Abstract
Background. A major goal in influenza vaccine development is induction of serological memory and cellular responses to confer long-term protection and limit virus spread after infection. Here, we investigate induction of long-lived immunity against the 2009 H1N1 virus after skin vaccination.
Methods. BALB/c mice received a single dose of 5 μg inactivated A/California/04/09 virus via coated metal microneedles (MN) applied to skin or via subcutaneous injection.
Results. MN or subcutaneous vaccination elicited similar serum IgG and hemagglutination inhibition titers and 100% protection against lethal viral challenge 6 weeks after vaccination. Six months after vaccination, the subcutaneous group exhibited a 60% decrease in functional antibody titers and extensive lung inflammation after challenge with 10 × LD50 of homologous virus. In contrast, the MN group maintained high functional antibody titers and IFN-γ levels, inhibition of viral replication, and no signs of lung inflammation after challenge. MN vaccination conferred complete protection against lethal challenge, whereas subcutaneous vaccination induced only partial protection. These findings were further supported by high numbers of bone marrow plasma cells and spleen antibody-secreting cells detected in the MN group.
Conclusions. A single skin vaccination with MN induced potent long-lived immunity and improved protection against the 2009 H1N1 influenza virus, compared with subcutaneous injection.
Vaccination against influenza could benefit from new vaccine formulations and vaccine delivery methods capable of inducing serological memory and strong cellular responses [1, 2]. This could result in better protection and decreased morbidity and mortality associated with influenza. The main aim of vaccination is the induction of long-lived immunity that will enable a rapid protective immune response after encounter with the pathogen by activating the appropriate mechanisms of the immune system. This will increase the magnitude and the quality of vaccine-induced immune response and result in long-term protection against influenza virus [3].
Recent studies have demonstrated that intramuscular administration of the vaccine is not the most efficient method of vaccine delivery [4–7]. The low concentration of dendritic cells and macrophages and the lack of MHC class II–expressing cells in the muscles result in limited activation of T lymphocytes, leading to reduced humoral and cellular responses and T cell–dependent B cell activation [6]. As an alternative site for vaccination, the skin contains a rich network of antigen-presenting cells (APCs), including macrophages, Langerhans cells (LCs), and dermal dendritic cells (DCs) [8, 9]. Delivery of an antigen through the skin activates innate immune mechanisms, including pattern recognition receptors (PRCs) that induce the production of cytokines and chemokines responsible for the initiation of the immune response [9–11]. After encounter with the antigen, LCs and DCs differentiate and migrate to the draining lymph nodes where they will activate naive T and B cells [12, 13].
We previously reported that new routes of influenza vaccine delivery via the skin using antigen-coated metal microneedle patches or dissolving microneedles encapsulating the antigen elicit strong humoral and cellular immune responses that can confer at least equal protection, compared with the conventional intramuscular route of delivery, against seasonal influenza [14–17].
The recent H1N1 pandemic illustrated the actual threat of influenza virus and that vaccine induced protective immunity against it is essential [18]. According to the Centers for Disease Control and Prevention, the recent H1N1 A/California/04/09 virus continues to be the predominant circulating strain worldwide, with younger age groups being more affected by it [19]. Therefore, vaccines that exhibit strongly protective immunity against the virus continue to be urgently needed [20].
In this study we showed, for the first time to our knowledge, the efficacy of a single-dose skin vaccination with inactivated virus using microneedles to elicit robust serological memory and cellular responses, resulting in improved viral clearance and protection after infection with the novel 2009 H1N1 influenza virus. Maintenance of high serum functional antibody titers and strong recall responses could reduce morbidity and mortality rates by conferring long-term protection against influenza virus after a single vaccine dose with microneedle patches.
MATERIALS AND METHODS
Microneedle Fabrication and Coating
As described elsewhere [14], metal microneedles were fabricated by laser-etching stainless steel sheets (McMaster-Carr). Microneedles were prepared in rows of 5 microneedles. Each microneedle measured 700 μm long, with a cross sectional area of 170 μm by 55 μm at the base and tapering to a sharp tip. Microneedles were dip-coated using a coating solution formulated with 1% (w/v) carboxymethylcellulose (Carbo-Mer), 0.5% (w/v) Lutrol F - 68NF (BASF), 15% (w/v) D-(+)-trehalose dihydrate (Sigma), and 5 μg/mL inactivated H1N1 2009 virus vaccine [21].
Cells and Viruses
Madin-Darby canine kidney cells (ATCC CCL 34, American Type Culture Collection) were maintained in Dulbecco's Modified Eagle's Medium (Mediatech) containing 10% fetal bovine serum (Hyclone; ThermoFisher Scientific). Influenza virus stocks (A/California/04/09, H1N1) were prepared, purified, and inactivated, as described elsewhere [22]. Hemagglutination (HA) activity was determined using turkey red blood cells (LAMPIRE Biological Laboratories) [23]. The mouse-adapted virus was obtained using 5 serial passages in lungs of BALB/c mice. The LD50 was calculated using the Reed-Muench formula, and viral titer was determined by plaque assay [14].
