the PnO is significantly decreased during both NREM sleep and REM sleep compared to concentrations measured during wakefulness (Fig. 5). Similarly, in the pontine reticular formation of cat, the concentration of GABA is significantly decreased below waking levels during both REM sleep (Vanini et al. 2011) and the loss of wakefulness caused by the general anesthetic isoflurane (Vanini et al. 2008). There are species differences between cat and rat with respect to NREM sleep-specific changes in GABA. The concentration of GABA in cat pontine reticular formation is not significantly different between wakefulness and NREM sleep (Vanini et al. 2011), whereas the present study revealed that the concentration of GABA in rat PnO is significantly lower during NREM sleep than during wakefulness (Fig. 5b). The reasons for these species differences are not understood. Different sleep responses to administering GABAA receptor antagonists (Sanford et al. 2003, Xi et al. 1999) and muscarinic cholinergic receptor agonists (Lydic & Baghdoyan 2008) into the pontine reticular formation of rat and cat are well documented.
The present findings are consistent with reports demonstrating that changing GABAergic signaling within rat PnO alters states of sleep and wakefulness. Increasing endogenous GABA by blocking GABA uptake with nipecotic acid causes a significant increase in wakefulness and a significant decrease in NREM sleep and REM sleep (Watson et al. 2008). Decreasing GABA levels in the PnO by selectively inhibiting GABA synthesis with 3-mercaptopropionic acid significantly decreases wakefulness and significantly increases NREM sleep and REM sleep (Watson et al. 2008). These GABA-induced alterations in sleep and wakefulness likely occur via the GABAA receptor. Microinjecting muscimol, a GABAA receptor agonist, into the pontine reticular formation of mouse (Flint et al. 2010), rat (Camacho-Arroyo et al. 1991), and cat (Xi et al. 1999) increases wakefulness. Futhermore, microinjection of bicuculline, a GABAA receptor antagonist, into the pontine reticular formation increases REM sleep (Flint et al. 2010, Camacho-Arroyo et al. 1991, Marks et al. 2008, Xi et al. 1999, Sanford et al. 2003). These data support the interpretation that endogenous GABA within the PnO promotes wakefulness.
The present results demonstrate for the first time that the concentration of glutamate in the PnO changes in a state-specific manner (Figs. 5-7). Previous reports measuring glutamate by HPLC have shown no state-specific changes in the concentration of glutamate within the pontine reticular formation (Hasegawa et al. 2003, Kodama et al. 1998). Using CE-LIF, the present study has shown that the concentration of glutamate within the PnO is significantly higher during wakefulness than during NREM sleep and REM sleep. Pharmacological data also indicate that glutamatergic transmission contributes to the regulation of sleep and wakefulness. Systemic administration of ketamine, an NMDA receptor antagonist, disrupts the normal sleep cycle of cat and creates a dissociated behavioral state (Lydic & Baghdoyan 2002).
Local administration of glutamate or glutamate receptor antagonists to the pontine reticular formation has been shown to alter certain traits (acetylcholine levels, respiratory rate, startle response, and REM sleep latency) that characterize states of sleep and wakefulness. Administration of NMDA receptor antagonists to the pontine reticular formation decreases acetylcholine release and breathing in cat (Lydic & Baghdoyan 2002), and acetylcholine release in cat pontine reticular formation is greatest during REM sleep (Leonard & Lydic 1997). In the caudal portion of rat pontine reticular formation, AMPA/kainate and NMDA receptors attenuate the fear-potentiated or acoustic startle response (Fendt et al. 1996, Krase et al. 1993). Direct administration of glutamate to rat pedunculopontine tegmental nucleus also decreases startle response by exciting inhibitory pedunculopontine tegmental neurons that project to the caudal part of the pontine reticular formation (Simon et al. 2011). Microinjection of glutamate into cat pontine reticular formation shows a trend for decreasing REM sleep latency (Baghdoyan et al. 1989). Together, these data support the interpretation that glutamatergic signaling within sleep-regulating regions of the pons modulates behavioral state.
