Abstract
The Gram-negative bacterium Burkholderia pseudomallei is the causative agent of melioidosis, a major cause of lethal sepsis and morbidity in endemic areas of Southeast Asia and a potential bioterrorism threat. We have used susceptible BALB/c mice to evaluate the potential of targeting vaccination and generic immunotherapy to the lung for optimal protection against respiratory challenge. Intranasal vaccination with live attenuated B. pseudomallei increased survival and induced interferon-γ–secreting T cells in the lung. Intranasal delivery of CpG oligodeoxynucleotides also provided significant protection; however, combining preexposure vaccination with CpG treatment at the time of infection or up to 18 hours after infection, provided significantly greater protection than either treatment alone. This combination prolonged survival, decreased bacterial loads by >1000-fold, and delayed the onset of sepsis. This novel approach may be applicable to other potential biodefense agents for which existing countermeasures are not fully effective.
The Gram-negative, saprophytic bacterium Burkholderia pseudomallei is the causative agent of melioidosis, a major cause of morbidity and mortality in endemic areas of Southeast Asia and tropical Australia [1, 2]. Melioidosis has a broad spectrum of disease presentation, ranging from fatal septicemia to chronic abscess formation or asymptomatic latent infection, which can reactivate after many decades from an as-yet-unknown tissue reservoir [3]. Natural infection can occur through the inhalation or subcutaneous inoculation of bacteria [1, 2]. Although virtually any organ can be affected, pulmonary infection is common and associated with the highest mortality rates [4–6]. Given its infectivity via the respiratory route, B. pseudomallei is also a potential biowarfare and bioterrorism agent [1].
There is no vaccine for melioidosis, and antibiotic treatment is not fully effective, with frequent relapses despite long courses of multiple drug therapy [1, 2]. Antibiotic efficacy varies considerably depending on whether infection is localized or bacteremic. Septicemic melioidosis is associated with high mortality rates despite aggressive antibiotic treatment and intensive care management [7, 8], whereas chronic infection is more successfully treated by the surgical removal of abscesses and/or antibiotic treatment [1]. Interventions that prevent or delay the onset of sepsis, thereby increasing the treatment window in which conventional antibiotics are more successful, are an important objective of ongoing research.
To date, experimental immunotherapies for melioidosis have targeted either the innate or adaptive immune responses by systemic administration of generic immunostimulants or vaccines. In murine models, systemic administration of synthetic CpG oligodeoxynucleotide (ODN) protects against a wide variety of intracellular pathogens [9, 10]. In the case of B. pseudomallei, partial protection was observed by intramuscular treatment with a B-class CpG but only when it was given several days before infection, a strategy that is not realistic in a biodefense setting [11]. Numerous vaccine strategies have also been attempted, each providing partial protection but again focusing mostly on systemic administration and analysis of splenic rather than pulmonary immune responses [12–15]. Evidence from other airborne pathogens such as Mycobacterium tuberculosis suggests that pulmonary vaccination can be superior to immunization at other sites for protection against respiratory infection [16, 17]. In the present study, we evaluated the potential of combining pulmonary vaccination against respiratory challenge with B. pseudomallei with the administration of CpG to boost innate immunity at the time of, or after, infection. We chose to use C class CpG, because these agents induce a broader range of immune responses than B class ODN [18]. This novel approach provided superior benefits for survival and delayed the onset of sepsis in a high-dose challenge infection model. Combining prophylactic immunization with postexposure generic immunotherapy may also be applicable to other potential biowarfare agents for which existing countermeasures are not fully effective.
MATERIALS AND METHODS
Treatment of Mice and Bacterial Infections
Female BALB/c mice (Charles River), aged 7–10 weeks, were used. Animals were maintained with access to food and water ad libitum under Animal Biohazard Containment Level 3 conditions. All animal experiments were performed in accordance with the Animals (Scientific Procedures) Act 1986 and were approved by the London School of Hygiene and Tropical Medicine Ethical Review Committee.
B. pseudomallei strain 576 was isolated from a patient with a fatal case of human melioidosis in Thailand. B. pseudomallei 576 2D2 was generated by signature-tagged mutagenesis, as described by Atkins [19], and contains an insertion in the Ilv1 gene required for synthesis of branch chain amino acids. Stocks were prepared in tryptone soy broth and stored at –80°C. Intranasal or intraperitoneal infections and tissue or blood bacterial loads were determined as described elsewhere, with a limit of detection of 100 colony forming units (CFU)/lung or 10 CFU/mL blood [15]. Mice were vaccinated with a single dose of either 105 or 106 CFU of B. pseudomallei 2D2 via the intranasal or intraperitoneal route, respectively, and challenged after 5–8 weeks with B. pseudomallei 576.
The C class CpG ODNs 10101 and 2395 and the negative control ODN 2137 [20] were provided by Dr Art Krieg (Coley Pharmaceutical, recently acquired by Pfizer). Unless indicated, ODNs were diluted in phosphate-buffered saline (PBS) together with the bacterial inoculum and administered simultaneously. Optimal concentrations were determined, to reduce potential toxicity.
Measuring Cytokine and Chemokine Protein Concentrations in Lung Homogenates and Plasma
Lungs were harvested into cold PBS containing protease inhibitors (Complete Mini Protease Inhibitor Cocktail; Roche) and homogenized as described elsewhere [21]. Cells were lysed with 0.1% Triton X100 (Sigma) before centrifugation to pellet cell debris. Blood samples were centrifuged for 10 minutes at 4°C and plasma assayed for interferon (IFN)–γ, interleukin (IL)–12, tumor necrosis factor (TNF)–α, IL-6, IL-10, and monocyte chemoattractant protein (MCP)–1 using CBA Mouse Inflammation Kits (BD Biosciences).