Vaccinations and Characterization of Immune Responses
Female BALB/c mice (Charles River Laboratory; 30 mice per group; age, 6–8 weeks) received 1 dose (5 μg) of the vaccine administered with microneedles or subcutaneously, which is the route most closely related to skin vaccination. For microneedle delivery, the mice were treated by manual insertion of the microneedles into skin on the dorsal surface for 5 min [14, 16]. A placebo group was treated in the same way with uncoated metal microneedles. Unimmunized mice were used as an additional negative control. Animals were bled retro-orbitally 2, 4, and 24 weeks after vaccination under systemic anesthesia [14]. Six weeks and 24 weeks after vaccination, mice (n = 5) were challenged with 10× LD50 dose of live mouse-adapted H1N1 virus and were monitored for 14 days for signs of morbidity (body weight changes, fever, and hunched posture) and mortality. A weight loss of >25% was used as the experimental endpoint, at which mice were euthanized according to the Institutional Animal Care and Use Committee guidelines. Four days after challenge of an independent cohort, blood samples were collected to determine humoral immune responses and lung samples were collected to determine virus and antibody titers, cytokine expression levels, and histopathological changes. All serum samples and lung homogenates were individually processed to determine humoral immune responses (total IgA, IgG, IgG isotypes, and hemagglutination inhibition [HAI] titers) [14]. Cellular immune responses were estimated using cytokine enzyme-linked immunosorbent assay (ELISA) [14, 22]. Finally, a cohort of immunized unchallenged mice (n = 5 per group) was euthanized 12 weeks after vaccination, and spleen and bone marrow were collected for the measurement of influenza-specific IgA and IgG antibody-secreting cells (ASC) and plasma cells by enzyme linked immunospot assay (ELISPOT) [24–26]. All animal studies had approval of the Emory University's Institutional Animal Care and Use Committee.
Histopathological Examination
Lung, liver, and spleen tissue samples were collected and fixed in 100% formalin solution, embedded in paraffin, sectioned, and stained with hematoxylin and eosin [27]. The stained samples were examined for signs of pathological changes under light microscopy.
Statistics
The statistical significance of the differences was calculated by 2-tailed unpaired Student's t test and 1-way analysis of variance (ANOVA; including Bonferronis's multiple comparison test) or 2-way ANOVA. Values of P ≤ .05 were considered to be statistically significant. Unless otherwise stated, independent experiments were run at least in triplicates.
RESULTS
Generation of Humoral Immune Responses After Skin Vaccination
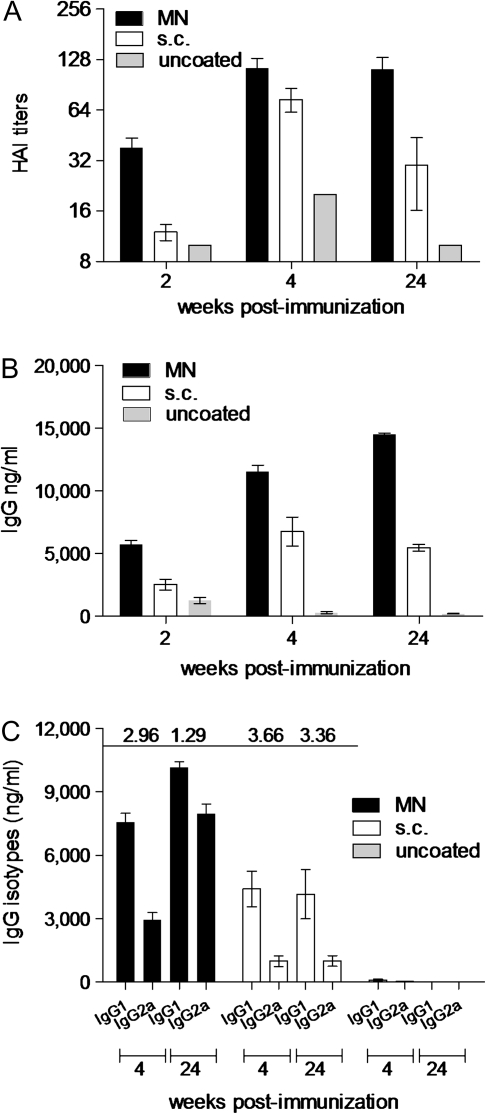
To compare the efficacy of vaccination routes, we measured the levels of functional antibody titers induced after vaccination. The HAI is a commonly used correlate for detecting the functional antibody responses against the HA protein of the virus, which is the most important antigen in inducing protective immunity. As early as week 2, mice vaccinated in the skin with H1N1-coated microneedles had 3-fold higher HAI (HAI, 38) titers than did mice vaccinated subcutaneously (Figure 1A). Although at week 4, the microneedle group showed only 1.5 times higher HAI titers than the subcutaneous group (114 vs 74; P = .063), at the end of the 24-week period, there was a dramatic decrease (60%) in HAI titers in the subcutaneously vaccinated mice whereas there was no change in the microneedle-vaccinated animals, which was almost 4-fold higher than in the subcutaneous group (112 vs 30; P = .009). These results demonstrate that microneedle vaccination with 1 dose of inactivated H1N1 influenza virus induces and sustains higher functional antibody titers over time, compared with subcutaneous vaccination.
Figure 1.
Induction of humoral immune responses. Serum samples from mice bled 2, 4, and 24 weeks after vaccination were analyzed for the levels of functional antibody titers against A/California/04/09 by HAI (A), total serum IgG titers (B), and their isotype profile, IgG1, and IgG2a (C) by quantitative ELISA. IgG1:IgG2a ratios are shown above the graph. MN, microneedle-vaccinated group; s.c., subcutaneously vaccinated group; uncoated, placebo group treated with uncoated microneedles. Data represent the mean ± standard error of the mean (SEM).
We then measured the levels of influenza-specific IgG antibodies in serum samples. At week 2, we observed a 2-fold greater difference in IgG titers in the microneedle group (Figure 1B), compared with the subcutaneous group (P < .001). Despite the increases of serum antibody levels over time in both vaccinated groups, IgG levels in the microneedle group also remained higher than those in the subcutaneous group (P < .001). Of interest, the levels of IgG in the subcutaneous group showed a 20% reduction from week 4–24, whereas there was a 25% increase in the microneedle group during the same period. These findings indicate that, in contrast to subcutaneous vaccination, a single microneedle vaccination with inactivated swine-origin H1N1 virus elicits high serum antibody titers that are being maintained at least 6 months after a single dose of the vaccine.