Microinjection of glutamate into the pontine peri-locus coeruleus α or nucleus magnocellularis of decerebrate cat causes muscle atonia (Lai & Siegel 1988), suggesting that the concentration of endogenous glutamate in the PnO might increase during REM sleep. However, AMPA/kainate receptor agonists suppress muscle tone and NMDA receptor agonists increase muscle tone when these drugs are administered directly into the pontine peri-locus coeruleus α and nucleus magnocellularis of decerebrate cat (Lai & Siegel 1991). This finding suggests that within these brain regions, the effects of glutamate on muscle atonia are receptor-subtype specific, and does not support or oppose the present finding of a decrease in the concentration of endogenous glutamate within the PnO during NREM sleep and REM sleep. Furthermore, the dialysis sites in the present study are not homologous to the sites used in the glutamate microinjection studies (Lai & Siegel 1988, Lai & Siegel 1991). The present study measured glutamate in an area ventral to the region in which glutamate caused atonia in the cat. Additional studies are needed to determine the synaptic mechanisms by which glutamatergic signaling within the PnO regulates sleep and wakefulness.
Comparison of Methods for Quantifying Changes in Amino Acid Concentrations Across Behavioral States
Biosensors (Dash et al. 2009, John et al. 2008) as well as in vivo microdialysis coupled to HPLC (Azuma et al. 1996, Kekesi et al. 1997, Kodama & Honda 1999, Kodama et al. 1998, Lopez-Rodriguez et al. 2007) or CE (Léna et al. 2005) have been used to measure state-specific changes in glutamate concentration. Changes in the concentration of GABA across states of wakefulness and sleep have been measured using HPLC (Kekesi et al. 1997, Nitz & Siegel 1997b, Nitz & Siegel 1997a, Nitz & Siegel 1996, Vanini et al. 2011). The main advantage of using biosensors to monitor glutamate is temporal resolution. Glutamate biosensors have sampling rates ranging from 1 to 4 s (Dash et al. 2009, John et al. 2008). The main disadvantages of biosensors are that they are currently limited to monitoring a single analyte and biosensors are not available for every analyte of interest. HPLC offers the advantage of monitoring multiple analytes simultaneously, but has a disadvantage in the time domain. The mass detection limits associated with HPLC measurements require sampling times ranging from 1 to 10 min. In animal models with long-duration sleep episodes, such as cat, these sampling times are adequate (Kekesi et al. 1997, Kodama & Honda 1999, Kodama et al. 1998, Lopez-Rodriguez et al. 2007, Nitz & Siegel 1997b, Nitz & Siegel 1997a, Nitz & Siegel 1996, Vanini et al. 2011). For animal models with short-duration sleep episodes, these sampling times are inadequate. Rodents have relatively short-duration (less than 1 min) episodes of NREM sleep and REM sleep. Thus, achieving adequate sample volumes involves either not distinguishing between NREM sleep and REM sleep (Azuma et al. 1996), mixing samples from multiple sleep episodes, or discarding samples comprised of less than 80% of one state. If microdialysis is coupled off-line to CE (Léna et al. 2005), the sample volume requirements (2 μL) and sampling times (2 min) are similar to HPLC. However, if in vivo microdialysis is coupled on-line to CE-LIF, the advantages of HPLC (multiple analytes) and biosensors (fast sampling) are achieved. The present study used in vivo microdialysis coupled on-line with CE to measure changes in concentrations of amino acids across states of sleep and wakefulness. With the current CE system, analyte separation time was 11 s (Fig. 2). When sample loading and injection times are included, it was possible to simultaneously measure concentrations of six amino acids once every 15 s. Thus, sampling was performed on a time scale that approaches the time resolution of biosensors while the ability to quantify multiple analytes comprising each sample was retained.
