Abstract
Background. Accelerated immunization schedules may help gain early control of influenza pandemics. We investigated different schedules of an AS03A-adjuvanted H5N1 vaccine.
Methods. This phase II, open-label, 6-month study randomized participants (aged 18–64 years) to 2 vaccine doses administered 21 (standard schedule), 14, or 7 days apart, or on the same day. Coprimary end points were that the lower limit of the 98.75% confidence interval 14 days after the last dose must be (1) >40% for seroconversion rate (SCR) (Center for Biologics Evaluation and Research [CBER] criterion) and (2) >50% for seroprotection rate (SPR) (attainment rate for reciprocal hemagglutination inhibition titers ≥40, protocol-defined criterion) for the vaccine homologous strain (A/Indonesia/5/2005). European Committee for Human Medicinal Products (CHMP) immunogenicity criteria were also evaluated.
Results. Coprimary end points were achieved (lower 98.75% confidence intervals exceeded defined values). Titers were highest with the standard schedule. Nevertheless, CBER SCR, protocol-defined SPR, and CHMP criteria were met with all schedules for the A/Indonesia/5/2005 strain. There were no significant differences between age groups (18–40 vs 41–64 years). Immune response was robust against drift variants A/turkey/Turkey/1/2005 and A/Vietnam/1194/2004.
Conclusions. The AS03A-adjuvanted H5N1 vaccine in accelerated schedules offers a robust immune response against vaccine homologous and drift variant strains, allowing consideration of compressed vaccination intervals.
Clinical Trials Registration. NCT00695669.
The avian H5N1 influenza virus remains a serious threat to human health, and human cases continue to be reported [1, 2]. The 2009 H1N1 influenza pandemic illustrates that pandemics may arise rapidly with an unexpected viral source. It is therefore essential to continue diligent preparations to deal with potential pandemic strain viruses, including H5N1.
Pandemic modeling data suggest that the peak disease incidence could occur in the United Kingdom and United States 50–85 days after the first national case is identified, assuming no intervention [3, 4]. In the 2009 H1N1 pandemic, the first cases were reported in mid-April 2009, and the World Health Organization announced Pandemic Alert Phase 6 on 11 June 2009. A vaccine based on a matching pandemic strain is unlikely to be produced quickly enough to immunize the required number of people to protect communities. An alternative strategy is to vaccinate before the pandemic occurs or when it is in its very early stages with a prepandemic formulation containing antigens that are not strain matched to the actual pandemic virus [3]. Prepandemic vaccines must induce a high and long-lasting cross-reactive immune response so that the immune system is primed to mount a rapid response to infection and/or to vaccination with the strain-matched pandemic vaccine. In addition, it is important that prepandemic and pandemic vaccines should be antigen sparing, because production of enough vaccine to meet global needs will rely on the availability of sufficient quantities of the prepandemic and pandemic virus antigen [5, 6].
Populations naive to the H5 hemagglutinin (HA) antigen may respond poorly to vaccination, and it has been shown elsewhere that up to 90 μg of HA antigen is needed to produce a satisfactory immune response with conventional H5N1 vaccines [7]. The use of adjuvants has been highlighted as an important strategy to gain antigen-sparing and improved immune responses in the development of pandemic and prepandemic vaccines [5]. An H5N1 vaccine containing as little as 3.75 μg of HA, adjuvanted with AS03A (a tocopherol oil-in-water emulsion based adjuvant system), is licensed to be used in Europe in the event of an imminent H5N1 pandemic. Immunogenicity against vaccine homologous strains and cross-reactivity have been demonstrated in other studies, together with an acceptable reactogenicity and safety profile [8–11]. Initial development of the AS03A-adjuvanted vaccine was based on the A/Vietnam/1194/2004 clade 1 strain, though the latest vaccines have used antigen from more recent H5N1 drift variants, such as A/Indonesia/5/2005 (clade 2.1) and A/turkey/Turkey/1/2005 (clade 2.2) [12].
Besides antigen-sparing and cross-reactive properties, prepandemic vaccines should provide flexibility in vaccination schedules to offer more public health response options. To date, most studies of prepandemic vaccines have used a 2-dose schedule with a 21–28-day interval. Other studies have demonstrated the immunogenicity of longer intervals using homologous and heteroclade antigen vaccine formulations [10]. However, modeling data suggest that rapid deployment of a vaccine is an effective strategy to gain early control of a pandemic [4]. Therefore the ability to accelerate vaccination schedules is advantageous. The purpose of the present study was to evaluate immunogenicity of an AS03A-adjuvanted vaccine using the A/Indonesia/5/2005 strain, administered in accelerated 2-dose immunization schedules using intervals between doses of 14, 7, and 0 days along with the usual interval of 21 days as the reference.