We previously reported that the route of vaccination influences the isotype profile of the immune response being generated [14, 16, 22]. Four weeks after vaccination, the microneedle- vaccinated group had an IgG1:IgG2a ratio of 2.96 and the subcutaneous group had a ratio of 3.66 (Figure 1C). The ratio of IgG1:IgG2a observed is suggestive of immune responses that are predominantly T helper type 2 (Th2). These numbers suggest that, initially, both routes induce a bias toward Th2 responses. When the IgG ratio was examined at 24 weeks, we observed no changes in the subcutaneous group, but the microneedle group shifted to a more balanced IgG1:IgG2a ratio (Figure 1C). These results indicate that microneedle delivery of the inactivated A/California/04/09 virus induces a switch toward Th1 responses over time and a more balanced Th1 and Th2 response.
Protective Efficacy Against Swine-Origin H1N1 Lethal Challenge 6 Weeks After Vaccination
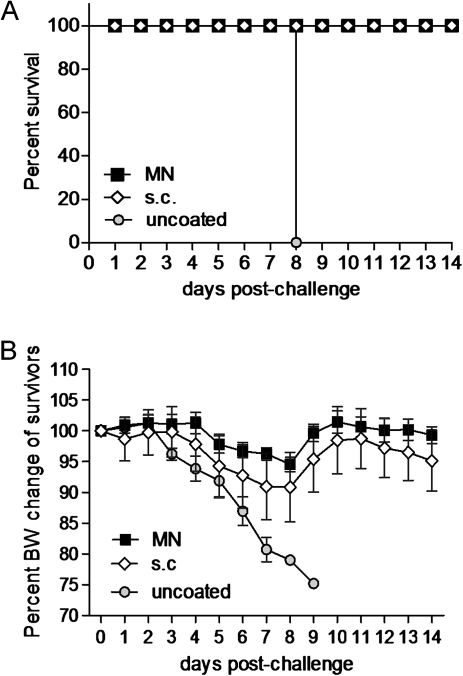
To evaluate the ability of the vaccine to confer protection, mice were challenged with 10× LD50 of mouse-adapted H1N1 virus 6 weeks after vaccination. All mice vaccinated subcutaneously or with microneedles in the skin survived the challenge, whereas all the naive mice died by day 8 (Figure 2A). Although the survival rates were similar, the microneedle-vaccinated animals fared better than the subcutaneously vaccinated animals, because they showed less body weight loss (5% peak body weight loss in the microneedle group vs 10% in the subcutaneous group) or morbidity signs (Figure 2B). These findings indicate that the microneedle route of vaccination confers moderately better protective immunity against lethal challenge than does subcutaneous vaccination.
Figure 2.
Protective efficacy against swine-origin H1N1 influenza infection 6 weeks after vaccination. Survival (A) after challenge with 10×LD50 of live mouse-adapted virus. Body weight (BW) changes of challenged mice (B) were recorded during the infection period. Microneedle (MN) or subcutaneously (s.c.) vaccinated mice and placebo (uncoated) mice were monitored for 14 days after challenge. Data represent the mean ± standard error of the mean (SEM).
Recall Systemic and Mucosal Humoral Immune Responses
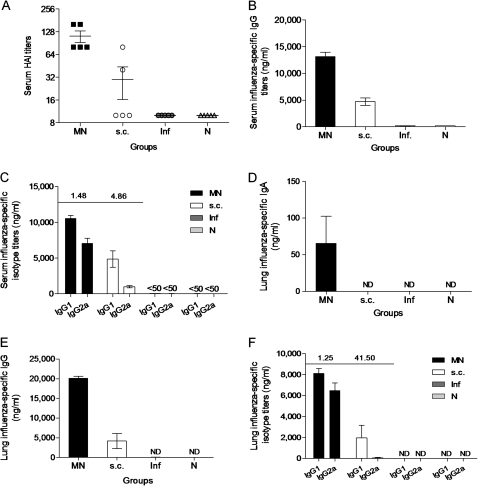
Twenty-four weeks after receiving a single dose of the vaccine, mice from all groups were challenged with 10× LD50 of mouse-adapted A/California/04/09 virus. Mice vaccinated in the skin with the microneedle route demonstrated robust recall serum humoral immune responses 4 days after challenge, with ∼3-fold higher HAI titers in the microneedle group, compared with the subcutaneously vaccinated animals (P = .009) (Figure 3A). Similar to this, we measured 3-fold higher influenza-specific IgG titers among microneedle-vaccinated animals, compared with subcutaneously vaccinated animals (P = .0001) (Figure 3B). The isotype profile data were similar to the prechallenge findings (Figure 3C). The high systemic antibody titers observed after challenge in the microneedle group indicate the presence of strong recall systemic humoral responses 6 months after vaccination, compared with the subcutaneously vaccinated group.
Figure 3.
Recall systemic and mucosal immune responses. The recall humoral immune responses in serum and lung suspensions were determined in vaccinated mice 24 weeks after vaccination and 4 days after challenge in MN and subcutaneously vaccinated mice. Naive (N) uninfected and naive infected mice (Inf) were used as control groups. Total serum HAI (A), IgG titers (B), antibody isotype profile IgG1 and IgG2a (C), lung IgA (D), lung IgG (E), and lung isotype profile (F). Titers and isotype profiles were determined by ELISA. IgG1:IgG2a ratios are shown above the graphs. Data represent the mean ± standard error of the mean (SEM).