Limitations and Conclusions
The strengths and limitations of microdialysis have been reviewed elsewhere (Watson et al. 2006), and include the inability to identify the cellular source(s) of glutamate and GABA. Sources can include neurons, glia, and cellular metabolism (van der Zeyden et al. 2008). GABAeric input to the PnO arises from multiple sources, including the hypothalamus (Rodrigo-Angulo et al. 2008), thalamus (Rodrigo-Angulo et al. 2008), basal forebrain (Rodrigo-Angulo et al. 2008), amygdala (Fung et al. 2011), contralateral PnO (Liang & Marks 2009), and ventral lateral periaqueductal gray (Boissard et al. 2003, Sapin et al. 2009). Signaling by glutamate and GABA includes synaptic and volume transmission (Del Arco et al. 2003), because both of these amino acids function as neurotransmitters and as gliotransmitters (Angulo et al. 2008, Halassa & Haydon 2010). Evidence suggests that glutamate and GABA released inside the synapse may not spill out into the extracellular space; instead, diffusion of synaptic glutamate and GABA may be tightly controlled by glia ensheathing the synapse (Del Arco et al. 2003). The organization and interaction between presynaptic, postsynaptic, and astrocytic membranes has lead to the idea of the tripartite synapse (Del Arco et al. 2003). In this model, neurotransmitters that spill out of the synaptic cleft activate receptors on astrocytes. Astrocytes, in turn, release transmitters into the synapse to regulate synaptic signaling or into the extracellular space to regulate volume transmission (Del Arco et al. 2003). In addition to gliotransmission and neurotransmission, extrasynaptic glutamate and GABA may be derived from cellular metabolism. Because there are multiple sources of extrasynaptic glutamate and GABA, determining the source of glutamate or GABA measured by any technique is a challenge. The multiple sources of glutamate and GABA may help explain the findings (Fig. 6) that certain episodes of NREM sleep and REM sleep were associated with increases in glutamate or GABA.
In conclusion, although the sources of sampled glutamate and GABA are not known, the present data demonstrate that during normal sleep, glutamatergic and GABAergic tone in the pontine reticular formation decreases. Furthermore, these state-specific decreases were selective for GABA and glutamate, and did not occur for amino acids in the pontine reticular formation that are not thought to play a major role in the regulation of sleep and wakefulness, including taurine, serine, glycine, and aspartate. These data provide novel support for the hypothesis that endogenous glutamate and GABA in the PnO contribute to the regulation of sleep-episode duration.
Acknowledgments
This study was not supported by industry, and the authors have no financial conflicts of interest. This study was supported by National Institutes of Health grants MH45361 (HAB), HL40881 (RL), HL65272 (RL), and by the Department of Anesthesiology. For expert assistance we thank M.A. Norat, K.A. Norton, and S.L. Watson of the Department of Anesthesiology, and K.B. Welch of the University of Michigan Center for Statistical Consultation and Research.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid
- CE
capillary electrophoresis
- EMG
electromyogram
- LIF
laser-induced fluorescence
- NREM
non-rapid eye movement
- PnO
pontine reticular formation, oral part
- REM
rapid eye movement
References
- Alam MN, Mallick BN. Role of lateral preoptic area alpha-1 and alpha-2 adrenoceptors in sleep-wakefulness and body temperature regulation. Brain Res Bull. 1994;35:171–177. doi: 10.1016/0361-9230(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Angulo MC, Le Meur K, Kozlov AS, Charpak S, Audinat E. GABA, a forgotten gliotransmitter. Prog Neurobiol. 2008;86:297–303. doi: 10.1016/j.pneurobio.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Azuma S, Kodama T, Honda K, Inoue S. State-dependent changes of extracellular glutamate in the medial preoptic area in freely behaving rats. Neurosci Lett. 1996;214:179–182. doi: 10.1016/0304-3940(96)12918-9. [DOI] [PubMed] [Google Scholar]