In Vitro Cell Stimulation and IFN-γ Analysis
Lung or spleen cell suspensions were prepared as described elsewhere [21], plated in 96-well plates (1 × 106 cells/well), and stimulated in triplicate with either medium, 1 × 105 CFU of γ-irradiated B. pseudomallei 576, or γ-irradiated bacteria and cyclosporin A (2.5 μg/mL; Sigma). For flow cytometry, after overnight incubation at 37°C, cells were treated with brefeldin A (10 μg/mL; Sigma) for 3 hours and stained using anti-CD4 (RM4-5; 2 μg/mL; Caltag Laboratories) and anti-CD8 (53–6.7; 1 μg/mL; BD Biosciences) or anti-IFN-γ (0.05 μg/mL; XMG1.2; BD Biosciences) antibodies [21]. Acquisition was performed on a BD Biosciences FACSCalibur flow cytometer; ≥100000 events were acquired per sample and analyzed using CellQuest software gated on lymphocytes defined by forward and side scatter. Enzyme-linked immunosorbent assays of supernatants harvested after incubation at 37°C for 3 days were performed using AN18 and biotinylated R46A2 antibodies (Mabtech) [21].
Statistical Analysis
Kaplan-Meier survival curves were compared by log-rank analysis. Median values of bacterial loads and cytokine levels were compared by nonparametric Mann-Whitney test. All other data were analyzed using Student’s unpaired t test. Differences were considered significant at P < .05.
RESULTS
Intranasal Vaccination to Increase Resistance Against Pulmonary Challenge and Prime B. pseudomallei Reactive T Cells in the Lung
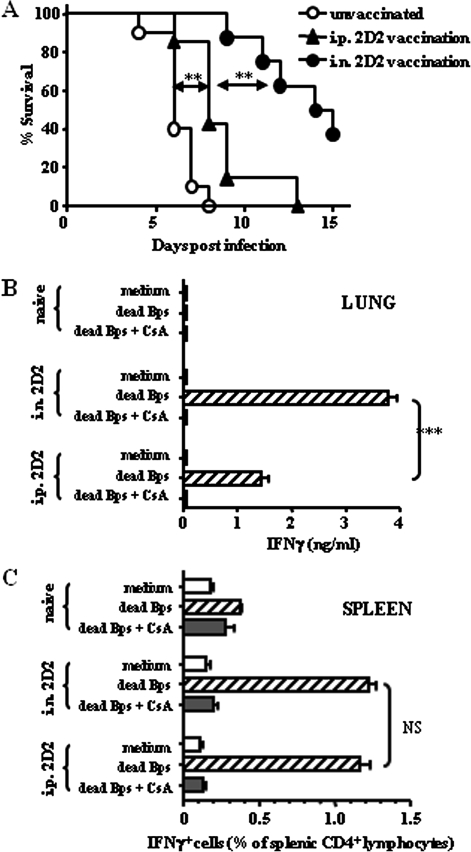
We have shown elsewhere that intraperitoneal vaccination with the attenuated B. pseudomallei auxotroph, 2D2, protected against intraperitoneal challenge with wild-type B. pseudomallei 576 in susceptible BALB/c mice [19, 22]. To assess its potential as a respiratory vaccine, we first demonstrated that 2D2 is also significantly attenuated via the intranasal route (>100000 times less virulent than wild type) and is cleared from lungs and spleens within 1 week (data not shown). Mice were then immunized with an optimized single dose of 2D2 via the intraperitoneal (106 CFU) or intranasal (105 CFU) route, challenged intranasally with 102 CFU B. pseudomallei 576, and monitored for survival. Intraperitoneally immunized mice were only marginally protected against pulmonary challenge compared with unvaccinated controls, with median survival times (MSTs) of 8 and 6 days, respectively (P = .003), whereas intranasally immunized mice survived significantly longer, with an MST of 14.5 days (P = .001) (Figure 1).
Figure 1.
Intranasal vaccination with live-attenuated Burkholderia pseudomallei mutant 2D2 provides optimal protection against virulent pulmonary infection associated with the production of local and systemic interferon (IFN)–γ–producing T cells. A, BALB/c mice (n = 6–8) were immunized with B. pseudomallei 2D2 via the intranasal (i.n.) or intraperitoneal (i.p.) route at a dose of 105 or 106 colony-forming units (CFU), respectively. After 5 weeks, mice were challenged intraperitoneally with 106 CFU of wild-type B. pseudomallei 576 and monitored for survival. Statistical significance was determined using Kaplan–Meier analysis; **P < .005. B, C, BALB/c mice (n = 3) were immunized with B. pseudomallei 2D2 via the intranasal or intraperitoneal route and boosted with a second immunization at the same dose and route after 2 weeks. Five weeks after the first vaccination, lung and spleen cells were stimulated in vitro with 105 dead B. pseudomallei with or without cyclosporin A. B, IFN-γ was detected in lung cell culture supernatants by enzyme-linked immunosorbent assay. ***P < .001. C, IFN-γ+CD4+ spleen cells were identified by flow cytometry after gating on viable lymphocytes by forward and side scatter profiles. Data are from triplicate samples, are presented as means ± standard errors, and represent 2 independent experiments. Statistical significance was determined using Student’s unpaired t test; NS, not significant.
IFN-γ is important for resistance against B. pseudomallei via the activation of macrophages [2, 22, 23]. Here, IFN-γ was undetectable in lung cells from naive mice stimulated with dead B. pseudomallei, whereas lung cells from 2D2-immunized mice produced IFN-γ on restimulation in a cyclosporin (ie, T-cell receptor)–dependent manner [24], regardless of vaccination route (Figure 1). Pulmonary T-cell responses were ∼2-fold greater in mice vaccinated intranasally than in those vaccinated intraperitoneally, a difference correlating with greater protection against pulmonary challenge (P < .001) (Figure 1). Intranasal vaccination also generated detectable CD4+ (Figure 1) and CD8+ (data not shown) T-cell responses in the spleen, as assessed by flow cytometry; these were equivalent in magnitude to responses induced by intraperitoneal vaccination, for which CD4+ but not CD8+ T cells mediate protection in vivo [22].