To evaluate the induction of mucosal immune responses, we measured the influenza-specific IgA and IgG titers in the lungs of mice 24 weeks after vaccination and 4 days after challenge. We found detectable levels of IgA titers in microneedle-vaccinated mice (Figure 3D), although the subcutaneous group had no detectable titers, similar to the naive animals. In the lungs, we detected high IgG titers in the microneedle-vaccianted mice, which were 4-fold greater, compared with the subcutaneously vaccinated animals (P = .0002) (Figure 3E). Of note, although the microneedle group had a similar isotype profile to that observed systemically, the subcutaneous group produced mostly Th2 responses with weak Th1 responses (Figure 3F). These data demonstrate strong local mucosal recall responses in the lungs of microneedle-vaccinated mice induced after a single vaccination and a more balanced Th1 and Th2 immune response, compared with subcutaneous injection.
Cellular Immune Responses
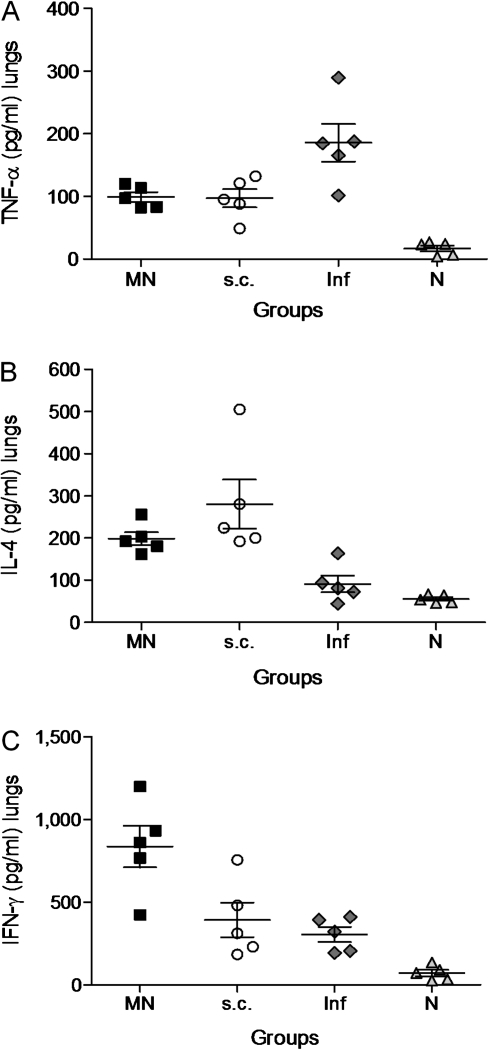
The levels of TNF-α, IL-4, and IFN-γ were measured as indicators of cellular immune responses in the lung suspensions of mice challenged 24 weeks after vaccination. We observed at least 2-fold higher levels of TNF-α in the unvaccinated infected mice (Figure 4A) than in either microneedle or subcutaneously vaccinated groups (P < .03), indicating the presence of strong inflammation and cell death in their lungs. The levels of IL-4, similar in both vaccinated groups (Figure 4B), were significantly higher than in the unvaccinated infected mice (P = .003 for microneedles group and P = .015 for the subcutaneous group). Because IL-4 inhibits the production of different pro-inflammatory cytokines, including TNF-α, its presence in the lungs of vaccinated mice indicates the inhibition of the inflammatory process. The most striking difference in the cytokine release after challenge was the significant increase of IFN-γ levels observed in the microneedle group (Figure 4C). IFN-γ production was 3-fold higher than in the subcutaneous group (P = .03) or the unvaccinated infected group (P = .004). These results demonstrate that vaccination of skin with microneedles induces robust cellular immune responses and increased production of IFN-γ in the lungs of microneedle-vaccinated mice, reflecting effective viral clearance after infection.
Figure 4.
Cellular immune responses in lung suspensions after virus challenge. TNF-α (A), IL-4 (B), and IFN-γ (C) were measured in lung suspensions of MN and subcutaneously vaccinated animals 24 weeks after vaccination and 4 days after challenge with 10× LD50 of live virus. Groups are as described in Figure 4 legend. Data represent the mean ± standard error of the mean (SEM).
Evaluation of Viral Replication After Challenge
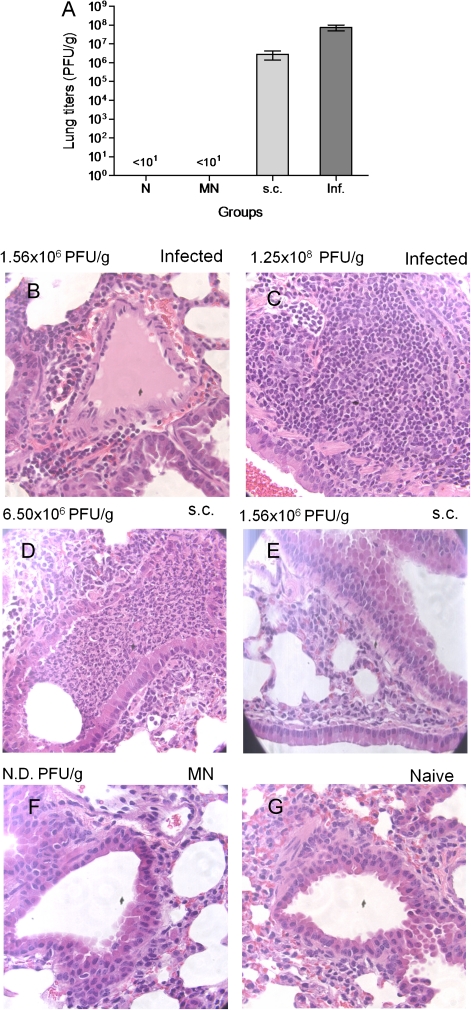
To further evaluate the protective immune responses induced by microneedle vaccination, we assessed the efficiency of virus clearance from the lungs of challenged vaccinated and naive mice 24 weeks after vaccination. Four days after challenge, the lungs of unvaccinated infected mice had viral titers 7.5 ×x 107 pfu/g, indicating a high replication rate of the virus (Figure 5A). In the subcutaneous group, the viral titers were only 1.4 log lower (2.8 ×x 106 pfu/g) than in the unvaccinated infected group. In contrast, no viral titers were detected in mice vaccinated with coated microneedle arrays, suggesting the presence of strong cellular responses and serological memory that contributed to the inhibition of viral replication in the lungs. These findings demonstrate that delivery of the antigen to skin enhances viral clearance from the respiratory compartment, compared with subcutaneous delivery, even 6 months after a single vaccination.