METHODS
Study Objectives
The primary objectives of the study were to demonstrate that the accelerated immunization schedules elicit an immune response against the vaccine homologous strain (A/Indonesia/5/2005) 14 days after the last vaccine dose of a 2-dose schedule that meets or exceeds the following criteria: (1) US Food and Drug Administration Center for Biologics Evaluation and Research (CBER) criterion for seroconversion rate (SCR) and (2) a protocol-defined, potentially clinically meaningful criterion for seroprotection rate (SPR) (attainment rate for reciprocal hemagglutination inhibition [HI] titers ≥40).
Secondary objectives were to (1) demonstrate that the immune response against the A/Indonesia/5/2005 strain meets the CBER criterion for SCR, the protocol-defined criterion for SPR 21 days after the last vaccine dose, and European Committee for Human Medicinal Products (CHMP) criteria at 14 and 21 days after the last vaccine dose; (2) describe the immune response against the drift-variant strains, A/Vietnam/1194/2004 and A/turkey/Turkey/1/2005, at 7, 14, and 21 days after the last vaccine dose; (3) describe the kinetics of the immune response between the first and last vaccinations and up to 6 months after the first vaccine dose; and (4) describe the safety and reactogenicity of the vaccination schedules.
Study Design and Vaccine
This was a phase II, open-label, randomized, parallel-group, 6-month study conducted in 3 centers in Canada between June and December 2008 (NCT00695669). Healthy male and nonpregnant female participants aged 18–64 years were included in the study. Exclusion criteria are shown in the supplementary material. The study was conducted in accordance with the current version of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. The protocol was approved by the independent ethics committee or institutional review board of each study center and written informed consent was obtained from each participant.
The random allocation list was generated by GlaxoSmithKline Biologicals. Investigators enrolled participants. Participants were randomized 1:1:1:1 and assigned to 1 of 4 study groups, according to an Internet-based randomization blocking scheme. The randomization algorithm used a minimization procedure accounting for center and age (18–40 or 41–64 years). There were 4 study groups, which received 2 vaccine doses: (1) 21 days apart on days 0 and 21 (group 0/21); (2) 14 days apart on days 0 and 14 (group 0/14); (3) 7 days apart on days 0 and 7 (group 0/7); (4) 2 doses on day 0 (group 0/0). All participants attended study visits at days 0, 21, 42, and 182. Additionally, visits were scheduled at 7, 14, and 21 days after the last vaccine dose. Telephone contact was made with all participants on day 51.
The H5N1-inactivated split-virion recombinant vaccine (GlaxoSmithKline Biologicals) contained 15 μg/mL HA of the A/Indonesia/5/2005 PR8-IBCDC-RG2 strain obtained from the Centers for Disease Control and Prevention. When mixed 1:1 with AS03A, an oil-in-water emulsion-based adjuvant system containing 11.86 mg DL-α-tocopherol [8, 9], each vaccine dose (0.5 mL) provided 3.75 μg of HA. The vaccine was administered into the deltoid region, the first dose into the nondominant arm and the last into the dominant arm.
Study End Points and Procedures
Blood samples were taken from all participants at each study visit, and the following parameters were derived from HI titers: (1) geometric mean titer (GMT), (2) SCR, (3) protocol-defined SPR, and (4) geometric mean fold rise (GMFR). The SCR was defined as the percentage of participants with either (1) a prevaccination titer <1:10 and a postvaccination titer ≥1:40 or (2) a prevaccination titer ≥1:10 and a minimum 4-fold increase in postvaccination titer. The SPR was defined as the percentage of participants who attained reciprocal HI titers of ≥40. The GMFR was defined as the geometric mean of the within-subject ratios of the postvaccination-prevaccination reciprocal HI titer.
Participants recorded solicited local and general adverse events (AEs) in diary cards on the day of vaccination and for 6 days afterward. They recorded unsolicited symptoms until the following visit. Investigators asked about AEs and serious AEs (SAEs) at each study visit and at the telephone contact on day 51. In addition, abnormal laboratory findings were recorded as AEs or SAEs if they met the definition. Definitions of SAEs and local and general solicited AEs are provided in the supplementary material.
Statistics
A cutoff value for HI titers was defined as 1:10. Seronegative participants was defined as those having an antibody titer below the cutoff value; seropositive participants, those having a titer greater than or equal to the cutoff value. Antibody titers below the assay cutoff value were given an arbitrary value of 5 (half the cutoff value) for the GMT calculation. A point estimate and the associated 2-sided exact 98.75% confidence interval (CI) were calculated for the coprimary end points of SCR and protocol-defined SPR [13, 14]. A 2-sided exact 95% CI was used for the secondary objective evaluation, calculated by the same method. The exact 98.75% or 95% CI for a proportion within a group was calculated with ProcStatXact software [13].