Effect of Intranasal 2D2 Vaccination on Lung Bacterial Loads After Pulmonary B. pseudomallei Challenge
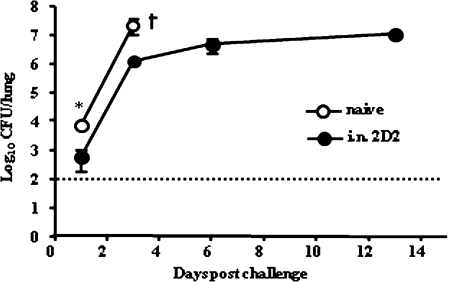
An assessment of the impact of vaccination on postchallenge pulmonary bacterial loads demonstrated that control of infection occurred in 2 phases. In vaccinated mice, there was a 12.7-fold reduction in the number of bacteria in the lungs within 1 day after challenge, compared with unvaccinated controls (P = .03) (Figure 2). The increase in bacterial burden then proceeded at the same rate in both groups until day 3; after this point, unvaccinated mice succumbed to infection, but in 2D2-vaccinated animals bacterial loads were maintained at between 106 and 107 CFUs until day 13 (Figure 2). This maintenance phase was associated with the formation of a few large abscesses within the lung and spleen compartments, which contrasted with the numerous small, miliary foci observed in unvaccinated mice with similar bacterial burdens at day 3 after infection (data not shown).
Figure 2.
Intranasal (i.n.) vaccination with live-attenuated Burkholderia pseudomallei mutant 2D2 results in control of bacterial growth in the lung after virulent pulmonary infection. BALB/c mice (n = 5–6) were vaccinated intranasally with 105 colony-forming units (CFU) of B. pseudomallei 2D2. After 5 weeks vaccinated mice and naive controls were challenged intranasally with 102 CFU of B. pseudomallei 576. Lung bacterial loads were determined over the first 2 weeks after challenge. Dashed line indicates limit of detection. Statistical significance was determined using Student’s unpaired t test; * P < .05.
Intranasal CpG for Protection From Lethal Pulmonary Infection With B. pseudomallei and Promotion of Inflammatory Cytokine Responses
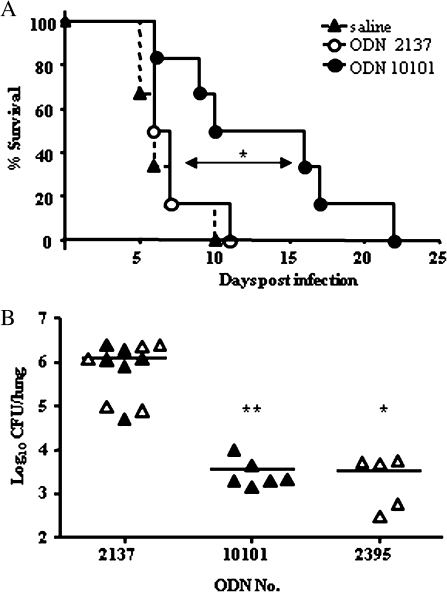
Our next objective was to determine the protective efficacy of the generic immunostimulant CpG ODN in the context of pulmonary infection. Intranasal treatment with 75 μg of CpG ODN 10101 alone, at the time of bacterial challenge, extended the MST to 13 days, compared with 6 and 6.5 days in mice treated with saline or control ODN, respectively (P = .04) (Figure 3). This protection was equal to a preinfection treatment regime, with dosing 10 and 3 days before infection with the same CpG ODNs (data not shown). Treatment with either C class CpG, ODN 10101 or ODN 2395, improved early bacterial clearance in the lung compared with control mice within 1 day of challenge (data not shown), and by day 3 bacterial loads were reduced 348- or 333-fold with CpG ODN 10101 (P = .004) (Figure 3) or CpG ODN 2395, respectively (P = .01) (Figure 3).
Figure 3.
Intranasal treatment with CpG oligodeoxynucleotide (ODN) protects mice from intranasal Burkholderia pseudomallei infection. A, BALB/c mice (n = 6) were treated intranasally with either CpG ODN 10101 (75 μg), control ODN 2137 (75 μg), or saline, at the time of intranasal infection with 102 colony-forming units (CFU) of B. pseudomallei and monitored for survival. Statistical significance determined using Kaplan-Meier analysis; *P < .05. B, Mice were treated intranasally with optimal doses of CpG ODN 10101 (75 μg), CpG ODN 2395 (12.5 μg), or equivalent control ODN 2137 at the time of infection, and bacterial loads in the lung were determined after 3 days. Data represent 2 independent experiments (open and closed triangles). Statistical significance was determined using Student’s unpaired t test; *P < .05; **P < .005
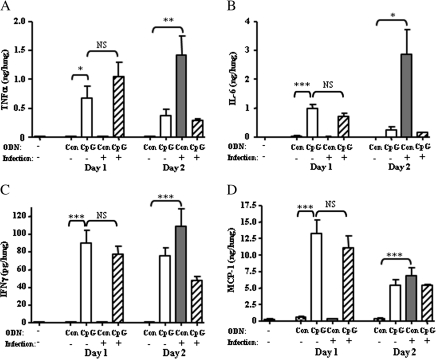
In uninfected mice, the administration of intranasal CpG ODNs induced TNF-α (P = .04), IL-6 (P < .001), MCP-1 (22-fold; P < .001), and, to a lesser extent, IFN-γ (P < .001) (Figure 4) in the lung within 1 day of treatment, compared with undetectable levels in control ODN-treated or untreated mice. These responses were mostly transient, decreasing by day 2 after treatment, except for the IFN-γ response, for which the difference was not statistically significant. In contrast, in B. pseudomallei–infected mice receiving no CpG, the same cytokine responses were not apparent until day 2 after infection, with large increases in levels of TNF-α (P = .004), IL-6 (P = .03), and IFN-γ (P < .001) and a 17-fold increase in MCP-1 (P < .001) (Figure 4) compared with uninfected mice. CpG treatment of infected mice did not exacerbate the tissue cytokine response. Accompanying these cytokine changes, CpG treatment of infected mice also resulted in the earlier recruitment of CD11bint/F480int macrophages and natural killer cells compared with controls (data not shown). Thus, delivery of CpG to the lung at the time of intranasal B. pseudomallei challenge brings about an earlier, but controlled, induction of proinflammatory cytokine and chemokine responses and the recruitment of potentially protective inflammatory cells.