Figure 5.
Lung viral titers and lung histopathological examination after lethal infection. Lung viral titers were measured as an indicator of protection after challenge with 10 × LD50 of live virus 6 months after vaccination (A). Data represent the mean ± standard error of the mean (SEM). Lung tissue sections from naive infected (B,C), subcutaneously vaccinated (D,E), microneedle (MN)-vaccinated (F), and naive (G) mice were stained with hematoxylin and eosin stain. Groups are as described in Figure 4. N.D., titers not detected.
Histopathological Analysis
The histopathology of the tissue samples collected after challenge of vaccinated or unvaccinated mice 24 weeks after vaccination did not show any signs of inflammation in spleen or liver samples, suggesting that the infection is restricted to the lungs without significant extrapulmonary involvement (data not shown). In contrast, we saw clear signs of profound pulmonary inflammation in the unvaccinated infected (Figures 5B and 5C) or subcutaneously vaccinated animals (Figures 5D and 5E). Peribronchial and intra-alveolar inflammation was accompanied by significant pulmonary edema and cellular infiltration, mainly consisting of neutrophils, although this histopathological picture was less intense in the subcutaneously vaccinated group. The group of mice vaccinated with microneedles did not show any signs of inflammation (Figure 5F), with a histological picture similar to the naive uninfected group of mice (Figure 5G). These findings are consistent with the lung viral titers and correlate well with the recall responses observed in the microneedle group 6 months after vaccination. These findings further indicate the presence of long-term protective immunity induced by microneedle vaccination in the skin.
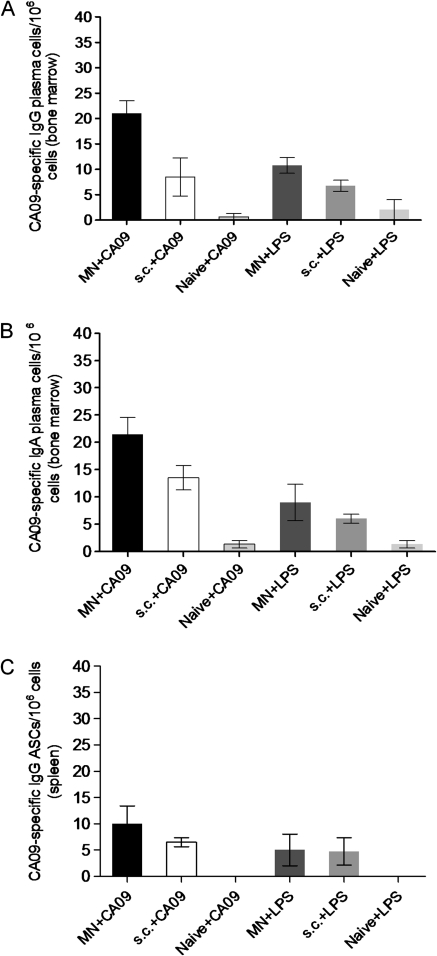
Evaluation of Long-lived Bone Marrow Plasma Cells and Spleen Antibody-Secreting Cells
To evaluate the efficacy of microneedle vaccination to induce and sustain long-lived immune responses, we investigated the presence of long-lived plasma cells in the bone marrow and ASCs in the spleen 12 weeks after a single vaccination, which is an efficient period for the establishment of memory. Anti-influenza-specific IgG plasma cells in the bone marrow were elevated in the microneedle-vaccinated group, compared with the naive group (P < .001). The largest number of plasma cells in the bone marrow was detected in the microneedle-vaccinated group (Figure 6A), with a 2-fold difference, compared with subcutaneously vaccinated mice (P = .033). We also detected increased numbers of IgA plasma cells in the bone marrow in both microneedle and subcutaneously vaccinated groups (Figure 6B), compared with naive mice (P < .003) but with no statistically significant difference between these 2 vaccinated groups (P < .08).
Figure 6.
Evaluation of bone marrow plasma cells and spleen antibody-secreting cells. Numbers of influenza-specific IgG (A) and IgA (B) plasma cells in the bone marrow and IgG ASCs in the spleen (C) were determined 3 months after a single vaccination. The cells were cultured in the presence of either 4 μg/mL purified inactivated A/California/04/09 virus or with 1 μg/ml LPS which is a polyclonal stimulator as a positive control. Sixteen hours later, the supernatants were transferred to ELISPOT plates coated with purified inactivated A/California/04/09 virus (4 μg/mL). ASC and plasma cell numbers of vaccinated mice were considered positive if the numbers of spots were higher than the sum of naive infected group spots plus 3 times the standard deviation [25]. Data represent the mean ± standard error of the mean (SEM).