- Baghdoyan HA, Lydic R, Callaway CW, Hobson JA. The carbachol-induced enhancement of desynchronized sleep signs is dose dependent and antagonized by centrally administered atropine. Neuropsychopharmacology. 1989;2:67–79. doi: 10.1016/0893-133x(89)90009-2. [DOI] [PubMed] [Google Scholar]
- Boissard R, Fort P, Gervasoni D, Barbagli B, Luppi PH. Localization of the GABAergic and non-GABAergic neurons projecting to the sublaterodorsal nucleus and potentially gating paradoxical sleep onset. The European journal of neuroscience. 2003;18:1627–1639. doi: 10.1046/j.1460-9568.2003.02861.x. [DOI] [PubMed] [Google Scholar]
- Boissard R, Gervasoni D, Schmidt MH, Barbagli B, Fort P, Luppi PH. The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study. Eur J Neurosci. 2002;16:1959–1973. doi: 10.1046/j.1460-9568.2002.02257.x. [DOI] [PubMed] [Google Scholar]
- Bowser MT, Kennedy RT. In vivo monitoring of amine neurotransmitters using microdialysis with on-line capillary electrophoresis. Electrophoresis. 2001;22:3668–3676. doi: 10.1002/1522-2683(200109)22:17<3668::AID-ELPS3668>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Brevig HN, Watson CJ, Lydic R, Baghdoyan HA. Hypocretin and GABA interact in the pontine reticular formation to increase wakefulness. Sleep. 2010;33:1285–1293. doi: 10.1093/sleep/33.10.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010;363:2638–2650. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho-Arroyo I, Alvarado R, Manjarrez J, Tapia R. Microinjections of muscimol and bicuculline into the pontine reticular formation modify the sleep-waking cycle in the rat. Neurosci Lett. 1991;129:95–97. doi: 10.1016/0304-3940(91)90728-c. [DOI] [PubMed] [Google Scholar]
- Cape EG, Jones BE. Effects of glutamate agonist versus procaine microinjections into the basal forebrain cholinergic cell area upon gamma and theta EEG activity and sleep-wake state. Eur J Neurosci. 2000;12:2166–2184. doi: 10.1046/j.1460-9568.2000.00099.x. [DOI] [PubMed] [Google Scholar]
- Dash MB, Douglas CL, Vyazovskiy VV, Cirelli C, Tononi G. Long-term homeostasis of extracellular glutamate in the rat cerebral cortex across sleep and waking states. J Neurosci. 2009;29:580–589. doi: 10.1523/JNEUROSCI.5486-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Patterson EH, Spoley EE. Excitation of the pedunculopontine tegmental NMDA receptors induces wakefulness and cortical activation in the rat. J Neurosci Res. 2001a;66:109–116. doi: 10.1002/jnr.1202. [DOI] [PubMed] [Google Scholar]
- Datta S, Spoley EE, Mavanji VK, Patterson EH. A novel role of pedunculopontine tegmental kainate receptors: a mechanism of rapid eye movement sleep generation in the rat. Neuroscience. 2002;114:157–164. doi: 10.1016/s0306-4522(02)00250-6. [DOI] [PubMed] [Google Scholar]
- Datta S, Spoley EE, Patterson EH. Microinjection of glutamate into the pedunculopontine tegmentum induces REM sleep and wakefulness in the rat. Am J Physiol Regul Integr Comp Physiol. 2001b;280:R752–R759. doi: 10.1152/ajpregu.2001.280.3.R752. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Segovia G, Fuxe K, Mora F. Changes in dialysate concentrations of glutamate and GABA in the brain: an index of volume transmission mediated actions? J Neurochem. 2003;85:23–33. doi: 10.1046/j.1471-4159.2003.01692.x. [DOI] [PubMed] [Google Scholar]