Figure 4.
Intranasal CpG treatment induces the rapid production of proinflammatory cytokines and chemokines in the lung. BALB/c mice (n = 5) were treated intranasally with CpG oligodeoxynucleotide (ODN) 2395 or control (Con) ODN 2137 at the time of intranasal challenge with 102 colony-forming units of Burkholderia pseudomallei 576. Uninfected mice were also treated with either CpG or control ODN or received no treatment. Lungs were harvested at day 1 and 2 after infection/treatment, and lung homogenates were assayed for pulmonary cytokine and chemokine levels by cytometric bead array. A, Tumor necrosis factor (TNF)–α. B, Interleukin (IL)–6. C, Interferon (IFN)–γ. D, Monocyte chemoattractant protein (MCP)–1. Data are presented as means ± standard errors and represent 2 experiments of similar design. Statistical significance was determined using Student’s unpaired t test; * P < .05; ** P < .005; *** P < .001; NS, not significant.
Combination Immunotherapy as Optimal Protection Against Pulmonary Challenge With B. pseudomallei
Because either vaccination or CpG treatment alone produced substantial reductions in bacterial burden, we used a more stringent high-dose challenge model (3-fold increase in challenge inoculum from previous experiments) to assess the potential of a combination approach. Untreated naive mice rapidly succumbed to infection, with an MST of 6 days, whereas mice given either single immunotherapy were partially protected from this higher challenge, with MSTs of 8 days after CpG treatment (P = .002) and 12.5 days after 2D2 vaccination (P < .001) (Table 1). In each case this protection was associated with a >10-fold decrease in lung bacterial loads compared with untreated controls (P = .004) (Table 1). However, mice receiving the combination of 2D2 vaccination and CpG immunotherapy (2D2 + CpG) survived significantly longer than those given either treatment alone, with an MST of 19 days (P = .03 vs each single treatment and P = .004 vs no treatment) (Table 1). Lung bacterial loads in the mice treated with 2D2 plus CpG were 69- and 109-fold lower, respectively, than those in mice given CpG or 2D2 alone (P = .004), and >1000-fold lower than mice receiving no treatment (P = .004) (Table 1).
Table 1.
Intranasal CpG Treatment in Mice Previously Vaccinated With B. pseudomallei Mutant 2D2 Provides Increased Protection Against Virulent Pulmonary Infection
| Vaccination and CpG treatment | MST, days | Lung bacterial load, mean ± SE, CFU |
| No vaccination | ||
| No CpG | 6.0a,b | 3,430,000 ± 1,060,000a |
| CpG | 8.0a | 21,3385 ± 64,531a |
| Vaccination | ||
| No CpG | 12.5b,c | 337,832 ± 91,815a |
| CpG | 19.0c | 3100 ± 1850a |
NOTE. BALB/c mice (n = 6–8) were vaccinated intranasally with 105 colony-forming units (CFU) of B. pseudomallei 2D2. After 5 weeks vaccinated and naive mice were treated intranasally with 12 μg of either CpG oligodeoxynucleotide (ODN) 2395 or control ODN 2137 and challenged intranasally with 3 × 102 CFU of B. pseudomallei 576. Mice were either monitored for survival or culled to determine bacterial loads in the lung on day 3 after challenge. Survival data are expressed as median survival time (MST). Bacterial loads are presented as means ± standard errors (SEs) from data pooled from 2 independent, identically designed experiments. Statistical significance was determined using Student’s unpaired t test.
P < .005.
P < .001.
P < .05.
Effect of Combination Therapy on Onset of Sepsis
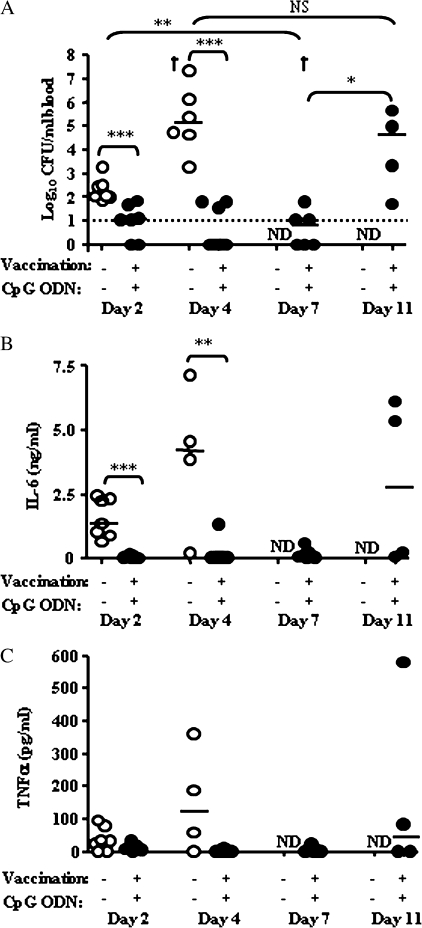
Given the clinical importance of sepsis as a predictor of death in human melioidosis, we then evaluated the efficacy of combination immunotherapy in the prevention of sepsis in our high-dose challenge model. Sepsis was defined as the appearance of bacteria and proinflammatory cytokines (TNF and IL-6) in the blood, concordant with the clinical definitions of sepsis used in general terms and for stratifying human melioidosis cases [25, 26]. All infected but untreated mice were bacteremic within 2 days, and after 4 days the median blood bacterial load was >105 CFU/mL (Figure 5). In mice treated with 2D2 plus CpG, blood bacterial loads were significantly lower, with 2 of 7, 5 of 8, and 3 of 6 mice culture negative at day 2, 4, and 7 after infection, respectively, and all remaining mice harboring <102 CFU/mL blood (P < .001 for days 2 and 4). However, by day 11 after infection, bacteria were detected in the blood of all of these treated mice (P > .05 for comparison with naive mice at days 2 and 4), consistent with previous observations that these animals will succumb from day 12 onward (Table 1 and data not shown).