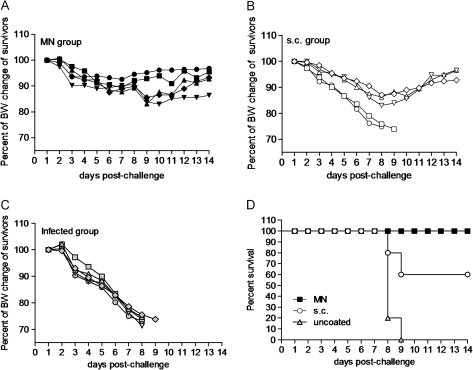
Figure 7.
Protective efficacy against H1N1 A/California/04/09 infection 6 months after vaccination. Body weight changes were recorded after lethal challenge with 10× LD50 of live A/California/04/09 virus in microneedle (MN)- vaccinated (A), subcutaneously vaccinated (B), and naive (N) mice (C); post-challenge survival rates (D). Data represent the mean ± standard error of the mean (SEM).
In the spleen, influenza-specific IgG ASCs were increased in both microneedle and subcutaneously vaccinated groups (Figure 6C), compared with naive mice (P < .02) but with no statistically significant difference between these 2 vaccinated groups (P > .05). No statistically significant difference was observed between vaccinated and naive mice in levels of virus-specific IgA ASCs in the spleen (data not shown).
Protection Against Swine-Origin H1N1 Lethal Challenge 6 Months After Vaccination
To evaluate the longevity and efficacy of the induced H1N1 influenza-specific antibodies to confer protection after a single vaccination, mice were challenged with 10 × LD50 of mouse-adapted virus 24 weeks later. Body weight changes and survival were registered daily, as previously described. Mice that were vaccinated with microneedles in the skin were fully protected against lethal challenge with a maximum body weight loss of 10%. In contrast, subcutaneously vaccinated mice were only partially protected, with a mortality rate of 40% and a mean body weight loss of 15% for the mice that survived. These findings demonstrate that the microneedle route of delivery induces long-lived immunity capable of conferring complete survival against lethal challenge even 6 months after a single vaccination.
DISCUSSION
The recent H1N1 influenza pandemic is a good example of the unpredictability of influenza virus evolution and emphasizes the necessity of developing improved vaccination methods and vaccination strategies capable of inducing serological memory and strong cellular responses. This approach would enable a better protection of the population, decreasing morbidity and mortality rates [28]. The conventional routes of vaccine administration may limit the magnitude and the quality of the immune response by targeting and activating less immunologically active sites [7, 28]. In contrast, administration of the vaccine through the skin appears to target and activate the appropriate innate mechanisms and immunologically active cells that could induce a more robust and long-lasting immune response [29].
Recent studies have demonstrated that keratinocytes and dermal endothelial cells are the only cells that can express all 10 Toll-like receptor genes (TLRs) and respond to respective ligands [30, 31]. In addition, the high concentration of dermal DCs, macrophages, and LCs expressing MHC class II after activation, prime T lymphocytes initiating the immune responses [13]. Naive T cells that are activated after encounter with the antigen will undergo clonal expansion and differentiate into effector and memory T cells [32]. This direct activation of T cells may be responsible for the proliferation of T helper cells into Th1 and Th2 cells, resulting in the activation of both humoral and cellular compartments eliciting robust humoral antibody responses; however, of more importance, it enhances T cell–mediated immunity inducing long-lasting protection against the pathogen [28, 33, 34]. The higher production of IFN-γ that we observed in mice vaccinated through the microneedle route of delivery could be attributed to the activation of these cellular mechanisms. The role of IFN-γ in the inhibition of viral replication is well established [35, 36]. IFN-γ activates macrophages and promotes cell-mediated immunity against different pathogens. Both CD4 and CD8 T cells produce IFN-γ after the development of antigen-specific immunity [14, 37].
Priming of T cells may also influence the quality and quantity of B cell responses. Differences in the intensity and quality of T cell and B cell immune responses that originate from different contribution of DCs and macrophages during T cell priming between intramuscular and intradermal administration of an antigen have been recently observed [38]. Naive B cells, after T-cell dependent antigenic activation [31], differentiate into memory B cells and plasmablasts that later fully differentiate into long-lived plasma cells that reside in the bone marrow and are crucial for the maintenance of circulating antibody levels [39–41]. Serological memory to an antigen is essential for long-term antibody-mediated immunity [25, 42]. This type of immunity is crucial for controlling and clearing the infection [25]. The higher number of influenza-specific plasma cells detected in the bone marrow of microneedle-vaccinated mice could explain the maintenance of high serum circulating total and neutralizing influenza-specific antibodies measured even 6 months after delivery of a single dose of inactivated influenza vaccine and the induction of strong recall responses observed after challenge [43, 44]. The levels of circulating antibodies induced after microneedle vaccination correlated well with the higher number of IgG plasma cells found in the bone marrow and the IgG ASCs in the spleen.
In this study, we report that a single microneedle vaccination with inactivated A/California/04/09 virus was successful in inducing long-lived serological memory against a pandemic strain. Challenge of mice with high doses of mouse adapted virus revealed complete protection 6 months after a single vaccination and improved inhibition of viral replication in the lungs of infected mice. We hypothesize that serological memory in combination with the higher production of IFN-γ induced after delivery of the antigen through the skin contribute to the rapid clearance of the virus after influenza infection.