The immune response was evaluated according to CBER, protocol-defined, and CHMP criteria. The criteria to meet the coprimary and secondary end points are described in Table 1. For the coprimary end points, the lower limit of the 98.75% CI for SCR had to be >40% (CBER criterion), and the lower limit of the 98.75% CI for SPR had to be >50% (protocol-defined criterion). The primary immunogenicity analysis was based on the according-to-protocol (ATP) cohort, which included all participants who met eligibility criteria, complied with the protocol, received 2 vaccine doses at the correct interval for their assigned treatment regimen, and had a complete data set for the coprimary end points. The safety analysis was based on the total vaccinated cohort (TVC), which included all vaccinated participants for whom safety data were available.
Table 1.
Criteria for the Evaluation of Primary and Secondary End Points
| Lower limit of CI,a % |
CHMP, point estimates |
||||
| End points | CBER (SCR) | Protocol defined (SPR) | SCR, % | SPR, % | GMFR |
| Coprimary end points | |||||
| 14 days after last dose for A/Indonesia/5/2005 | >40 | >50 | — | — | — |
| Secondary end points | |||||
| 21 days after last dose for A/Indonesia/5/2005 | >40 | >50 | >40 | >70 | >2.5 |
| 14 and 21 days after last dose for A/Vietnam/1194/2004 and A/turkey/Turkey/1/2005 | >40 | >50 | >40 | >70 | >2.5 |
NOTE. The seroconversion rate (SCR) was defined as the percentage of participants with either a prevaccination titer <1:10 and a postvaccination titer ≥1:40 or a prevaccination titer ≥1:10 and a minimum 4-fold increase in postvaccination titer. The seroprotection rate (SPR) was defined as the percentage of participants who attained reciprocal hemagglutination inhibition (HI) titers of ≥40. The geometric mean fold rise (GMFR) was defined as the geometric mean of the within-subject ratio of postvaccination/prevaccination reciprocal HI titers. CBER, Center for Biologics Evaluation and Research; CHMP: European Committee for Human Medicinal Products.
For coprimary end points, 98.75% confidence intervals (CIs) were used; for secondary end points, 95% CIs were used.
The overall study power was 81.5% to reach the coprimary objectives in any of the vaccine groups. Assuming a 10% dropout or unevaluable subject rate, a sample size of 78 participants per group was required to reach 70 evaluable subjects in each group to meet the study coprimary objectives with the designed power.
RESULTS
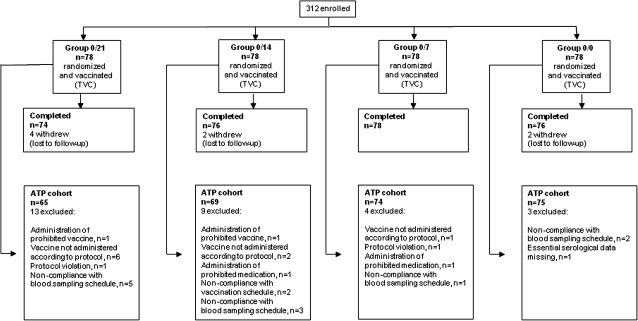
Of the 312 participants who were enrolled and vaccinated, all but 8 completed the study (Figure 1). The ATP cohort for the immunogenicity analysis consisted of 283 participants (90.7% of the TVC); reasons for exclusion are shown in Figure 1. Demographic characteristics were similar in each study group and between the TVC and ATP cohort; in the TVC, the mean age was 40.3 years, 53.2% of participants were female, and 87.8% were of white/European heritage. A total of 148 (47.4%) were in the younger age stratum (18–40 years; mean age, 27.8 years), and 164 (52.6%) were in the older stratum (41–64 years; mean age, 51.7 years).
Figure 1.
Disposition of participants. ATP, according-to-protocol cohort; TVC, total vaccinated cohort.
Immunogenicity Analysis
Immune Response Against the Vaccine Homologous Strain in All Participants.
The study met its coprimary end points: Both the CBER criterion for SCR and the protocol-defined criterion for SPR were achieved 14 days after the last vaccine dose with all administration schedules (Tables 2 and 3). High levels of SCR and SPR were achieved with all schedules 14 days after the last vaccine dose, although rates were higher in groups 0/21 and 0/14 than in groups 0/7 and 0/0 (Tables 2 and 3). Responses in group 0/14 were similar to those in group 0/21, with point estimates higher for group 0/21. Similar findings were observed 21 days after the last vaccine dose (Tables 2 and 3), although point estimates were somewhat higher in the 0/7 group than in the 0/0 group. Although CIs overlapped, trends were for peak antibody responses to occur 14 days after the last dose in participants receiving the vaccine doses at 21- or 14-day intervals versus peak responses occurring 21 days after the last dose in those receiving the vaccine doses 7 days apart or on the same day.
Table 2.