- Fendt M, Koch M, Schnitzler HU. NMDA receptors in the pontine brainstem are necessary for fear potentiation of the startle response. Eur J Pharmacol. 1996;318:1–6. doi: 10.1016/s0014-2999(96)00749-2. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Shou M, Samaha AN, Watson CJ, Kennedy RT, Robinson TE. The rate of intravenous cocaine administration alters c-fos mRNA expression and the temporal dynamics of dopamine, but not glutamate, overflow in the striatum. Brain Res. 2008;1209:151–156. doi: 10.1016/j.brainres.2008.02.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint RR, Chang T, Lydic R, Baghdoyan HA. GABA(A) receptors in the pontine reticular formation of C57BL/6J mouse modulate neurochemical, electrographic, and behavioral phenotypes of wakefulness. J Neurosci. 2010;30:12301–12309. doi: 10.1523/JNEUROSCI.1119-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung SJ, Xi M, Zhang J, Torterolo P, Sampogna S, Morales FR, Chase MH. Projection neurons from the central nucleus of the amygdala to the nucleus pontis oralis. J Neurosci Res. 2011;89:429–436. doi: 10.1002/jnr.22554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene RW, Carpenter DO. Actions of neurotransmitters on pontine medial reticular formation neurons of cat. J Neurophysiol. 1985;54:520–531. doi: 10.1152/jn.1985.54.3.520. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010;72:335–355. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T, Kohyama J, Honda K. Amino acid release in the rat oral pontine reticular nucleus across various vigilance states. Sleep Biol Rhythms. 2003;1:195–198. [Google Scholar]
- John J, Ramanathan L, Siegel JM. Rapid changes in glutamate levels in the posterior hypothalamus across sleep-wake states in freely behaving rats. Am J Physiol-Reg I. 2008;295:R2041–R2049. doi: 10.1152/ajpregu.90541.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Itoh K, Shigemoto R, Mizuno N. Glutaminase-like immunoreactivity in the lower brainstem and cerebellum of the adult rat. Neuroscience. 1989;32:79–98. doi: 10.1016/0306-4522(89)90109-7. [DOI] [PubMed] [Google Scholar]
- Kekesi KA, Dobolyi A, Salfay O, Nyitrai G, Juhasz G. Slow wave sleep is accompanied by release of certain amino acids in the thalamus of cats. Neuroreport. 1997;8:1183–1186. doi: 10.1097/00001756-199703240-00025. [DOI] [PubMed] [Google Scholar]
- Kodama T, Honda Y. Acetylcholine and glutamate release during sleep-wakefulness in the pedunculopontine tegmental nucleus and norepinephrine changes regulated by nitric oxide. Psychiat Clin Neuros. 1999;53:109–111. doi: 10.1046/j.1440-1819.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- Kodama T, Lai YY, Siegel JM. Enhanced glutamate release during REM sleep in the rostromedial medulla as measured by in vivo microdialysis. Brain Res. 1998;780:178–181. [PMC free article] [PubMed] [Google Scholar]
- Krase W, Koch M, Schnitzler HU. Glutamate antagonists in the reticular formation reduce the acoustic startle response. Neuroreport. 1993;4:13–16. doi: 10.1097/00001756-199301000-00003. [DOI] [PubMed] [Google Scholar]
- Lada MW, Vickroy TW, Kennedy RT. High temporal resolution monitoring of glutamate and aspartate in vivo using microdialysis on-line with capillary electrophoresis with laser-induced fluorescence detection. Anal Chem. 1997;69:4560–4565. doi: 10.1021/ac970518u. [DOI] [PubMed] [Google Scholar]
- Lai YY, Clements JR, Siegel JM. Glutamatergic and cholinergic projections to the pontine inhibitory area identified with horseradish peroxidase retrograde transport and immunohistochemistry. J Comp Neurol. 1993;336:321–330. doi: 10.1002/cne.903360302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY, Siegel JM. Medullary regions mediating atonia. J Neurosci. 1988;8:4790–4796. doi: 10.1523/JNEUROSCI.08-12-04790.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY, Siegel JM. Pontomedullary glutamate receptors mediating locomotion and muscle tone suppression. J Neurosci. 1991;11:2931–2937. doi: 10.1523/JNEUROSCI.11-09-02931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léna I, Parrot S, Deschaux O, Muffat-Joly S, Sauvinet V, Renaud B, Suaud-Chagny M-F, Gottesman C. Variations in extracellular levels of dopamine, noradrenaline, glutamate, and aspartate across the sleep-wake cycle in the medial prefrontal cortex and nucleus accumbens of freely moving rats. J Neurosci Res. 2005;81:891–899. doi: 10.1002/jnr.20602. [DOI] [PubMed] [Google Scholar]