The pandemic H1N1 A/California/04/09 influenza virus currently continues to be the predominant circulating strain with low genetic variation of the virus (antigenic drift). Groups at high risk of influenza, including the elderly population, infants [45] and children [20], or immunocompromised individuals [46], could benefit from the immunologic advantages induced by delivery of the vaccine to the skin. Maintenance of high serum antibody titers would be expected to the provide increased long-term protection [1] until encounter with the virus and could also induce higher responses after revaccination [41, 47, 48].
Skin-based vaccination has received less attention than other routes of administration largely because of the difficulty to perform intradermal injections using conventional hypodermic needles [49]. In this study, we used microneedles to simply and reliably target vaccination in the skin. Microneedles, therefore, may serve as an enabling technology to make skin-based vaccination a clinically viable alternative, which as this study shows, offers a number of immunologic advantages; microneedle delivery also offers other logistical advantages that make this method attractive for influenza vaccination, such as inexpensive manufacturing, small size for easy storage and distribution, and simple administration that might enable self-vaccination to increase patient coverage [50]. Overall, delivery of an antigen through the skin using microneedles appears to be a very promising approach for vaccination and, in the future, could be expanded to delivering other vaccines as well.
Funding
The work was supported in part by the US National Institutes of Health (NIH; EB006369 and AI074579) and MdPM was supported by the National Institute of Allergy and Infectious Diseases, NIH (IPIRC contract 5 HHSN266200700006C).
Acknowledgments
We thank Dr. Richard J. Webby from St. Jude Children's Research Hospital for providing us with the H1N1 2009 influenza virus. We thank Dianne Alexis from the Pathology Core of Winship Cancer Institute at Emory University for the H+E staining of tissue samples.
References
- 1.Subbarao K, Murphy BR, Fauci AS. Development of effective vaccines against pandemic influenza. Immunity. 2006;24:5–9. doi: 10.1016/j.immuni.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Subbramanian RA, Basha S, Brady RC, Hazenfeld S, Shata MT, Bernstein DI. Age-related changes in magnitude and diversity of cross-reactive CD4(+) T-cell responses to the novel pandemic H1N1 influenza hemagglutinin. Hum Immunol. 2010;71:957–63. doi: 10.1016/j.humimm.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Castellino F, Galli G, Del Giudice G, Rappuoli R. Generating memory with vaccination. Eur J Immunol. 2009;39:2100–5. doi: 10.1002/eji.200939550. [DOI] [PubMed] [Google Scholar]
- 4.Charest AF, McDougall J, Goldstein MB. A randomized comparison of intradermal and intramuscular vaccination against hepatitis B virus in incident chronic hemodialysis patients. Am J Kidney Dis. 2000;36:976–82. doi: 10.1053/ajkd.2000.19099. [DOI] [PubMed] [Google Scholar]
- 5.Egemen A, Aksit S, Kurugol Z, Erensoy S, Bilgic A, Akilli M. Low-dose intradermal versus intramuscular administration of recombinant hepatitis B vaccine: a comparison of immunogenicity in infants and preschool children. Vaccine. 1998;16:1511–5. doi: 10.1016/s0264-410x(98)80006-6. [DOI] [PubMed] [Google Scholar]
- 6.Raz E, Carson DA, Parker SE, et al. Intradermal gene immunization: the possible role of DNA uptake in the induction of cellular immunity to viruses. Proc Natl Acad Sci U S A. 1994;91:9519–23. doi: 10.1073/pnas.91.20.9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berzofsky JA, Ahlers JD, Belyakov IM. Strategies for designing and optimizing new generation vaccines. Nat Rev Immunol. 2001;1:209–19. doi: 10.1038/35105075. [DOI] [PubMed] [Google Scholar]
- 8.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 9.Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol. 2004;4:211–22. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abadie V, Bonduelle O, Duffy D, Parizot C, Verrier B, Combadiere B. Original encounter with antigen determines antigen-presenting cell imprinting of the quality of the immune response in mice. PLoS One. 2009;4:e8159. doi: 10.1371/journal.pone.0008159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bos JD, Kapsenberg ML. The skin immune system: progress in cutaneous biology. Immunol Today. 1993;14:75–8. doi: 10.1016/0167-5699(93)90062-P. [DOI] [PubMed] [Google Scholar]
- 12.Glenn GM, Kenney RT. Mass vaccination: solutions in the skin. Curr Top Microbiol Immunol. 2006;304:247–68. doi: 10.1007/3-540-36583-4_14. [DOI] [PubMed] [Google Scholar]
- 13.Angel CE, George E, Ostrovsky LL, Dunbar PR. Comprehensive analysis of MHC-II expression in healthy human skin. Immunol Cell Biol. 2007;85:363–9. doi: 10.1038/sj.icb.7100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koutsonanos DG, del Pilar Martin M, Zarnitsyn VG, et al. Transdermal influenza immunization with vaccine-coated microneedle arrays. PLoS One. 2009;4:e4773. doi: 10.1371/journal.pone.0004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Q, Zarnitsyn VG, Ye L, et al. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc Natl Acad Sci U S A. 2009;106:7968–73. doi: 10.1073/pnas.0812652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan SP, Koutsonanos DG, Del Pilar Martin M, et al. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 2010;16:915–20. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Improved influenza vaccination in the skin using vaccine coated microneedles. Vaccine. 2009;27:6932–8. doi: 10.1016/j.vaccine.2009.08.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen J, Enserink M. Swine flu. After delays, WHO agrees: the 2009 pandemic has begun. Science. 2009;324:1496–7. doi: 10.1126/science.324_1496. [DOI] [PubMed] [Google Scholar]