Seroconversion Rate (SCR) 14 and 21 Days After Administration of the Last Vaccine Dose
| SCR (95% CI)a |
|||
| Group and interval after last dose | A/Indonesia/5/2005 | A/Vietnam/1194/2004 | A/turkey/Turkey/1/2005 |
| 14 days | |||
| Group 0/21 (n = 65) | 96.9 (86.9–99.8) (n = 63) | 76.9 (64.8–86.5) (n = 50) | 83.1 (71.7–91.2) (n = 54) |
| Group 0/14 (n = 69) | 92.8 (81.2–98.3) (n = 64) | 59.4 (46.9–71.1) (n = 41) | 75.4 (63.5–84.9) (n = 52) |
| Group 0/7 (n = 74) | 71.6 (56.8–83.7) (n = 53) | 33.8 (23.2–45.7) (n = 25) | 51.4 (39.4–63.1) (n = 38) |
| Group 0/0 (n = 75) | 72.0 (57.3–83.9) (n = 54) | 33.3 (22.9–45.2) (n = 25) | 42.7 (31.3–54.6) (n = 32) |
| 21 days | |||
| Group 0/21 (n = 62) | 95.2 (86.5–99.0) (n = 59) | 66.1 (53.0–77.7) (n = 41) | 83.9 (72.3–92.0) (n = 52) |
| Group 0/14 (n = 69) | 92.8 (83.9–97.6) (n = 64) | 49.3 (37.0–61.6) (n = 34) | 71.0 (58.8–81.3) (n = 49) |
| Group 0/7 (n = 72) | 80.6 (69.5–88.9) (n = 58) | 26.4 (16.7–38.1) (n = 19) | 51.4 (39.3–63.3) (n = 37) |
| Group 0/0 (n = 74) | 74.3 (62.8–83.8) (n = 55) | 31.1 (20.8–42.9) (n = 23) | 50.0 (38.1–61.9) (n = 37) |
NOTE. Data are presented for according-to-protocol cohort (participants aged 18–64 years). Vaccine doses were administered at intervals of 21 days (group 0/21), 14 days (group 0/14), or 7 days (group 0/7) or on the same day (group 0/0). The SCR was defined as the percentage of participants with either a prevaccination titer <1:10 and a postvaccination titer ≥1:40 or a prevaccination titer ≥1:10 and a minimum 4-fold increase in postvaccination titer; n = number of participants with data available (stub column) or numbers of respondents (with SCR values).
For the A/Indonesia/5/2005 strain at 14 days, 98.75% confidence intervals (CIs) are shown; all other CIs are 95%.
Table 3.
Seroprotection Rate (SPR) 14 and 21 Days After Administration of the Last Vaccine Dose
| SPR (95% CI)a |
|||
| Group and interval after last dose | A/Indonesia/5/2005 | A/Vietnam/1194/2004 | A/turkey/Turkey/1/2005 |
| 14 days | |||
| Group 0/21 (n = 65) | 96.9 (86.9–99.8) (n = 63) | 78.5 (66.5–87.7) (n = 51) | 92.3 (83.0–97.5) (n = 60) |
| Group 0/14 (n = 69) | 92.8 (81.2–98.3) (n = 64) | 59.4 (46.9–71.1) (n = 41) | 82.6 (71.6–90.7) (n = 57) |
| Group 0/7 (n = 74) | 74.3 (59.7–85.9) (n = 55) | 35.1 (24.4–47.1) (n = 26) | 58.1 (46.1–69.5) (n = 43) |
| Group 0/0 (n = 75) | 74.7 (60.2–86.1) (n = 56) | 37.3 (26.4–49.3) (n = 28) | 52.0 (40.2–63.7) (n = 39) |
| 21 days | |||
| Group 0/21 (n = 62) | 95.2 (86.5–99.0) (n = 59) | 67.7 (54.7–79.1) (n = 42) | 93.5 (84.3–98.2) (n = 58) |
| Group 0/14 (n = 69) | 92.8 (83.9–97.6) (n = 64) | 49.3 (37.0–61.6) (n = 34) | 79.7 (68.3–88.4) (n = 55) |
| Group 0/7 (n = 72) | 81.9 (71.1–90.0) (n = 59) | 30.6 (20.2–42.5) (n = 22) | 61.1 (48.9–72.4) (n = 44) |
| Group 0/0 (n = 74) | 77.0 (65.8–86.0) (n = 57) | 36.5 (25.6–48.5) (n = 27) | 58.1 (46.1–69.5) (n = 43) |
NOTE. Data are presented for according-to-protocol cohort (participants aged 18–64 years). Vaccine doses were administered at intervals of 21 days (group 0/21), 14 days (group 0/14), or 7 days (group 0/7) or on the same day (group 0/0). The SPR was defined as the attainment rate for reciprocal hemagglutination inhibition titers ≥40; n = number of participants with data available (stub column) or numbers of respondents (with SPR values).
For the A/Indonesia/5/2005 strain at 14 days, 98.75% confidence intervals (CIs) are shown; all other CIs are 95%.