Figure 5.
Intranasal CpG treatment in mice previously vaccinated with Burkholderia pseudomallei mutant 2D2 delays the onset of sepsis. BALB/c mice (n = 4–8) were vaccinated intranasally with B. pseudomallei 2D2. Vaccinated mice treated intranasally with CpG oligodeoxynucleotide (ODN) 2395 and unvaccinated mice treated with control ODN 2137 were challenged with 3 × 102 colony-forming units (CFU) of B. pseudomallei 576, and heparinized blood was harvested on days 2, 4, 7, and 11 after challenge. A, Bacterial loads from whole blood. Dashed line indicates limit of detection. B and C. Plasma levels of proinflammatory cytokines were determined by CBA assay. B, Interleukin (IL)–6. C, Tumor necrosis factor (TNF)–α. ND, not done (severity of infection in untreated mice did not permit sampling at these later time points); dagger indicates that a mouse died before blood was harvested. Statistical significance was determined using the Mann–Whitney test; *P < .05; **P < .005; ***P < .001; NS, not significant.
In control mice, plasma concentrations of IL-6 were elevated 2 days after challenge compared with uninfected mice (<30 pg/mL) and exceeded 3.5 ng/mL in 3 of 4 mice by day 4 (Figure 5). In contrast, IL-6 concentrations in plasma from mice treated with both 2D2 vaccination and CpG remained at baseline levels in 6, 7, and 4 of 8 animals at days 2, 4, and 7 after infection, respectively, and by day 7 remained significantly lower than those in control mice at day 2 after infection (P < .001) (Figure 5). In mice receiving combination treatment, plasma TNF-α also remained at baseline levels for a week after challenge and increased only at day 11 as mice succumbed to infection (Figure 5). Thus, combination immunotherapy with pulmonary CpG treatment and 2D2 vaccination delayed the onset of sepsis by almost a week, even though residual bacteria were present in multiple organs.
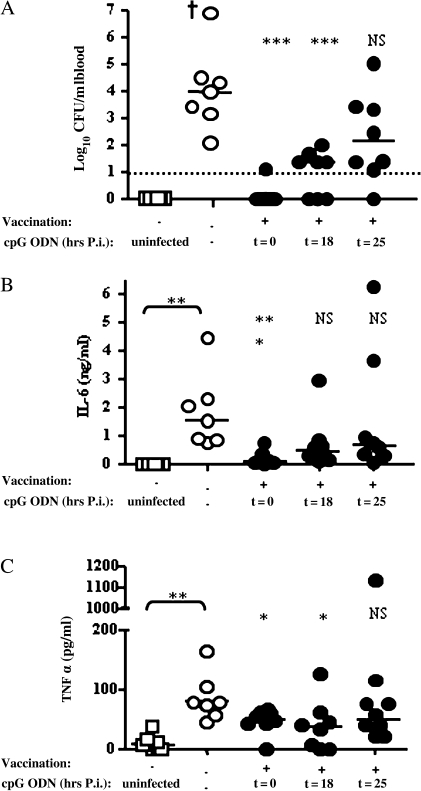
Most importantly, CpG treatment at 18 hours after infection still significantly reduced bacteremia, with 3 of 8 mice blood culture negative on day 4 compared with 0 of 8 in the untreated group (P < .001 for blood bacterial load), with a 100-fold reduction in bacterial loads in all remaining mice and decreased plasma concentrations of IL-6 and TNF-α (Figure 6). However, statistically significant protection was not observed when CpG treatment was delayed until 25 hours after challenge. Thus, in mice that have previously been vaccinated, there is a short but significant window of time when postinfection treatment with CpG is effective in delaying the onset of lethal sepsis.
Figure 6.
Intranasal CpG treatment after infection in mice previously vaccinated with Burkholderia pseudomallei mutant 2D2. BALB/c mice (n = 7–8) were either left untreated or vaccinated intranasally with B. pseudomallei mutant 2D2 and after 5 weeks treated with CpG oligodeoxynucleotide (ODN) 2395 (intranasal) either at the time of challenge with 3 × 102 CFU B. pseudomallei 576 (t = 0) or at 18 hours (t = 18) or 25 hours (t = 25) after infection (p.i.). Heparinized blood from all mice was harvested at day 4 after challenge. A, Bacterial loads from whole blood. The dashed line indicates the limit of detection. B, C, Plasma cytokine levels were determined by CBA assay. B, Interleukin (IL)–6. C, Tumor necrosis factor (TNF)–α. Baseline plasma cytokine levels were obtained from saline-treated uninfected mice. Dagger indicates a mouse died before blood harvest. Statistical significance was determined using the Mann–Whitney test; ** P < .005; *** P < .001; NS, not significant.
DISCUSSION
Pulmonary exposure to B. pseudomallei occurs in endemic regions and is the most likely potential route of infection in a biodefense scenario. The most susceptible route of infection in murine models of melioidosis is also by intranasal instillation or inhalation of bacteria [27, 28], and in genetically susceptible BALB/c mice provides the most stringent test of any candidate intervention. Using the well-characterized live-attenuated B. pseudomallei mutant 2D2, which we have shown elsewhere to protect against intraperitoneal challenge [19, 22], we now demonstrate that intranasal vaccination elicits significant protection against intranasal B. pseudomallei challenge. Importantly, 2D2 vaccination via the lung was superior to the intraperitoneal vaccination for eliciting protection against a subsequent respiratory challenge. This is consistent with previous B. pseudomallei studies showing that systemic vaccination was not effective against pulmonary challenge [12, 29].