- 19.Garten RJ, Davis CT, Russell CA, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325:197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glezen WP. Containing the novel influenza A (H1N1) virus. Clin Infect Dis. 2010;50:869–70. doi: 10.1086/650751. [DOI] [PubMed] [Google Scholar]
- 21.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J Control Release. 2010;142:187–95. doi: 10.1016/j.jconrel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skountzou I, Quan FS, Jacob J, Compans RW, Kang SM. Transcutaneous immunization with inactivated influenza virus induces protective immune responses. Vaccine. 2006;24:6110–9. doi: 10.1016/j.vaccine.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Compans RW. Hemagglutination-inhibition: rapid assay for neuraminic acid-containing viruses. J Virol. 1974;14:1307–9. doi: 10.1128/jvi.14.5.1307-1309.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Czerkinsky CC, Nilsson LA, Nygren H, Ouchterlony O, Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983;65:109–21. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- 25.Slifka MK, Ahmed R. Long-lived plasma cells: a mechanism for maintaining persistent antibody production. Curr Opin Immunol. 1998;10:252–8. doi: 10.1016/s0952-7915(98)80162-3. [DOI] [PubMed] [Google Scholar]
- 26.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods. 2004;286:111–22. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Itoh Y, Shinya K, Kiso M, et al. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature. 2009;460:1021–5. doi: 10.1038/nature08260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Combadiere B, Siberil S, Duffy D. Keeping the memory of influenza viruses. Pathol Biol (Paris) 2010;58:e79–86. doi: 10.1016/j.patbio.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Agace WW. Tissue-tropic effector T cells: generation and targeting opportunities. Nat Rev Immunol. 2006;6:682–92. doi: 10.1038/nri1869. [DOI] [PubMed] [Google Scholar]
- 30.Fitzner N, Clauberg S, Essmann F, Liebmann J, Kolb-Bachofen V. Human skin endothelial cells can express all 10 TLR genes and respond to respective ligands. Clin Vaccine Immunol. 2008;15:138–46. doi: 10.1128/CVI.00257-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elgueta R, de Vries VC, Noelle RJ. The immortality of humoral immunity. Immunol Rev. 2010;236:139–50. doi: 10.1111/j.1600-065X.2010.00924.x. [DOI] [PubMed] [Google Scholar]
- 32.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–62. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 33.Graham CM, Christensen JR, Thomas DB. Differential induction of CD94 and NKG2 in CD4 helper T cells. A consequence of influenza virus infection and interferon-gamma? Immunology. 2007;121:238–47. doi: 10.1111/j.1365-2567.2007.02563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKinstry KK, Strutt TM, Swain SL. The potential of CD4 T-cell memory. Immunology. 2010;130:1–9. doi: 10.1111/j.1365-2567.2010.03259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doherty PC, Kelso A. Toward a broadly protective influenza vaccine. J Clin Invest. 2008;118:3273–5. doi: 10.1172/JCI37232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss ID, Wald O, Wald H, et al. IFN-gamma treatment at early stages of influenza virus infection protects mice from death in a NK cell-dependent manner. J Interferon Cytokine Res. 2010;30:439–49. doi: 10.1089/jir.2009.0084. [DOI] [PubMed] [Google Scholar]
- 37.Vogt A, Mahe B, Costagliola D, et al. Transcutaneous anti-influenza vaccination promotes both CD4 and CD8 T cell immune responses in humans. J Immunol. 2008;180:1482–9. doi: 10.4049/jimmunol.180.3.1482. [DOI] [PubMed] [Google Scholar]
- 38.Abadie V, Badell E, Douillard P, et al. Neutrophils rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes. Blood. 2005;106:1843–50. doi: 10.1182/blood-2005-03-1281. [DOI] [PubMed] [Google Scholar]
- 39.Wrammert J, Ahmed R. Maintenance of serological memory. Biol Chem. 2008;389:537–9. doi: 10.1515/bc.2008.066. [DOI] [PubMed] [Google Scholar]
- 40.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 41.Traggiai E, Puzone R, Lanzavecchia A. Antigen dependent and independent mechanisms that sustain serum antibody levels. Vaccine. 2003;21(Suppl 2):S35–7. doi: 10.1016/s0264-410x(03)00198-1. [DOI] [PubMed] [Google Scholar]
- 42.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–72. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 43.Ahuja A, Anderson SM, Khalil A, Shlomchik MJ. Maintenance of the plasma cell pool is independent of memory B cells. Proc Natl Acad Sci U S A. 2008;105:4802–7. doi: 10.1073/pnas.0800555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DiLillo DJ, Hamaguchi Y, Ueda Y, et al. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J Immunol. 2008;180:361–71. doi: 10.4049/jimmunol.180.1.361. [DOI] [PubMed] [Google Scholar]
- 45.Edwards KM. The burden of influenza in children: time for prevention. Clin Infect Dis. 2009;49:1022–4. doi: 10.1086/605571. [DOI] [PubMed] [Google Scholar]
- 46.Zimmerman RK, Lauderdale DS, Tan SM, Wagener DK. Prevalence of high-risk indications for influenza vaccine varies by age, race, and income. Vaccine. 2010;28:6470–7. doi: 10.1016/j.vaccine.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pinna D, Corti D, Jarrossay D, Sallusto F, Lanzavecchia A. Clonal dissection of the human memory B-cell repertoire following infection and vaccination. Eur J Immunol. 2009;39:1260–70. doi: 10.1002/eji.200839129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 49.Lambert PH, Laurent PE. Intradermal vaccine delivery: will new delivery systems transform vaccine administration? Vaccine. 2008;26:3197–208. doi: 10.1016/j.vaccine.2008.03.095. [DOI] [PubMed] [Google Scholar]
- 50.Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. Curr Top Microbiol Immunol. 2009;333:369–93. doi: 10.1007/978-3-540-92165-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]