The CHMP criteria for SCR and SPR were met with all schedules at 14 and 21 days after the last vaccine dose (Tables 2 and 3). In addition, CHMP criteria were met for GMFR with all schedules at both time points: Values were 119.4 (95% CI, 84.0–169.7), 65.0 (45.6–92.5), 14.2 (10.0–20.1), and 11.6 (8.0–16.7) for groups 0/21, 0/14, 0/7, and 0/0, respectively, 14 days after the last dose. Compared with the results at 14 days after the second dose, GMFR values at 21 days after the last dose were slightly lower in groups 0/21 and 0/14 but slightly higher in groups 0/7 and 0/0: 97.3 (95% CI, 67.1–141.2), 57.6 (41.3–80.3), 18.1 (12.8–25.8), and 13.5 (9.2–19.6), respectively.
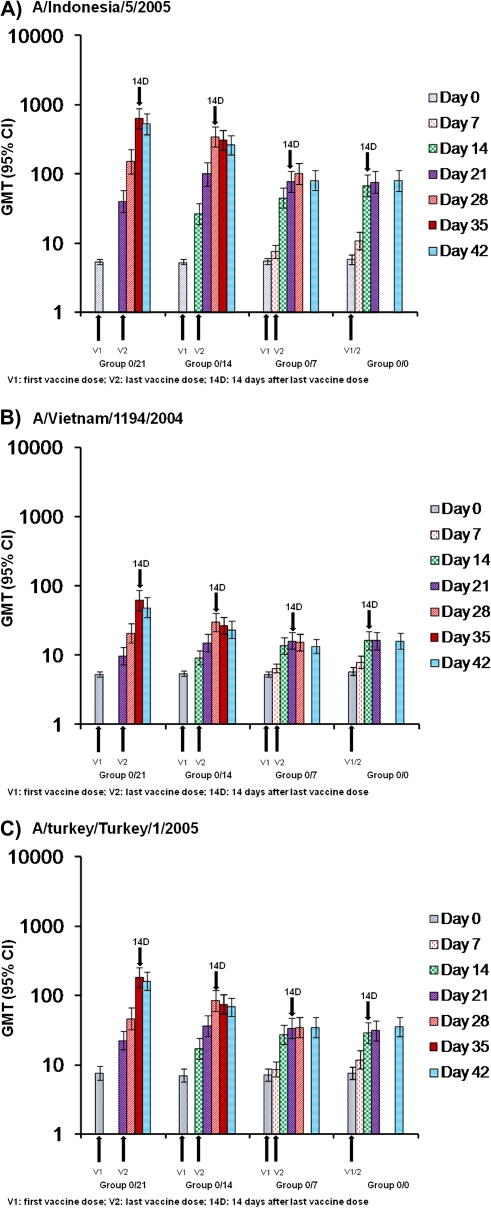
GMTs were highest in group 0/21, followed by group 0/14; they were lower in groups 0/7 and 0/0 (Figure 2, A–C). All groups exhibited an increase in titers after each dose, with a larger increase after the last dose. The antibody response was sustained at 6 months after vaccination, although antibody titers declined compared with earlier values (data shown in supplementary material). At 6 months, the CBER criterion for SCR was achieved in groups 0/21, 0/14, and 0/0, and the protocol-defined criterion for SPR was achieved in groups 0/21 and 0/14 (data shown in supplementary material).
Figure 2.
Hemagglutination inhibition geometric mean titers (GMTs) against (A) A/Indonesia/5/2005 H5N1, (B) A/Vietnam/1194/2004 H5N1, and (C) A/turkey/Turkey/1/2005 H5N1 at day 0 through 42. Data are presented for the according-to-protocol cohort (participants aged 18–64 years). Vaccine doses were administered at intervals of 21 days (group 0/21), 14 days (group 0/14), or 7 days (group 0/7) or on the same day (group 0/0). CI, confidence interval.
Immune Response Against the Vaccine Homologous Strain According to Age.
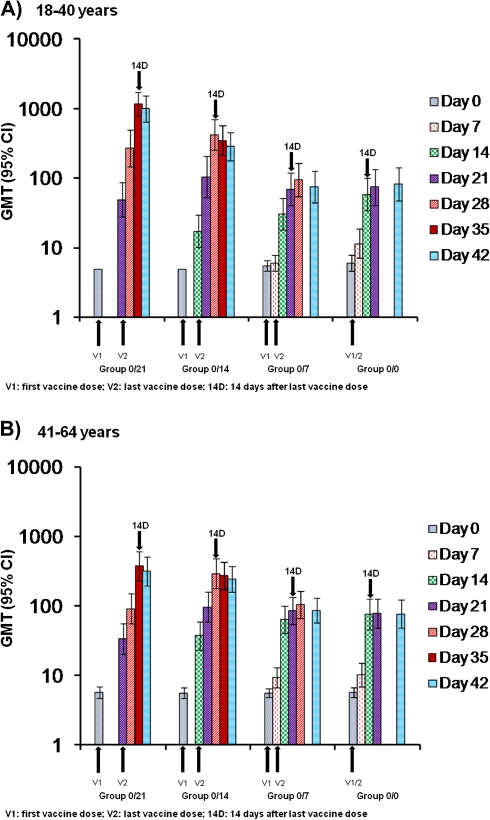
As with the overall analysis, the immune response within each age group (18–40 and 41–64 years) was higher in groups 0/21 and 0/14 than in groups 0/7 and 0/0 (Figure 3a and b). Values for SCR, SPR, and GMT did not differ greatly by age. However, GMTs in the 0/21 group were somewhat higher in younger participants (age, 18–40 years) than older participants (age, 41–64 years), and a similar pattern was observed in the 0/14 group.