Dannenberg and Scott and Breitbach et al [30, 31] have described the potential of pulmonary vaccination against respiratory challenge with B. pseudomallei, but until now the nature of the immune responses generated have not been defined. Previous work from our laboratory has shown that intraperitoneal 2D2 immunization primes B. pseudomallei–reactive T cells in the spleen, with depletion studies and adoptive transfer experiments demonstrating that CD4+ T cells are essential for protection [22]. In the present study we go on to demonstrate that intranasal 2D2 immunization also generates B. pseudomallei–reactive, IFN-γ–producing T cells in the lung and spleen and, importantly, that the magnitude of the T-cell responses in the lung correlates with the extent of protection. Other vaccination studies in experimental models of pulmonary M. tuberculosis have also shown that intranasal vaccination conferred optimal protection compared with other routes and was associated with the ability to prime specific T-cell responses in the lung or lung lymph nodes [16, 17]. The ability of 2D2 vaccine–primed T cells to secrete IFN-γ is consistent with the importance of this cytokine for macrophage activation and killing of intracellular B. pseudomallei [23, 32].
Pretreatment with CpG ODNs alone have been shown to elicit protection against primary intraperitoneal B. pseudomallei infection [11], as well as infection with a range of other bacterial pathogens [9, 10, 33–35]. CpG also has an important role as an adjuvant in vaccination strategies against B. pseudomallei [14]. However, we have addressed 2 novel approaches to CpG treatment that are more applicable to a bioterrorism setting: targeting treatment directly to the lung and delivering CpG at the time of or shortly after bacterial challenge. We demonstrate that CpG treatment delivered to the lung conferred protection against pulmonary B. pseudomallei infection, associated with accelerated proinflammatory cytokine and chemokine responses (including TNF-α and IFN-γ), increased recruitment of natural killer cells and macrophages, and enhanced bacterial clearance from the tissues. Our results clearly demonstrate the protective effects of intranasal CpG treatment in B. pseudomallei infection and support the growing evidence for the efficacy of this route of administration against pulmonary infection with other bacterial and viral pathogens [35, 36] Importantly, we have shown that mice were protected from B. pseudomallei infection when CpG ODNs were delivered at the time of challenge, in contrast to previous B. pseudomallei and B. mallei survival studies in which significant protection was obtained only after a preinfection treatment regimen [10, 11]. Until now, CpG treatment administered at the time of challenge, or after infection, has been successful only in controlling slower-growing pulmonary pathogens [34]. It seems likely that targeting CpG ODN to the lung and inducing protective immune responses directly at the site of infection allows CpG treatment to be effective more rapidly [36].
We then asked if the protection offered by a partially effective vaccine could be enhanced by CpG given at the time of bacterial challenge. This involved local administration of immunostimulatory CpG ODN, not as an adjuvant [14] but to boost the innate pulmonary immune response in vaccinated mice at the time of challenge. Combining intranasal 2D2 vaccination and CpG treatment improved protection against a high-dose respiratory challenge in terms of extending survival, decreasing early pulmonary bacterial loads, and, most importantly, delaying by up to a week the onset of bacteremia and the systemic cytokine release (IL-6 and TNF-α) known to contribute to the immunopathology of sepsis [26, 37]. The latter is likely to be of particular clinical importance, because the success rate of antibiotic treatment is much higher when disease is localized and has not spread systemically. Remarkably, despite using the most susceptible route of exposure in a genetically susceptible mouse strain, CpG treatment could also be withheld for up to 18 hours after challenge in 2D2-vaccinated mice and still delay the onset of bacteremia and the systemic proinflammatory cytokine response. Nevertheless, under these conditions late breakthrough bacteremia still occurred, showing that our combination approach was successful but not sterilizing.
The ability to treat successfully after exposure is particularly applicable to a biodefense setting. Other combination therapies are being investigated for other highly virulent bioterrorism agents, such as Bacillus anthracis and Francisella tularensis, in mouse and nonhuman primate models [38, 39]. These have combined either vaccination or cytokine therapy with antibiotic treatment. The combination of vaccination and local CpG treatment has been shown to be an effective treatment in experimental models of melanoma [40]. However, our study provides a proof of principle demonstration that this strategy targeting both the innate and adaptive immune response in the lung is effective in controlling infectious diseases. Given that CpG ODN treatment alone is also partially effective against other select agents ([9, 10, 36] reviewed in [18]), a combined approach may also be broadly applicable for the control of other pathogens, such as F. tularensis, for which current vaccines may prove unreliable in the bioterrorism setting [41, 42]. Recently, combining antibiotics and cationic liposome-DNA complex mediated immunotherapy has also proved successful in a murine model of melioidosis [43].
Finally, these findings also have important implications for disease management after the deliberate release of B. pseudomallei or other biodefense pathogens. Theoretically, combining prior vaccination and postexposure CpG treatment provides a window of time for potentially infected individuals to present themselves for treatment when bacterial growth is still contained within the tissues, and the prognosis for antibiotic treatment is good. From a public health perspective, this increased window for effective treatment, along with the ease of applying CpG ODNs via the lung compared with by injection, is essential given the logistics of treating people on a large scale, as would occur after a potential mass exposure event. Combining the benefits of our vaccine plus CpG approach together with antibiotic treatment may eventually provide the best long-term protection against disease.
Funding
This work was supported by the Defence Science and Technology Laboratory (grants RD013-0931417 and RD032-0469 to G. J. B.) and the National Institutes of Health (grant UO1 AI061363-05 to G. J. B.).