- Leonard TO, Lydic R. Pontine nitric oxide modulates acetylcholine release, rapid eye movement sleep generation, and respiratory rate. J Neurosci. 1997;17:774–785. doi: 10.1523/JNEUROSCI.17-02-00774.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang CL, Marks GA. A novel GABAergic afferent input to the pontine reticular formation: the mesopontine GABAergic column. Brain Res. 2009;1297:32–40. doi: 10.1016/j.brainres.2009.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rodriguez F, Medina-Ceja L, Wilson CL, Jhung D, Morales-Villagran A. Changes in extracellular glutamate levels in rat orbitofrontal cortex during sleep and wakefulness. Arch Med Res. 2007;38:52–55. doi: 10.1016/j.arcmed.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Lydic R, Baghdoyan HA. Ketamine and MK-801 decrease acetylcholine release in the pontine reticular formation, slow breathing, and disrupt sleep. Sleep. 2002;25:617–622. [PubMed] [Google Scholar]
- Lydic R, Baghdoyan HA. Acetylcholine modulates sleep and wakefulness: a synaptic perspective. In: Monti JM, Pandi-Perumal SR, Sinton CM, editors. Neurochemistry of Sleep and Wakefulness. Cambridge Univeristy Press; Cambridge, NY: 2008. pp. 109–143. [Google Scholar]
- Mallick BN, Kaur S, Saxena RN. Interactions between cholinergic and GABAergic neurotransmitters in and around the locus coeruleus for the induction and maintenance of rapid eye movement sleep in rats. Neuroscience. 2001;104:467–485. doi: 10.1016/s0306-4522(01)00062-8. [DOI] [PubMed] [Google Scholar]
- Marks GA, Sachs OW, Birabil CG. Blockade of GABA, type A, receptors in the rat pontine reticular formation induces rapid eye movement sleep that is dependent upon the cholinergic system. Neuroscience. 2008;156:1–10. doi: 10.1016/j.neuroscience.2008.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitz D, Siegel J. GABA release in the dorsal raphe nucleus: role in the control of REM sleep. Am J Physiol. 1997a;273:R451–R455. doi: 10.1152/ajpregu.1997.273.1.R451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitz D, Siegel JM. GABA release in posterior hypothalamus across sleep-wake cycle. Am J Physiol. 1996;271:R1707–R1712. doi: 10.1152/ajpregu.1996.271.6.R1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitz D, Siegel JM. GABA release in the locus coeruleus as a function of sleep/wake state. Neuroscience. 1997b;78:795–801. doi: 10.1016/s0306-4522(96)00549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal D, Mallick BN. GABA in pedunculopontine tegmentum increases rapid eye movement sleep in freely moving rats: possible role of GABA-ergic inputs from substantia nigra pars reticulata. Neuroscience. 2009;164:404–414. doi: 10.1016/j.neuroscience.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th Ed. Academic Press; New York: 2007. [Google Scholar]
- Pettit HO, Justice JB. Dopamine in the nucleus accumbens during cocaine self-administration as studied by in vivo microdialysis. Pharmacol Biochem Behav. 1989;34:899–904. doi: 10.1016/0091-3057(89)90291-8. [DOI] [PubMed] [Google Scholar]
- Rodrigo-Angulo ML, Heredero S, Rodriguez-Veiga E, Reinoso-Suarez F. GABAergic and non-GABAergic thalamic, hypothalamic and basal forebrain projections to the ventral oral pontine reticular nucleus: their implication in REM sleep modulation. Brain Res. 2008;1210:116–125. doi: 10.1016/j.brainres.2008.02.095. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Tang X, Xiao J, Ross RJ, Morrison AR. GABAergic regulation of REM sleep in reticularis pontis oralis and caudalis in rats. J Neurophysiol. 2003;90:938–945. doi: 10.1152/jn.00993.2002. [DOI] [PubMed] [Google Scholar]