Figure 3.
Hemagglutination inhibition geometric mean titers (GMTs) against A/Indonesia/5/2005 H5N1 at day 0 through 42 according to age: (A) 18–40 years vs (B) 41–64 years. Data are presented for according-to-protocol cohort. Vaccine doses were administered at intervals of 21 days (group 0/21), 14 days (group 0/14), or 7 days (group 0/7) or on the same day (group 0/0). CI, confidence interval.
Immune Response Against Drift-Variant Strains.
The immune response against the drift variants was relatively robust, although, as expected, lower than against the vaccine homologous strain (Tables 2 and 3). The immune response against the clade 2 A/turkey/Turkey/1/2005 strain was greater than against the clade 1 A/Vietnam/1194/2004 strain.
The CBER criterion for SCR was reached for both strains in group 0/21 and 0/14 at 14 days after the last vaccine dose, although this was not the case for the A/Vietnam/1194/2004 strain in group 0/14 at 21 days after the last dose (Table 2). The protocol-defined criterion for SPR was achieved in group 0/21 for both strains at both time points. For group 0/14, it was achieved for the A/turkey/Turkey/1/2005 strain at both time points (Table 3).
The CHMP criterion for SCR was met with all vaccination schedules for the A/turkey/Turkey/1/2005 strain and in groups 0/21 and 0/14 for the A/Vietnam/1194/2004 strain at both time points (Table 2). For SPR, the CHMP criterion was met at 14 days after the last dose in groups 0/21 and 0/14 for the A/turkey/Turkey/1/2005 strain and in group 0/21 for the A/Vietnam/1194/2004 strain (Table 3). However, at 21 days after the last dose, only the A/turkey/Turkey/1/2005 strain met the CHMP criterion for SPR in groups 0/21 and 0/14 (Table 3).
Safety Analysis
There were no clinically relevant differences between study groups in the reactogenicity or general safety profile of the vaccine (data shown in supplementary material). A total of 8 SAEs were reported by 5 participants: 1 participant reported exacerbated irritable bowel syndrome (group 0/21); 1 died as a result of injury (group 0/7); 1 experienced syncope (group 0/7); 1 experienced exacerbated diabetes mellitus, myocardial infarction, cellulitis, and congestive heart failure, and was withdrawn from the study (group 0/7); and 1 experienced phlebitis (group 0/0). All SAEs were considered unrelated to the vaccine by the investigators.
DISCUSSION
The study showed that the AS03A-adjuvanted vaccine produced a robust immune response with all the accelerated schedules evaluated. The study met its primary end point, with the vaccine eliciting an immune response against the vaccine homologous strain with all schedules at 14 days after the last vaccine dose that exceeded the CBER criterion for SCR and a protocol-defined criterion for SPR that was considered potentially clinically meaningful.
Mathematical modeling studies have suggested that a stockpiled vaccine not strain matched to the pandemic virus may nevertheless have a considerable impact on containing the pandemic [3, 15], because protection could develop not only against the vaccine homologous strain but also against drifted strains. Another model has predicted that vaccinating a certain number of people with 1 dose is more effective at mitigating a pandemic than providing maximal protection with 2 vaccine doses to half that number [4]. Some schedule flexibility may help to ease the inevitable logistic pressures associated with delivering a pandemic or prepandemic vaccination program [5]. In particular, vaccination with only 1 dose will simplify programs [16]. The H1N1 pandemic has shown that a pandemic can indeed arise very rapidly from an unexpected source. Timely actions are needed to contain a pandemic, and vaccination strategies that prioritize rapid mass immunization are crucial from a public health perspective.
Licensing authority criteria are based on threshold levels of immune response that are likely to offer protection against infection to individuals. However, it could be important that health authorities also consider vaccination strategies that may offer less than maximal protection to individuals but provide greater benefits to the population as a whole because they help contain the pandemic. It was in this context that we developed the protocol-defined criterion specifying that the lower limit of the CI for SPR had to be >50%. Although there is no known correlate of protection for H5N1, we assumed that potentially useful clinical protection would be achieved if the lower limit of the SPR exceeded 50%. This assumption was based on modeling results predicting that a vaccine that achieved 30% efficacy against viral susceptibility and 50% efficacy against infectiousness may be a useful part of a pandemic containment strategy [15]. Thus, attaining a titer potentially able to modify susceptibility or transmission in 50% of participants within 2 weeks of completing immunization seemed a reasonable goal.