References
- 1.Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18:383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiersinga WJ, van der Poll T, White NJ, Day NP, Peacock SJ. Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat Rev Microbiol. 2006;4:272–82. doi: 10.1038/nrmicro1385. [DOI] [PubMed] [Google Scholar]
- 3.Ngauy V, Lemeshev Y, Sadkowski L, Crawford G. Cutaneous melioidosis in a man who was taken as a prisoner of war by the Japanese during World War II. J Clin Microbiol. 2005;43:970–2. doi: 10.1128/JCM.43.2.970-972.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ip M, Osterberg LG, Chau PY, Raffin TA. Pulmonary melioidosis. Chest. 1995;108:1420–4. doi: 10.1378/chest.108.5.1420. [DOI] [PubMed] [Google Scholar]
- 5.Currie BJ, Fisher DA, Howard DM, et al. Endemic melioidosis in tropical northern Australia: a 10-year prospective study and review of the literature. Clin Infect Dis. 2000;31:981–6. doi: 10.1086/318116. [DOI] [PubMed] [Google Scholar]
- 6.Puthucheary SD, Vadivelu J, Wong KT, Ong GS. Acute respiratory failure in melioidosis. Singapore Med J. 2001;42:117–21. [PubMed] [Google Scholar]
- 7.Chan KP, Low JG, Raghuram J, Fook-Chong SM, Kurup A. Clinical characteristics and outcome of severe melioidosis requiring intensive care. Chest. 2005;128:3674–8. doi: 10.1378/chest.128.5.3674. [DOI] [PubMed] [Google Scholar]
- 8.Puthucheary SD, Parasakthi N, Lee MK. Septicaemic melioidosis: a review of 50 cases from Malaysia. Trans R Soc Trop Med Hyg. 1992;86:683–5. doi: 10.1016/0035-9203(92)90191-e. [DOI] [PubMed] [Google Scholar]
- 9.Elkins KL, Rhinehart-Jones TR, Stibitz S, Conover JS, Klinman DM. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J Immunol. 1999;162:2291–8. [PubMed] [Google Scholar]
- 10.Waag DM, McCluskie MJ, Zhang N, Krieg AM. A CpG oligonucleotide can protect mice from a low aerosol challenge dose of Burkholderia mallei. Infect Immun. 2006;74:1944–8. doi: 10.1128/IAI.74.3.1944-1948.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wongratanacheewin S, Kespichayawattana W, Intachote P, et al. Immunostimulatory CpG oligodeoxynucleotide confers protection in a murine model of infection with Burkholderia pseudomallei. Infect Immun. 2004;72:4494–502. doi: 10.1128/IAI.72.8.4494-4502.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson M, Prior JL, Lever MS, Jones HE, Atkins TP, Titball RW. Evaluation of lipopolysaccharide and capsular polysaccharide as subunit vaccines against experimental melioidosis. J Med Microbiol. 2004;53:1177–82. doi: 10.1099/jmm.0.45766-0. [DOI] [PubMed] [Google Scholar]
- 13.Healey GD, Elvin SJ, Morton M, Williamson ED. Humoral and cell-mediated adaptive immune responses are required for protection against Burkholderia pseudomallei challenge and bacterial clearance postinfection. Infect Immun. 2005;73:5945–51. doi: 10.1128/IAI.73.9.5945-5951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YS, Hsiao YS, Lin HH, Yen CM, Chen SC, Chen YL. Immunogenicity and anti-Burkholderia pseudomallei activity in Balb/c mice immunized with plasmid DNA encoding flagellin. Vaccine. 2006;24:750–8. doi: 10.1016/j.vaccine.2005.08.069. [DOI] [PubMed] [Google Scholar]
- 15.Jones SM, Ellis JF, Russell P, Griffin KF, Oyston PC. Passive protection against Burkholderia pseudomallei infection in mice by monoclonal antibodies against capsular polysaccharide, lipopolysaccharide or proteins. J Med Microbiol. 2002;51:1055–62. doi: 10.1099/0022-1317-51-12-1055. [DOI] [PubMed] [Google Scholar]
- 16.Goonetilleke NP, McShane H, Hannan CM, Anderson RJ, Brookes RH, Hill AV. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guerin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J Immunol. 2003;171:1602–9. doi: 10.4049/jimmunol.171.3.1602. [DOI] [PubMed] [Google Scholar]
- 17.Giri PK, Sable SB, Verma I, Khuller GK. Comparative evaluation of intranasal and subcutaneous route of immunization for development of mucosal vaccine against experimental tuberculosis. FEMS Immunol Med Microbiol. 2005;45:87–93. doi: 10.1016/j.femsim.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–84. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 19.Atkins T, Prior RG, Mack K, et al. A mutant of Burkholderia pseudomallei, auxotrophic in the branched chain amino acid biosynthetic pathway, is attenuated and protective in a murine model of melioidosis. Infect Immun. 2002;70:5290–4. doi: 10.1128/IAI.70.9.5290-5294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vicari AP, Schmalbach T, Lekstrom-Himes J, et al. Safety, pharmacokinetics and immune effects in normal volunteers of CPG 10101 (ACTILON), an investigational synthetic Toll-like receptor 9 agonist. Antivir Ther. 2007;12:741–51. [PubMed] [Google Scholar]
- 21.Easton A, Haque A, Chu K, Lukaszewski R, Bancroft GJ. A critical role for neutrophils in resistance to experimental infection with Burkholderia pseudomallei. J Infect Dis. 2007;195:99–107. doi: 10.1086/509810. [DOI] [PubMed] [Google Scholar]
- 22.Haque A, Chu K, Easton A, et al. A live experimental vaccine against Burkholderia pseudomallei elicits CD4+ T cell-mediated immunity, priming T cells specific for 2 type III secretion system proteins. J Infect Dis. 2006;194:1241–8. doi: 10.1086/508217. [DOI] [PubMed] [Google Scholar]