- Sapin E, Lapray D, Berod A, et al. Localization of the brainstem GABAergic neurons controlling paradoxical (REM) sleep. PLoS One. 2009;4:e4272. doi: 10.1371/journal.pone.0004272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman JG, Watson CJ, Kennedy RT. High-throughput automated post-processing of separation data. J Chromatogr A. 2004;1040:273–282. doi: 10.1016/j.chroma.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Simon C, Wallace-Huitt T, Thapa P, Skinner RD, Garcia-Rill E. Effects of glutamate receptor agonists on the p13 auditory evoked potential and startle response in the rat. Front Neurol. 2011;2:3. doi: 10.3389/fneur.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Watson CJ, Frantz KJ, Eppler B, Kennedy RT, Peris J. Differential increase in taurine levels by low-dose ethanol in the dorsal and ventral striatum revealed by microdialysis with on-line capillary electrophoresis. Alcohol Clin Exp Res. 2004;28:1028–1038. doi: 10.1097/01.alc.0000131979.78003.34. [DOI] [PubMed] [Google Scholar]
- Stevens DR, McCarley RW, Greene RW. Excitatory amino acid-mediated responses and synaptic potentials in medial pontine reticular formation neurons of the rat in vitro. J Neurosci. 1992;12:4188–4194. doi: 10.1523/JNEUROSCI.12-11-04188.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zeyden M, Oldenziel WH, Rea K, Cremers TI, Westerink BH. Microdialysis of GABA and glutamate: analysis, interpretation and comparison with microsensors. Pharmacol Biochem Behav. 2008;90:135–147. doi: 10.1016/j.pbb.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Vanini G, Torterolo P, McGregor R, Chase MH, Morales FR. GABAergic processes in the mesencephalic tegmentum modulate the occurrence of active (rapid eye movement) sleep in guinea pigs. Neuroscience. 2007;145:1157–1167. doi: 10.1016/j.neuroscience.2006.12.051. [DOI] [PubMed] [Google Scholar]
- Vanini G, Wathen BL, Lydic R, Baghdoyan HA. Endogenous GABA levels in the pontine reticular formation (PRF) are greater during wakefulness than during REM sleep. J Neurosci. 2011;31 doi: 10.1523/JNEUROSCI.5674-10.2011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanini G, Watson CJ, Lydic R, Baghdoyan HA. Gamma-aminobutyric acid-mediated neurotransmission in the pontine reticular formation modulates hypnosis, immobility, and breathing during isoflurane anesthesia. Anesthesiology. 2008;109:978–988. doi: 10.1097/ALN.0b013e31818e3b1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CJ, Lydic R, Baghdoyan HA. Sleep and GABA levels in the oral part of rat pontine reticular formation are decreased by local and systemic administration of morphine. Neuroscience. 2007;144:375–386. doi: 10.1016/j.neuroscience.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CJ, Lydic R, Baghdoyan HA. Pontine reticular formation (PnO) administration of hypocretin-1 increases PnO GABA levels and wakefulness. Sleep. 2008;31:453–464. doi: 10.1093/sleep/31.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CJ, Venton BJ, Kennedy RT. In vivo measurements of neurotransmitters by microdialysis sampling. Anal Chem. 2006;78:1391–1399. doi: 10.1021/ac0693722. [DOI] [PubMed] [Google Scholar]
- Wigren HK, Schepens M, Matto V, Stenberg D, Porkka-Heiskanen T. Glutamatergic stimulation of the basal forebrain elevates extracellular adenosine and increases the subsequent sleep. Neuroscience. 2007;147:811–823. doi: 10.1016/j.neuroscience.2007.04.046. [DOI] [PubMed] [Google Scholar]
- Xi M-C, Morales FR, Chase MH. Evidence that wakefulness and REM sleep are controlled by a GABAergic pontine mechanism. J Neurophysiol. 1999;82:2015–2019. doi: 10.1152/jn.1999.82.4.2015. [DOI] [PubMed] [Google Scholar]