The reference schedule with a 21-day interval between the first and last vaccine doses produced the highest immune response against the vaccine homologous strain, followed by the schedule with a 14-day interval. The response was less for the schedules with shorter intervals (7-day interval and same-day administration), but these schedules nevertheless produced an adequate immune response based on CBER SCR, protocol-defined SPR, and CHMP (SCR, SPR, and GMFR) criteria 14 days after the last dose. Values with the 21- and 14-day schedules tended to be higher in younger participants (18–40 years) than in older participants (41–64 years), but there were no relevant differences between the immune responses of younger versus older adults. The vaccine has also been evaluated in the elderly (≥61 years) and can be given at the same dose and schedule as in younger adults (3.75-μg doses given 21 days apart) [17]. Recent studies suggest a negative impact of prior seasonal influenza vaccination on seroresponses to H5N1 vaccines [18]. However, we did not evaluate any potential effect of seasonal vaccination in this study.
Antibodies persisted above baseline levels 6 months after vaccine administration; although levels had declined compared with earlier in the study, CBER and CHMP criteria for SCR were met with the 21-day, 14-day, and same-day schedules. Nevertheless, the study was not powered to detect differences between the different schedules in terms of the magnitude of response at 6 months.
Previous studies have shown that the AS03A-adjuvanted H5N1 vaccine produces a cross-reactive immune response to drift variant strains [8–10]. The immune response against the drift variant strains, A/Vietnam/1194/2004 and A/turkey/Turkey/1/2005, was robust, particularly against the A/turkey/Turkey/1/2005 strain, which is genetically more closely related to the vaccine homologous strain. As with the vaccine homologous strain, the response was higher with the 21- and 14-day intervals. Nevertheless, some level of response was observed with all schedules, with CBER, protocol-defined, and CHMP criteria being met in several cases.
The observed peak immune response occurred consistently at 14 days after the last vaccine dose with the 21- and 14-day schedules, regardless of strain or age. However, no clear peak was observed with the 7-day and same-day schedules, although the trend was for the peak response to occur at 21 days after the second vaccine dose. This suggests that reducing the interval between vaccinations not only induces lower immune responses but also generates a slower increase in the response.
As mentioned elsewhere, a mathematical model has predicted that vaccinating a certain number of people with 1 dose provides more effective pandemic mitigation than vaccinating half that number with 2 doses [4]. In our study, GMTs against the vaccine homologous strain in the 21-day interval group were 40.2 at 21 days after a single vaccine dose, compared with 640.0 at 14 days after the second dose. Similarly, in the 14-day interval group, GMTs were 26.8 at 14 days after a single dose, compared with 345.0 at 14 days after the second dose. The same pattern was seen in the 7-day interval group: 7.6 at 7 days after a single dose compared with 77.7 at 14 days after the second dose. Thus, a relatively low immune response was observed after a single dose, and therefore the effect on pandemic mitigation after only 1 dose of H5N1 vaccine is uncertain.
The safety profile of the vaccine was acceptable and there appeared to be no impact of administration schedule on its reactogenicity profile. Reactogenicity was no more severe when the vaccine was administered twice on the same day compared with the other schedules.
In conclusion, the present study shows that the vaccine given in an accelerated schedule elicits a potent immune response against the vaccine homologous strain, offers a rapid immune response evident after only 1 double dose, and elicits a cross-reactive response against drift variant strains. This profile may provide flexibility for health authorities to consider more temporally compressed dosing schedules with an antigen-sparing vaccine in the event of an emergency H5N1 outbreak. The data presented suggest that such shortened regimens could be implemented.
Supplementary data
Supplementary data are available at The Journal of Infectious Diseases online.
Funding
Study funding was provided by the Public Health Agency of Canada. GlaxoSmithKline Biologicals also provided funding, was involved in all stages of the study conduct and analysis, and assumed all costs associated with the development and publishing of this article.
Supplementary Material
Acknowledgments
We are indebted to the participating study volunteers, clinicians, coordinators and laboratory technicians at the study sites and the sponsor’s project staff for their support and contributions throughout the study, in particular Caroline Gesualdi and Astrid Beriaux for study coordination, Nancy Bouveret, Louis Fries, and Bruce Innis for contributions to study design, and Stephanie Sharp for preparation of the study protocol and related study documentation. We are grateful to Thierry Ollinger for specimen coordination and Roger Bernhard and his team who performed the serologic laboratory work. Finally, we thank Dr Mary Greenacre (independent, United Kingdom, on behalf of GlaxoSmithKline Biologicals), who provided medical writing services to develop the manuscript. All authors critically reviewed the proposed drafts of the manuscript, and their comments were taken into account and incorporated. All authors approved the content of the final version of the manuscript before it was submitted by the corresponding author. We thank Dr Isabelle Gautherot (GlaxoSmithKline Biologicals) and Dr Geraldine Verplancke (Keyrus Biopharma, on behalf of GlaxoSmithKline Biologicals), who provided support to coordinate the circulation of the manuscript to all coauthors, collect comments received from coauthors, and make sure that International Committee of Medical Journal Editors recommendations were fulfilled.