- 23.Miyagi K, Kawakami K, Saito A. Role of reactive nitrogen and oxygen intermediates in gamma interferon-stimulated murine macrophage bactericidal activity against Burkholderia pseudomallei. Infect Immun. 1997;65:4108–3. doi: 10.1128/iai.65.10.4108-4113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lertmemongkolchai G, Cai G, Hunter CA, Bancroft GJ. Bystander activation of CD8+ T cells contributes to the rapid production of IFN-gamma in response to bacterial pathogens. J Immunol. 2001;166:1097–105. doi: 10.4049/jimmunol.166.2.1097. [DOI] [PubMed] [Google Scholar]
- 25.Stevens DL. Bacterial shock: clinical definitions, clinical trials and the dynamics of the host response. Curr Opin Infect Dis. 2001;14:247–9. doi: 10.1097/00001432-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Simpson AJ, Smith MD, Weverling GJ, et al. Prognostic value of cytokine concentrations (tumor necrosis factor-alpha, interleukin-6, and interleukin-10) and clinical parameters in severe melioidosis. J Infect Dis. 2000;181:621–5. doi: 10.1086/315271. [DOI] [PubMed] [Google Scholar]
- 27.Liu B, Koo GC, Yap EH, Chua KL, Gan YH. Model of differential susceptibility to mucosal Burkholderia pseudomallei infection. Infect Immun. 2002;70:504–11. doi: 10.1128/IAI.70.2.504-511.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leakey AK, Ulett GC, Hirst RG. BALB/c and C57Bl/6 mice infected with virulent Burkholderia pseudomallei provide contrasting animal models for the acute and chronic forms of human melioidosis. Microb Pathog. 1998;24:269–75. doi: 10.1006/mpat.1997.0179. [DOI] [PubMed] [Google Scholar]
- 29.Levine HB, Maurer RL. Immunization with an induced avirulent auxotrophic mutant of Pseudomonas pseudomallei. J Immunol. 1958;81:433–8. [PubMed] [Google Scholar]
- 30.Dannenberg AM, Jr, Scott EM. Melioidosis: pathogenesis and immunity in mice and hamsters. III. Effect of vaccination with avirulent strains of Pseudomonas pseudomallei on the resistance to the establishment and the resistance to the progress of respiratory melioidosis caused by virulent strains; all-or-none aspects of this disease. J Immunol. 1960;84:233–46. [PubMed] [Google Scholar]
- 31.Breitbach K, Kohler J, Steinmetz I. Induction of protective immunity against Burkholderia pseudomallei using attenuated mutants with defects in the intracellular life cycle. Trans R Soc Trop Med Hyg. 2008;102(Suppl 1):S89–94. doi: 10.1016/S0035-9203(08)70022-1. [DOI] [PubMed] [Google Scholar]
- 32.Breitbach K, Klocke S, Tschernig T, van Rooijen N, Baumann U, Steinmetz I. Role of inducible nitric oxide synthase and NADPH oxidase in the early control of Burkholderia pseudomallei infection in mice. Infect Immun. 2006;74:6300–9. doi: 10.1128/IAI.00966-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krieg AM, Love-Homan L, Yi AK, Harty JT. CpG DNA induces sustained IL-12 expression in vivo and resistance to Listeria monocytogenes challenge. J Immunol. 1998;161:2428–34. [PubMed] [Google Scholar]
- 34.Juffermans NP, Leemans JC, Florquin S, et al. CpG oligodeoxynucleotides enhance host defense during murine tuberculosis. Infect Immun. 2002;70:147–52. doi: 10.1128/IAI.70.1.147-152.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng JC, Moore TA, Newstead MW, Zeng X, Krieg AM, Standiford TJ. CpG oligodeoxynucleotides stimulate protective innate immunity against pulmonary Klebsiella infection. J Immunol. 2004;173:5148–55. doi: 10.4049/jimmunol.173.8.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rees DG, Gates AJ, Green M, et al. CpG-DNA protects against a lethal orthopoxvirus infection in a murine model. Antivir Res. 2005;65:87–95. doi: 10.1016/j.antiviral.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Friedland JS, Suputtamongkol Y, Remick DG, et al. Prolonged elevation of interleukin-8 and interleukin-6 concentrations in plasma and of leukocyte interleukin-8 mRNA levels during septicemic and localized Pseudomonas pseudomallei infection. Infect Immun. 1992;60:2402–8. doi: 10.1128/iai.60.6.2402-2408.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vietri NJ, Purcell BK, Lawler JV, et al. Short-course postexposure antibiotic prophylaxis combined with vaccination protects against experimental inhalational anthrax. Proc Natl Acad Sci U S A. 2006;103:7813–6. doi: 10.1073/pnas.0602748103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pammit MA, Budhavarapu VN, Raulie EK, Klose KE, Teale JM, Arulanandam BP. Intranasal interleukin-12 treatment promotes antimicrobial clearance and survival in pulmonary Francisella tularensis subsp. novicida infection. Antimicrob Agents Chemother. 2004;48:4513–9. doi: 10.1128/AAC.48.12.4513-4519.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tormo D, Ferrer A, Bosch P, et al. Therapeutic efficacy of antigen-specific vaccination and Toll-like receptor stimulation against established transplanted and autochthonous melanoma in mice. Cancer Res. 2006;66:5427–35. doi: 10.1158/0008-5472.CAN-06-0399. [DOI] [PubMed] [Google Scholar]
- 41.Burke DS. Immunization against tularemia: analysis of the effectiveness of live Francisella tularensis vaccine in prevention of laboratory-acquired tularemia. J Infect Dis. 1977;135:55–60. doi: 10.1093/infdis/135.1.55. [DOI] [PubMed] [Google Scholar]
- 42.Dennis DT, Inglesby TV, Henderson DA, et al. Tularemia as a biological weapon: medical and public health management. JAMA. 2001;285:2763–3. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- 43.Propst KL, Troyer RM, Kellihan LM, Schweizer HP, Dow SW. Immunotherapy markedly increases the effectiveness of antimicrobial therapy for treatment of Burkholderia pseudomallei infection. Antimicrob Agents Chemother. 2010;54:1785–92. doi: 10.1128/AAC.01513-09. [DOI] [PMC free article] [PubMed] [Google Scholar]