References
- 1.World Health Organization. Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO. 6 May 2010. Available at: http://www.who.int/csr/disease/avian_influenza/country/cases_table_2010_05_06/en/index.html. Accessed 22 July 2010. [Google Scholar]
- 2.Melidou A, Gioula G, Exindari M, Chatzidimitriou D, Diza-Mataftsi E. Influenza A(H5N1): an overview of the current situation. Eurosurveillance. 2009;14:1–4. doi: 10.2807/ese.14.20.19216-en. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature. 2006;442:448–52. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Germann TC, Kadau K, Longini IM, Macken CA. Mitigation strategies for pandemic influenza in the United States. Proc Natl Acad Sci U S A. 2006;103:5935–40. doi: 10.1073/pnas.0601266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jennings LC, Monto AS, Chan PK, Szucs TD, Nicholson KG. Stockpiling prepandemic influenza vaccines: a new cornerstone of pandemic preparedness plans. Lancet Infect Dis. 2008;8:650–8. doi: 10.1016/S1473-3099(08)70232-9. [DOI] [PubMed] [Google Scholar]
- 6.Stephenson I, Gust I, Pervikov Y, Kieny MP. Development of vaccines against influenza H5. Lancet Infect Dis. 2006;6:458–60. doi: 10.1016/S1473-3099(06)70528-X. [DOI] [PubMed] [Google Scholar]
- 7.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated adjuvanted whole-virion influenza A (H5N1) vaccine. N Engl J Med. 2006;354:1345–51. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 8.Leroux-Roels I, Bernhard R, Gerard P, Drame M, Hanon E, Leroux-Roels G. Broad clade 2 cross-reactive immunity induced by an adjuvanted clade 1 rH5N1 pandemic influenza vaccine. PLoS One. 2008;3:e1665. doi: 10.1371/journal.pone.0001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leroux-Roels I, Borkowski A, Vanwolleghem T, et al. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine. Lancet. 2007;370:580–9. doi: 10.1016/S0140-6736(07)61297-5. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz TF, Horacek T, Knuf N, et al. Single dose vaccination with AS03A-adjuvanted H5N1 vaccines in a randomized trial induces strong and broad immune responsiveness to booster vaccination in adults. Vaccine. 2009;27:6284–90. doi: 10.1016/j.vaccine.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 11.Rümke HC, Bayasb JM, de Juanesc JR, et al. Safety and reactogenicity profile of an adjuvanted H5N1 pandemic candidate vaccine in adults within a phase III safety trial. Vaccine. 2008;26:2378–88. doi: 10.1016/j.vaccine.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Antigenic and genetic characteristics of H5N1 viruses and candidate H5N1 vaccine viruses developed for potential use as pre-pandemic vaccines. March 2007. Available at: http://www.who.int/csr/disease/avian_influenza/guidelines/summaryH520070403.pdf. Accessed 22 July 2010. [PubMed] [Google Scholar]
- 13.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of binomial. Biometrika. 1934;26:404–13. [Google Scholar]
- 14.Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods (method 5) Statist Med. 1998;17:873–90. doi: 10.1002/(sici)1097-0258(19980430)17:8<873::aid-sim779>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 15.Longini IM, Jr, Nizam A, Xu S, et al. Containing pandemic influenza at the source. Science. 2005;309:1083–7. doi: 10.1126/science.1115717. [DOI] [PubMed] [Google Scholar]
- 16.Neuzil KM. Pandemic influenza vaccine policy: considering the early evidence. New Engl J Med. 2009;361:e59. doi: 10.1056/NEJMe0908224. [DOI] [PubMed] [Google Scholar]
- 17.Heijmans S, De Meulemeester M, Reynders P, et al. Immunogenicity Profile of a 3.75-microg Hemagglutinin Pandemic rH5N1 Split Virion AS03A-Adjuvanted Vaccine in Elderly Persons: A Randomized Trial. The Journal of Infectious Diseases. 2011;203:1054–62. doi: 10.1093/infdis/jiq174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nolan T, Richmond PC, Formica NT, et al. Safety and immunogenicity of a prototype adjuvanted inactivated split-virus influenza A (H5N1) vaccine in infants and children. Vaccine. 2008;26:6383–91. doi: 10.1016/j.vaccine.2008.08.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.